Abstract
This study investigated the modulatory effects of alkaloid extracts of Cannabis sativa (CSAE), Datura stramonium (DSAE), Nicotiana tabacum (NTAE) and male Carica papaya (CMAE) on neurotransmitter, neurotrophic and neuro-inflammatory systems linked to anxiety and depression. Male Wistar rats were orally administered the alkaloid extracts in doses of 5, 50, 500, and 2000 mg/kg for 90 days. On day 91, neurobehavioural studies were evaluated, rats were sacrificed, brain hippocampus removed and tissue homogenate prepared. Biochemical, cytokine and neurotransmitter metabolisms were estimated in the hippocampus. Expressions of genes linked to anxiety and depression were evaluated by RT-qPCR. Results showed CSAE, NTAE and CMAE act as anxiolytic and antidepressant agents by depleting TNF-α, IL-1β and reactive oxygen species concentrations, and monoamine oxidase, angiotensin 1-converting enzyme and acetylcholinesterase activities while elevating IL-10 and dopamine concentrations and glutamate dehydrogenase activity at doses of 5, 50 and 500. Same doses of CSAE, NTAE and CMAE also depleted the gene expressions of GSK3β, JNK, NF-ĸB, and Nesfatin-1 while increasing expressions of CREB, BDNF, serotonin and Nrf2. However, administration of DSAE and 2000 mg/kg CSAE, NTAE and CMAE had adverse modulatory effects on the neurochemical concentrations and activities as well as the gene expressions of the evaluated neurotransmitter, neurotrophic and inflammatory systems. In conclusion, the study established the sub-chronic instrumentalization potential of CSAE, CMAE, and NTAE for anxiolytic and anti-depressive moods, though their use may be associated with dependence and addiction, which may result in more detrimental effects than any therapeutic potential they may proffer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is the world’s largest mental-health disorder that affects one in six individuals globally irrespective of age, gender, or location (Mineur et al. 2013; Singkhorn et al. 2021). It is characterized by an aversion to activities that affect individual behavior, thoughts, emotions, and sense of wellbeing. Depressed individuals are often anxious, sad, hopeless, empty, worthless, helpless, irritable, guilty, restless, and ashamed (Perviz et al. 2016). Altered feeding patterns, depleted libido or energy levels, disrupted daily and ultradian tempos of activities, body temperature, or several endocrinal functions, and hypersomnia or insomnia are physical changes associated with depression cascades (Tondo et al. 2003). While imbalance in neurotransmitters (acetylcholine, serotonin, dopamine, etc.) systems in the hippocampus, cortex and subcortical regions of the brain indicate the progression of neuronal depressive symptomatology (Yates, 2011). Therefore, drugs that elevate these neurotransmitters levels in the central nervous system are termed, antidepressant agents. Several features of depressive disorder have been observed to overlap with anxiety disorder, with both disorders accounting for about 20 million global suicide attempts every year (Calvó-Perxas et al. 2016; Gadassi and Mor, 2016; Muhammad et al. 2013). About 350 and 264 million people are suffering from depression and anxiety disorder, respectively, ranking them among the leading cause of disability globally (WHO 2017).
Depression is a feature of the psychiatric syndrome, though it may also be a result of a typical response to bereavement, isolation, ailments, drugs, or individual stress load. Other predisposing factors to depression are foods rich in advanced glycation end products, stress, and neuro-inflammation (Stein et al. 2018). Interestingly, neuro-inflammation interacts with neurobiological correlates of depression and anxiety, such as alteration of the hypothalamus–pituitary–adrenal (HPA) axis, dysregulation of the continuous production of dentate gyrus adult-generated neurons, and serotonin depletion in the hippocampus (Troubat et al. 2020). Furthermore, alterations in inflammatory markers (C-reactive protein, tumor necrosis factor α (TNF-α), interleukins 1β, and interleukin 6), have been implicated to enhance depression (Haapakoski et al. 2015; Li et al. 2019). More so, excessive stress is implicated with elevated corticotropin-releasing factor secretion, reactive oxygen species (ROS) levels, and amygdala activities that elicit depressive episodes (Hennessy et al. 2017).
Several people with anxiety and depression disorders most times develop coping tactics to self-control and manage the resulting symptoms as most anxiety and depression disorders’ synthetic drugs have been reported to cause severe adverse effects (Houle et al. 2013; Fedotova et al. 2017; Stelzer et al. 2019). Cogent of the self-management strategy is the use of psychoactive substances to deal with anxiety, hyper-arousal, loss of motivation, and depressive moods (Müller et al. 2021; Picciotto et al. 2015). This type of psychoactive substance use is classified under drug instrumentalization, which is different from the recreational use of the drugs for primarily hedonistic motives. However, the long-term use of drug instrumentalization has been associated with drug dependence and addiction that may require medical action (Müller et al. 2020). Drug instrumentation has been assumed for several psychoactive substances, provided they are used in a nonaddictive approach (Müller et al. 2021). Examples of plant-based psychoactive substances in Nigeria that are employed in drug instrumentalization are Cannabis sativa, Datura stramonium, Nicotiana tabacum, and Carica papaya (male) (Olley, 2007; Jaffe et al. 2013; Perviz et al. 2016; Berlowitz et al. 2020; Fasakin et al. 2021; Shi et al. 2021).
Male rats were preferred for this study due to retrospective observations that males engage in recreational substance use in a ratio of 3:1 compared to their female counterparts in Nigeria (UNODC, 2018). Nevertheless, how the two sexes will differ in response during exposure to C. sativa alkaloid extract (CSAE), D. stramonium alkaloid extract (DSAE), N. tabacum alkaloid extract (NTAE) and C. papaya (male) alkaloid extract (CMAE) certainly need future study. Meanwhile, the ability of antidepressants or depressants to alleviate or induce anxiety-like and depression-like states in healthy subjects has been established (Sarubin et al. 2014), an indication that the alkaloids in the present study will be able to alleviate or induce anxiety-like and depression-like states in normal experimental animals. Additionally, the effects of antidepressants are often delayed, requiring a period of 1–2 months of administration to show efficacy (Jaffe et al. 2013; Lipton et al. 2016). Therefore, the study was designed for 90 days. The ability of CSAE, DSAE, NTAE, and CMAE to modulate anxiety and the depressive-like mood was established in the present study using different behavioral models as well as their ability to modulate the levels of cytokines, neurotransmitters, and proteins linked to anxiety and depression in the hippocampus of experimental rats during a sub-chronic exposure.
