Abstract
Background
Inflammation is a key biological reaction that comprises a complex network of signals that both initiate and stop the inflammation process.
Purpose
This study targets to evaluate the anti-inflammatory potential of the leaves of the Plectranthus rugosus (P. rugosus) plant involving both in vitro and in vivo measures. The current available drugs exhibit serious side effects. Traditional medicines impart an essential role in drug development. P. rugosus is a plant used in traditional medicine of Tropical Africa, China, and Australia to treat various diseases.
Methods
Lipopolysaccharide (LPS), an endotoxin, kindles macrophages to discharge huge quantities of pro-inflammatory cytokines like TNF-α and IL-6. So, clampdown of macrophage stimulation may have a beneficial potential to treat various inflammatory disorders. The leaves of the P. rugosus are used for swelling purpose by local population; however, its use as an anti-inflammatory agent and associated disorders has no scientific evidence.
Results
The extracts of the plant Plectranthus rugosus ethanolic extract (PREE), Plectranthus rugosus ethyl acetate extract (PREAF), and the compound isolated (oleanolic acid) suppress the pro-inflammatory cytokines (IL-6 and TNF-α) and nitric oxide (NO), confirming its importance in traditional medicine.
Conclusion
The pro-inflammatory cytokines are inhibited by P. rugosus extracts, as well as an isolated compound oleanolic acid without compromising cell viability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation occurs when cells in an organism respond to a threat, such as an infection or tissue injury, by producing inflammatory cytokines. It seeks to get rid of pathogens, pinpoint the damage, and get normal tissue function back (Chen et al. 2018). Even though adaptive immunity may not be directly triggered during inflammation, it is nonetheless predicated on the activation of innate immune mechanisms. In most cases, it progresses swiftly, stays contained, and disappears with the elimination of the causative agents. Inflammation can have beneficial effects in most situations, but in extreme cases (serious infection or trauma, poor regulation), it can have detrimental effects, including death (Barton 2008). Cancer, metabolic syndrome, diabetes, atherosclerosis, and various forms of neurodegeneration are only few of the clinical disorders for which there is mounting evidence that inflammatory processes play a central role (Libby 2002; Karin and Greten 2005; Schett 2006; Affendi Raja Ali and John Egan 2011; Scharl and Rogler 2012; Mir et al. 2021). This emphasizes the need for research into effective anti-inflammatory medicines and the significance of gaining a detailed understanding of the mechanisms underlying inflammation. Two types of inflammation are recognized: acute inflammation and chronic inflammation. The body’s first line of defense against foreign invaders and damaged tissues is acute inflammation, which speed up the delivery of plasma and leukocytes to the infected area. Acute inflammation is characterized by a rapid onset of redness, heat, discomfort, edema, and dysfunction (Charles et al. 2008; Hortelano 2009). In contrast, chronic inflammation develops when the body’s own defenses against infection are unable to restore balance. Continual exposure to chemicals, the persistence of foreign bodies, and repeated attacks of acute inflammation are also major contributors to this condition (Perrone et al. 2016). Furthermore, inflammation can play a supporting role in a wide variety of illnesses. In the case of atherosclerosis, or hardening of the arteries, for instance, plaque formation in the arteries can develop from persistent inflammation of the walls of the blood vessels. There is mounting evidence that chronic inflammation contributes to not only pain but also poor sleep, obesity, physical impairment, and a general decline in quality of life (Cotran 1999).
Macrophages are the body’s first line of defense against bacteria and other pathogens (Dunn et al. 1987). These cells use pattern recognition receptors to identify molecular patterns associated with pathogens. Monocytes and macrophages make up what is called the mononuclear phagocyte system (Geissmann et al. 2010). Miniature white blood cells called monocytes are found in the circulation and are produced from myeloid progenitor cells. Once released into the bloodstream, monocytes undergo a process of differentiation into macrophages, which are bigger and exhibit a greater variety of immunological receptors. In addition to their activities in innate and adaptive immunity, macrophages play numerous other roles in tissue homeostasis, such as the removal of damaged cells and the synthesis of vital enzymes. Recent research has shown that in addition to their pro-inflammatory action, macrophages play a crucial role in the regulation of inflammation (Lawrence and Fong 2010; Fujiwara and Kobayashi 2005). Inflammation is a multifaceted phenomenon affecting the whole organism, yet it has its origins in the inflammatory responses of certain cells. Suppressing the inflammatory activation of macrophages may be an effective treatment strategy against numerous diseases due to their central role in both acute and chronic inflammation. Numerous medications, including non-steroidal anti-inflammatory medicines (NSAIDs), corticosteroids, immunosuppressants, and biologicals, are used to treat inflammatory illnesses. Although effective, toxicities to the digestive tract, the kidneys, and the cardiovascular system are among the most often reported unwanted effects of these medications (Rainsford 1999; Whelton 2000; Rao and Knaus 2008; Sibilia 2003; Barnes 2006; Duncan et al. 2007). These drawbacks of existing anti-inflammatory medications highlight the need for the research and development of new anti-inflammatory medications. Natural products, such as those found in plants, play a preeminent role in the creation of medicines for the treatment of human diseases, and plants are a rich supply of such compounds (Folmer et al. 2008; Newman and Cragg 2007; Shah et al. 2021). Therefore, at the moment, studies are focusing primarily on locating chemicals in plants that can treat various illnesses.
