Abstract
A chronic inflammatory disorder, rheumatoid arthritis (RA) is an autoimmune and systemic disease characterized by progressive and prolonged destruction of joints. This results in increased mortality, physical disability and destruction. Cardiovascular disorders are one of the primary causes of mortality in patients with RA. It is multifactorial in nature and includes genetic, environmental and demographic factors which contribute to the severity of disease. Endothelin-1 (ET-1) is a peptide which acts as a potent vasoconstrictor and is generated through vascular smooth muscle and endothelial cells. Endothelins may be responsible for RA, as under certain circumstances they produce reactive oxygen species which further promote the production of pro-inflammatory cytokines. This enhances the production of superoxide anion, which activates pro-inflammatory cytokines, resulting in RA. The aim of this review is to elucidate the role of endothelin in the progression of RA. This review also summarizes the natural and synthetic anti-inflammatory drugs which have provided remarkable insights in targeting endothelin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
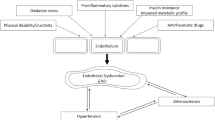
Rheumatoid arthritis (RA) is a prototypical autoimmune disorder that is chronic in nature and mainly affects the lining of the synovial joints, further leading to progressive disability and associated with socioeconomic burden and death. The chronic inflammation results in progressive joint destruction which leads to leucocyte infiltration. This causes blood cell formation inside the synovial membrane which leads to bone and cartilage damage, ultimately resulting in incapacitating pain. Further progression causes destruction of surrounding cartilage as well as joints. Proliferation in the synovium results in pannus formation, causing invasion and erosion, further leading to progressive disability, systemic complications and even death. The specific cause of RA is not yet known; however, with progressive advancement, numerous factors have been elucidated, making it a multifactorial disease. It occurs due to the interaction of numerous factors including genetic, lifestyle and environmental aspects (Behl et al. 2020; Chadha et al. 2021, 2020). The severity and duration of disease are both regulated by individual factors. Disordered innate immunity results in altered complex-induced adaptive immune response against self-antigens, which causes progression of disease. The hallmarks of RA are associated with infiltration of inflammatory cells inside the synovial membrane which results in increased cell density and tissue inflammation as a result of the interaction between macrophages, lymphocytes and endothelium as well as synovial fibroblasts. Attenuated matrix metalloproteinase (MMP) levels, DNA histones and micro-RNAs are associated with the process of gene translation and thus can lead to RA (Kaur et al. 2020; McInnes and Schett 2011). Endothelin 1 (ET-1) acts as an endogenously present vasoconstrictor that is secreted from endothelial cells and binds to two receptors, endothelin receptor type A (ETA) and B (ETB). ET-1 also exerts its action by causing fibrosis of vascular cells, which in turn promotes the generation of reactive oxygen species (ROS). ET-1 stimulates pro-inflammatory processes by enhancing secretion of cytokines and superoxide anions (D'Orléans-Juste et al. 2019; Wang and Dashwood 2011; Alvarez-Cienfuegos et al. 2019). ET-1 promotes activation of nuclear factor kappa B (NF-κB) (acting as transcription factor) and pro-inflammatory cytokines (including interleukin-1 [IL-1], tumour necrosis factor alpha [TNF-α] and IL-6) (Mourouzis et al. 2020; Zhang and Lin 2019; Liang et al. 2014). It is a polypeptide, containing 21 amino acids and two intramolecular disulphide bonds. It amplifies in vitro proliferation of fibroblasts, vascular smooth muscle cells and mesangial cells. Factors like transforming growth factor beta (TGF-β), IL-1 and thrombin enhance the production of endothelins that exert their effect in RA. This shows that endothelins can be produced locally around joints and thus can lead to joint destruction (Davenport et al. 2016; Zarpelon et al. 2013; Elisa et al. 2015; Olender et al. 2016). The main aim of this review is to explore mechanistic pathways through which endothelins produce ROS and pro-inflammatory cytokines and numerous factors and conditions under which endothelins can cause RA. This review also explores the natural and synthetic components having the potential to reduce the risk of RA.
Overview of endothelins
Endothelins are naturally occurring peptides which have a significant impact on body vasculature, and serve both a regulatory and developmental role in the process of normal physiology (including water balance maintenance, respiratory development and salt balance). Numerous studies have shown that overexpression of endothelins is related to systemic rheumatic disease such as pulmonary arterial hypertension and ischaemic digital ulcer. Endothelins play a role in numerous diseases by acting and binding to endothelin receptors, which were isolated, purified and sequenced by Japanese investigators in the 1980s (Olender et al. 2016; Parida and Nayak 2013; Bdolah 2016). The three major isoforms of endothelin protein were isolated and termed ET-1, ET-2 and ET-3, and are closely related to sarafotoxins (a potent vasoconstrictor peptide obtained from snake venom) and produced through unique genes. Each endothelin exerts its own specific function and tissue localization. Various evidence has shown that endothelins exert their effect inside the respiratory, urogenital, cardiovascular, gastrointestinal, endocrine and central nervous systems (Bdolah 2016; Ohtaki 2016). The most significant forms of endothelins in humans are ET-1, ET-2 and ET-3, which are abundantly present inside the brain and are thus called neural endothelin. ET-1 is a 21-amino-acid peptide and a potent vasoconstrictor comprising G protein-coupled receptors (GPCRs), mainly ETA and ETB receptors. The transduction of numerous processes is carried out via ET-1, and the ETB receptor assists in its role as clearance receptor. ET-1 is synthesized in the form of pre-pro-ET-1, which undergoes cleavage to form precursor big ET-1 (pro-ET-1). Under the influence of endothelin-converting enzyme 1, pro-ET-1 is converted into ET-1 (Zhan and Rockey 2011; Bakrania et al. 2017; Bourque et al. 2011). ET-1 and nitric oxide act as an antagonist and maintain vascular homeostasis by balancing vasoconstriction and vasodilation (Chester and Yacoub 2014). Disturbance or attenuation between these factors can result in altered conditions which may lead to hypertension, and overexpression of ET-1 inside endothelial cells results in systemic hypertension (Rautureau and Schiffrin 2012; Jing et al. 2015).
Endothelins are not stored inside cells, as their production is controlled at the transcriptional level. The numerous factors that inhibit or stimulate the release of ET-1 are shown in Table 1. Expression of endothelin production is dependent on calcium-dependent protein kinase C (PKC). Numerous stimulating elements including chemical and physical factors like hypoxia, low shear stress, cytokines, angiotensin II and growth factors enhance endothelin expression (Bogdanov 2015; Kawanabe and Nauli 2011). The suppression of genetic expression is achieved by nitric oxide (NO), amplified blood flow, prostacyclin and natriuretic peptides. The preliminary precursor of ET-1 is pre-pro-ET-1 which is a 212-residue peptide. The precursor undergoes cleavage twice: initially via endopeptidase (a peptide with 38 amino acids) and subsequently by endothelin-converting enzyme. Endothelins released from the endothelial cells have lower plasma concentration (1 mol/ml), as well as shorter half-life in blood (4–7 min), acting as a local hormone and binding rapidly to smooth muscle cells (Kawanabe and Nauli 2011; Stow et al. 2011; Lozano et al. 2010).
Relation of endothelin with inflammation
Endothelin is mainly responsible for vasoconstriction, and its overexpression is related to short- and long-term physiological and pathological processes including tissue remodelling, fibrosis, mitogenesis, inflammation and vascular hypertrophy. Regulated expression of endothelin is required for maintenance, growth and development of numerous physical systems including respiratory health, water balance and cardiovascular homeostasis. Endothelins act as a powerful potentiator of cholinergic neuronal activity, which results in amplified formation and enhanced neurohormonal expression including norepinephrine and angiotensin II (Guarda et al. 1993; Shrestha et al. 2009; Yoshida et al. 1998). They stimulate adrenaline cells, leading to production of cortisol, aldosterone and corticosterone. Phospholipase A2 activation results in enhanced metabolism of arachidonic acid, which elevates prostaglandin and thromboxane production. Thromboxane, in turn, attenuates platelet aggregation and contributes to vasodilation. Owing to its fibrotic, inflammatory and mitogenic action, thromboxane is significantly involved in rheumatic disease due to overexpression of endothelin. Endothelins are associated with cellular replication of smooth muscle cells, myocytes and fibroblasts, which enhances the mitogenic action of numerous growth factors linked with profibrotic activity (Del Rio et al. 2011; Leask 2010).