Materials and methods
Sample collection and preparation
Cannabis sativa L., Nicotiana tabacum L. and Carica papaya L. leaves, and Datura stramonium L. seeds were purchased from Akure South Local Government Area of Ondo State in Nigeria. The D. stramonium seeds and C. sativa, N. tabacum and C. papaya leaves were authenticated and identified at the Centre for Research and Development (CERAD) unit of the Federal University of Technology, Akure, Nigeria. Plant samples were deposited and voucher numbers 0346, 0347, and 0348 were obtained. After cleansing the leaves and seeds from debris, stalks, and other undesirable materials, they were powered with the aid of an electric blender.
Chemical and reagents
Acetylthiocholine iodide, Hydrogen peroxide, Ellman’s reagent, dopamine hydrochloride, bovine serum albumin (BSA), α-ketoglutarate, glutamic acid, and Coomassie brilliant blue were purchased from Sigma-Aldrich (Germany). Nuclease-free water was purchased from VWR life science (Solon, USA). Primers were purchased from Inqaba Biotechnology (Hatfield, South Africa). TRI Reagent® was obtained from Zymo Research (USA). Luna Universal qPCR Master Mix and ProtoScript II First Strand cDNA Synthesis Kit were bought from BioLabs (New England). All other reagents and chemicals used in this study were purchased from standard sources and of analytical grades.
Alkaloid extracts preparation
Alkaloid extracts from the leaves of Cannabis sativa, Nicotiana tabacum, and Carica papaya and seeds of Datura stramonium were prepared according to standard methods (Fasakin et al. 2021). The alkaloid extracts were named CSAE, DSAE, NTAE, and CMAE to indicate Cannabis sativa, Datura stramonium, Nicotiana tabacum, and male Carica papaya alkaloid extracts respectively.
Experimental protocol
Adult male Wistar Albino rats (181 ± 13 g) were purchased from the Department of Biochemistry Animal House, Federal University of Technology, Akure, Ondo State, Nigeria. Their use and handling were approved at the Centre for Research and Development (CERAD) of the Federal University of Technology, Akure by the Animal Ethical Committee while an ethical number (FUTA/ETH/2020/016) was also given. Wistar Albino rats were housed in stainless steel cages in a room with 12 h light/dark cycle, room temperature (25–27 °C), and relative humidity (60–70%) all through the experiment period. Six rats were randomly housed in each cage to indicate a group and were sectioned as follows: Group 1 served as the normal control. Groups 2 to 5 were orally administered CSAE once daily at doses of 5, 50, 500, and 2000 mg/kg bwt respectively. Groups 6 to 9 were orally administered DSAE once daily at doses of 5, 50, 500, and 2000 mg/kg bwt respectively. Groups 10 to 13 were orally administered NTAE once daily at doses of 5, 50, 500, and 2000 mg/kg bwt respectively. Groups 14 to 17 were orally administered CMAE once daily at doses of 5, 50, 500, and 2000 mg/kg bwt respectively (WHO 2000; Fasakin et al. 2022). Experimental animals were allowed free access to water and rat chow ad libitum for the whole period of the experiment.
Behavioral tests
Experimental animals were exposed to Elevated Plus-Maze, Forced Swim and Tail suspension Tests before the commencement of the experiment to ascertain their cognitive status. After 90 days of CSAE, DSAE, NTAE, and CMAE oral administration, the experimental animals were re-exposed to the aforementioned behavioral tests to ascertain the anxiolytic and antidepressant ability of the extracts.
Elevated plus-maze test
For the elevated plus maze test (EPM), the number and time of entries of experimental rats in closed and open arms were documented. This behavioral model for anxiety is based on the hypothesis that rodents will naturally avoid high and open spaces (Lister 1987; Pellow et al. 1985). EPM test was carried out using four arms (30 cm by 7 cm) black Plexiglas apparatus elevated 50 cm above ground level. Two arms were enclosed (using 20 cm high walls) while the other two arms were opened. Wistar rats were positioned at the center of the EPM initially and allowed to freely explore the four arms for 5 min. Ethyl alcohol (70%) was used to purify the apparatus to minimize potential unspecific scents before testing. All EPM sessions were video-recorded and analyzed thereafter to evaluate the anxiousness of each experimental animal using the number of entries and time spent in the open arms.
Tail suspension test (FST)
Tail suspension test (FST) was carried out to evaluate the antidepressant potentials of CSAE, DSAE, NTAE, and CMAE according to modified methods of Cryan et al. (2005). Experimental rats were hung from mounted blunt hooks via adhesive tape at 1 cm from the rat tail’s tip with the rats’ nose positioned at about 50 cm from padded chamber floor and investigated for 6 min. TST sessions were video-recorded and analyzed thereafter to evaluate immobility time for each experimental animal. Immobility was evaluated as the sum of total time the rat hanged passively during the last 4 min of the experimental 6 min (total time) of monitoring.
Forced swimming test (FST)
Forced swimming test (FST) was carried out to evaluate the antidepressant potentials of CSAE, DSAE, NTAE, and CMAE according to modified methods of Porsolt et al. (1977). A glass vessel of 25 cm (height) by 15 cm (diameter) was half-filled with water at room temperature and kept for 24 h before the 5 min forced swimming test. All FST sessions were video-recorded and analyzed thereafter to evaluate immobility time and swimming activity for each experimental animal. Experimental animals were considered immobile when they stop efforts in attempting escape (desperation) though still engage in movements required to keep the animals' heads above water level (survival). After each experiment, the experimental animals were cloth-dried and returned to their respective cages.
Tissue homogenate preparation
After 91 days of oral alkaloid administration, CSAE, DSAE, NTAE, and CMAE exposed animals were anesthetized with mild diethyl ether. The experimental animals were then sacrificed while the whole brain was excised, washed quickly with a cold saline solution while the hippocampus was carefully sectioned out, and stored at − 80 °C for consequent analysis.
Gene expression analysis via real-time quantitative polymerase (RT-qPCR) reaction
TRI Reagent® was employed to extract total RNA from the hippocampal tissues. cDNA was synthesized by reverse transcriptase reaction using 1 μg of the extracted RNA samples in a ProtoScript II First Strand cDNA Synthesis Kit (BioLabs, New England) via a 3-step reaction condition: 65 °C for 5 min, 42 °C for 60 min, and 80 °C for 5 min. Primers to rat cDNA used for PCR were obtained from Inqaba Biotec, Hatfield, SA, and are listed in Table 1. Real-time quantitative PCR (qPCR) was executed using the Luna Universal qPCR Master Mix (BioLabs, New England) on a StepOnePlus Applied Biosystem qPCR System following the manufacturer’s procedures. PCR settings were as follows: 95 °C for 180 s, 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s. The method of comparative cycle threshold (DDCT) was employed to quantify the relative cDNA amount while the β-actin gene was employed to standardize the relative expression level of the respective gene.
