Abstract
Curcuma longa extract and its marker curcuminoids have potential use in inflammatory conditions. However, curcuminoids solubility and bioavailability are major hindrances to their bioactivity. The current study investigated green extraction-based curcuminoids-enriched extract (CRE) prepared from C. longa and its cyclodextrin inclusion complexes, i.e., binary inclusion complexes (BC) and ternary inclusion complexes (TC), in complete Freund’s adjuvant (CFA)-induced mice for their comparative anti-arthritic efficacy. CRE, BC, and TC (2.5 and 5 mg/kg) with the standard drug diclofenac sodium (13.5 mg/kg) were orally administered to mice for 4 weeks. Variations in body weight, hematological and biochemical parameters, along with gene expression analysis of arthritis biomarkers, were studied in animals. The histopathological analysis and radiographic examination of joints were also performed. CRE, BC and TC treatment significantly restored the arthritic index, histopathology and body weight changes. The concentration of C-reactive protein, rheumatoid factor and other liver function parameters were significantly recovered by curcuminoids formulations. The pro-inflammatory cytokines (NF-κB, COX-2, IL-6, IL-1β, and TNF-α) gene expression was considerably (p < 0.001) downregulated, while on the other side, the anti-inflammatory genes IL-4 and IL-10 were upregulated by the use of CRE and its complexes. The concentration of antioxidant enzymes was considerably (P < 0.001) recovered by CRE, BC and TC with marked decrease in lipid peroxidation, erosion of bone, inflammation of joints and pannus formation in comparison to arthritic control animals. Therefore, it is concluded that green CRE and its cyclodextrin formulations with enhanced solubility could be considered as an applicable therapeutic choice to treat chronic polyarthritis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation or degeneration of joints is the hallmark of arthritis, a disease that affects joints (Smolen et al. 2023). This disorder has a dynamic epidemiology and background all over the world. There are several types of osteoarthritis each with distinct characteristics and affected joints (Jang et al. 2022). The progression and severity of osteoarthritis can vary widely from person to person (Li et al. 2023). The development of osteoarthritis is influenced by factors such as genetics, age, joint use, and other underlying health conditions. This disorder is more prevalent in women than in men (Sanchez-Lopez et al. 2022). Approximately, 54 million adults and 300,000 offspring have arthritis, but the exact numbers are not known because several people with the disease do not take the treatment until their condition becomes severe (Allen et al. 2022). In the US, 8 million adults complain their limited ability to work is due to arthritis; they may have difficulty in walking from a parking deck to their workplace or climbing stairs (Kazeminasab et al. 2022). In the US, 1 in 4 children has this disease (Ardalan et al. 2022). According to the National Center for Chronic Disease Prevention and Health Promotion, a number of guidelines and approaches can be used to cure arthritis through active lifestyles, weight management, joint care and protection, consultation with specialists, and participation in self-management training programs (Hawk et al. 2012). Indications of joint inflammation are aching, pain, swelling, and stiffness in or around the joints.
This condition is symptomatically treated with nonsteroidal anti-inflammatory drugs that relieve inflammation and reduce pain, but also raise the risk of adverse effects such as hypertension, stroke, heart attack, and trigger stomach irritation (Gu et al. 2017). Current medications like corticosteroids reduce pain, inflammation, and reduce the joint damage; however, these medications are also associated with adverse effects such as thinning of bones, weight gain, diabetes, and the risk of infections (Ebada et al. 2022). Therefore, a current necessary clinical need is for safe alternative therapy options and natural treatment methods. Despite the advancements in a medical field, the necessity for plant-based treatments is growing worldwide. The majority of plants displayed impressive phytochemical components that are unique, exceptional source of efficient, and secure anti-arthritic agents (Farooq et al. 2022).
Turmeric is conventionally used as a regular food in developing countries, holding remarkable therapeutic potential against lethal diseases (Tsuda 2018). Turmeric has different organic effects including antitumor, antimicrobial, anti-inflammatory, and anti-hyperlipidemic activity (Gul and Bakht 2015; Farjam et al. 2014; Mehrabani et al. 2015). Curcumin (as the very unique component of turmeric) is an amazing typical therapeutic agent against inflammation, making it possible to be used as a protective agent for the development of novel anti-arthritic and anti-inflammatory agents (Babu and Srinivasan 1997; Sharma et al. 2005). It has a prolonged practice history in conventional medicines and is used to cure inflammatory disorders. Curcumins modulated the pro-inflammatory marker and the signaling pathways. Its therapeutic efficacy is reduced due to its weak bioavailability, and it has lower water miscibility in acidic and neutral mediums. Pharmacokinetic studies of curcumin show that it has rapid metabolism and low intestinal absorption, which limits its efficacy. The curcuminoids-rich extract (CRE) was prepared by the green extraction method (88% curcuminoids contents). In order to enhance curcuminoids’ therapeutic efficacy and solubility, CRE, hydroxypropyl-cyclodextrin (binary) and CRE, hydroxypropyl-cyclodextrin, and polyvinylpyrrolidone K30 (ternary) inclusion complexes of cyclodextrin (CD) were developed (Gould et al. 2005; Lateh et al. 2019b).