The use of herbs as a therapy modality has a solid basis in herbal medicine. For inflammation, many people turn to herbal remedies, many of which have their origins in traditional Chinese medicine, and Ayurvedic medicine. India has an abundance of plants that can be used to treat various illnesses (Ernst 2000). Ancient Indian medical traditions including Ayurveda, Unani, and Tibbi list more than 2000 plants of therapeutic significance. More than 2000 plant species used for therapeutic purposes have been documented for use in Ayurveda alone, the oldest medical system in India and the Indian subcontinent. Some plants used in Ayurvedic medicine, called Rasayana Drugs, are thought to delay the aging process, enhance life expectancy and improve resistance to disease by strengthening the immune system. Some additional plants, like Ashwagandha, are extensively utilized as a cure for a variety of maladies and employed as ingredients in various formulations tailored for a wide range of musculoskeletal issues, enhancing health and lifespan, and preventing disease in athletes, pregnant women, and the elderly. Similarly, the Siddha and Unani medical traditions both make use of a variety of indigenous Indian herbs (Patwardhan 2005; Mir et al. 2022). Due to a lack of written records and relatively low revenue in these traditions, traditional knowledge systems have begun to fade over time. In recent years, belief in herbal medicine has grown due to its perceived less adverse effects compared to allopathic medication. This has led to a renaissance of interest in medicinal plants (Patwardhan and Gautam 2005). Drugs derived from therapeutic plants are in high demand, with yearly growth rates of 15–25 percent. The World Health Organization predicts that by 2050, the market for such plants would be worth more than $5 trillion. It has been estimated that India’s annual medicinal plant trade is worth around $1 billion (Kala et al. 2004).
Traditional uses of some plants believed to have immunomodulatory or anti-inflammatory properties have been verified through global research efforts. Their potential is being investigated, evaluated, and utilized in new areas, and many have been reassigned to do so. Some instances include the following: Turbinaria ornata is a type of brown algae that is found all over the south and east coasts of India, and it has been found to have strong anti-inflammatory effects in living organisms. Inhibition of nitric oxide and carrageenan-induced paw edema was observed (Ananthi et al. 2010; Mir and Masoodi 2020). Similarly, Zataria multiflora extracts from the plant’s aerial parts have showed anti-inflammatory benefits in animal models of both acute and chronic inflammation (Hosseinzadeh et al. 2000). Saffron, Crocus sativus (Iridaceae), is utilized in many traditional medicines and is cultivated widely from Kashmir to the northern United States, Greece, and Spain. Crocetin and carotenoids, two of the components of saffron stigma, have been linked to their anti-inflammatory properties (Hosseinzadeh and Younesi 2002). The correct screening of biologically active natural products leads to the discovery of several bioactive compounds and medicines (Grabley and Sattler 2003; Lee 2010; Gautam and Jachak 2009; Li and Lou 2018; Nance 2015; Freire and Van Dyke 2013). So, at the moment, scientists can only focus on finding plant-based compounds to treat various illnesses.
In this respect, we have looked into the mechanism behind the anti-inflammatory effects of P. rugosus, which has been reported to have a wide range of therapeutic effects (Ahmad et al. 2014; Khan and Khatoon 2007; Adnan et al. 2012a; Shuaib et al. 2014; Lukhoba et al. 2006; Weyerstahl et al. 1983a; Akhtar et al. 2013). The flowering plant P. rugosus (Lamiaceae) can be found in many different places of the world, but it is most commonly associated with Asia, Tropical Africa, and Australia. Its typical range in India ranges from Kashmir to Garhwal, at an average elevation of 900–2800 m. P. rugosus is a wild plant that stands tall (between 160 and 180 cm) and releases a distinct scent (Shuaib et al. 2015; Akhtar et al. 2013; Sabeen and Ahmad 2009; Tuchscherer et al. 2012; Adnan et al. 2012b). Despite widespread anecdotal reports of success in treating inflammation and associated disorders, there is currently no scientific evidence to support the use of P. rugosus leaves for any of these conditions. The current investigation seeks to assess anti-inflammatory potential by analyzing several in vitro and in vivo parameters. We did a preliminary phytochemical analysis of the ethanolic leaf extract and its components. To the author's knowledge, no one has yet tried to investigate the in vitro and in vivo anti-inflammatory activities of P. rugosus. Taking this into account, as well as the therapeutic value of P. rugosus, the current research intends to investigate the anti-inflammatory properties of both the ethanolic (PREE) and ethyl acetate (PREAF) extracts of the plant, as well as the isolated component (PROA).
Materials and methods
Collection of plant material
The leaf part of P. rugosus was collected from Tangmarg area (Kashmir), India in 2018 and identified at department of taxonomy at Kashmir University with specimen number 2685-(KASH) by Prof. Akhtar H. Malik. For future use, a sample specimen of the obtained material was placed in the herbarium. The aerial parts were instantly shade dried and powdered. 4 kg of the powdered material was extracted with ethanol, filtered with Whatman filter paper, and concentrated in a rotary evaporator. Hexane, ethyl acetate, and n-butanol were used for the ethanolic extract's liquid–liquid extraction (LLE). To find the secondary metabolites, preliminary phytochemical screening was done on the plant material.
Cells and cell culture
In Dulbecco’s Eagle’s medium with fetal bovine serum supplemented at 10% with 1% Streptomycin and Penicillin, respectively. The RAW 264.7 cell line was cultured after being bought from ATCC, USA.
Chemicals used
In the current investigation, all of the chemicals and reagents are of the highest quality. PBS and DMEM were procured from Sigma, UK. Invitrogen (USA) supplied the ELISA kits. LPS from Escherichia coli was purchased from Calbiochem (USA), and fetal bovine serum was purchased from GIBCO (USA). The Griess Reagent and MTT were acquired from respective manufacturers, Promega, and Calbiochem, respectively. In addition, all of the substances utilized in the investigation were of laboratory quality. Chloroform, glacial acetic acid, and ferric chloride were all acquired from the CDH (central drug house). From Merck, we obtained ascorbic acid, methanol, sodium chloride, DPPH, sucrose, and trichloroacetic acid. Hydrochloric acid, peroxide, and potassium dihydrogen phosphate were all acquired from Qualigens. Butylated hydroxytoluene was acquired from Sisco research laboratories (SRL), and ethyl acetate and hexane were purchased from Rankem.