Biochemical analysis
Hippocampal total protein content was evaluated using Coomassie blue method using bovine serum albumin as standard (Bradford 1976). Monoamine oxidase (MAO) activity was evaluated using 0.0125 mol/L semi-carbazides, 0.025 mol/L phosphate buffer (pH 7.0), 10 mmol/L benzylamines, acetic acid, 2, 4-Dinitrophenylhydrazine, benzene, and 0.1 N NaOH, while the developed orange–yellow color was assessed at 450 nm (Green and Haughton 1961). Dopamine concentrations were measured using 5 mM FeCl3, 5 mM potassium ferricyanide, and phosphate buffer (pH 4.0). Dopamine concentration was extrapolated from the dopamine hydrochloride calibration curve by plotting measured absorbance at 735 nm against varying dopamine hydrochloride concentrations (Guo et al. 2009). Angiotensin 1-converting enzyme (ACE) activity was evaluated using the substrate, HHL (hippuryl-l-histidyl l-leucine, while the resulting hippuric acid (Bz-Gly) concentrations were evaluated to assess the activity of ACE at 228 nm (Cushman and Cheung 1971). Acetylcholinesterase (AChE) activity was evaluated using 5,5ʹ-dithiobis (2-nitrobenzoic) acid (3.3 mM) prepared by 0.1 M buffer (pH 7.0) constituting NaHCO3. Substrate used was AChE while absorbance was assessed at 412 nm at intervals of 30, 60, 90, 120, 150, and 180 secs (Perry et al. 2000). Glutamate dehydrogenase (GDH) activity was evaluated using Tris–HCl (pH 8.3), nicotinamide adenine dinucleotide phosphate reduced, ammonium chloride and α-ketoglutarate while absorbance was measured at 340 nm at intervals of 60, 120, 180, 240 and 300 secs (Abdel-Zaher et al. 2011). Reactive oxygen species (ROS) concentrations were assessed using ferrous sulfate (4.37 µM) prepared in 0.1 M sodium acetate (pH 4.8) and N-N-diethyl-para-phenylenediamine (DEPPD) (6 mg/ml). H2O2 production was employed to measure ROS concentrations at 505 nm (Hayashi et al. 2007). The concentrations of interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and interleukin 10 (IL-10) in the hippocampus of the experimental animals were evaluated using commercially available Enzyme-Linked Immunosorbent Assay (ELISA) kits (Phoenix Pharmaceuticals Inc, Burlingame, CA, USA) as described in the manufacturer’s manual.
Gas chromatograph–mass spectrometry analysis of alkaloid-rich extracts
Qualitative characterization analyses of probable compounds in the alkaloid extracts of C. sativa, D. stramonium, N. tabacum and C. papaya (male) were carried out via a Varian 3800/4000 GCMS furnished with an Agilent equipped with a capillary column DB5ms (30.0 m × 0.25 mm, 0.25 μm film thickness) as previously described by Ademiluyi et al. (2016).
Statistical analysis
In the present study, experimental data were checked for normality patterns using Kolmogorov–Smirnov test before statistical analysis. The data were then analyzed by one-way ANOVA using GraphPad version 8.0.2. followed by Tukey’s test. Results were expressed at mean ± SEM (standard error of the mean) (n = 6) and considered significantly different at p ˂ 0.05.
Results
The gas chromatograph–mass spectrometry analysis of CSAE, DSAE, NTAE, and CMAE
Figures 1, 2, 3, 4 revealed the GC–MS characterization of the alkaloid-rich extract from Cannabis sativa, Datura stramonium, Nicotiana tabacum and Carica papaya. The observed alkaloid constituents of the alkaloid-rich extracts are listed in Tables 1, 2, 3, 4. Alkaloid extracts of Cannabis sativa revealed the presence of Choline, Trigonelline, Dopamine, Hordenine, Cannabidiol, Δ-9-tetrahydrocannabinol, Pyridine, 3-(1-methyl-2-pyrrolidinyl)-, (S)-, Morphine, D-Lysergic acid, Boldine, Strychnine, Anhydrocannabisativine, Cannabisativine, Cannabimine C and Aconitine. Alkaloid extracts of Datura stramonium revealed the presence of 2-Pyrrolidinone, 8-Methyl-8-azabicyclo[3.2.1]octane-2,6-diol, Ecgonine ethyl ester, Benzeneacetic acid, Dasycarpidan-1-methanol, acetate (ester), Anisodine, Littorine, Hyoscyamine, Atropine, 2,7-Diphenyl-1,6-dioxopyridazino[4,5:2,3] pyrrolo[4,5-d]pyridazine, 17-(1,5-Dimethylhexyl)-10,13-dimethyl-4-vinylhexadecahydrocyclopenta[a]phenanthren-3-ol, 3-(1,5-Dimethyl-hexyl)3a,10,10,12b-tetramethyl1,2,3,3a4,6,8,9,10,10a,11,12,12a,12b-tetradecahydro-benzo[4,5] Cyclohept, 3,8,8-Trimethoxy-3-piperidyl-2,2-benaphthalene-1,1,4,4-tetrone, Ethyl iso-allocholate, Scopolamine, [5β]Pregnane-3,20beta-diol, 14alpha,18alpha-[4-methyl-3-oxo-(1-oxa-4-azabutane-1,4-diyl)]-, and diacetate, 1-Monolinolein. Alkaloid extracts of Nicotiana tabacum revealed the presence of Pyridine, Anatabine, Quinoline, Nicotine, Nicotyrine, Nornicotyrine, 2,2'-Bipyridine, Anabasine, 2,3'-Dipyridyl, Pyridine, 3-(1-methyl-2-pyrrolidinyl)-, (S)-, Cotinine, N-Nitrosonornicotine, Nornicotine, N-formyl, 2-Pyrrolidinone and Nornicotine, N-acetyl. Alkaloid extracts of male Carica papaya revealed the presence of choline, 8-Methyl-8-azabicyclo [3.2.1] octane-2,6-diol, Benzylthiourea, 3,3-Diethyl-5-methyl piperidine-2,4,6-trione, Anonaine, Trigonelline, +)-Laurotetanine, Pseudocarpaine, Carpaine, Alstoniaphylline C, ( +)-alstonisine, Dehydrocarpaine I and Dehydrocarpaine II (Table 5).