Endothelins can induce and produce fibronectin by modulating its release through epithelial cells. Fibronectin further stimulates fibroblast chemotaxis, whose progression results in enhanced fibroblast collagen, thus defining the chief role of endothelins in synthesis and remodelling of extracellular matrix. The endothelin-mediated stimulation of neutrophils facilitates the release of elastase, which activates mast cells and permits monocytes to release numerous cytokines including TNF, interleukins, TGF and macrophage colony-stimulating factors. The mechanism of action of endothelin is dependent on numerous and abundant factors including concentration of endothelins, followed by tissue site, pathological state of tissue and the receptor on which binding takes place (Lattmann et al. 2005; Motte et al. 2006; Nakano et al. 1994).
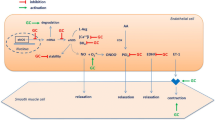
The receptors to which endothelin binds are referred to as endothelin receptors, which are members of the superfamily of seven-transmembrane GPCRs. Mammals have two separate endothelin receptors that exert antagonizing effects, namely ETA and ETB. The response of the ETA receptor towards ET-1 is specific and selective, whereas the ETB receptor is selective towards ET-1, ET-2 and ET-3. The cell membrane comprises both receptors, and its regulation is carried out in the same way as that of endothelin (Channick et al. 2001; Squadrito et al. 2002; Vercauteren et al. 2017). Factors such as epidermal growth factor (EGF), cyclic adenosine monophosphate (cAMP), hypoxia-inducible factor and basic fibroblast growth factor aid in upregulating the concentrations of ETA receptor in various tissues, whereas TGF and PDGF downregulate ETA receptor production. ETA receptors are predominantly located on the surface of smooth muscle cells, inducing vasoconstriction by increasing intracellular calcium through a biphasic mechanism. The primitive transient peak of Ca2+ appears as a result of binding of endothelin, further leading to cleavage of phospholipase C into activated phosphatidylinositol 4,5-bisphosphate (PIP2). This PIP2 is converted to inositol 1,4,5-trisphopshate (IP3) and diacylglycerol (DAG). IP3 acts on IP3-sensitive Ca2+ channels, resulting in rapid release of calcium through intracellular stores (Bagnato et al. 1997; Finsnes et al. 2001; Houde et al. 2016; Sánchez et al. 2014). Successive calcium upregulation takes place due to the influx of Ca2+ across the plasma membrane via calcium channels by direct binding of endothelin to their receptor. ETB receptors are predominantly present on vascular smooth muscle cells and stimulate the process of vasoconstriction. Under certain conditions, vasodilation is also observed due to the release of NO and prostacyclin. Some mediating action of endothelins can occur due to accumulation of DAG and Ca2+, which in turn mediates PKC activation. This results in vasoconstriction and cell proliferation, as shown in Fig. 1. In normal tissues, the effect of endothelin is vasodilatory, but pathogenic conditions can lead to vasoconstriction. Studies have shown that a disease state can result in dysregulated endothelin levels—for example, the level of ETB receptors is increased relative to ETA receptors in the case of chronic heart failure (Finsnes et al. 2001; Vercauteren et al. 2017). In individuals with myocardial ischaemia and hypertension, vasodilation is observed. In scleroderma-related fibrotic lung disease, elevated levels of ETB receptors relative to ETA were observed.
Action of endothelin by binding with endothelin A receptor, which is a G protein-stimulatory receptor (Gq), on vascular smooth muscle cells (VSMC), leading to cleavage of phospholipase C (PLC) into activated phosphatidylinositol 4,5-bisphosphate (PIP2), which is further converted into inositol 1,4,5-trisphopshate (IP3) and diacylglycerol (DAG). IP3 acts on IP3-sensitive Ca2+ channels and results in quick release of calcium through intracellular stores in the sarcoplasmic reticulum (SR), which in turn leads to their action at the nuclear level
ET-1 in production of pro-inflammatory mediators resulting in rheumatoid arthritis
ET-1 is implicated as an inflammatory mediator and demonstrates this role in numerous autoimmune diseases such as scleroderma and RA. Elevated ET-1 concentration is reported (in both synovial fluids and serum) in patients with RA compared with normal individuals. ET-1 is produced by synovial macrophages, and interacts with inflammatory cytokines like TNF-α and IL-1β. It regulates the production of adhesion proteins including vascular cell adhesion molecule-1 (VCAM-1), CD44, CD106 and intercellular adhesion molecule-1 (ICAM-1) (Elisa et al. 2015; Tatar et al. 2016). ET-1 plays a significant role in inflammatory responses via recruitment of neutrophils and causes their infiltration around inflamed tissues in patients with arthritis. Increased levels of local ET-1 are correlated with extra-articular manifestation in patients with RA. IL-6 is an abundantly present pro-inflammatory cytokine inside the serum and synovial joints of RA patients. IL-6 can exhibit direct induction of pre-pro-ET-1 messenger RNA (mRNA) expression inside the fibrotic kidney of an individual suffering from angiotensin II-induced hypertension (Narazaki et al. 2017; Wermuth et al. 2016; Yuzugulen 2016). ET-1 exerts its effect on synovial inflammation, as articular chondrocytes do not express ET-1 by themselves, but under circumstances like diseased conditions and natural ageing, its expression is observed. The concentration of ET receptors on articular chondrocytes is prominently affected by age, growth factors, cytokines (including TGF-a, IL-1β, TNF-a) and PDGF-BB. IL-1β, PDGF-BB and TNF-α may cause increased responsiveness of articular chondrocytes towards ET-1 (Amin et al. 1995; Del Rio et al. 2011; Kuryliszyn-Moskal et al. 2006). Elevated ET-1 levels result in enhanced MMP 1 and 13 expression via osteoarthritic chondrocytes (enzyme largely responsible for damaging cartilaginous matrix in RA and osteoarthritis) (Felx et al. 2006; Sin et al. 2015). Overexpression of endothelial ET-1 can result in exaggerated hypertrophic differentiation of articular chondrocytes, which can lead to thinning of the cartilage, resulting in structural damage to cartilage. ET-1 can promote the production of collagen through articular chondrocytes in a dose-dependent manner. Moreover, it can also lead to dysfunction of disc cells in the lumbar region. Endothelins demonstrate their role in inflammation and the pain it induces. Subcutaneous injection of ET-1 can induce pain by activating nociceptive C fibres in a dose-dependent manner (Fernandes et al. 2020; Wong et al. 2012).
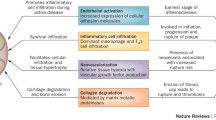
It was found that endothelin type A receptor antagonist significantly reduced joint pain in rats suffering from RA. The ETA receptor antagonist, when given in combination with bradykinin receptor antagonists, reduces pain and protects the knee joint in patients with arthritis (Guarda et al. 1993). Inflammatory and mechanical stimuli enhance expression and manifestation of nerve growth factor (NGF) by activating articular chondrocytes present at the osteochondral junction, thus serving a major role in arthritic joint pain. Although the exact reason is unknown, through radiographic techniques, bone marrow lesions were observed which exhibited a direct relation with the severity of pain. Moreover, higher concentrations of inflammatory cytokines such as IL-6, IL-1β and TGF-β1 can lead to defective osteoblasts, as shown in Fig. 2, causing alterations in phenotypic characteristics of normal articular chondrocytes towards hypertrophic differentiation. This occurs due to suppression of parathyroid hormone-related protein (PTHrP) as well as MMP elevation (Hughes et al. 1996; Tchetina et al. 2005).