Antioxidant activity
ABTS radical cation scavenging activity
Radical cation decolorization experiment was used to determine whether or not PREAF, PREAE, and compound (PROA were effective in scavenging ABTS (Re et al. 1999). Aliquots (0.1 ml) of PREE, PREAF, and PROA compound at various concentrations (20, 40, 60, 80, and 100 g/ml) were mixed with 2.9 ml of ABTS, incubated at 300 °C for 20 min, using ascorbic acid as a standard, and absorbance was measured at 734 nm. To determine PREE, PREAF, and PROA's reducing potential to the ABTS cation radical, the following formula was utilized:
DPPH activity
PREE, PREAF, and PROA were tested for their ability to scavenge free radicals using the oxidizing agent (DPPH). Different plant extract concentrations (20, 40, 60, 80, and 100) were added to a 3 ml (DPPH) solution, and the absorbance at 520 nm was determined with methanol as a control and ascorbic acid as a reference after 30 min. The following formula was used to determine the percentage of inhibition of free radicals by the extracts and compounds (Braca et al. 2001).
Cell viability by MTT assay
Cell survival was measured using an MTT reduction test. Briefly, 96-well plates were seeded with RAW 264.7 cells at a density of 16000 cells/well, and the plates were placed in a CO2 incubator at 37 °C for 24 h to promote cell adhesion. Once the cells had been incubated for 24 h, they were treated with the extract (0–100 M/ml) and isolated compounds for additional 24 h with LPS. 20 μL of MTT reagent were then added, and the mixture was incubated for an additional four hours at 37 °C, and DMSO 100 µL was applied to each well. Absorbance at 570 nm was determined using a Synergy Mx plate reader. There were three replicates for each treatment (Xu et al. 2014).
Nitric oxide assay
The quantity of nitrite in the supernatant was quantified as an indication of NO generation in RAW 264.7 cells using the Griess reaction. The RAW 264.7 macrophage cells (2 × 105 cells/well) were cultured for 24 h before LPS treatment and then treated with varying doses of extract and compound 1 h before LPS treatment. The use of dexamethasone in varying dosages served as a positive control. The absorbance was checked at 540 nm with the use of a Synergy Mx plate reader. Concentration of nitrite was computed with reference to the standard sodium nitrite concentration curve (NaNo2) (Joo et al. 2014).
NO % inhibition was estimated using this formula.
Cytokines production in RAW 264.7 cells
The ELISA kit was used to measure the PREE, PREAF, and PROA’s ability to suppress cytokine production. RAW 264.7 cells were seeded at a density of 2 × 105 cells/well into a 96-well plate, and the plate was then incubated overnight. Afterward, the cells were treated with PREE, PREAF, and PROA for 1 h before being stimulated with LPS for 24 h to generate inflammation. Once the desired concentration of TNF-α and IL-6 was reached, the culture plate was centrifuged at 1500 rpm to collect the supernatant, which was then tested in accordance with the manufacturer’s (Invitrogen) protocol. The entire study was replicated three times.
Molecular docking analysis
Using the (MOE) 2019.01 docking program, we positioned oleanolic acid and dexamethasone on IL-1β (PDB ID: 3O4O) and TNF-α (PDB ID: 2AZ5). All the cracks in the structures of TNF-α and IL-1β were patched once they were retrieved from Protein database. The partial charges were introduced into the proteins after they had been desiccated and hydrogen substituted. Minimizing their three-dimensional protein structures in MOE-2019.01 with the help of the OPLS force field allowed for the best possible results. Oleanolic acid and dexamethasone’s three-dimensional structure was obtained from the NCBI PubChem database, translated to the mol2 format using Open Babel 2.4.1. Co-crystallization of TNF-α with ligands oleanolic acid and dexamethasone was achieved after protein and ligand synthesis. For IL-1β, the binding site was discovered using the Meta Pocket 2.0 server. Docking of oleanolic acid and dexamethasone at the designated location was performed after scoring function and literature-based confirmation of the binding site. Using RMSD and scoring, we generated 100 solutions for both the TNF-α and IL-1β docking situations and subsequently clustered them. The most populous group was chosen to have a representative member displayed. Oleanolic acid and dexamethasone interact with tumor necrosis factor-α and interleukin-1β, as depicted by the docking position. Cyscore 2.0 was used to determine oleanolic acid’s and dexamethasone's binding affinities with tumor necrosis factor-α and interleukin-1β. Our in vitro results corroborate the findings from docking score and cyscore analysis, showing that oleanolic acid has a higher affinity for TNF-α and IL-1β than dexamethasone.
In vivo anti-inflammatory activity
Animals
In this study, BALB/c mice (female 7–8 weeks old, weight 20–25 g) were used. The protocol for the experiment was approved by the IAEC (Registration No. 801/GO/Re/2003/CPCSEA). The animals were housed in accordance with accepted laboratory practices (12/12 h light and dark cycles) and fed with standard pellet diet, as well as water ad libitum. By higher inhalation dose of diethyl ether, the animals were subjected to euthanasia and finally disposed of by incineration after experimentation.