The modulatory effects of CSAE, DSAE, NTAE, and CMAE in rat neurobehavioural models of depression and anxiety
Results showed that experimental rats orally administered CSAE, NTAE, and CMAE at 5, 50 and 500 mg/kg bwt spent significantly (P < 0.0001; F(16, 85) = 60.35) longer time in the open arms of EPM in comparison to the control group rats (Fig. 5a). In parallel with time spent in open arms of EPM, there were also higher entries’ frequencies (P < 0.0001; F(16, 85) = 24.39) into the open arms compared to control rats (Fig. 5b). The EPM result of the present study may indicate the anxiolytic effects of the alkaloid extracts. However, exposure to DSAE had induced opposite effects by reducing time spent and the number of entries into open arms compared to the control group. During TST, a significant decrease in immobility time (P < 0.0001; F(16, 85) = 125.4) was observed after sub-chronic exposure to CSAE, NTAE, and CMAE at 5, 50, and 500 mg/kg bwt (Fig. 6). However, at 2000 mg/kg bwt and exposure to DSAE, the experimental animals showed higher immobility time compared to normal control rats. FST was also employed, and we observed a significant decrease in immobility time and an increase in swimming time (P < 0.0001; F(16, 85) = 57.51) and (P < 0.0001; F(16, 85) = 71.43) after a sub-chronic exposure to CSAE, NTAE, and CMAE at 5, 50, and 500 mg/kg bwt (Fig. 7a, b) which were absent in normal control rats. However, exposure to DSAE and 2000 mg/kg bwt of CSAE, NTAE, and CMAE increased the immobility time and decreased the swimming time. This suggested that 90 days of exposure to CSAE, DSAE, NTAE, and CMAE can alter the depressive-like behaviors of experimental animals.
a Effects of CSAE, DSAE, NTAE, and CMAE on Time Spent in Open Arms (secs) of Elevated Plus Maze. Results are expressed as mean ± SEM (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the normal control group. CSAE5—orally administered alkaloid extracts of C. sativa at 5 mg/kg bwt; CSAE50—orally administered alkaloid extracts of C. sativa at 50 mg/kg bwt; CSAE500—orally administered alkaloid extracts of C. sativa at 500 mg/kg bwt; CSAE2000—orally administered alkaloid extracts of C. sativa at 2000 mg/kg bwt; DSAE5—orally administered alkaloid extracts of D. stramonium at 5 mg/kg bwt; DSAE50—orally administered alkaloid extracts of D. stramonium at 50 mg/kg bwt; DSAE500—orally administered alkaloid extracts of D. stramonium at 500 mg/kg bwt; DSAE2000—orally administered alkaloid extracts of D. stramonium at 2000 mg/kg bwt; NTAE5—orally administered alkaloid extracts of N. tabacum at 5 mg/kg bwt; NTAE50—orally administered alkaloid extracts of N. tabacum at 50 mg/kg bwt; NTAE500—orally administered alkaloid extracts of N. tabacum at 500 mg/kg bwt; NTAE2000—orally administered alkaloid extracts of N. tabacum at 2000 mg/kg bwt; CMAE5—orally administered alkaloid extracts of C. papaya at 5 mg/kg bwt; CMAE50—orally administered alkaloid extracts of C. papaya at 50 mg/kg bwt; CMAE500—orally administered alkaloid extracts of C. papaya at 500 mg/kg bwt; CMAE2000—orally administered alkaloid extracts of C. papaya at 2000 mg/kg bwt. b Effects of CSAE, DSAE, NTAE, and CMAE on Number of Entries (frequency) of Elevated Plus Maze. Results are expressed as mean ± SEM (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the normal control group. CSAE5—orally administered alkaloid extracts of C. sativa at 5 mg/kg bwt; CSAE50—orally administered alkaloid extracts of C. sativa at 50 mg/kg bwt; CSAE500—orally administered alkaloid extracts of C. sativa at 500 mg/kg bwt; CSAE2000—orally administered alkaloid extracts of C. sativa at 2000 mg/kg bwt; DSAE5—orally administered alkaloid extracts of D. stramonium at 5 mg/kg bwt; DSAE50—orally administered alkaloid extracts of D. stramonium at 50 mg/kg bwt; DSAE500—orally administered alkaloid extracts of D. stramonium at 500 mg/kg bwt; DSAE2000—orally administered alkaloid extracts of D. stramonium at 2000 mg/kg bwt; NTAE5—orally administered alkaloid extracts of N. tabacum at 5 mg/kg bwt; NTAE50—orally administered alkaloid extracts of N. tabacum at 50 mg/kg bwt; NTAE500—orally administered alkaloid extracts of N. tabacum at 500 mg/kg bwt; NTAE2000—orally administered alkaloid extracts of N. tabacum at 2000 mg/kg bwt; CMAE5—orally administered alkaloid extracts of C. papaya at 5 mg/kg bwt; CMAE50—orally administered alkaloid extracts of C. papaya at 50 mg/kg bwt; CMAE500—orally administered alkaloid extracts of C. papaya at 500 mg/kg bwt; CMAE2000—orally administered alkaloid extracts of C. papaya at 2000 mg/kg bwt
Effects of CSAE, DSAE, NTAE, and CMAE on Immobility Time during Tail Suspension Test. Results are expressed as mean ± SEM (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the normal control group. CSAE5—orally administered alkaloid extracts of C. sativa at 5 mg/kg bwt; CSAE50—orally administered alkaloid extracts of C. sativa at 50 mg/kg bwt; CSAE500—orally administered alkaloid extracts of C. sativa at 500 mg/kg bwt; CSAE2000—orally administered alkaloid extracts of C. sativa at 2000 mg/kg bwt; DSAE5—orally administered alkaloid extracts of D. stramonium at 5 mg/kg bwt; DSAE50—orally administered alkaloid extracts of D. stramonium at 50 mg/kg bwt; DSAE500—orally administered alkaloid extracts of D. stramonium at 500 mg/kg bwt; DSAE2000—orally administered alkaloid extracts of D. stramonium at 2000 mg/kg bwt; NTAE5—orally administered alkaloid extracts of N. tabacum at 5 mg/kg bwt; NTAE50—orally administered alkaloid extracts of N. tabacum at 50 mg/kg bwt; NTAE500—orally administered alkaloid extracts of N. tabacum at 500 mg/kg bwt; NTAE2000—orally administered alkaloid extracts of N. tabacum at 2000 mg/kg bwt; CMAE5—orally administered alkaloid extracts of C. papaya at 5 mg/kg bwt; CMAE50—orally administered alkaloid extracts of C. papaya at 50 mg/kg bwt; CMAE500—orally administered alkaloid extracts of C. papaya at 500 mg/kg bwt; CMAE2000—orally administered alkaloid extracts of C. papaya at 2000 mg/kg bwt