These curcuminoids-rich extract and its binary and ternary inclusion complexes exhibited remarkable pharmacological activities like antioxidant, anti-inflammatory, and anticancer (Lateh et al. 2022). Sani et al. (2021) reported the therapeutic potential of these curcuminoids formulations in muscle atrophy through downregulation of Atrogin-1 expression and MuRF-1 retarding protein degradation and upregulation of protein synthesis through Phospho-Akt pathway stimulation. Pengjam et al. (2021a) reported the inhibitory effect of curcuminoids ternary inclusion complexes on RANKL-induced osteoclastogenesis in RAW 264.7 cells. These complexes retarded the production of multinucleated osteoclasts, and raised the F4/80 contents, and suppressed the linked pathways of NF-κB and ERK components. Pengjam et al. (2021b) also reported the inhibitory effect of water-soluble curcuminoids ternary inclusion complexes on osteoclastogenesis through downregulation in mRNA expression of miR-21 through modulation of NFκB pathway. However, due to robust antioxidant and anti-inflammatory pursuits, in the haloperidol-induced Parkinson’s disease rat model, administration of these curcuminoid formulations markedly mitigated the catalepsy, cognitive decline, akinesia and also recovered the motor functions in other neurodegenerative disorders (Saleem et al. 2023). Therefore, in the light of reported work and due to curcuminoids formulations have remarkable effectiveness in osteoporosis, muscular atrophy, and movement disorders, this study was designed to investigate the therapeutic effect of curcuminoids and their formulations in complete Freund adjuvant (CFA)-induced rheumatoid arthritis (RA) in rodent model.
Materials and methods
CRE and cyclodextrin complexes preparation
The curcuminoid extracts, including CRE, BC, and TC, used in this study were previously prepared at Phytomedicines and Pharmaceutical Biotechnology Excellence Centre, Faculty of Pharmaceutical Sciences, Prince of Songkla University, Thailand (Lateh 2018). CRE extract was prepared using green solvent ethanol by microwave-assisted extraction technique, and semi-purified after treatment with Diaion HP-20 bio-degradable resin, and eluted by hydro-ethanol (Lateh et al. 2019a). Curcuminoids binary complex was prepared using CRE and hydroxypropyl-β-cyclodextrin and ternary complex was prepared by mixing CRE, hydroxypropyl-β-cyclodextrin and polyvinyl pyrrolidine K30 to increase the solubility, bioavailability, and ultimately therapeutic efficacy. Based on HPLC analysis, the curcuminoids content of CRE (88%), BC (27%), and TC (14%) was estimated (Lateh et al. 2022).
Animal husbandry
Healthy mice were kept in the animal house at Government College University in Faisalabad, Pakistan. The laboratory was set up at the recommended conditions of 25 ± 2 °C and 60% relative humidity. The mice were kept in cages made of polypropylene, provided free access of diet and water with 12 h dark/light cycles. The Institutional Review Board of Government College University in Faisalabad approved the recommendation of the Ethical Committee with Reference No. GCUF/ERC/106. The animals were acclimated for 2 weeks before the research began (Prager et al., 2011).
Experimental design
Nine groups of five healthy mice, each weighing 27–30 g was randomly assigned (n = 9). Mice outside the control group were given an intradermal 100 µL CFA on day 0 to cause polyarthritis. Vernier calipers and an electrical weighing balance were used to measure the animal’s body weight and paw diameter prior to the CFA injection. Oral treatment with an extract and its formulation rich in curcuminoids began after 1 week of CFA administration and continued for 4 weeks.
Healthy animals were kept in the control group and received only distilled water (Arham Shabbir et al. 2016). Animals in the arthritic control group received intradermal CFA injection on day 0 only. Arthritic animals in the standard group received diclofenac sodium 13.5 mg/kg/daily. Curcuminoids treatment to six groups of arthritic animals was given as 2.5 and 5 mg/kg of each formulation CRE, BC, and TC.
Arthritis scoring
Before starting the study, a Vernier caliper was used to measure the thickness of the animals’ paws. Similarly, their weights were recorded before applying the CFA injection. Paw edema was assessed on days 7, 14, 21, and 28 after CFA infusion to induce acute inflammation. Each treated group’s percentage inhibition of paw edema was calculated.
According to the arthritic scoring system, paw erythema and edema were scored from 0 to 4, and the arthritic index was calculated as follows: (Qing Zhang et al. 2018)
0 = no inflammation and swelling.
1 = toe joint inflammation.
2 = inflammation of toe joints and toes.
3 = ankle joint inflammation.
4 = inflammation of the entire paw.
Hematological and biochemical markers evaluation
After a cardiac puncture, blood samples were taken on the 29th day after anesthesia before the animals were killed. Using a widely available kit, the levels of hematologic parameters, including the total blood count, hemoglobin, and erythrocyte sedimentation rate, were estimated (Elabsciences). Serum samples were collected to investigate the level of C-reactive proteins, alkaline phosphate, rheumatoid factor, liver, and kidney function tests using commercial kit according to the manufacturer’s protocol by a biochemical analyser (Microlab 300®, Germany).
Histopathological assessment
10% formalin solution was used to preserve the isolated ankle and right rear paw joints. Treatment with 10% EDTA for 1 month helped to decalcify the joints. Joint sections were examined under a microscope for histopathological alterations following hematoxylin and eosin staining. The appearances of pannus, bone erosion, and inflammation were looked for on the slides 10 X magnification (Shabbir et al. 2018).
Radiographic assessment
After the completion of 28 days, animals were killed under anesthesia, and hind limbs were isolated for radiographic analysis (Uttra and Hasan 2017).
Estimation of pro-inflammatory cytokines by ELISA
Using commercial kits (Elabsciences Biotechnology® China, IL-6 (Catalog No: E-EL-R0015), TNF-α (Catalog No: E-EL-R0019), serum samples were analyzed to determine the presence of pro-inflammatory biomarkers TNF-α and IL-6 in accordance with vendor procedure.
Estimation of real-time quantitative polymerase chain reaction (RT-PCR)
EDTA-containing tubes were used to gather blood samples for the quantitative estimation of arthritis biomarkers. Blood was treated with RBC's lysis buffer before RNA was removed using the Trizol technique. Nanodrop was used to measure the extracted RNA yield. The manufacturer’s instructions were followed when using the Revert Aid cDNA Synthesis Kit to convert RNA to cDNA. For analyzing gene expression, real-time PCR equipment (the Bio-Rad® system) was employed, and 40 successions of quantitative thermal cycling were run. As a maintenance or internal control gene, glyceraldehyde-3-phosphate dehydrogenase was used. A list of genes used with expected product length is presented in Table 1. The threshold cycle (CT) method was used by Realplex software to estimate the relative quantification of reference and target genes. By the Livak method, fold changes were calculated (Ammara Saleem et al. 2020).