Animal study design
Healthy BALB/c mice were selected and were acclimatized for a period of 7 days. A total of ten groups were created with each group containing 5 animals. Group I receives only normal saline and serves as a negative control. Group II which is a control group receives LPS (20 µg/mice i.p). Group III receives dexamethasone (10 mg/kg) and serves as a positive control. Group IV receives L-NAME (10 mg/kg). Groups V and VI receive PREE (10 and 30 mg/kg). Groups VII and VIII receive PREAF (10 mg/kg and 30 mg/kg). Groups IX and X receive PROA (10 and 30 mg/kg). Six hours after LPS injection, retro-orbital punctures were used to collect blood samples and centrifuged for 10 min at 5000 rpm at 4 ℃ in order to separate the serum for determination of (NO). The survival state of each group was recorded at different intervals. After experimentation, liver tissue was sliced and washed in saline. Then in 10% formalin, the samples were kept for a period of 24 h. With different concentrations of ethanol (70, 80, 85, 95, and 100%), they were dehydrated and finally embedded in paraffin to obtain a section of 5 µM thickness by using microtome. On glass microscope slides, hematoxylin and eosin (H and E) were used to stain the samples after sectioning. Finally, light microscope under 20X magnification the tissue sections were observed for histopathological changes.
Statistical analysis
All the experiments were conducted in triplicates (both in vitro and in vivo). The histograms were plotted using GraphPad Prism (version 5.01, California Corporation, USA) and one-way analysis of variance (ANOVA) was performed, followed by Tukey’s multiple comparison test. P < 0.05 was considered statistically significant.
Results
Preliminary phytochemical screening
A wide range of different phytochemicals were found in the P. rugosus extracts (Fig. 1) that were studied for their phytochemical potential. As shown in Table 1, the following key components can be found in hexane, ethyl acetate, and n-butanol extracts.
Isolation of compounds from Plectranthus rugosus
The ethyl acetate extract (PREAF, 25 g) was put to chromatography on silica gel (60–120) and run in n-hexane and ethyl acetate (from 0 to 100%). Six fractions (Fig. 2) were collected and were analyzed on TLC with n-hexane–ethyl acetate in altered ratios (7:3, 6:4, 1:1). Fractions which exhibited related TLC profile were combined and were further subjected to Silica gel (230–400) chromatography and eluted with hexane:ethyl acetate. The fraction obtained with hexane:ethyl acetate (6:4) was refined by recrystallization in ethanol to get oleanolic acid (Fig. 3) (C30H48O3), having purity more than 95% as shown in (Fig. 4). ESI–MS m/z 456 [M + H] +:1H NMR (400 MHz, CDCl3, δ, ppm, J/Hz):5.31(H-12), 3.18(H-3), 2.79(H-12), 0.89(H-23), 0.69 (CH3-24), 0.68 (CH3-25), 0.91 (CH3-26), 1.21 (CH3-27),0.89 (CH3-29), 0.88 (CH3-30). 13C NMR (100 MHz, CDCl3, δ, ppm): 184.19 (C-28), 142.59 (C-13), 123.58 (C-12), 78.09 (C-3), 54.19 (C-5), 46.71 (C-9), 45.61 (C-17), 44.06 (C-19), 40.71 (C-14), 41.89 (C-18), 38.09 (C-8), 37.07 (C-1), 38.19 (C-10), 34.90 (C-21), 34.11 (C-29), 33.61 (C-22), 31.61 (C-7), 32.77 (C-20), 29.89 (C-23), 26.07 (C-15), 26.09 (C-2), 24.33 (C-27), 22.91 (C-30), 24.55 (C-16), 24.89 (C-11), 19.22 (C-6), 16.27 (C-26), 16.23 (C-25), 14.83 (C-24).
Antioxidant activity
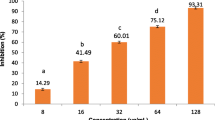
The ABTS radical scavenging activity of PREE, PREAF, and PROA is shown in (Fig. 5a). With respect to standard ascorbic acid (91.02 ± 3.21), the extracts PREE (57.93 ± 3), PREAF (60.99 ± 3.55), and compound PROA (72.13 ± 4) reveal substantial ABTS radical scavenging activity at a concentration of 100 µg/ml. The ethanolic extract (PREE), ethyl acetate extract (PREAF), and isolated compound (PROA) of P. rugosus also showed dose-dependent scavenging of DPPH free radical. It was observed that in ethyl acetate fraction the maximum DPPH scavenging was highest at 87.23 ± 2.5%, followed by ethanolic extract (84.32 ± 1.3%) and the isolated compound (55.43 ± 1.6%) at concentration of 100 µg/ml, whereas ascorbic acid shows percentage inhibition of 94.23 ± 4 at the same concentration as shown in Fig. 5b.
a: ABTS activity by PREE, PREAF, and PROA measured at 517 nm. Each value represents Mean ± SEM. ns (non-significant) P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001. b: DPPH free radical by PREE, PREAF, PROA, and ascorbic acid measured at 517 nm. Each value represents Mean ± SEM. ns (non-significant) P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001
Effect on cell viability
Raw cells were exposed to diverse concentrations (1-100 µM) of PREE, PREAF, and PROA for 48 h. At low concentrations (1–10 µM), cell viability was greater than 80% (Fig. 6). So, to explore the anti-inflammatory activity of PREE, PREAF, and PROA, our data are non-toxic at a (1–10 µM) concentration.
Nitric oxide in RAW 264.7 cells stimulated by LPS
The nitric oxide production was suppressed by extracts (PREE, PREAF) and the isolated compound (PROA) more extensively (Fig. 7). For nitric oxide release, highest inhibition was witnessed in PREE (58.33% ± 3) followed by PROA (55.23% ± 2) and PREAF (51.4% ± 4) at 10 µM.