Various intracellular factors which disrupt the formation of osteoclasts and thus can cause bone resorption. Wnt wingless-related integration site, NFATc 1 nuclear factor of activated T cells 1, OPG osteoprotegerin, COX-2 cyclooxygenase 2, TGF-β1 transforming growth factor beta 1, CTGF connective tissue growth factor, Klf Krüppel-like family of transcription factors
Role of endothelins in generation/production of ROS
The stimulatory effect of ET-1 on ROS production primarily enhances superoxide anions and leads to enhanced oxidative stress. Vasodilation of blood vessels is observed through intravenous injection, followed by longer-lasting contraction. Increased production takes place in numerous cells and occurs through the COX, NF-κB and NADPH oxidase-dependent pathways (Hughes et al. 1996; Loomis et al. 2005). Activation of NF-κB is correlated with increased levels of pro-inflammatory cytokines like IL-1, TNF-α and IL-6. The pro-inflammatory cytokines produced thus stimulate ET-1 production , subsequently enhancing/amplifying the synthesis of TNF-α inside monocytes and macrophages. These cytokines result in increased inflammatory responses through processes such as phagocytosis and chemotaxis of neutrophils and monocytes. Excessive levels of pro-inflammatory cytokines can activate and enhance the generation of prostaglandins in multiple cells, such as vascular smooth muscle cells and vascular endothelial cells. COX is also referred to as prostaglandin endo-peroxide synthase, which serves as a key enzyme responsible for prostaglandin production and exists in two forms, COX-1 and COX-2. Phospholipase A2 accelerates the release of arachidonic acid through membrane phospholipids. COX in turn catalyses the transformation of arachidonic acid into prostaglandins. Of the two isoforms of COX, COX-1 is expressed constitutively in the presence of normal physiological conditions and is responsible for maintenance of vascular homeostasis as well as physiological responses. COX-2 is not detectable under normal conditions, but exerts its expression inside inflammatory cells induced through endotoxins, growth factors and cytokines. COX-2 plays a major role in the expression of inflammatory responses such as vascular inflammation and leads to the production of potent inflammatory mediators, causing neurodegeneration. ET-1 promotes enhanced production of COX-2 as well as prostaglandin E2 (PGE2) via the mitogen-activated protein kinase (MAPK) and NF-κB pathways, which results in inflammation. ET-1 activates ET receptors (ETA/ETB), which are a heterotrimer GPCR and can stimulate numerous processes. The mechanism responsible is carried out via ETB receptor-coupling Gq proteins, which ultimately causes phospholipase C activation. Further hydrolysis of phosphoinositide leads to the formation of IP3 and DAG, which causes an increase in the concentration of PKC as well as Ca2+. Activation of Gq and Gi protein receptors can take place through signalling pathways resulting in stimulation of MAPKs. ET-1 stimulates MAPK in order to regulate different cellular responses, such as cell growth, proliferation and survival. The MAPK activation is associated with elevated regulation of COX-2. Agonists of GPCR (such as thrombin, bradykinin and sphingosine-1-phosphate) can lead to stimulation of NF-κB and MAPK activation, which amplifies COX-2 expression. ET-1 promotes translational regulation of COX-2, which is mediated through the ETB receptor and leads to PGE2 synthesis, further producing pro-inflammatory mediators (Peng et al. 2008; Phull et al. 2018). Moreover, enhanced expression of adhesion molecules is observed on the surface of endothelial cells, which aggregates to form polymorphonuclear neutrophils (PMNs). These PMNs contribute to endothelial dysfunction as well as inflammation via production of ROS and arachidonic acid metabolites. ET-1 also stimulates arterial VCAM-1, which causes adhesion of inflammatory cells. ET-1 stimulates the production of macrophages and monocytes in order to release IL-6 and TNF-α in a dose-dependent manner. Evidence supports the association of ET-1 with stimulation of alveolar macrophages, which results in increased arachidonic acid, in turn enhancing cytokine release, as mentioned above, and the whole process is depicted in the form of a flow chart in Fig. 3 (Yu et al. 2021).
Inflammatory effects of ET-1 via its actions on numerous cells and its role in the generation of inflammatory cytokines which can cause bone degradation, thus resulting in rheumatoid arthritis. TNF-α tumour necrosis factor alpha, IL-1 interleukin-1, TGF-β transforming growth factor beta, COX-2 cyclooxygenase 2, VEGF vascular endothelial growth factor, iNOS inducible nitric oxide synthase, NO nitric oxide, PGE2 prostaglandin E2, MMP matrix metalloproteinase
ET-1 in rheumatoid arthritis and inflammation
RA is associated with articular and extra-articular manifestations. Investigations have shown that patients with RA develop atherosclerotic lesions at a faster rate than that in the general population. Endothelins are key mediators involved in the development of RA and atherosclerosis. Endothelin dysfunction was first identified in RA patients in 2002 by Bergholm and co-workers. ET-1 leads to activation of numerous inflammatory and fibrotic signalling pathways and thus can cause hypertension, fibrosis of the heart, lungs, kidney and skin, and even diabetes. The blockade of endothelin signalling pathways results in decreased secretion of TGF-β (inside fibroblasts), attenuating fibrosis. TGF-β1 promotes ET-1 production via a Smad2/3-dependent mechanism, and this can be attenuated by the ALK5/c-Jun N-terminal kinase signalling pathway. ET-1 has been implicated in autoimmune disorders like RA and scleroderma. It has been observed that synovial macrophages lead to the production of ET-135, which interacts with numerous inflammatory cytokines including TNF-α and IL-1β to regulate the production of ICAM-1 (Hans et al. 2008; Yu et al. 2021). ET-1 is involved in mediating inflammatory responses of synovitis, which occurs via recruitment of neutrophils and endothelial cells, leading to inflammation inside the tissues. An increased concentration of ET-1 has been linked with extra-articular manifestations of RA and hypertension. IL-6 is the most prevalent and abundant pro-inflammatory cytokine present in the synovial joints of patients with RA. IL-6 induces the expression of pre-pro-ET-1 mRNA, which acts directly on articular chondrocytes, leading to fibrosis and synovial inflammation. Conditions like growth factors, age, and cytokines including PDGF-BB/IL-1β affect the expression of ET receptors on articular chondrocytes. ET-1 is involved in stimulating increased levels of MMP-1 and MMP-13 via osteoarthritic chondrocytes. An elevated level of ET-1 leads to hypertrophic differentiation of articular chondrocytes, resulting in thickening of calcified cartilage, which damages the hyaline cartilage. ET-1 also stimulates and enhances collagen and proteoglycan production in a dose-dependent manner. In RA, higher levels of inflammatory cytokines (such as TGF-β1, IL-6, IL-1β, and PGE2) indicate the presence of defective osteoblasts. These defective osteoblasts alter the phenotype of articular chondrocytes; the same occurs by ameliorating the expression of parathyroid hormone (PTH) and increasing MMP concentration (Shinagawa et al. 2017; Zhao et al. 2016).
Evidence supporting the relation between endothelins and rheumatoid arthritis
Various supporting evidence is reported in reference to endothelins and their ascribed role in RA. Helset and co-workers studied the impact of endothelins on the release of interleukins and TNF-α. Increased levels of endothelins suggested their role in the pathophysiology of pro-inflammatory mediators and reactions. The effects of ET-1 were investigated with respect to the release kinetics of cytokines from monocyte-derived macrophages, and a time-dependent release was observed. It was determined that ET-1 in a concentration of 0.01–1 nM could lead to a 200–400% increase in the level of cytokine release. The study concluded that increased ET-1 stimulates monocytes and macrophages, leading to the release of pro-inflammatory cytokines to exert inflammatory responses (Kowalczyk et al. 2015).