Tissue homogenate preparation
Using a tissue homogenizer, liver homogenates (10%) were made in a solution of phosphate buffer, which has a 7.4 pH. A sterile tube was taken to isolate the supernatant layer following centrifugation at 3000 rpm for 20 min at 4 0C (Akhtar et al. 2016b, 2016a).
Appraisal of superoxide dismutase level
The superoxide dismutase activity was determined after taking 0.1 mL of tissue homogenate and combining it with 1.2 mL of 0.05 M sodium phosphate solution at a 7.4 pH. Then, this combination was mixed with phenazine methosulphate (0.1 mL) and nitro blue tetrazolium (0.3 mL) and incubated for 90 min at 30°C. After vigorous stirring, 0.4 mL of n-butanol and glacial acetic acid (0.1 mL) were combined. After centrifugation, the absorbance at 560 nm of the supernatant layer was determined, and the concentration of protein was noted as units/mg (Mir et al. 2019).
Catalase level
Phosphate buffer (1.95 mL) and 30 mM hydrogen peroxide (1 mL) were mixed with 0.05 mL of tissue homogenate to find out the catalase level. At 240 nm, the optical density was recorded, and the following formula was applied (Bhangale and Acharya 2016):
Catalase level = OD/E × sample (mL) × protein (mg).
Malondialdehyde level
The determination of lipid peroxidation level in brain homogenate was carried out by measuring the level of malondialdehyde. Tissue homogenate (0.2 mL) was added to 200 μl of sodium dodecyl sulfate, acetic acid, (1.5 mL) and 1.5 mL of thiobarbituric acid. This mixture was mixed with 4 mL purified water and warmed up to 90°C in a water bath for about 1 h. After cooling, 5 mL n-butanol and 1 mL of distilled water were added until a yellow color appeared. For 10 min, centrifugation of the mixture was performed at 4000 rpm. The supernatant was separated and the value of absorbance was noted at 532 nm and the MDA level was expressed as μg/mg protein (Saleem et al. 2014).
The concentration of MDA is measured using the formula:
Here, Vt is the mixture volume, 1.56 × 105 = molar extinction coefficient, and W is the weight of test compound.
Statistical data study
GraphPad Prism version 5 was applied to analyze the data, and the final values were represented in terms of mean ± SEM. One-way ANOVA tailed by the Tukey test was applied for statistical analysis.
Results
Measurement of paw inflammation
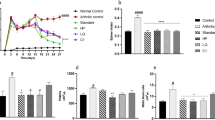
All treatment groups showed obvious swelling symptoms after 1 week of CFA, excluding normal control animals. The considerable increase (p < 0.001) in paw diameter of arthritic control group was noted after 1 week, and it continued till the last day of the study as compared to animals of normal control. The 13th day of the trial revealed excessive inflammation. Animals treated with CRE, and its CD inclusion complexes expressively (P < 0.001) decreased inflammation in contrast to arthritic rodents (Fig. 1). Ternary cyclodextrin inclusion complexes at a dose 2.5 mg/kg markedly decreased the paw inflammation compared to all other treatment groups (Table 2).
Body weight determination
With the progression of arthritis, the body weight of animals was markedly decreased. A momentous (p < 0.001) reduction in weight after 1 week until last day of the study was observed in all arthritic animals, CRE, and complexes treated animal, while the treatment groups and the standard group showed dose-dependent considerable improvement in rebuilt the weight during the last week of the study (Table 3).
Arthritic scoring
No swelling or inflammation was observed in the control group, but the arthritic index increased continuously in the disease group. CRE and its CD inclusion complexes and diclofenac sodium markedly decreased arthritic index values from the 16th day to the end of the study as compared to animals in the disease group. The maximum arthritic index was seen at the end of the study on the 28th day in disease group animals. Figure 2 displays the effects of test compounds on the paw diameter of mice paws before slaughtering.
Histopathological assessment
Histopathological examination revealed joint degenerative hallmarks as the articular cartilage changes, formation of subchondral bone cysts, synovial hyperplasia, sclerosis, osteophyte formation, ligament hypertrophy and chondrocyte cluster in arthritic control group (Fig. 3).
However, treatment with curcuminoid rich extract, binary and ternary cyclodextrin inclusion complexes dose dependently (5 mg/kg ˃ 2.5 mg/kg) mitigated the pathological changes: bone erosion, hypergenesis of synovial space, pannus development, and indefinite migration of inflammatory cells similar to diclofenac sodium-treated animals (Fig. 4).
Histopathological examination of ankle joints. A Control; B disease control; C diclofenac sodium treated; D and E CRE 2.5 mg/kg and 5 mg/kg, F and G BC 2.5 mg/kg and 5 mg/kg; H and I TC 2.5 mg/kg and 5 mg/kg. a: Synovial hyperplasia, b: sclerosis, c: aggregation of chondrocytes, d: calcification, e: infiltration of inflammatory mediators and scale bar of 100 μm
Radiographic image analysis
Radiographic image analysis revealed the acute soft tissue swelling, hardness of connective tissue, obvious joint space shrinkage, critical bone erosion, joint resorption, and joint deformity symptoms in arthritic mice compared to healthy control animals. Diclofenac sodium-treated animals showed the recovery from all symptoms such as inflammation of soft tissues, bone erosion, no joint spacing, and marked development of the muscles across the joints. Animals taking the standard drug exhibited joint gap narrowing, marked connective tissue development, erosion, and resorption of bones. Figure 5 shows dose-dependent recovery with CRE, BC, and TC recover the pathological features similar to those of healthy animals and diclofenac-treated groups.