Determination of pro-inflammatory cytokines production in RAW 264.7 cells
In RAW 264.7 cells, stimulated by LPS, the extracts and the isolated compound were examined for TNF-α and IL-6. From the results (Fig. 8a, b), it is apparent that PROA exhibited highest TNF-α inhibition of 67% at 10 µM concentration. Besides, PROA displayed the highest IL-6 suppression up to 57% at 10 µM concentration. For PREAF, the highest TNF-α and IL-6 inhibition of 51 and 47%, respectively, were observed at 10 µM concentration. For PREE, TNF-α 48% and IL-6 45% were observed at 10 µM concentration (Ammon 2010; Lin and Lin 2010).
a: Suppression of TNF-α production in RAW 264.7 cells. Each value represents Mean ± SEM. ns (non-significant) P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001. b: Suppression of IL-6 production in RAW 264.7 cells. Each value represents Mean ± SEM. ns (non-significant) P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001
Molecular docking
Oleanolic acid and dexamethasone interact with TNF-α and IL-1β, as shown in the docking position (Fig. 9). Cyscore 2.0 was utilized to ascertain the binding affinity of oleanolic acid and dexamethasone with TNF-α and IL-1β (Fig. 10). Oleanolic acid was found to have higher affinity for TNF-α and IL-1 than dexamethasone, as measured by docking score and cyscore analysis (Table 2). This finding was further supported by in vitro data.
Histopathology
Liver hepatocyte architecture was found to be normal upon microscopic inspection in vehicle-treated mice. In LPS-treated animals, the liver showed accumulation of inflammatory cells, infiltration of neutrophils, and bleak necrotic reaction. However, PREE, PREAF, and PROA ameliorate LPS-induced tissue injury particularly neutrophil infiltration, degeneration of parenchymal cells in hepatic lobules, and necrosis in a dose-dependent manner (5 mg, 10 mg, and 30 mg doses (Fig. 11), respectively. All the treated groups (PREE, PREAF, PROA) manifested rejuvenation of hepatocytes, followed by normalization of necrosis process and less infiltration of neutrophils. The hepatoprotective potency of PROA at 30 mg was comparable to that of dexamethasone and L-Nitroarginine methyl ester (L-NAME), respectively.
Histological analysis of liver stained with hematoxylin and eosin (20X). All the treated groups (PREE, PREAF, PROA) manifested rejuvenation of hepatocytes, followed by normalization of necrosis process and less infiltration of neutrophils. A Normal BALB/c mice liver section. B LPS-stimulated BALB/c mice liver section. C Dexamethasone (10 mg/kg) + LPS-stimulated BALB/c mice liver section (D) L-NAME (10 mg/kg) + LPS-stimulated BALB/c mice liver section. E PREE extract (5 mg/kg) + LPS-stimulated BALB/c mice liver section. F PREE extract (10 mg/kg) + LPS-stimulated BALB/c mice liver section. G PREE extract (30 mg/kg) + LPS-stimulated BALB/c mice liver section. H PREAF (5 mg/kg) + LPS-stimulated BALB/c mice liver section (I) PREAF (10 mg/kg) + LPS-stimulated BALB/c mice liver section (J) PREAF (30 mg/kg) + LPS-stimulated BALB/c mice liver section. K PROA (5 mg/kg) + LPS-stimulated BALB/c mice liver section (L) PROA (10 mg/kg) + LPS-stimulated BALB/c mice liver section (M) PROA (30 mg/kg) + LPS-stimulated BALB/c mice liver section
Discussion
Inflammation, a key process in the host defense system, is spatially and temporally restricted by a tightly controlled regulatory mechanism. Rheumatoid arthritis, chronic inflammatory bowel disease, neurological problems, and septic shock syndrome are only few of the illnesses that might result from a lack of control (Nathan and Ding 2010; Stark and Massberg 2021; Hou et al. 2021). The prevalence of inflammatory illnesses is rising in the world's aging populations. Anti-inflammatory medications used in clinical practice have the drawback of unpleasant side effects and high treatment costs (Ho et al. 2018; Moore et al. 2006). To address this issue, researchers are focusing on finding more potent anti-inflammatory drugs with fewer or no negative effects. Multiple target-oriented approaches, such as those used to create herbal medicine or to isolate an active component, are necessary for the successful development of anti-inflammatory medications derived from plant resources (Shaikh et al. 2016; Mahesh et al. 2021). Despite this, choosing the right plant for pharmacological research is a crucial and significant phase in the process. There is also the possibility of creating structural analogs with enhanced pharmacological activity and reduced negative effects by semi-synthesis methods of novel compounds generated by molecular alteration of the functional groups of lead drugs (Wahyuni et al. 2022). Inflammation and the consequences are rapidly becoming one of the humanity's most pressing health concerns on a global scale. It is the most common sickness that affects many different parts of the body. Therapeutic options for persons with inflammation-related diseases have been revolutionized as our understanding of their pathophysiology has grown. The inflammatory response can be slowed down with the help of non-steroidal anti-inflammatory drugs (NSAIDs). Aside from the direct consequences for NSAIDs therapy, a few NSAIDs components cause substantial side effects such as vulnerability to common and opportunistic infection, demyelinating illness, cancer, and blood count fluctuation. There is a pressing need for the rapid development of safer and more effective medications, particularly those of herbal/natural origin, in response to this problem, which carries a high socio-economic cost and no complete treatment with major irreversible bad effects. Therefore, we examined the effects of P. rugosus extracts on LPS-stimulated RAW 264.7 murine macrophages' production of pro-inflammatory mediators in the present study. Because of the plant's potential importance as a medicine for treating a wide range of conditions, this study was carried out (Weyerstahl et al. 1983b; Razdan et al. 1982). P. rugosus has a long history of medicinal usage, including as a remedy for bronchitis, toothaches, wounds, rheumatism, and fever. In addition, there is evidence that it can combat free radicals and slow the aging process in the field of contemporary medicine (Singh et al. 2019; Irshad et al. 2012; Tiwari et al. 2008). However, no attempts have been undertaken to investigate its anti-inflammatory properties. The current study therefore provides with an evaluation of P. rugosus’s anti-inflammatory properties.