Another study conducted by Agata and co-workers aimed at identifying the impact of ET-1 in inflammatory responses. The study revealed that ET-1 acts as a potent endogenous vasoconstrictor which enhances the formation of ROS and further intensifies pro-inflammatory mechanisms. ET-1 receptor antagonists can be employed for prediction of numerous disorders. In a study conducted by Nobuyuki, the generation of ET-1 was observed in patients suffering from inflammatory arthritis. Using radioimmunoassay, ET-1 activity was measured in the synovial fluid of arthritic patients, and from observations it was concluded that ET-1 contributes significantly to synovial proliferation, which occurs in an autocrine manner (Soldano et al. 2017).
The relationship/expression between ET-1 and adhesion molecules located on fibroblast-like synovial cells was studied experimentally. The study investigated the expression of adhesion molecules CD44, VCAM-1 and ICAM-1 via fibroblast-like synoviocytes. Fibroblasts were treated using ET-1 in the presence and absence of C1306, which acts as an antagonist of ETA. The cell expression was studied using immunofluorescence. Increased expression of ICAM-1, CD44 and VCAM-1 was experimentally observed on the surface of fibroblast-like synoviocytes. ET-1 also enhances the production of IL-1. This effect could be blocked by C-1306, which acts as a selective ETA receptor antagonist and thus inhibits its activity. From this study, it was concluded that ET-1 exerts its immunoregulatory functions in the recruitment of cells inside inflamed tissues, and this can be inhibited by treatment with a selective antagonist (Donate et al. 2012).
Anti-inflammatory natural drugs targeting endothelin
Dietary components or nutraceuticals are essential agents to protect against disease states in the human body and are known for their appreciable medicinal properties. One of the most commonly used pharmaceuticals for inflammation is aspirin i.e., a COX inhibitor, which originally was derived from a natural product (Maroon et al. 2010). In line with this, multiple plant species and phytoconstituents have been shown to exhibit anti-inflammatory potential and have been utilized in different groups of disorders and ailments.
Isonahocol E3, obtained from Sargassum siliquastrum (brown algae), has been found to play both an anti-inflammatory and anti-angiogenic role by antagonizing the actions of the ET-1 receptor (Sah et al. 2013). Cell division and release of inflammatory mediators such as TNF-α, IL-6 and IL-8 and pro-angiogenic factors such as metalloproteinases has been reported to be inhibited by isonahocol E3 in human keratinocytes (Sah et al. 2013). Furthermore, ETA and ETB receptor levels, along with ET-1-mediated phosphorylation of extracellular signal-regulated kinase (ERK), are also found to be suppressed by isonahocol E3 (Sah et al. 2013).
The major ingredient of turmeric, curcumin, is an anti-inflammatory agent that has been shown to exhibit neuroprotective action by protecting the neurons of the hippocampus from degeneration via ET-1, reportedly producing a twofold increase in the level of c-Jun, according to immunoblot analysis results (Stankowska et al. 2017). However, co-treatment with curcumin mitigated the release of c-Jun, mediated by ET-1. Furthermore, the release of cleaved fodrin, cleaved caspase-3 and caspase-7 was also attenuated by curcumin treatment, which in turn induced neuroprotection in Alzheimer’s disease (Stankowska et al. 2017).
Flavonoids are the largest category of polyphenolic compounds, and exhibit multiple functions owing to their wide biological activity. Investigations have assessed the role of flavonoids as endothelin antagonists in carrying out potential functions in the body, such as the role of apigenin in ET-1–mediated collagen gel contraction, as observed in an in vitro model of extracellular matrix remodelling. Apigenin was found to block the ET-1–induced contraction of collagen gel, due to which it was reported to be given as a dietary supplement in retarding skin fibrosis in patients with systemic sclerosis (Jun et al. 2010).
Naringenin, a flavonoid, has been reported to inhibit superoxide ion-induced COX-2 mRNA expression. COX-2 has been found to be closely associated with cytokines, which are further related to ET-1. The antagonists of the ET-1 receptor inhibit the hyperalgesia mediated by cytokines (Manchope et al. 2016). The cytokines promote the pre-pro-ET-1 mRNA expression as well as production of ET-1. Therefore, besides alleviating the production of cytokines, naringenin has also been reported to inhibit the pre-pro-ET-1 mRNA expression and COX-2 levels, thereby blocking superoxide ion-induced inflammatory pain (Manchope et al. 2016).
Luteolin is another anti-inflammatory flavonoid compound that has been found to alleviate the secretion and genetic expression of ET-1 in porcine aortic endothelial cells (Kozakai et al. 2005).
Diosgenin is an anti-inflammatory pharmaceutical agent that possesses a steroidal ring (steroidal saponin), and has been reported to inhibit ET-1 production in endothelial cells and restore the loss of insulin-induced vasodilation in the presence of palmitate (Liu et al. 2012).
Similarly, another steroidal compound, withaferin A also, attenuates ET-1 levels and restores impaired vasodilation, mediated by endothelium, in isolated aortic preparations (Batumalaie et al. 2016). Such results illustrate the potential of withaferin A to block the production of ROS as well as inflammation to restore disturbed insulin resistance in cultured endothelial cells and modulate the impairment of these cells in rat aortic rings (Batumalaie et al. 2016).
Finally, an anti-inflammatory nutraceutical, resveratrol, has been found to inhibit the secretion of ET-1 and ET-1 mRNA concentration in endothelial cells in humans. In addition, the endothelin-converting enzyme-1 mRNA concentration was inhibited by resveratrol and its analogue, to further mitigate the enzyme concentration (Coppa et al. 2011).
Anti-inflammatory synthetic drugs targeting endothelin
The ET-1 receptor activates transcription factors such as NF-κB and pro-inflammatory cytokines such as IL-6, IL-1 and TNF-α, which stimulate the body’s inflammatory responses (Urbanowicz et al. 2005). Bosentan, a mixed ETA and ETB receptor antagonist, was found to potentially attenuate both inflammation and swelling in knee joints, and also reduced inflammatory mechanical hyperalgesia (Imhof et al. 2011). Moreover, chronic administration of this drug prevented destruction of joints during antigen-induced arthritis flare-up reactions (Imhof et al. 2011). An ETA receptor antagonist, BQ-123, was shown to ameliorate the concentration of TNF-α and IL-1β in the lung tissue of a rat model during oxidative stress conditions mediated by the intraperitoneally administered extract of cigarette smoke (Urbanowicz et al. 2004). Studies have investigated the role of BQ-123 in activating polymorphonuclear myeloid-derived suppressor cells in mice, which inhibited acute inflammation in a T-cell-dependent manner, thus providing a reliable approach for treating conditions of acute inflammation (Chen et al. 2021). Another study demonstrated lowering of TNF-α levels when patients with optic nerve damage were administered BQ-788, an endothelin antagonist (Tonari et al. 2012). Another agent, ambrisentan, an ETA antagonist, was found to be useful in patients with pulmonary arterial hypertension and to mitigate the progression of cancer metastasis (Kappes et al. 2020). ABT-627, a novel selective antagonist of the ETA receptor, exhibited antinociceptive activity in a streptozotocin (STZ)-induced diabetic rat model of neuropathic pain (Jarvis et al. 2000). Multiple studies have also evaluated the role of endothelin and platelet-activating factor (PAF) in the body’s inflammatory responses (Sato et al. 2013). Substantial evidence has suggested a primary role of ET-3, but not ET-1 and ET-2, receptors in attenuating the inflammation induced by PAF, via direct interaction with the peptide Tyr-Lys-Asp (YKD) region to the factor along with its metabolite (Sato et al. 2013). This is then followed by blockage of the association between PAF and its receptor. In addition, the peptide encompassing the YKD sequence might be a suitable therapeutic anti-inflammatory candidate for attenuating the progression of inflammatory disorders by targeting the functions mediated by endothelin and PAF (Sato et al. 2013). An anti-inflammatory ETA antagonist drug, avosentan, was investigated in a randomized, placebo-controlled, parallel-design, double blind investigation, which evaluated the impact of avosentan on the urinary excretion rate of albumin in patients with diabetic nephropathy (Wenzel et al. 2009). It was observed that the excretion rate was decreased in patients upon administration of avosentan with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) (Wenzel et al. 2009). Tezosentan, a non-selective ETA and ETB receptor antagonist, was reported to mitigate lipopolysaccharide-induced liver injury by retarding intrahepatic neutrophil infiltration in rat models. The drug also increased survival in the rats (Urbanowicz et al. 2004).