Hematologic and biochemical examination
A cardiac puncture was used to collect blood samples for hematologic and biochemical analyses. We used manufacturer’s protocols and an automated biochemistry analyser to analyze C-reactive protein, rheumatoid factor, alkaline transaminases, alkaline phosphatases, alanine aminotransferases, creatinine, CBC, and urea levels. The CFA injection considerably (P < 0.001) decreased the hemoglobin and RBCs count (Figs. 6 and 7) in arthritic rodents in contrast to the animals in the control group. Healthy animals in a control group as compared to arthritic animals had significantly (p < 0.001) higher concentrations of CRP, WBCs, ALT, RF, AST, and ALP (Fig. 8). Anyhow, test formulations significantly ameliorated the abnormal changes in hematologic and biochemical markers dose dependently. Furthermore, arthritic control animals that received CFA injections had significantly higher levels of blood urea nitrogen and creatinine than those that received other treatments.
Effects of curcuminoids-rich extract CRE, cyclodextrin inclusion complexes BC and TC on hemoglobin level, RBCs, WBCs, AST ALT, RA factor, CRP, and ALP level. Data are represented as mean ± SEM, *** (P < 0.001), ** (P < 0.01), and * (P < 0.05) in contrast to arthritic control, ^^^ (P < 0.001), ^^ (P < 0.01), and ^ (P < 0.05) compared to healthy animals
Effects of curcuminoids-rich extract (CRE) and its formulations on tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) inflammatory biomarkers. Data are shown as mean ± SEM, *** (P < 0.001), ** (P < 0.01), and *(P < 0.05) compared to arthritic control, ^^^ (P < 0.001), ^^ (P < 0.01), and ^ (P < 0.05) in contrast to the control group
Effects of curcuminoids-rich extract (CRE) and its formulations on mRNA expression of arthritic biomarkers. Data are represented in the form of mean ± SEM, *** (P< 0.001), ** (P < 0.01), and *(P < 0.05) as compared to the arthritic control, ^^^ (P < 0.001), ^^ (P < 0.01), and ^ (P < 0.05) control group
Identifying first-line antioxidant biomarkers
In the CFA injection model, first-line antioxidant enzymes decreased, an indicative of oxidative stress. As compared to control animals, arthritic control animals had considerably (p < 0.001) lower levels of superoxide dismutase (SOD) and catalase (CAT). Due to oxidative stress, the concentration of malondialdehyde (MDA) significantly increased in arthritic animals compared to all other treatment groups. Treatment with CRE, BC, and TC cyclodextrin inclusion complexes notably (p < 0.001) decreased the level of lipid peroxidation and MDA level in contrast to the disease control group, but was similar to diclofenac-treated animals’ dose dependently (Table 4).
Estimation of inflammatory biomarkers
The serum level of pro-inflammatory cytokines TNF-α and IL-6 was significantly raised in arthritic control animals compared to control group. However, treatment with curcumminoid rich extract and formulations markedly decreased the raised level of inflammatory cytokines compared to arthritic control group.
Measurement of gene expression level by QRT-PCR
After treatment with RBC lysis buffer, WBC pellets were isolated from whole blood samples, and mRNA expression of arthritis biomarkers was estimated. The mRNA expression of inflammatory biomarkers, IL-beta, TNF-α, NF-κB, IL-6, and COX-2, was markedly (p < 0.001) upregulated in rodents with disease as compared to healthy animals, while the expression of anti-inflammatory biomarkers such as IL-4 and IL-10 was considerably downregulated in arthritic control group in contrast to other treatment groups. However, treatment with CRE, BC, and TC significantly recovered the raised levels of arthritis biomarkers in all treatment groups compared to arthritic group and approximated to standard drug-treated and healthy animals in the control group (Fig. 8).
Discussion
Turmeric oriented curcuminoids-rich extract, binary and ternary cyclodextrin inclusion complexes were prepared by green solvent microwave extraction to increase water solubility, bioavailability, and therapeutic effectiveness. These curcuminoids formulations exhibited multiple pharmacological activities such as antioxidant, anti-inflammatory, anticancer, and many other medicinal activities (Ahmad et al. 2020; Pengjam et al. 2021a, 2021c). Curcuminoids are associated with robust anti-inflammatory, antioxidant, and antitumor activities (Hay et al. 2019). By keeping in view the promising health beneficial effects of curcuminoids, the current study as a pilot effort was designed to assess the efficacy of curcuminoids formulation in RA. RA is a medical problem characterized by the production of autoantibodies, systemic inflammation, pannus development, chronic arthromeningitis, and extreme injury to cartilage and bone (McInnes and Schett 2011). This disease may cause joint dysfunction, pulmonary and cardiovascular diseases. In the pathogenesis of RA, a series of complex interactions are found between the environment and genes. Most genetic factors are involved in the pathogenesis of RA (Gibofsky 2012). Environmental factors include smoking, alcohol intake, poor education, more manual or laborious occupations, increased birth weight, and low or lack of breastfeeding after birth (Harris Jr 1990).
The current therapeutic options include NSAIDS, disease-modifying anti-rheumatic drugs, and corticosteroids, all of which have serious side effects and poor compliance from patients (Gaffo et al. 2006). Therefore, the development of novel and effective therapeutic strategies of natural origin to treat RA is an unfulfilled instant clinical need. Most of the remarkable therapeutic effects of curcumin are reported, but their role in the mitigation of arthritis is the least reported (Lestari and Indrayanto 2014).