The effects of P. rugosus extracts on lipopolysaccharide (LPS)-induced TNF-α and IL-6 production in RAW 264.7 murine macrophages were analyzed. After LPS stimulation, RAW 264.7 cells secrete IL-6 and TNF-α. LPS, a component of the cell wall of Gram-negative bacteria, activates macrophages and monocytes, which play a crucial role in the innate immune response. LPS stimulation of RAW 264.7 cells causes a cascade of intracellular processes that result in the release of cytokines and other inflammatory mediators, which together make up the pro-inflammatory response. Pro-inflammatory cytokines (IL-6 and TNF-α) are downregulated in RAW 264.7 cells after pretreatment with P. rugosus extracts at different doses and followed by a 24 h LPS treatment. Both the extracts and the isolated substance demonstrated significant cytokine inhibition at 10 g/ml. Cells that had not been exposed to LPS had undetectable amounts of IL-6 and TNF-α, making them useful as a control. In addition, the MTT assay demonstrated that the extracts did not have any effect on the viability of RAW 264.7 cells at concentrations up to 10 g/ml. Further, NO generation is inhibited by both the extracts (PREE, PREAF) and the isolated molecule (PROA), which is also generated during inflammation to activate different macrophages. The inhibitory potential of extracts and the isolated molecule was investigated in relation to the gold standard medication dexamethasone. In addition, data analysis using docking scores and cyscores further confirmed that oleanolic acid (PROA) binds to TNF-α and IL-1β with higher affinity than dexamethasone.
Conclusion
Plants provide a rich resource of novel bioactive secondary metabolites, and natural products have long played a major role in the discovery and development of medicines to treat human illnesses. In this regard, we have studied how P. rugosus exerts its anti-inflammatory effects, which has been reported to possess various therapeutic activities. Based on this strategy our research group investigated the anti-inflammatory properties of both the ethanolic (PREE) and ethyl acetate (PREAF) extracts of the P. rugosus, as well as the isolated component (PROA). The pro-inflammatory cytokines (IL-6 and TNF-α) and NO are inhibited by P. rugosus extracts, as well as an isolated chemical without compromising cell viability. These results were also confirmed by docking studies. So, our research demonstrated that P. rugosus leaf extracts have anti-inflammatory properties, supporting its use in traditional medicine. The findings further promote research into the underlying molecular pathways, further supporting the molecular basis of the anti-inflammatory activity. The results provide an ideal natural plant to treat various inflammatory diseases.
Data availability
All data generated or analyzed during this study are included in this article.
References
Adnan M, Begum S, Khan AL, Tareen AM, Lee I-J (2012a) Medicinal plants and their uses in selected temperate zones of Pakistani Hindukush-Himalaya. J Med Plants Res 6:4113–4127
Adnan M, Begum S, Latif A, Tareen AM, Lee L (2012b) Medicinal plants and their uses in selected temperate zones of Pakistani Hindukush-Himalaya. J Med Plants Res 6:4113–4127
Affendi Raja Ali R, John EL (2011) How to manage the risk of colorectal cancer in ulcerative colitis. Curr Drug Targets 12:1424–1432
Ahmad M, Sultana S, Fazl-I-Hadi S, Ben Hadda T, Rashid S, Zafar M, Khan MA, Khan MPZ, Yaseen G (2014) An ethnobotanical study of medicinal plants in high mountainous region of Chail valley (District Swat-Pakistan). J Ethnobiol Ethnomed 10:36
Akhtar N, Rashid A, Murad W, Bergmeier E (2013) Diversity and use of ethno-medicinal plants in the region of Swat, North Pakistan. J Ethnobiol Ethnomed 9:25
Ammon H (2010) Modulation of the immune system by Boswellia serrata extracts and boswellic acids. Phytomedicine 17:862–867
Ananthi S, Raghavendran HRB, Sunil AG, Gayathri V, Ramakrishnan G, Vasanthi HR (2010) In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornata (Marine Brown Alga). Food Chem Toxicol 48:187–192
Barnes PJ (2006) How corticosteroids control inflammation: quintiles prize lecture 2005. Br J Pharmacol 148:245–254
Barton GM (2008) A calculated response: control of inflammation by the innate immune system. J Clin Invest 118:413–420
Braca A, de Tommasi N, di Bari L, Pizza C, Politi M, Morelli I (2001) Antioxidant principles from bauhinia t arapotensis. J Nat Prod 64:892–895
Charles JF, Humphrey MB, Zhao X, Quarles E, Nakamura MC, Aderem A, Seaman WE, Smith KD (2008) The innate immune response to Salmonella enterica serovar Typhimurium by macrophages is dependent on TREM2-DAP12. Infect Immun 76:2439–2447
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L (2018) Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9:7204
Cotran R (1999) Cellular pathology I: cell injury and cell death. In: Robbins pathologic basis of disease, pp 23–25
Duncan FJ, Wulff BC, Tober KL, Ferketich AK, Martin J, Thomas-Ahner JM, Allen SD, Kusewitt DF, Oberyszyn TM, Vanbuskirk AM (2007) Clinically relevant immunosuppressants influence UVB-induced tumor size through effects on inflammation and angiogenesis. Am J Transplant 7:2693–2703