Future perspective
The above-mentioned studies support the extensive network and role of ET-1 resulting in inflammation and arthritis. The synthesis of endothelins is stimulated through Ca2+ via a number of stimuli (including mechanical/chemical, along with various factors mentioned above). Therefore, ET-1 can be considered one of the modulating factors responsible for RA, and its treatment can be designed on this basis. The endothelin receptor antagonist may be one such particular target for treatment of endothelin-associated RA. In an investigation conducted by Paula and co-workers, the action of bosentan was checked in reference to RA. Bosentan is an endothelin receptor antagonist and thus can be useful for treating collagen-induced arthritis. The evaluation assessment showed clinical aspects and application of bosentan in order to treat hyperalgesia and paw swelling. Bosentan was also shown to reduce the level of pro-inflammatory cytokines, leukocyte infiltration, and pain as well as joint damage. In addition, it enhanced the expression of pre-pro-ET mRNA and inhibited TNF-α (Muller et al. 2000; Conte et al. 2008).
In another investigation conducted by Conte and co-workers, the action of lipoxin A in zymosan-induced arthritis was assessed. Lipoxin A4 (LXA4) acts as lipid mediator and serves a role in resolution of inflammation. Endothelins exhibit a major role in articular responses, and treatment was performed using LXA4. The assessed data and observations showed that administration of LXA4 resulted in inhibition of neutrophil infiltration and was associated with reduced levels of ET-1-related neutrophil chemotaxis. Thus, LXA4 can serve as a preferred treatment for patients with RA due to elevated ET-1 levels. Another ETA receptor antagonist, ambrisentan, has emerged as useful for treating pulmonary arterial hypertension, and can also be employed for cancer and RA. Endothelin A is most prominently involved in tumour formation via the process of metastasis. Ambrisentan, along with an antihypertensive effect, exerts an inhibitory effect on numerous tumour cells. It acts via invasion and migration inside tumour cells and is useful for the treatment of ovarian cancer, metastatic pancreatic adenocarcinoma, pro-myelocytic leukaemia and breast adenocarcinoma. It also acts by inhibiting the process of metastasis and thus can be used for RA (Conte et al. 2010). Various other drugs are mentioned in Table 2 which can be useful for RA treatment.
Clinical outcome
ET-1 signalling frequently affects cells of the joints including osteoblasts, synoviocytes and articular chondrocytes. Thus, liberation of ROS and pro-inflammatory cytokines can lead to RA. The initial effect on the osteoblast will be an anabolic effect, leading to inhibition of osteoclasts, followed by enzymatic degradation of articular cartilage. It further progresses through recruitment of pro-inflammatory mediators (monocytes, neutrophils and macrophages) around the inflamed synovial tissues, thereby leading to local/systemic inflammatory pain. Increased levels of ET-1 in RA patients are further related to extra-articular manifestations. The in vitro proliferation of fibroblasts, mesangial cells and vascular smooth muscle cells along with factors such as TGF-β, interleukin-1 and thrombin enhances the production of endothelins. Enhanced endothelins can exert their effect in RA. ET-1 exerts its role in mediating inflammation and inducing pain. Subcutaneous injection of ET-1 can induce pain by activating the nociceptive C-receptors in a dose-dependent manner. Mechanical and inflammatory stimuli activate NGF at the osteochondral junction, which results in arthritic joint pain. ET-1 exerts its role in mediating pain by activating receptors at the osteochondral junction. This shows that endothelin can be produced locally around the joints and thus can lead to joint destruction. Taken together, the information discussed in this review highlights the effects of endothelin signalling on the inflammatory signalling cascade, suggesting a key role of this peptide in RA. Thus, treatment aimed at blocking the effects of ET-1 represents a promising and innovative pharmaceutical approach for preventing cartilage destruction in RA.
Data availability
Not applicable.
References
Alvarez-Cienfuegos A, Cantero-Nieto L, García-Gómez JA, Ríos-Fernández R, Martín J, González-Gay MA, Ortego-Centeno N. (2019). Endothelin-1 serum levels in women with rheumatoid arthritis. Acta Reumatol Port 44:250–257. http://hdl.handle.net/10261/226842
Amin AR, Di Cesare PE, Vyas P, Attur M, Tzeng E, Billiar TR, Stuchin SA, Abramson SB (1995) The expression and regulation of nitric oxide synthase in human osteoarthritis-affected chondrocytes: evidence for up-regulated neuronal nitric oxide synthase. J Exp Med 182:2097–2102. https://doi.org/10.1084/jem.182.6.2097
Bagnato A, Tecce R, Di Castro V, Catt KJ (1997) Activation of mitogenic signaling by endothelin 1 in ovarian carcinoma cells. Cancer Res 57:1306–1311
Bakrania B, Duncan J, Warrington JP, Granger JP (2017) The endothelin type A receptor as a potential therapeutic target in preeclampsia. Int J Mol Sci 18:522. https://doi.org/10.3390/ijms18030522
Batumalaie K, Amin MA, Murugan DD, Sattar MZ, Abdullah NA, Withaferin A (2016) Protects against palmitic acid-induced endothelial insulin resistance and dysfunction through suppression of oxidative stress and inflammation. Sci Rep. https://doi.org/10.1038/srep27236 (PMID: 27250532; PMCID: PMC4890118)
Mackessy SP (2021) 14 Sarafotoxins, the Snake Venom homologs of the endothelins. Handbook of venoms and toxins of reptiles. London, New York
Behl T, Chadha S, Sachdeva M, Kumar A, Hafeez A, Mehta V, Bungau S (2020) Ubiquitination in rheumatoid arthritis. Life Sci 261:118459. https://doi.org/10.1016/j.lfs.2020.118459
Bogdanov G, Metodieva R, Boyadjieva N (2015) New research on endothelins and their receptors. S’rdechno-S’ Dovi Zabolyavaniya/medical Review-Cardiovascular Diseases 46:3–8
Bourque SL, Davidge ST, Adams MA (2011) The interaction between endothelin-1 and nitric oxide in the vasculature: new perspectives. Am J Physiol Regul Integr Comp Physiol 300:R1288–R1295. https://doi.org/10.1152/ajpregu.00397.2010
Chadha S, Behl T, Kumar A, Khullar G, Arora S (2020) Role of Nrf2 in rheumatoid arthritis. Curr Res Transl Med 68:171–181. https://doi.org/10.1016/j.retram.2020.05.002
Chadha S, Behl T, Bungau S, Kumar A, Kaur R, Venkatachalam T, Gupta A, Kandhwal M, Chandel D (2021) Focus on the multimodal role of autophagy in rheumatoid arthritis. Inflammation 44:1–2. https://doi.org/10.1007/s10753-020-01324-8
Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, Badesch DB, Roux S, Rainisio M, Bodin F, Rubin LJ (2001) Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebocontrolled study. Lancet 358:1119–1123. https://doi.org/10.1016/S0140-6736(01)06250-X
Chen Z, Zhang X, Lv S, Xing Z, Shi M, Li X, Chen M, Zuo S, Tao Y, Xiao G, Liu J, He Y (2021) Treatment with endothelin-A receptor antagonist BQ123 attenuates acute inflammation in mice through T cell-dependent polymorphonuclear myeloid-derived suppressor cell activation. Front Immunol. https://doi.org/10.3389/fimmu.2021.641874 (PMID: 33828553; PMCID: PMC8019801)
Chester AH, Yacoub MH (2014) The role of endothelin-1 in pulmonary arterial hypertension. Glob Cardiol Sci Pract 2014:29. https://doi.org/10.5339/gcsp.2014.29
Claudino RF, Leite DF, Bento AF, Chichorro JG, Calixto JB, Rae GA (2017) Potential role for ET-2 acting through ETA receptors in experimental colitis in mice. Inflamm Res 66:141–155. https://doi.org/10.1007/s00011-016-1001-7
Clozel M, Salloukh H (2005) Role of endothelin in fibrosis and anti-fibrotic potential of bosentan. Ann Med 37:2–12. https://doi.org/10.1080/07853890410018925
Conte FD, Barja-Fidalgo C, Verri WA Jr, Cunha FQ, Rae GA, Penido C, Henriques MD (2008) Endothelins modulate inflammatory reaction in zymosan-induced arthritis: participation of LTB4, TNF-α, and CXCL-1. J Leukoc Biol 84:652–660. https://doi.org/10.1189/jlb.1207827
Conte FP, Menezes-de-Lima O Jr, Verri WA Jr, Cunha FQ, Penido C, Henriques MG (2010) Lipoxin A4 attenuates zymosan-induced arthritis by modulating endothelin-1 and its effects. Br J Pharm 161:911–924. https://doi.org/10.1111/j.1476-5381.2010.00950.x
Coppa T, Lazzè MC, Cazzalini O, Perucca P, Pizzala R, Bianchi L, Stivala LA, Forti L, Maccario C, Vannini V, Savio M (2011) Structure-activity relationship of resveratrol and its analogue, 4,4’-dihydroxy-trans-stilbene, toward the endothelin axis in human endothelial cells. J Med Food 10:1173–1180. https://doi.org/10.1089/jmf.2010.0272 (Epub 2011 May 9 PMID: 21554123)
Davenport AP, Hyndman KA, Dhaun N, Southan C, Kohan DE, Pollock JS, Pollock DM, Webb DJ, Maguire JJ (2016) Endothelin. Pharm Rev 68:357–418. https://doi.org/10.1124/pr.115.011833
Del Rio R, Moya EA, Iturriaga R (2011) Differential expression of pro-inflammatory cytokines, endothelin-1 and nitric oxide synthases in the rat carotid body exposed to intermittent hypoxia. Brain Res 1395:74–85. https://doi.org/10.1016/j.brainres.2011.04.028
Denault AY, Pearl RG, Michler RE, Rao V, Tsui SS, Seitelberger R, Cromie M, Lindberg E, D’Armini AM (2013) Tezosentan and right ventricular failure in patients with pulmonary hypertension undergoing cardiac surgery: the TACTICS trial. J Cardiothorac Vasc Anesth 27:1212–1217. https://doi.org/10.1053/j.jvca.2013.01.023
Donate PB, Cunha TM, Verri WA, Junta CM, Lima FO, Vieira SM, Peres RS, Bombonato-Prado KF, Louzada P, Ferreira SH, Donadi EA (2012) Bosentan, an endothelin receptor antagonist, ameliorates collagen-induced arthritis: the role of TNF-α in the induction of endothelin system genes. Inflamm Res 61:337–348. https://doi.org/10.1007/s00011-011-0415-5
D’Orléans-Juste P, Ndunge OB, Desbiens L, Tanowitz HB, Desruisseaux MS (2019) Endothelins in inflammatory neurological diseases. Pharmacol Ther 194:145–160. https://doi.org/10.1016/j.pharmthera.2018.10.001
Elisa T, Antonio P, Giuseppe P, Alessandro B, Giuseppe A, Federico C, Marzia D, Ruggero B, Giacomo M, Andrea O, Daniela R (2015) Endothelin receptors expressed by immune cells are involved in modulation of inflammation and in fibrosis: relevance to the pathogenesis of systemic sclerosis. J Immunol Res. https://doi.org/10.1155/2015/147616
Fattori V, Serafim KG, Zarpelon AC, Borghi SM, Pinho-Ribeiro FA, Alves-Filho JC, Cunha TM, Cunha FQ, Casagrande R, Verri WA Jr (2017) Differential regulation of oxidative stress and cytokine production by endothelin ETA and ETB receptors in superoxide anion-induced inflammation and pain in mice. J Drug Target 25:264–274. https://doi.org/10.1080/1061186X.2016.1245308
Felx M, Guyot MC, Isler M, Turcotte RE, Doyon J, Khatib AM, Leclerc S, Moreau A, Moldovan F (2006) Endothelin-1 (ET-1) promotes MMP-2 and MMP-9 induction involving the transcription factor NF-κB in human osteosarcoma. Clin Sci 110:645–654. https://doi.org/10.1080/1061186X.2016.1245308
Fernandes TL, Gomoll AH, Lattermann C, Hernandez AJ, Bueno DF, Amano MT (2020) Macrophage: a potential target on cartilage regeneration. Front Immunol 11:111. https://doi.org/10.3389/fimmu.2020.00111
Finsnes F, Lyberg T, Christensen G, Skjønsberg OH (2001) Effect of endothelin antagonism on the production of cytokines in eosinophilic airway inflammation. Am J Physiol Lung Cell Mol Physiol 280:L659–L665. https://doi.org/10.1152/ajplung.2001.280.4.L659
Guarda E, Katwa LC, Myers PR, Tyagi SC, Weber KT (1993) Effects of endothelins on collagen turnover in cardiac fibroblasts. Cardiovasc Res 27:2130–2134. https://doi.org/10.1093/cvr/27.12.2130
Hans G, Deseure K, Adriaensen H (2008) Endothelin-1-induced pain and hyperalgesia: a review of pathophysiology, clinical manifestations and future therapeutic options. Neuropeptides 42:119–132. https://doi.org/10.1016/j.npep.2007.12.001
Houde M, Desbiens L, D’Orléans-Juste P (2016) Endothelin-1: biosynthesis, signaling and vasoreactivity. Adv Pharmacol 77:143–175. https://doi.org/10.1016/bs.apha.2016.05.002
Hughes AK, Stricklett PK, Padilla E, Kohan DE (1996) Effect of reactive oxygen species on endothelin-1 production by human mesangial l cells. Kidney Int 49:181–189. https://doi.org/10.1038/ki.1996.25
Imhof AK, Glück L, Gajda M, Bräuer R, Schaible HG, Schulz S (2011) Potent anti-inflammatory and antinociceptive activity of the endothelin receptor antagonist bosentan in monoarthritic mice. Arthritis Res Ther. https://doi.org/10.1186/ar3372 (PMID: 21689431; PMCID: PMC3218912)
Jarvis MF, Wessale JL, Zhu CZ, Lynch J. (2000) ABT-627 An endothelin ETA receptor selective antagonist, attenuates tactile allodynia in a diabetic rat model of neuropathic pain. Eur J Pharmacol. https://www.sciencedirect.com/science/article/abs/pii/S0014299999008651
Jing J, Dou TT, Yang JQ, Chen XB, Cao HL, Min M, Cai SQ, Zheng M, Man XY (2015) Role of endothelin-1 in the skin fibrosis of systemic sclerosis. Eur Cytokine Netw 26:10–14. https://doi.org/10.1684/ecn.2015.0360
Jun JB, Na YI, Kim TH et al (2010) Dietary flavonoid apigenin inhibits endothelin-1-induced contraction of collagen gel. Rheumatol Int 30:1695–1697. https://doi.org/10.1007/s00296-009-1285-9
Kappes L, Amer RL, Sommerlatte S, Bashir G, Plattfaut C, Gieseler F, Gemoll T, Busch H, Altahrawi A, Al-Sbiei A, Haneefa SM, Arafat K, Schimke LF, Khawanky NE, Schulze-Forster K, Heidecke H, Kerstein-Staehle A, Marschner G, Pitann S, Ochs HD, Mueller A, Attoub S, Fernandez-Cabezudo MJ, Riemekasten G, Al-Ramadi BK, Cabral-Marques O (2020) Ambrisentan, an endothelin receptor type a-selective antagonist, inhibits cancer cell migration, invasion, and metastasis. Sci Rep 10(1):15931. https://doi.org/10.1038/s41598-020-72960-1