CRE was prepared by green extraction and enriched with macroporous resin (Diaion® HP-20) column, containing 89% w/w curcuminoids. Binary and ternary inclusion complexes were prepared by solvent extraction method and characterized. The water solubility of curcuminoids was increased to 70.3 µg/mL, and therefore, have more therapeutic efficacy compared to rich extract against cancer cell lines (Lateh et al. 2022). These curcuminoids formulations exhibit robust anticancer activity equal to marker curcuminoids compounds, and have the nutraceutical industrial advantage of being low cost, simple, and environmental friendly (Lateh et al. 2019a). The CRE binary inclusion complexes exhibited sufficient water solubility to have the therapeutic applications in the treatment of osteoporosis.
Due to the phenolic compounds present in C. longa, it has anti-inflammatory and anti-arthritic effects. As a result of curcumin’s ability to inhibit pro-inflammatory biomarkers such as IL-6 and TNF-a, as well as delay the activation of NF-kB and TNF-a, pain is lessened. Curcuminoids can reduce the body’s amount of C-reactive protein and slow down the production of IL-6 and NO (Saleem et al. 2023; Paliwal and Paliwal). The CRE binary inclusion complexes exhibiting sufficient water solubility and have therapeutic applications in the treatment of osteoporosis. These formulations at a dose of 5 mg/mL raised the activity of alkaline phosphatase in MC3T3-E1 and C3H10T1/2 cells. Curcuminoids formulations upregulated the mRNA and protein expression of Bmp-2, Runx2, and Collagen 1a dose dependently. Therefore, it is helpful to treat osteoporosis through modulation of Wnt/b-catenin and BMP signaling pathways (Pengjam et al. 2021c). It is reported that arthritis pathology starts when protein denaturation occurs at the CFA administration site, which causes auto-antigens that induce arthritic symptoms such as inflammation, joint pain, and paw edema (Harris Jr 1990). The CFA injection causes two stages of arthritis: the induction phase and the chronic phase. The first phase continues for only 10 days, during which the immune system secretes serotonin, histamine, and prostaglandin G without any indication of synovial inflammation. The second phase can continue for 11–28-day period, where pro-inflammatory mediators showed the interruption and abstraction in normal physiology that results in bone erosion, synovial inflammation, and excessive growth of cells (Prabhu et al. 2011). A reduction in paw inflammation and diameter due to a decline in liberation and production of inflammatory cytokines is the desired action of anti-inflammatory medications. Similar to previously reported anti-inflammatory activity of CRE and inclusion complexes, current findings also augmented the supressant effect on inflammation through modulation of cytokines mediators. During the process of inflammation, macrophages and neutrophils are produced by the lysosomal components that are capable of killing the neighboring tissues and cells. Medications that have the anti-inflammatory properties prevent the process of inflammation by inhibiting the lysosomal enzymes and stabilize the membrane.
Findings from current work elaborated that CRE and its CD inclusion complex have significant dose-dependent anti-arthritic potential. Ternary inclusion complexes (TC) were found to be more effective against arthritic hallmarks compared to CRE and BC complexes.
In an in vitro human red blood cell assay, the membrane stabilizing action of CRE and its CD inclusion complex against membrane destructive aspects was reported (Oyeleke et al. 2018). Current findings annul the anti-inflammatory effect of CRE and inclusion complexes through reduction in paw inflammation and paw diameter dose dependent.
Oxidative stress subsidizes the pathogenesis of arthritis, cancer, and neurodegenerative disorders (Khojah et al. 2016). Therefore, compounds like curcuminoids exhibiting robust antioxidant potential can be considered reasonable therapeutic choices to treat arthritis (Adjimani and Asare 2015). CRE and its formulations have beneficial effects on the body weight of arthritic animals, and through their suppression of bone and cartilage deformation in the arthritis model caused by CFA were obvious. CRE, BC, and TC dose dependently through their anti-inflammatory action reduced the paw inflammation, arthritic index, and paw edema. The oxidative stress induced by CFA injection was also mitigated by curcuminoids formulation administration to arthritic animals. A reduction in weight is associated with the progression of rheumatoid arthritis (Thite et al. 2014). CRE and other cyclodextrin complex formulations rebuilt the body weight of animals and reduced the paw inflammation and arthritic index in treatment groups compared to arthritic animals.
One of the key features of RA is a decreased level of hemoglobin and erythrocytes as in anemia, while WBC’s count is increased in rheumatoid arthritis (Quintão et al. 2012). The hematological testing showed that CRE and its CD inclusion complex appreciably restored levels of hemoglobin, RBC’s and WBC’s when compared to arthritic control group.
Liver damage is also related to arthritis, and higher level of ALP is a sign of osteoporosis and bone erosion. From the cells of impaired organs, AST was released which showed increased levels of AST. An incredible decrease in the ALP, AST, and ALT was observed after treatment with curcuminoids formulations, manifesting the hepato-protective, anti-inflammatory, and anti-arthritic activity of CRE and its CD inclusion complex (Rajkapoor et al. 2009). Arthritic score decreased after treatment with CRE and inclusion complexes. Advancement in disease stage and systemic inflammation are assessed by measurement of ESR rate, rheumatoid factor, and C-reactive protein values in serum (Arntz et al. 2018). Levels of these above mentioned markers were markedly raised in arthritic control group, however, CRE, BC, and TC recovered the level of these markers (Mbiantcha et al. 2017). Downregulation of mediators of pro-inflammation and decreased liberation at the site of injury prevented the destruction of joints (Voon et al. 2017). CRE and its formulations showed downregulation of many pro-inflammatory genes. Similar to previous studies that predicted the anti-arthritic activity of catechin and quercetin, these curcuminoids formulations also showed a notable anti-inflammatory and anti-arthritic effect (Abd Rani et al. 2018; Lee et al. 2021).