Dunn DL, Barke RA, Ewald DC, Simmons RL (1987) Macrophages and translymphatic absorption represent the first line of host defense of the peritoneal cavity. Arch Surg 122:105–110
Ernst E (2000) Prevalence of use of complementary/alternative medicine: a systematic review. Bullet World Health Organ 78:258–266
Folmer F, Jaspars M, Dicato M, Diederich M (2008) Marine natural products as targeted modulators of the transcription factor NF-κB. Biochem Pharmacol 75:603–617
Freire MO, van Dyke TE (2013) Natural resolution of inflammation. Periodontol 2000(63):149–164
Fujiwara N, Kobayashi K (2005) Macrophages in inflammation. Curr Drug Targets-Inflam Allergy 4:281–286
Gautam R, Jachak SM (2009) Recent developments in anti-inflammatory natural products. Med Res Rev 29:767–820
Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K (2010) Development of monocytes, macrophages, and dendritic cells. Science 327:656–661
Grabley S, Sattler I (2003) Natural products for lead identification: nature is a valuable resource for providing tools. In: Hillisch A, Hilgenfeld R (eds) Modern methods of drug discovery. Springer, Basel
Ho KY, Gwee KA, Cheng YK, Yoon KH, Hee HT, Omar AR (2018) Nonsteroidal anti-inflammatory drugs in chronic pain: implications of new data for clinical practice. J Pain Res. https://doi.org/10.2147/JPR.S168188
Hortelano S (2009) Molecular basis of the anti-inflammatory effects of terpenoids. Inflamm Allergy-Drug Targets 8:28–39
Hosseinzadeh H, Younesi HM (2002) Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol 2:7
Hosseinzadeh H, Ramezani M, Salmani G-A (2000) Antinociceptive, anti-inflammatory and acute toxicity effects of Zataria multiflora Boiss extracts in mice and rats. J Ethnopharmacol 73:379–385
Hou J, Karin M, Sun BJNRCO (2021) Targeting cancer-promoting inflammation—have anti-inflammatory therapies come of age? Nat Rev Clin Oncol 18:261–279
Irshad M, Aziz S, Habib-Ur-Rehman Hussain H (2012) GC-MS analysis and antifungal activity of essential oils of angelica glauca, plectranthus rugosus, and Valeriana wallichii. J Essent Oil Bear Plants 15:15–21
Joo T, Sowndhararajan K, Hong S, Lee J, Park S-Y, Kim S, Jhoo J-W (2014) Inhibition of nitric oxide production in LPS-stimulated RAW 264.7 cells by stem bark of Ulmus pumila L. Saudi J Biol Sci 21:427–435
Kala CP, Farooquee NA, Dhar U (2004) Prioritization of medicinal plants on the basis of available knowledge, existing practices and use value status in Uttaranchal, India. Biodivers Conserv 13:453–469
Karin M, Greten FR (2005) NF-κB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 5:749–759
Khan SW, Khatoon S (2007) Ethnobotanical studies on useful trees and shrubs of Haramosh and Bugrote valleys in Gilgit northern areas of Pakistan. Pak J Bot 39:699–710
Lawrence T, Fong C (2010) The resolution of inflammation: anti-inflammatory roles for NF-κB. Int J Biochem Cell Biol 42:519–523
Lee KH (2010) Discovery and development of natural product-derived chemotherapeutic agents based on a medicinal chemistry approach. J Natl Prod 73:500–516
Li G, Lou HX (2018) Strategies to diversify natural products for drug discovery. Med Res Rev 38:1255–1294
Libby P (2002) Ridker PM, and Maseri A. Inflam Atheroscler Circ 105:1135–1143
Lin W-C, Lin J-Y (2010) Five bitter compounds display different anti-inflammatory effects through modulating cytokine secretion using mouse primary splenocytes in vitro. J Agric Food Chem 59:184–192
Lukhoba CW, Simmonds MS, Paton AJ (2006) Plectranthus: a review of ethnobotanical uses. J Ethnopharmacol 103:1–24
Mahesh G, Anil Kumar K, Reddanna P (2021) Overview on the discovery and development of anti-inflammatory drugs: should the focus be on synthesis or degradation of PGE2? J Inflam Res. https://doi.org/10.2147/JIR.S278514
Mir RH, Masoodi MHJCBC (2020) Anti-inflammatory plant polyphenolics and cellular action mechanisms. Curr Bioactive Compounds 16:809–817
Mir RH, Shah AJ, Mohi-Ud-din R, Pottoo FH, Dar M, Jachak SM, Masoodi MH (2021) Natural anti-inflammatory compounds as drug candidates in Alzheimer’s disease. Curr Med Chem 28:4799–4825
Mir RH, Banday N, Sabreen S, Shah AJ, Jan R, Wani TU, Farooq S, Bhat Z (2022) Resveratrol: a potential drug candidate with multispectrum therapeutic application. Stud Natl Prod Chem 73:99–137
Moore RA, Derry S, Phillips CJ, Mcquay H (2006) Nonsteroidal anti-inflammatory drugs (NSAIDs), cyxlooxygenase-2 selective inhibitors (coxibs) and gastrointestinal harm: review of clinical trials and clinical practice. BMC Musculoskelet Disord 7:1–13
Nance CL (2015) Clinical efficacy trials with natural products and herbal medicines. In: Ramzan I (ed) Phytotherapies: efficacy, safety and regulation. Wiley online library, Hoboken
Nathan C, Ding AJC (2010) Nonresolving inflammation. Cell 140:871–882
Newman DJ, Cragg GM (2007) Natural products as sources of new drugs over the last 25 years. J Nat Prod 70:461–477
Patwardhan B (2005) Ethnopharmacology and drug discovery. J Ethnopharmacol 100:50–52