Kaur I, Behl T, Bungau S, Kumar A, Mehta V, Setia D, Uddin MS, Zengin G, Aleya L, Arora S (2020) Exploring the therapeutic promise of targeting HMGB1 in rheumatoid arthritis. Life Sci 258:118164. https://doi.org/10.1016/j.lfs.2020.118164
Kawanabe Y, Nauli SM (2011) Endothelin. Cell Mol Life Sci 68:195–203. https://doi.org/10.1007/s00018-010-0518-0
Kowalczyk A, Kleniewska P, Kolodziejczyk M, Skibska B, Goraca A (2015) The role of endothelin-1 and endothelin receptor antagonists in inflammatory response and sepsis. Arch Immunol Ther Exp 63:41–52. https://doi.org/10.1007/s00005-014-0310-1
Kozakai T, Yamanaka A, Ichiba T, Toyokawa T, Kamada Y, Tamamura T, Ichimura T (2005) Luteolin inhibits endothelin-1 secretion in cultured endothelial cells. Biosci Biotechnol Biochem 69(8):1613–1615. https://doi.org/10.1271/bbb.69.1613
Kuryliszyn-Moskal A, Klimiuk PA, Sierakowski S, Ciolkiewicz M (2006) A study on vascular endothelial growth factor and endothelin-1 in patients with extra-articular involvement of rheumatoid arthritis. Clin Rheumatol 25:314–319. https://doi.org/10.1007/s10067-005-0007-2
Lattmann T, Hein M, Horber S, Ortmann J, Teixeira MM, Souza DG, Haas E, Tornillo L, Münter K, Vetter W, Barton M (2005) Activation of pro-inflammatory and anti-inflammatory cytokines in host organs during chronic allograft rejection: role of endothelin receptor signaling. Am J Transpl 5:1042–1049. https://doi.org/10.1111/j.1600-6143.2005.00807.x
Leask A (2010) Potential therapeutic targets for cardiac fibrosis: TGFβ, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res 106:1675–1680. https://doi.org/10.1161/CIRCRESAHA.110.217737
Liang L, Yu J, Zhou W, Liu N, L E, Wang DS, Liu H (2014) Endothelin-1 stimulates proinflammatory cytokine expression in human periodontal ligament cells via mitogen-activated protein kinase pathway. J Periodontol 85:618–626. https://doi.org/10.1902/jop.2013.130195
Liu K, Zhao W, Gao X, Huang F. (2012) Diosgenin ameliorates palmitate-induced endothelial dysfunction and insulin resistance via blocking IKKβ and IRS-1 pathways. Atherosclerosis. 2012 August. https://www.sciencedirect.com/science/article/abs/pii/S0021915012003875
Loomis ED, Sullivan JC, Osmond DA, Pollock DM, Pollock JS (2005) Endothelin mediates superoxide production and vasoconstriction through activation of NADPH oxidase and uncoupled nitric-oxide synthase in the rat aorta. J Pharmacol 315:1058–1064. https://doi.org/10.1124/jpet.105.091728
Lozano E, Segarra M, Corbera-Bellalta M, García-Martínez A, Espígol-Frigolé G, Plà-Campo A, Hernández-Rodríguez J, Cid MC (2010) Increased expression of the endothelin system in arterial lesions from patients with giant-cell arteritis: association between elevated plasma endothelin levels and the development of ischaemic events. Ann Rheum Dis 69:434–442. https://doi.org/10.1136/ard.2008.105692
Manchope MF, Calixto-Campos C, Coelho-Silva L, Zarpelon AC, Pinho-Ribeiro FA, Georgetti SR, Baracat MM, Casagrande R, Verri WA Jr (2016) Naringenin inhibits superoxide anion-induced inflammatory pain: role of oxidative stress, cytokines, Nrf-2 and the NO-cGMP-PKG-KATP channel signaling pathway. PLoS One 11(4):e0153015. https://doi.org/10.1371/journal.pone.0153015
Maroon JC, Bost JW, Maroon A (2010) Natural anti-inflammatory agents for pain relief. Surg Neurol Int 1:80. https://doi.org/10.4103/2152-7806.73804
McInnes IB, Schett G (2011) The pathogenesis of rheumatoid arthritis. N Engl J Med 365:2205–2219. https://doi.org/10.1056/NEJMra1004965
Motte S, McEntee K, Naeije R (2006) Endothelin receptor antagonists. Pharm Ther 110:386–414. https://doi.org/10.1016/j.pharmthera.2005.08.012
Mourouzis K, Oikonomou E, Siasos G, Tsalamadris S, Vogiatzi G, Antonopoulos A, Fountoulakis P, Goliopoulou A, Papaioannou S, Tousoulis D (2020) Pro-inflammatory cytokines in acute coronary syndromes. Curr Pharm Des 26:4624–4647. https://doi.org/10.2174/1381612826666200413082353
Muller DN, Mervaala EM, Schmidt F, Park JK, Dechend R, Genersch E, Breu V, Löffler BM, Ganten D, Schneider W, Haller H (2000) Effect of bosentan on NF-κB, inflammation, and tissue factor in angiotensin II–induced end-organ damage. Hypertension 36:282–290. https://doi.org/10.1161/01.HYP.36.2.282
Nakano J, Takizawa H, Ohtoshi T, Shoji S, Yamaguchi M, Ishii A, Yanagisawa M, Ito K (1994) Endotoxin and pro-inflammatory cytokines stimulate endothelin-I expression and release by airway epithelial cells. Clin Exp Allergy 24:330–336. https://doi.org/10.1111/j.1365-2222.1994.tb00243.x
Narazaki M, Tanaka T, Kishimoto T (2017) The role and therapeutic targeting of IL-6 in rheumatoid arthritis. Expert Rev Clin Immunol 13:535–551. https://doi.org/10.1080/1744666X.2017.1295850
Ohtaki HE (2016) In handbook of hormones. Academic Press, Cambrige, pp 326-e36
Olender J, Nowakowska-Zajdel E, Walkiewicz K, Muc-Wierzgoń M (2016) Endothelins and carcinogenesis. Postepy Hig Med Dosw 70:872–880. https://doi.org/10.5604/17322693.1214386
Parida A, Nayak V (2013) Endothelins: Their current status and future prospects. Int J Pharm Sci Rev Res 23:94–97
Peng H, Chen P, Cai Y, Chen Y, Wu QH, Li Y, Zhou R, Fang X (2008) Endothelin-1 increases expression of cyclooxygenase-2 and production of interlukin-8 in hunan pulmonary epithelial cells. Peptides 29:419–424. https://doi.org/10.1016/j.peptides.2007.11.015
Phull AR, Nasir B, ul Haq I, Kim SJ (2018) Oxidative stress, consequences and ROS mediated cellular signaling in rheumatoid arthritis. Chem Biol Interact 281:121–136. https://doi.org/10.1016/j.cbi.2017.12.024
Rautureau Y, Schiffrin EL (2012) Endothelin in hypertension: an update. Curr Opin Nephrol Hypertens 21:128–136. https://doi.org/10.1097/MNH.0b013e32834f0092
Ricciardi R, Schafer BK, Shah SA, Quarfordt SH, Banner BF, Wheeler SM, Donahue SE, Meyers WC, Chari RS (2001) Bosentan, an endothelin antagonist, augments hepatic graft function by reducing graft circulatory impairment following ischemia/reperfusion injury. J Gastrointest Surg 5:322–329. https://doi.org/10.1016/S1091-255X(01)80055-X
Sah SK, Kin B, Park GT, Kim S (2013) Novel isonahocol E3 exhibits anti-inflammatory and anti-angiogenic effects in endothelin-1-stimulated human keratinocytes. Eur J Pharm. https://www.sciencedirect.com/science/article/abs/pii/S0014299913007838
Sánchez A, Martínez P, Muñoz M, Benedito S, García-Sacristán A, Hernández M, Prieto D (2014) Endothelin-1 contributes to endothelial dysfunction and enhanced vasoconstriction through augmented superoxide production in penile arteries from insulin-resistant obese rats: role of ETA and ETB receptors. Br J Pharm 171:5682–5695. https://doi.org/10.1111/bph.12870