Curcuminoids-rich extracts, binary and ternary inclusion complexes through their modulatory effect on antioxidant pathways mitigated the oxidative stress-induced neurodegenerative disorders in Alzheimer’s disease (AD) and Parkinson’s disease (PD) disease models. These formulations downregulated the mRNA expression of neuroinflammatory markers such as IL-10, IL-1α, IL-1β, TNF-α, alpha-synuclein, and β-secretase (Anam Shabbir et al. 2022; Uzma Saleem et al. 2023). Ternary inclusion complexes of curcuminoids in liposomes formulation at dose of 20 μg/ml markedly inhibited the formation of osteoclasts RAW 264.7 cells induced by NF-κB receptor activator, through increasing F4/80 content, Tartrate-Resistant Acid Phosphatase Expression (TRAP) contents along with cathepsin K. curcuminoids treatment is effective to treat osteoporosis via suppression of NF-κB-p65, ERK, phospho-NF-κB-p65, and phospho-ERK pathways (Pengjam et al. 2021a). These formulations downregulated the mRNA expression of the miR-21 gene through modulation of NFκB-Akt-miR-21 pathway: de novo mechanism (Pengjam et al. 2021b).
The gene expression of anti-inflammatory and pro-inflammatory cytokines with transcription factors TNF-α, NF-κB, IL-4, COX-2, IL-10, IL-β and IL-6 was performed to perceive the moderating effect of CRE and its CD inclusion complex. CRE, BC, and TC markedly recover the raised mRNA of arthritis biomarkers. Therefore, these curcuminoids formulations can be considered promising anti-arthritic therapeutic choices with maximum bioavailability at a low cost.
The current study demonstrates promising findings regarding the potential benefits of C. longa extract and its curcuminoids inclusion complexes in treating inflammatory conditions including arthritis; however, there are several limitations that should be acknowledged. The current study addressed the issue of curcuminoids’ solubility and bioavailability by formulating their inclusion complexes, it is essential to conduct pharmacokinetic studies to assess how effectively these formulations are absorbed, distributed, metabolized, and eliminated in the body. The study examined the expression of various genes involved in arthritis. However, a more detailed understanding of the molecular mechanisms underlying the observed effects would provide valuable insights into how C. longa extract and its complexes exert their anti-arthritic effects. The study compared the effects of C. longa extract and its complexes with diclofenac sodium. It would be valuable to conduct further comparative studies with other established anti-arthritic drugs to assess the relative efficacy and safety of these formulations. While the animal model provides important preliminary data, clinical trials involving human subjects are necessary to validate the effectiveness and safety of these formulations in a real-world setting.
Conclusion
It is deduced from current experimental work that TC, and BC formulations in comparison to CRE prepared from C. longa ameliorated the pathological features of RA such as loss of body weight, paw edema, bone erosion, and pannus development better due to their enhanced solubility. These curcuminoids formulations with enhanced bioavailability decelerated the disease advancement, mitigated the joint inflammation, and progression of polyarthritis through downregulation of pro-inflammatory mRNA expression and upregulation of mRNA expression of anti-inflammatory mediators in treated arthritic animals. Based on the current preclinical results, it is recommended to conduct the clinical trials of TC and BC for their potential as a phytomedicine for arthritis.
Data availability
Data availability is provided on request for this journal.
References
Abd Rani NZ, Husain K, Kumolosasi E (2018) Moringa genus: a review of phytochemistry and pharmacology. Front Pharmacol 9:108
Adjimani JP, Asare P (2015) Antioxidant and free radical scavenging activity of iron chelators. Toxicol Rep 2:721–728
Ahmad RS, Hussain MB, Sultan MT, Arshad MS, Waheed M, Shariati MA et al (2020) Biochemistry, safety, pharmacological activities, and clinical applications of turmeric: a mechanistic review. Evid Based Compl Altern Med 2020(1):1
Akhtar MF, Ashraf M, Anjum AA, Javeed A, Sharif A, Saleem A et al (2016a) Textile industrial effluent induces mutagenicity and oxidative DNA damage and exploits oxidative stress biomarkers in rats. Environ Toxicol Pharmacol 41:180–186
Akhtar MF, Ashraf M, Javeed A, Anjum AA, Sharif A, Saleem A et al (2016b) Toxicity appraisal of untreated dyeing industry wastewater based on chemical characterization and short term bioassays. Bull Environ Contam Toxicol 96(4):502–507
Allen KD, Thoma LM, Golightly YM (2022) Epidemiology of osteoarthritis. Osteoarthritis Cartilage 30(2):184–195
Ardalan K, Lloyd-Jones DM, Schanberg LE (2022) Cardiovascular health in pediatric rheumatologic diseases. Rheum Dis Clin 48(1):157–181
Arntz OJ, Pieters BC, Thurlings RM, Wenink MH, Van Lent PL, Koenders MI et al (2018) Rheumatoid arthritis patients with circulating extracellular vesicles positive for IgM rheumatoid factor have higher disease activity. Front Immunol 9:2388
Babu PS, Srinivasan K (1997) Hypolipidemic action of curcumin, the active principle of turmeric (Curcuma longa) in streptozotocin induced diabetic rats. Mol Cell Biochem 166(1):169–175