Patwardhan B, Gautam M (2005) Botanical immunodrugs: scope and opportunities. Drug Discov Today 10:495–502
Perrone S, Lotti F, Geronzi U, Guidoni E, Longini M, Buonocore G (2016) Oxidative stress in cancer-prone genetic diseases in pediatric age: the role of mitochondrial dysfunction. Oxid Med Cell Long. https://doi.org/10.1155/2016/4782426
Rainsford K (1999) Profile and mechanisms of gastrointestinal and other side effects of nonsteroidal anti-inflammatory drugs (NSAIDs). Am J Med 107:27–35
Rao P, Knaus EE (2008) Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J Pharm Pharm Sci 11:81–110s
Razdan T, Kachroo V, Harkar S, Koul G, Dhart KJP (1982) Plectranthoic acid, acetylplectranthoic acid and plectranthadiol, three triterpenoids from Plectranthus rugosus. Phytochemistry 21:409–412
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med 26:1231–1237
Sabeen M, Ahmad SS (2009) Exploring the folk medicinal flora of Abbotabad city, Pakistan. Ethnobotanical Leaflets 2009:1
Scharl M, Rogler G (2012) Inflammatory bowel disease: dysfunction of autophagy? Dig Dis 30:12–19
Schett G (2006) Rheumatoid arthritis: inflammation and bone loss. Wien Med Wochenschr 156:34–41
Shah AJ, Mir RH, Pottoo FH, Masoodi MH, Bhat ZA (2021) Depression: an insight into heterocyclic and cyclic hydrocarbon compounds inspired from natural sources. Curr Neuropharmacol. https://doi.org/10.2174/1570159X19666210426115234
Shaikh RU, Pund MM, Gacche RN, Mediciney C (2016) Evaluation of anti-inflammatory activity of selected medicinal plants used in Indian traditional medication system in vitro as well as in vivo. J Tradit Complement Med 6:355–361
Shuaib M, Khan I, Sharifullah RK, Hashmatullah SM, Naz R (2014) Ethnobotanical studies of spring flora of Dir Lower, Khyber Pakhtunkhwa, Pakistan. Pak J Weed Sci Res 20:37–49
Shuaib M, Khan I, Sharifullah KM (2015) Study of medicinal plants of lower dir, Timergara, Tehsil Balambat, Khyber Paktunkhaw-Pakistan. Am Eurasian J Agric Environ Sci 15:2088–2094
Sibilia J (2003) Corticosteroids and inflammation. Rev Prat 53:495–501
Singh P, Kumar R, Prakash O, Pant AK, Kumar M, Isidorov VA, Szczepaniak LJ. 2019. Chemical composition, anti-inflammatory, analgesic, antipyretic, myorelaxant, antibacterial and antifungal activity of rabdosia rugosus wall.(Syn. Plectranthus rugosus Wall.). 5, 8–15.
Stark K, Massberg S (2021) Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol 18:666–682
Tiwari A, Padalia R, Mathela CJ (2008) Sesquiterpene rich essential oil from plectranthus rugosus wall. J Essent Oil BearPlants 11:58–61
Tuchscherer M, Otten W, Kanitz E, Gräbner M, Tuchscherer A, Bellmann O, Rehfeldt C, Metges CC (2012) Effects of inadequate maternal dietary protein: carbohydrate ratios during pregnancy on offspring immunity in pigs. BMC Vet Res 8:232
Wahyuni IS, Sufiawati I, Nittayananta W, Levita J (2022) Anti-inflammatory activity and wound healing effect of Kaempferia galanga L. Rhizome on the chemical-induced oral mucosal ulcer in wistar rats. J Inflam Res. https://doi.org/10.2147/JIR.S359042
Weyerstahl P, Kaul V, Meier N, Weirauch M, Marschall H (1983a) Volatile constituents of Plectranthus rugosus leaf oil. Planta Med 48:99–102
Weyerstahl P, Kaul V, Meier N, Weirauch M, Marschall HJPM (1983b) Volatile constituents of Plectranthus rugosus leaf oil. J Essential Oil Bear Plants 48:99–102
Whelton A (2000) Renal and related cardiovascular effects of conventional and COX-2-specific NSAIDs and non-NSAID analgesics. Am J Therap 7:63–74
Xu X, Yin P, Wan C, Chong X, Liu M, Cheng P, Chen J, Liu F, Xu J (2014) Punicalagin inhibits inflammation in LPS-induced RAW264. 7 macrophages via the suppression of TLR4-mediated MAPKs and NF-κB activation. Inflammation 37:956–965
Acknowledgements
Lamya Ahmed Al-Keridis extends her appreciation to Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2024R82), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Funding
This work is supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2024R82), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
RHM and MHM contributed to study concept and design, collected/analyzed data, performed project administration, and drafted the original manuscript. RM-ud-d, LAAl-K, BA, NA, MP, and MA contributed to methodology, data curation, investigation, visualization, analysis, review, and editing; RHM, MA, MHM, and RM-ud-d contributed to critical revision of the manuscript, methodology, validation, formal analysis, and study supervision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
The protocol for the experiment was approved by the Institutional Animal ethics committee (Registration No. 801/GO/Re/2003/CPCSEA), University of Kashmir, India.
Informed consent statement
Not applicable.
Conflicts of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mir, R.H., Mohi-ud-din, R., Al-Keridis, L.A. et al. Phytochemical profiling, antioxidant, cytotoxic, and anti-inflammatory activities of Plectranthus rugosus extract and fractions: in vitro, in vivo, and in silico approaches. Inflammopharmacol 32, 1593–1606 (2024). https://doi.org/10.1007/s10787-023-01419-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-023-01419-2