Effects of CSAE, DSAE, NTAE, and CMAE on Immobility Time during Force Swim Test. Results are expressed as mean ± SEM (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the normal control group. CSAE5—orally administered alkaloid extracts of C. sativa at 5 mg/kg bwt; CSAE50—orally administered alkaloid extracts of C. sativa at 50 mg/kg bwt; CSAE500—orally administered alkaloid extracts of C. sativa at 500 mg/kg bwt; CSAE2000—orally administered alkaloid extracts of C. sativa at 2000 mg/kg bwt; DSAE5—orally administered alkaloid extracts of D. stramonium at 5 mg/kg bwt; DSAE50—orally administered alkaloid extracts of D. stramonium at 50 mg/kg bwt; DSAE500—orally administered alkaloid extracts of D. stramonium at 500 mg/kg bwt; DSAE2000—orally administered alkaloid extracts of D. stramonium at 2000 mg/kg bwt; NTAE5—orally administered alkaloid extracts of N. tabacum at 5 mg/kg bwt; NTAE50—orally administered alkaloid extracts of N. tabacum at 50 mg/kg bwt; NTAE500—orally administered alkaloid extracts of N. tabacum at 500 mg/kg bwt; NTAE2000—orally administered alkaloid extracts of N. tabacum at 2000 mg/kg bwt; CMAE5—orally administered alkaloid extracts of C. papaya at 5 mg/kg bwt; CMAE50—orally administered alkaloid extracts of C. papaya at 50 mg/kg bwt; CMAE500—orally administered alkaloid extracts of C. papaya at 500 mg/kg bwt; CMAE2000—orally administered alkaloid extracts of C. papaya at 2000 mg/kg bwt. b Effects of CSAE, DSAE, NTAE, and CMAE on Swimming Time during Force Swim Test. Results are expressed as mean ± SEM (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the normal control group. CSAE5—orally administered alkaloid extracts of C. sativa at 5 mg/kg bwt; CSAE50—orally administered alkaloid extracts of C. sativa at 50 mg/kg bwt; CSAE500—orally administered alkaloid extracts of C. sativa at 500 mg/kg bwt; CSAE2000—orally administered alkaloid extracts of C. sativa at 2000 mg/kg bwt; DSAE5—orally administered alkaloid extracts of D. stramonium at 5 mg/kg bwt; DSAE50—orally administered alkaloid extracts of D. stramonium at 50 mg/kg bwt; DSAE500—orally administered alkaloid extracts of D. stramonium at 500 mg/kg bwt; DSAE2000—orally administered alkaloid extracts of D. stramonium at 2000 mg/kg bwt; NTAE5—orally administered alkaloid extracts of N. tabacum at 5 mg/kg bwt; NTAE50—orally administered alkaloid extracts of N. tabacum at 50 mg/kg bwt; NTAE500—orally administered alkaloid extracts of N. tabacum at 500 mg/kg bwt; NTAE2000—orally administered alkaloid extracts of N. tabacum at 2000 mg/kg bwt; CMAE5—orally administered alkaloid extracts of C. papaya at 5 mg/kg bwt; CMAE50—orally administered alkaloid extracts of C. papaya at 50 mg/kg bwt; CMAE500—orally administered alkaloid extracts of C. papaya at 500 mg/kg bwt; CMAE2000—orally administered alkaloid extracts of C. papaya at 2000 mg/kg bwt
The modulatory effects of CSAE, DSAE, NTAE, and CMAE on neurotransmitter systems linked to anxiety and depression
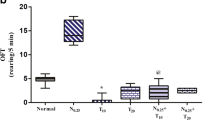
Results showed significant disturbances in neurotransmitter metabolism after sub-chronic exposure to CSAE, DSAE, NTAE, and CMAE. A significant alteration in the activities of MAO (P < 0.0001; F (16, 85) = 55.88), Glutamate dehydrogenase (P < 0.0001; F(16, 85) = 100.3), ACE (P < 0.0001; F(16, 85) = 266.0), and AChE (P < 0.0001; F(16, 85) = 348.0) were observed in the experimental animals after sub-chronic exposure to CSAE, DSAE, NTAE, and CMAE (Figs. 8, 9, 10, 11). Meanwhile, the level of dopamine (P < 0.0001; F(16, 85) = 132.1) showed a biphasic dose–response curve after sub-chronic exposure to CSAE, DSAE, NTAE, and CMAE (Fig. 12).
Effects of CSAE, DSAE, NTAE, and CMAE on Monoamine Oxidase Activities. Results are expressed as mean ± SEM (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the normal control group. CSAE5—orally administered alkaloid extracts of C. sativa at 5 mg/kg bwt; CSAE50—orally administered alkaloid extracts of C. sativa at 50 mg/kg bwt; CSAE500—orally administered alkaloid extracts of C. sativa at 500 mg/kg bwt; CSAE2000—orally administered alkaloid extracts of C. sativa at 2000 mg/kg bwt; DSAE5—orally administered alkaloid extracts of D. stramonium at 5 mg/kg bwt; DSAE50—orally administered alkaloid extracts of D. stramonium at 50 mg/kg bwt; DSAE500—orally administered alkaloid extracts of D. stramonium at 500 mg/kg bwt; DSAE2000—orally administered alkaloid extracts of D. stramonium at 2000 mg/kg bwt; NTAE5—orally administered alkaloid extracts of N. tabacum at 5 mg/kg bwt; NTAE50—orally administered alkaloid extracts of N. tabacum at 50 mg/kg bwt; NTAE500—orally administered alkaloid extracts of N. tabacum at 500 mg/kg bwt; NTAE2000—orally administered alkaloid extracts of N. tabacum at 2000 mg/kg bwt; CMAE5—orally administered alkaloid extracts of C. papaya at 5 mg/kg bwt; CMAE50—orally administered alkaloid extracts of C. papaya at 50 mg/kg bwt; CMAE500—orally administered alkaloid extracts of C. papaya at 500 mg/kg bwt; CMAE2000—orally administered alkaloid extracts of C. papaya at 2000 mg/kg bwt