Bhangale JO, Acharya SR (2016) Anti-Parkinson activity of petroleum ether extract of Ficus religiosa (L.) leaves. Adv Pharm Pharm Sci 2016:1
Dandawate PR, Vyas A, Ahmad A, Banerjee S, Deshpande J, Swamy KV et al (2012) Inclusion complex of novel curcumin analogue CDF and β-cyclodextrin (1: 2) and its enhanced in vivo anticancer activity against pancreatic cancer. Pharm Res 29:1775–1786
Ebada HM, Nasra MM, Nassra RA, Solaiman AA, Abdallah OY (2022) Cationic nanocarrier of rhein based on hydrophobic ion pairing approach as intra-articular targeted regenerative therapy for osteoarthritis. Colloids Surf, B 211:112285
Farjam M, Mehrabani D, Abbassnia F, Tanideh N, Imanieh MH, Pakbaz S et al (2014) The healing effect of Curcuma longa on liver in experimental acute hepatic encephalopathy of rat. Comp Clin Pathol 23(6):1669–1673
Farooq S, Shaheen G, Asif HM, Aslam MR, Zahid R, Rajpoot SR et al (2022) Preliminary phytochemical analysis: In-Vitro comparative evaluation of anti-arthritic and anti-inflammatory potential of some traditionally used medicinal plants. Dose-Res 20(1):15593258211069720
Gaffo A, Saag KG, Curtis JR (2006) Treatment of rheumatoid arthritis. Am J Health Syst Pharm 63(24):2451–2465
Gibofsky A (2012) Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. Am J Manag Care 18(13 Suppl):S295-302
Gould S, Scott RC (2005) 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): a toxicology review. Food Chem Toxicol 43(10):1451–1459
Gu Y-T, Chen J, Meng Z-L, Ge W-Y, Bian Y-Y, Cheng S-W et al (2017) Research progress on osteoarthritis treatment mechanisms. Biomed Pharmacother 93:1246–1252
Gul P, Bakht J (2015) Antimicrobial activity of turmeric extract and its potential use in food industry. J Food Sci Technol 52(4):2272–2279
Harris ED Jr (1990) Rheumatoid arthritis: pathophysiology and implications for therapy. N Engl J Med 322(18):1277–1289
Hawk C, Ndetan H, Evans MW Jr (2012) Potential role of complementary and alternative health care providers in chronic disease prevention and health promotion: an analysis of National Health Interview Survey data. Prev Med 54(1):18–22
Hay E, Lucariello A, Contieri M, Esposito T, De Luca A, Guerra G et al (2019) Therapeutic effects of turmeric in several diseases: An overview. Chem Biol Interact 310:108729
Jang S, Kwon E-J, Lee JJ (2022) Rheumatoid arthritis: pathogenic roles of diverse immune cells. Int J Mol Sci 23(2):905
Kazeminasab S, Nejadghaderi SA, Amiri P, Pourfathi H, Araj-Khodaei M, Sullman MJ et al (2022) Neck pain: global epidemiology, trends and risk factors. BMC Musculoskelet Disord 23(1):1–13
Khojah HM, Ahmed S, Abdel-Rahman MS, Hamza A-B (2016) Reactive oxygen and nitrogen species in patients with rheumatoid arthritis as potential biomarkers for disease activity and the role of antioxidants. Free Radical Biol Med 97:285–291
Lateh L, Yuenyongsawad S, Chen H, Panichayupakaranant P (2019a) A green method for preparation of curcuminoid-rich Curcuma longa extract and evaluation of its anticancer activity. Pharmacogn Mag 15(65):730–735
Lateh L, Yuenyongsawad S, Chen H, Panichayupakaranant P (2019b) A green method for preparation of curcuminoid-rich Curcuma longa extract and evaluation of its anticancer activity. Pharmacognosy Mag 15(65):730–735
Lateh L, Kaewnopparat N, Yuenyongsawad S, Panichayupakaranant P (2022) Enhancing the water-solubility of curcuminoids-rich extract using a ternary inclusion complex system: Preparation, characterization, and anti-cancer activity. Food Chem 30(368):130827
Lateh L (2018) Preparation and Study of nticancer Activity of Curcuminoid-Rich Curcuma longa Extracts (CRE) and CRE-Cyclodextrin Inclusion Complexes
Lee F, Bae KH, Ng S, Yamashita A, Kurisawa M (2021) Hyaluronic acid–green tea catechin conjugates as a potential therapeutic agent for rheumatoid arthritis. RSC Adv 11(24):14285–14294
Lestari ML, Indrayanto G (2014) Curcumin. Prof Drug Subst Excip Rel Methodol 39:113–204
Li ZA, Sant S, Cho SK, Goodman SB, Bunnell BA, Tuan RS et al (2023) Synovial joint-on-a-chip for modeling arthritis: Progress, pitfalls, and potential. Trends Biotechnol 41(4):511–527
Mbiantcha M, Almas J, Shabana SU, Nida D, Aisha F (2017) Anti-arthritic property of crude extracts of Piptadeniastrum africanum (Mimosaceae) in complete Freund’s adjuvant-induced arthritis in rats. BMC Complement Altern Med 17(1):1–16
McInnes IB, Schett G (2011) The pathogenesis of rheumatoid arthritis. N Engl J Med 365(23):2205–2219
Mehrabani D, Farjam M, Geramizadeh B, Tanideh N, Amini M, Panjehshahin MR (2015) The healing effect of curcumin on burn wounds in rat. World J Plastic Surg 4(1):29
Mir NT, Saleem U, Anwar F, Ahmad B, Ullah I, Hira S et al (2019) Lawsonia Inermis markedly improves cognitive functions in animal models and modulate oxidative stress markers in the brain. Medicina 55(5):192
Oyeleke SA, Ajayi AM, Umukoro S, Aderibigbe A, Ademowo OG (2018) Anti-inflammatory activity of Theobroma cacao L. stem bark ethanol extract and its fractions in experimental models. J Ethnopharmacol 222:239–248
Paliwal R, Paliwal SR. Advances in Nanochemoprevention
Pengjam Y, Panichayupakaranant P, Tanrattanakul V (2021a) Curcuminoid (CRE-Ter)/Liposome as delivery platform for anti-osteoclastogenesis via NF-κB/ERK pathways in RANKL-induced RAW 264.7 cells through PLA foams. Heliyon 7(9):e07823