Sato A, Ebina K (2013) Common mechanism in endothelin-3 and PAF receptor function for anti-inflammatory responses. Eur J Pharm. https://doi.org/10.1016/j.ejphar.2013.09.025 (Epub. PMID: 24055926)
Shinagawa S, Okazaki T, Ikeda M, Yudoh K, Kisanuki YY, Yanagisawa M, Kawahata K, Ozaki S (2017) T cells upon activation promote endothelin 1 production in monocytes via IFN-γ and TNF-α. Sci Rep 7:1–8. https://doi.org/10.1038/s41598-017-14202-5
Shrestha S, Gracias NG, Mujenda F, Khodorova A, Vasko MR, Strichartz GR (2009) Local antinociception induced by endothelin-1 in the hairy skin of the rat’s back. J Pain 10:702–714. https://doi.org/10.1016/j.jpain.2008.12.005
Sin A, Tang W, Wen CY, Chung SK, Chiu KY (2015) The emerging role of endothelin-1 in the pathogenesis of subchondral bone disturbance and osteoarthritis. Osteoarthr Cartil 23:516–524. https://doi.org/10.1016/j.joca.2014.11.002
Soldano S, Montagna P, Brizzolara R, Trombetta AC, Corallo C, Pizzorni C, Paolino S, Sulli A, Ghio M, Smith V, Giordano N (2017) AB0185 Endothelin-1 stimulates the profibrotic alternative activated phenotype in cultured human macrophages isolated from systemic sclerosis patients. Ann Rheum Dis 76:1111–1111. https://doi.org/10.1136/annrheumdis-2017-eular.5857
Squadrito F, Altavilla D, Morabito N, Crisafulli A, D’Anna R, Corrado F, Ruggeri P, Campo GM, Calapai G, Caputi AP, Squadrito G (2002) The effect of the phytoestrogen genistein on plasma nitric oxide concentrations, endothelin-1 levels and endothelium dependent vasodilation in postmenopausal women. Atherosclerosis 163:339–347. https://doi.org/10.1016/S0021-9150(02)00013-8
Stankowska DL, Krishnamoorthy VR, Ellis DZ, Krishnamoorthy RR (2017) Neuroprotective effects of curcumin on endothelin-1 mediated cell death in hippocampal neurons. Nutr Neurosci. https://doi.org/10.1080/1028415X.2015.1119377 (Epub 2015 Dec 12. PMID: 26651837)
Stow LR, Jacobs ME, Wingo CS, Cain BD (2011) Endothelin-1 gene regulation. FASEB J 25:16–28. https://doi.org/10.1096/fj.10-161612
Tatar A, Yayla M, Kose D, Halici Z, Yoruk O, Polat E (2016) The role of endothelin-1 and endothelin receptor antagonists in allergic rhinitis inflammation: ovalbumin-induced rat model. Rhinology 54:266–272. https://doi.org/10.4193/Rhino15.059
Tchetina EV, Squires G, Poole AR (2005) Increased type II collagen degradation and very early focal cartilage degeneration is associated with upregulation of chondrocyte differentiation related genes in early human articular cartilage lesions. J Rheumatol 32:876–886
Tonari M, Kurimoto T, Horie T et al (2012) Blocking endothelin-B receptors rescues retinal ganglion cells from optic nerve injury through suppression of neuroinflammation. Invest Ophthalmol vis Sci 53:3490–3500
Urbanowicz W, Sogni P, Moreau R (2005) Tezosentan, an endothelin receptor antagonist, limits liver injury in endotoxin challenged cirrhotic rats. Biosci Biotechnol Biochem 69(8):1613–1615. https://doi.org/10.1271/bbb.69.1613
Vercauteren M, Trensz F, Pasquali A, Cattaneo C, Strasser DS, Hess P, Iglarz M, Clozel M (2017) Endothelin ETA receptor blockade, by activating ETB receptors, increases vascular permeability and induces exaggerated fluid retention. J Pharm Exp Ther 361:322–333. https://doi.org/10.1124/jpet.116.234930
Wang R, Dashwood RH (2011) Endothelins and their receptors in cancer: identification of therapeutic targets. Pharm Res 63:519–524. https://doi.org/10.1016/j.phrs.2011.01.002
Wenzel RR, Littke T, Kuranoff S, Jürgens C, Bruck H, Ritz E, Philipp T, Mitchell A. (2009) SPP301 (Avosentan) endothelin antagonist Evaluation in Diabetic Nephropathy Study Investigators. Avosentan reduces albumin excretion in diabetics with macroalbuminuria. J Am Soc Nephrol 20(3):655–64 https://doi.org/10.1681/ASN.2008050482Epub 2009 Jan 14. PMID: 19144760; PMCID: PMC2653691
Wermuth PJ, Li Z, Mendoza FA, Jimenez SA (2016) Stimulation of transforming growth factor-β1-induced endothelial-to-mesenchymal transition and tissue fibrosis by endothelin-1 (ET-1): a novel profibrotic effect of ET-1. PLoS One 11:e0161988. https://doi.org/10.1371/journal.pone.0161988
Wong CT, Rowlands DK, Wong CH, Lo TW, Nguyen GK, Li HY, Tam JP (2012) Orally active peptidic bradykinin B1 receptor antagonists engineered from a cyclotide scaffold for inflammatory pain treatment. Angew Chem Int Ed 51:5620–5624. https://doi.org/10.1002/anie.201200984
Yoshida H, Imafuku Y, Ohhara M, Miyata M, Kasukawa R, Ohsumi K, Horiuchi J (1998) Endothelin-1 production by human synoviocytes. Ann Clin Biochem 35:290–294. https://doi.org/10.1177/000456329803500215
Yu J, Chen X, Li Y, Wang Y, Cao X, Liu Z, Shen B, Zou J, Ding X (2021) Pro-inflammatory cytokines as potential predictors for intradialytic hypotension. Ren Fail 43:198–205. https://doi.org/10.1080/0886022X.2021.1871921
Yuzugulen J. (2016) Characterisation of proendothelin-1 derived peptides and evaluation of their utility as biomarkers of vascular and renal pathologies (Doctoral dissertation, Queen Mary University of London)
Zarpelon AC, Cunha TM, Alves-Filho JC, Pinto LG, Ferreira SH, McInnes IB, Xu D, Liew FY, Cunha FQ, Verri WA Jr (2013) IL-33/ST 2 signalling contributes to carrageenin-induced innate inflammation and inflammatory pain: role of cytokines, endothelin-1 and prostaglandin E 2. Br J Pharm 169:90–101. https://doi.org/10.1111/bph.12110
Zhan S, Rockey DC (2011) Tumor necrosis factor α stimulates endothelin-1 synthesis in rat hepatic stellate cells in hepatic wound healing through a novel IKK/JNK pathway. Exp Cell Res 317:1040–1048. https://doi.org/10.1016/j.yexcr.2010.12.026
Zhang S, Lin X (2019) CARMA3: Scaffold protein involved in NF-κB signaling. Front Immunol. https://doi.org/10.3389/fimmu.2019.00176
Zhao Z, Li E, Cao Q, Sun J, Ma B (2016) Endothelin-1 concentrations are correlated with the severity of knee osteoarthritis. J Investig Med 64:872–874. https://doi.org/10.1002/jcla.22094
Funding
The present study received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization: AS and TB; investigation: AS, TB and SS; writing—original draft preparation: AS, NS and MA; figure-making: HAA and AMM; literature collection: AS and TB: editing: AS: proofreading: LA and SB.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
All authors have given consent for publication of the current article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sehgal, A., Behl, T., Singh, S. et al. Exploring the pivotal role of endothelin in rheumatoid arthritis. Inflammopharmacol 30, 1555–1567 (2022). https://doi.org/10.1007/s10787-022-01051-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-022-01051-6