Effects of CSAE, DSAE, NTAE, and CMAE on Glutamate Dehydrogenase Activities. Results are expressed as mean ± SEM (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the normal control group. CSAE5—orally administered alkaloid extracts of C. sativa at 5 mg/kg bwt; CSAE50—orally administered alkaloid extracts of C. sativa at 50 mg/kg bwt; CSAE500—orally administered alkaloid extracts of C. sativa at 500 mg/kg bwt; CSAE2000—orally administered alkaloid extracts of C. sativa at 2000 mg/kg bwt; DSAE5—orally administered alkaloid extracts of D. stramonium at 5 mg/kg bwt; DSAE50—orally administered alkaloid extracts of D. stramonium at 50 mg/kg bwt; DSAE500—orally administered alkaloid extracts of D. stramonium at 500 mg/kg bwt; DSAE2000—orally administered alkaloid extracts of D. stramonium at 2000 mg/kg bwt; NTAE5—orally administered alkaloid extracts of N. tabacum at 5 mg/kg bwt; NTAE50—orally administered alkaloid extracts of N. tabacum at 50 mg/kg bwt; NTAE500—orally administered alkaloid extracts of N. tabacum at 500 mg/kg bwt; NTAE2000—orally administered alkaloid extracts of N. tabacum at 2000 mg/kg bwt; CMAE5—orally administered alkaloid extracts of C. papaya at 5 mg/kg bwt; CMAE50—orally administered alkaloid extracts of C. papaya at 50 mg/kg bwt; CMAE500—orally administered alkaloid extracts of C. papaya at 500 mg/kg bwt; CMAE2000—orally administered alkaloid extracts of C. papaya at 2000 mg/kg bwt
Effects of CSAE, DSAE, NTAE, and CMAE on Angiotensin-1 Converting Enzyme Activities. Results are expressed as mean ± SEM (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the normal control group. CSAE5—orally administered alkaloid extracts of C. sativa at 5 mg/kg bwt; CSAE50—orally administered alkaloid extracts of C. sativa at 50 mg/kg bwt; CSAE500—orally administered alkaloid extracts of C. sativa at 500 mg/kg bwt; CSAE2000—orally administered alkaloid extracts of C. sativa at 2000 mg/kg bwt; DSAE5—orally administered alkaloid extracts of D. stramonium at 5 mg/kg bwt; DSAE50—orally administered alkaloid extracts of D. stramonium at 50 mg/kg bwt; DSAE500—orally administered alkaloid extracts of D. stramonium at 500 mg/kg bwt; DSAE2000—orally administered alkaloid extracts of D. stramonium at 2000 mg/kg bwt; NTAE5—orally administered alkaloid extracts of N. tabacum at 5 mg/kg bwt; NTAE50—orally administered alkaloid extracts of N. tabacum at 50 mg/kg bwt; NTAE500—orally administered alkaloid extracts of N. tabacum at 500 mg/kg bwt; NTAE2000—orally administered alkaloid extracts of N. tabacum at 2000 mg/kg bwt; CMAE5—orally administered alkaloid extracts of C. papaya at 5 mg/kg bwt; CMAE50—orally administered alkaloid extracts of C. papaya at 50 mg/kg bwt; CMAE500—orally administered alkaloid extracts of C. papaya at 500 mg/kg bwt; CMAE2000—orally administered alkaloid extracts of C. papaya at 2000 mg/kg bwt
Effects of CSAE, DSAE, NTAE, and CMAE on Acetylcholinesterase Activities. Results are expressed as mean ± SEM (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the normal control group. CSAE5—orally administered alkaloid extracts of C. sativa at 5 mg/kg bwt; CSAE50—orally administered alkaloid extracts of C. sativa at 50 mg/kg bwt; CSAE500—orally administered alkaloid extracts of C. sativa at 500 mg/kg bwt; CSAE2000—orally administered alkaloid extracts of C. sativa at 2000 mg/kg bwt; DSAE5—orally administered alkaloid extracts of D. stramonium at 5 mg/kg bwt; DSAE50—orally administered alkaloid extracts of D. stramonium at 50 mg/kg bwt; DSAE500—orally administered alkaloid extracts of D. stramonium at 500 mg/kg bwt; DSAE2000—orally administered alkaloid extracts of D. stramonium at 2000 mg/kg bwt; NTAE5—orally administered alkaloid extracts of N. tabacum at 5 mg/kg bwt; NTAE50—orally administered alkaloid extracts of N. tabacum at 50 mg/kg bwt; NTAE500—orally administered alkaloid extracts of N. tabacum at 500 mg/kg bwt; NTAE2000—orally administered alkaloid extracts of N. tabacum at 2000 mg/kg bwt; CMAE5—orally administered alkaloid extracts of C. papaya at 5 mg/kg bwt; CMAE50—orally administered alkaloid extracts of C. papaya at 50 mg/kg bwt; CMAE500—orally administered alkaloid extracts of C. papaya at 500 mg/kg bwt; CMAE2000—orally administered alkaloid extracts of C. papaya at 2000 mg/kg bwt
Effects of CSAE, DSAE, NTAE, and CMAE on Dopamine Level. Results are expressed as mean ± SEM (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the normal control group. CSAE5—orally administered alkaloid extracts of C. sativa at 5 mg/kg bwt; CSAE50—orally administered alkaloid extracts of C. sativa at 50 mg/kg bwt; CSAE500—orally administered alkaloid extracts of C. sativa at 500 mg/kg bwt; CSAE2000—orally administered alkaloid extracts of C. sativa at 2000 mg/kg bwt; DSAE5—orally administered alkaloid extracts of D. stramonium at 5 mg/kg bwt; DSAE50—orally administered alkaloid extracts of D. stramonium at 50 mg/kg bwt; DSAE500—orally administered alkaloid extracts of D. stramonium at 500 mg/kg bwt; DSAE2000—orally administered alkaloid extracts of D. stramonium at 2000 mg/kg bwt; NTAE5—orally administered alkaloid extracts of N. tabacum at 5 mg/kg bwt; NTAE50—orally administered alkaloid extracts of N. tabacum at 50 mg/kg bwt; NTAE500—orally administered alkaloid extracts of N. tabacum at 500 mg/kg bwt; NTAE2000—orally administered alkaloid extracts of N. tabacum at 2000 mg/kg bwt; CMAE5—orally administered alkaloid extracts of C. papaya at 5 mg/kg bwt; CMAE50—orally administered alkaloid extracts of C. papaya at 50 mg/kg bwt; CMAE500—orally administered alkaloid extracts of C. papaya at 500 mg/kg bwt; CMAE2000—orally administered alkaloid extracts of C. papaya at 2000 mg/kg bwt
The modulatory effects of CSAE, DSAE, NTAE, and CMAE on inflammatory markers linked to anxiety and depression
Results showed the modulatory effect of CSAE, DSAE, NTAE, and CMAE on pro-inflammatory (TNF-α and IL-1β) and anti-inflammatory (IL-10) cytokines after sub-chronic exposure (Figs. 13, 14, 15). Interestingly, CSAE, NTAE, and CMAE depleted TNF-α (P < 0.0001; F(16, 85) = 62.6) and IL-1β (P < 0.0001; F(16, 85) = 99.04) concentrations while increasing IL-10 concentrations (P < 0.0001; F(16, 85) = 64.77). However, DSAE elevated TNF-α (P < 0.0001; F(16, 85) = 62.6) and IL-1β (P < 0.0001; F(16, 85) = 99.04) concentrations while decreasing IL-10 concentrations (P < 0.0001; F(16, 85) = 64.77).