Pengjam Y, Prajantasen T, Tonwong N, Panichayupakaranant P (2021b) Downregulation of miR-21 gene expression by CRE-Ter to modulate osteoclastogenesis: De Novo mechanism. Biochem Biophys Rep 26:101002
Pengjam Y, Syazwani N, Inchai J, Numit A, Yodthong T, Pitakpornpreecha T et al (2021c) High water-soluble curcuminoids-rich extract regulates osteogenic differentiation of MC3T3-E1 cells: Involvement of Wnt/β-catenin and BMP signaling pathway. Chin Herb Med 13(4):534–540
Prabhu VV, Nalini G, Chidambaranathan N, Kisan SS (2011) Evaluation of anti-inflammatory and analgesic activity of Tridax procumbens Linn against formalin, acetic acid and CFA induced pain models. Int J Pharm Pharm Sci 3(2):126–130
Quintão NL, Antonialli CS, da Silva GF, Rocha LW, de Souza MM, Malheiros A et al (2012) Aleurites moluccana and its main active ingredient, the flavonoid 2 ″-O-rhamnosylswertisin, have promising antinociceptive effects in experimental models of hypersensitivity in mice. Pharmacol Biochem Behav 102(2):302–311
Rajkapoor B, Kavimani S, Ravichandiran V, Sekhar K, Kumar RS, Kumar MR et al (2009) Effect of Indigofera aspalathoides on complete Freund’s adjuvant-induced arthritis in rats. Pharm Biol 47(6):553–557
Saleem U, Ahmad B, Ahmad M, Hussain K, Bukhari NI (2014) Investigation of in vivo antioxidant activity of Euphorbia helioscopia latex and leaves methanol extract: a target against oxidative stress induced toxicity. Asian Pac J Trop Med 7:S369–S375
Saleem A, Saleem M, Akhtar MF (2020) Antioxidant, anti-inflammatory and antiarthritic potential of Moringa oleifera Lam: An ethnomedicinal plant of Moringaceae family. S Afr J Bot 128:246–256
Saleem U, Khalid S, Chauhdary Z, Anwar F, Shah MA, Alsharif I et al (2023) The curative and mechanistic acumen of curcuminoids formulations against haloperidol induced Parkinson’s disease animal model. Metab Brain Dis 38(3):1051–1066
Sanchez-Lopez E, Coras R, Torres A, Lane NE, Guma M (2022) Synovial inflammation in osteoarthritis progression. Nat Rev Rheumatol 18(5):258–275
Sani A, Hasegawa K, Yamaguchi Y, Panichayupakaranant P, Pengjam Y (2021) Inhibitory effects of curcuminoids on dexamethasone-induced muscle atrophy in differentiation of C2C12 cells. Phytomedicine plus 1(1):100012
Shabbir A, Shahzad M, Ali A, Zia-ur-Rehman M (2016) Discovery of new benzothiazine derivative as modulator of pro-and anti-inflammatory cytokines in rheumatoid arthritis. Inflammation 39(6):1918–1929
Shabbir A, Rehman K, Akash MSH, Akbar M, Chaudhary Z, Panichayupakaranant P et al (2022) Differential neuroprotective effect of curcuminoid formulations in aluminum chloride–induced Alzheimer’s disease. Environ Sci Pollut Res 29(45):67981–67996
Sharma R, Gescher A, Steward W (2005) Curcumin: the story so far. Eur J Cancer 41(13):1955–1968
Smolen JS, Landewé RB, Bergstra SA, Kerschbaumer A, Sepriano A, Aletaha D et al (2023) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis 82(1):3–18
Thite AT, Patil RR, Naik SR (2014) Anti-arthritic activity profile of methanolic extract of Ficus bengalensis: comparison with some clinically effective drugs. Biomed Aging Pathol 4(3):207–217
Tsuda T (2018) Curcumin as a functional food-derived factor: degradation products, metabolites, bioactivity, and future perspectives. Food Funct 9(2):705–714
Uttra AM, Hasan UH (2017) Anti-arthritic activity of aqueous-methanolic extract and various fractions of Berberis orthobotrys Bien ex Aitch. BMC Compl Altern Med 17(1):1–16
Voon F-L, Sulaiman MR, Akhtar MN, Idris MF, Akira A, Perimal EK et al (2017) Cardamonin (2′, 4′-dihydroxy-6′-methoxychalcone) isolated from Boesenbergia rotunda (L.) Mansf. inhibits CFA-induced rheumatoid arthritis in rats. Eur J Pharmacol 794:127–134
Zhang Q, Yu Y, Li J, Guan Y, Huang J, Wang Z et al (2018) Anti-arthritic activities of ethanol extracts of Circaea mollis Sieb. & Zucc. (whole plant) in rodents. J Ethnopharmacol 225:359–366
Zhang L, Yang S, Wong LR, Xie H, Ho PC-L (2020) In vitro and in vivo comparison of curcumin-encapsulated chitosan-coated poly (lactic-co-glycolic acid) nanoparticles and curcumin/hydroxypropyl-β-Cyclodextrin inclusion complexes administered intranasally as therapeutic strategies for Alzheimer’s Disease. Mol Pharm 17(11):4256–4269
Acknowledgements
This research was financially supported by Prince of Songkla University and the Ministry of Higher Education, Science, Research and Innovation under the Reinventing University Project (Grant Number REV65026).
Funding
This research was financially supported by Prince of Songkla University and Ministry of Higher Education, Science, Research and Innovation under the Reinventing University Project (Grant Number REV65026).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interest
The authors declare that they do not have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saleem, U., Chauhdary, Z., Bakhtawar, Z. et al. Curcuminoids-enriched extract and its cyclodextrin inclusion complexes ameliorates arthritis in complete Freund’s adjuvant-induced arthritic mice via modulation of inflammatory biomarkers and suppression of oxidative stress markers. Inflammopharmacol 31, 3047–3062 (2023). https://doi.org/10.1007/s10787-023-01370-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-023-01370-2