Effects of CSAE, DSAE, NTAE, and CMAE on Interleukin-1β (IL-1β) concentrations. Results are expressed as mean ± SEM (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the normal control group. CSAE5—orally administered alkaloid extracts of C. sativa at 5 mg/kg bwt; CSAE50—orally administered alkaloid extracts of C. sativa at 50 mg/kg bwt; CSAE500—orally administered alkaloid extracts of C. sativa at 500 mg/kg bwt; CSAE2000—orally administered alkaloid extracts of C. sativa at 2000 mg/kg bwt; DSAE5—orally administered alkaloid extracts of D. stramonium at 5 mg/kg bwt; DSAE50—orally administered alkaloid extracts of D. stramonium at 50 mg/kg bwt; DSAE500—orally administered alkaloid extracts of D. stramonium at 500 mg/kg bwt; DSAE2000—orally administered alkaloid extracts of D. stramonium at 2000 mg/kg bwt; NTAE5—orally administered alkaloid extracts of N. tabacum at 5 mg/kg bwt; NTAE50—orally administered alkaloid extracts of N. tabacum at 50 mg/kg bwt; NTAE500—orally administered alkaloid extracts of N. tabacum at 500 mg/kg bwt; NTAE2000—orally administered alkaloid extracts of N. tabacum at 2000 mg/kg bwt; CMAE5—orally administered alkaloid extracts of C. papaya at 5 mg/kg bwt; CMAE50—orally administered alkaloid extracts of C. papaya at 50 mg/kg bwt; CMAE500—orally administered alkaloid extracts of C. papaya at 500 mg/kg bwt; CMAE2000—orally administered alkaloid extracts of C. papaya at 2000 mg/kg bwt
Effects of CSAE, DSAE, NTAE, and CMAE on Tumor Necrosis Factor-α (TNF-α) concentrations. Results are expressed as mean ± SEM (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the normal control group. CSAE5—orally administered alkaloid extracts of C. sativa at 5 mg/kg bwt; CSAE50—orally administered alkaloid extracts of C. sativa at 50 mg/kg bwt; CSAE500—orally administered alkaloid extracts of C. sativa at 500 mg/kg bwt; CSAE2000—orally administered alkaloid extracts of C. sativa at 2000 mg/kg bwt; DSAE5—orally administered alkaloid extracts of D. stramonium at 5 mg/kg bwt; DSAE50—orally administered alkaloid extracts of D. stramonium at 50 mg/kg bwt; DSAE500—orally administered alkaloid extracts of D. stramonium at 500 mg/kg bwt; DSAE2000—orally administered alkaloid extracts of D. stramonium at 2000 mg/kg bwt; NTAE5—orally administered alkaloid extracts of N. tabacum at 5 mg/kg bwt; NTAE50—orally administered alkaloid extracts of N. tabacum at 50 mg/kg bwt; NTAE500—orally administered alkaloid extracts of N. tabacum at 500 mg/kg bwt; NTAE2000—orally administered alkaloid extracts of N. tabacum at 2000 mg/kg bwt; CMAE5—orally administered alkaloid extracts of C. papaya at 5 mg/kg bwt; CMAE50—orally administered alkaloid extracts of C. papaya at 50 mg/kg bwt; CMAE500—orally administered alkaloid extracts of C. papaya at 500 mg/kg bwt; CMAE2000—orally administered alkaloid extracts of C. papaya at 2000 mg/kg bwt
Effects of CSAE, DSAE, NTAE, and CMAE on Interleukin 10 (IL-10) concentrations. Results are expressed as mean ± SEM (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the normal control group. CSAE5—orally administered alkaloid extracts of C. sativa at 5 mg/kg bwt; CSAE50—orally administered alkaloid extracts of C. sativa at 50 mg/kg bwt; CSAE500—orally administered alkaloid extracts of C. sativa at 500 mg/kg bwt; CSAE2000—orally administered alkaloid extracts of C. sativa at 2000 mg/kg bwt; DSAE5—orally administered alkaloid extracts of D. stramonium at 5 mg/kg bwt; DSAE50—orally administered alkaloid extracts of D. stramonium at 50 mg/kg bwt; DSAE500—orally administered alkaloid extracts of D. stramonium at 500 mg/kg bwt; DSAE2000—orally administered alkaloid extracts of D. stramonium at 2000 mg/kg bwt; NTAE5—orally administered alkaloid extracts of N. tabacum at 5 mg/kg bwt; NTAE50—orally administered alkaloid extracts of N. tabacum at 50 mg/kg bwt; NTAE500—orally administered alkaloid extracts of N. tabacum at 500 mg/kg bwt; NTAE2000—orally administered alkaloid extracts of N. tabacum at 2000 mg/kg bwt; CMAE5—orally administered alkaloid extracts of C. papaya at 5 mg/kg bwt; CMAE50—orally administered alkaloid extracts of C. papaya at 50 mg/kg bwt; CMAE500—orally administered alkaloid extracts of C. papaya at 500 mg/kg bwt; CMAE2000—orally administered alkaloid extracts of C. papaya at 2000 mg/kg bwt
The modulatory effects of CSAE, DSAE, NTAE, and CMAE on redox stress
Results showed the ability of CSAE, DSAE, NTAE, and CMAE to modulate ROS production after sub-chronic exposure (Fig. 16). Interestingly, sub-chronic exposure to CSAE, NTAE, and CMAE inhibited ROS production at 5, 50 and 500 mg/kg bwt (P < 0.0001; F(16, 85) = 260.0). However, DSAE and 2000 mg/kg bwt of CSAE, NTAE, and CMAE elevated ROS production (P < 0.0001; F(16, 85) = 260.0).
Effects of CSAE, DSAE, NTAE, and CMAE on Reactive Oxygen Species (ROS) Production. Results are expressed as mean ± SEM (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the normal control group. CSAE5—orally administered alkaloid extracts of C. sativa at 5 mg/kg bwt; CSAE50—orally administered alkaloid extracts of C. sativa at 50 mg/kg bwt; CSAE500—orally administered alkaloid extracts of C. sativa at 500 mg/kg bwt; CSAE2000—orally administered alkaloid extracts of C. sativa at 2000 mg/kg bwt; DSAE5—orally administered alkaloid extracts of D. stramonium at 5 mg/kg bwt; DSAE50—orally administered alkaloid extracts of D. stramonium at 50 mg/kg bwt; DSAE500—orally administered alkaloid extracts of D. stramonium at 500 mg/kg bwt; DSAE2000—orally administered alkaloid extracts of D. stramonium at 2000 mg/kg bwt; NTAE5—orally administered alkaloid extracts of N. tabacum at 5 mg/kg bwt; NTAE50—orally administered alkaloid extracts of N. tabacum at 50 mg/kg bwt; NTAE500—orally administered alkaloid extracts of N. tabacum at 500 mg/kg bwt; NTAE2000—orally administered alkaloid extracts of N. tabacum at 2000 mg/kg bwt; CMAE5—orally administered alkaloid extracts of C. papaya at 5 mg/kg bwt; CMAE50—orally administered alkaloid extracts of C. papaya at 50 mg/kg bwt; CMAE500—orally administered alkaloid extracts of C. papaya at 500 mg/kg bwt; CMAE2000—orally administered alkaloid extracts of C. papaya at 2000 mg/kg bwt
The modulatory effects of CSAE, DSAE, NTAE, and CMAE on gene expression of proteins, enzymes and cytokines linked to anxiety and depression
Also, Figs. 17, 18, 19, 20, 21, 22, 23, 24 showed the modulatory effect of CSAE, DSAE, NTAE, and CMAE on proteins, enzymes and cytokines’ gene expressions during a sub-chronic study. Interestingly, CSAE, NTAE, and CMAE caused a significant increase in the mRNA expressions of serotonin (P < 0.0001; F(16, 68) = 74.38), BDNF (P < 0.0001; F(16, 68) = 64.40), CREB (P < 0.0001; F(16, 68) = 574.7), and Nrf2 (P < 0.0001; F(16, 68) = 313.4) while decreasing the mRNA expressions of NF-ĸB (P < 0.0001; F(16, 68) = 195.2), GSK3β (P < 0.0001; F(16, 68) = 177.9), JNK3 (P < 0.0001; F(16, 68) = 1663.0), and nesfatin-1 (P < 0.0001; F(16, 68) = 275.2) at 5, 50 and 500 mg/kg bwt sub-chronic exposure. However, DSAE sub-chronic administration caused a significant decrease in the mRNA expressions of serotonin (P < 0.0001; F(16, 68) = 74.38), BDNF (P < 0.0001; F(16, 68) = 64.40), CREB (P < 0.0001; F(16, 68) = 574.7), and Nrf2 (P < 0.0001; F(16, 68) = 313.4) while increasing the mRNA expressions of NF-ĸB (P < 0.0001; F(16, 68) = 195.2), GSK3β (P < 0.0001; F(16, 68) = 177.9), JNK3 (P < 0.0001; F(16, 68) = 1663.0), and nesfatin-1 (P < 0.0001; F(16, 68) = 275.2).
Effects of CSAE, DSAE, NTAE, and CMAE on Serotonin mRNA expression. Results are expressed as mean ± SEM (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the normal control group. CSAE5—orally administered alkaloid extracts of C. sativa at 5 mg/kg bwt; CSAE50—orally administered alkaloid extracts of C. sativa at 50 mg/kg bwt; CSAE500—orally administered alkaloid extracts of C. sativa at 500 mg/kg bwt; CSAE2000—orally administered alkaloid extracts of C. sativa at 2000 mg/kg bwt; DSAE5—orally administered alkaloid extracts of D. stramonium at 5 mg/kg bwt; DSAE50—orally administered alkaloid extracts of D. stramonium at 50 mg/kg bwt; DSAE500—orally administered alkaloid extracts of D. stramonium at 500 mg/kg bwt; DSAE2000—orally administered alkaloid extracts of D. stramonium at 2000 mg/kg bwt; NTAE5—orally administered alkaloid extracts of N. tabacum at 5 mg/kg bwt; NTAE50—orally administered alkaloid extracts of N. tabacum at 50 mg/kg bwt; NTAE500—orally administered alkaloid extracts of N. tabacum at 500 mg/kg bwt; NTAE2000—orally administered alkaloid extracts of N. tabacum at 2000 mg/kg bwt; CMAE5—orally administered alkaloid extracts of C. papaya at 5 mg/kg bwt; CMAE50—orally administered alkaloid extracts of C. papaya at 50 mg/kg bwt; CMAE500—orally administered alkaloid extracts of C. papaya at 500 mg/kg bwt; CMAE2000—orally administered alkaloid extracts of C. papaya at 2000 mg/kg bwt
Effects of CSAE, DSAE, NTAE, and CMAE on Brain-derived neurotrophic factor (BDNF) mRNA expression. Results are expressed as mean ± SEM (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the normal control group. CSAE5—orally administered alkaloid extracts of C. sativa at 5 mg/kg bwt; CSAE50—orally administered alkaloid extracts of C. sativa at 50 mg/kg bwt; CSAE500—orally administered alkaloid extracts of C. sativa at 500 mg/kg bwt; CSAE2000—orally administered alkaloid extracts of C. sativa at 2000 mg/kg bwt; DSAE5—orally administered alkaloid extracts of D. stramonium at 5 mg/kg bwt; DSAE50—orally administered alkaloid extracts of D. stramonium at 50 mg/kg bwt; DSAE500—orally administered alkaloid extracts of D. stramonium at 500 mg/kg bwt; DSAE2000—orally administered alkaloid extracts of D. stramonium at 2000 mg/kg bwt; NTAE5—orally administered alkaloid extracts of N. tabacum at 5 mg/kg bwt; NTAE50—orally administered alkaloid extracts of N. tabacum at 50 mg/kg bwt; NTAE500—orally administered alkaloid extracts of N. tabacum at 500 mg/kg bwt; NTAE2000—orally administered alkaloid extracts of N. tabacum at 2000 mg/kg bwt; CMAE5—orally administered alkaloid extracts of C. papaya at 5 mg/kg bwt; CMAE50—orally administered alkaloid extracts of C. papaya at 50 mg/kg bwt; CMAE500—orally administered alkaloid extracts of C. papaya at 500 mg/kg bwt; CMAE2000—orally administered alkaloid extracts of C. papaya at 2000 mg/kg bwt
Effects of CSAE, DSAE, NTAE, and CMAE on cAMP response element-binding protein (CREB) mRNA expression. Results are expressed as mean ± SEM (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the normal control group. CSAE5—orally administered alkaloid extracts of C. sativa at 5 mg/kg bwt; CSAE50—orally administered alkaloid extracts of C. sativa at 50 mg/kg bwt; CSAE500—orally administered alkaloid extracts of C. sativa at 500 mg/kg bwt; CSAE2000—orally administered alkaloid extracts of C. sativa at 2000 mg/kg bwt; DSAE5—orally administered alkaloid extracts of D. stramonium at 5 mg/kg bwt; DSAE50—orally administered alkaloid extracts of D. stramonium at 50 mg/kg bwt; DSAE500—orally administered alkaloid extracts of D. stramonium at 500 mg/kg bwt; DSAE2000—orally administered alkaloid extracts of D. stramonium at 2000 mg/kg bwt; NTAE5—orally administered alkaloid extracts of N. tabacum at 5 mg/kg bwt; NTAE50—orally administered alkaloid extracts of N. tabacum at 50 mg/kg bwt; NTAE500—orally administered alkaloid extracts of N. tabacum at 500 mg/kg bwt; NTAE2000—orally administered alkaloid extracts of N. tabacum at 2000 mg/kg bwt; CMAE5—orally administered alkaloid extracts of C. papaya at 5 mg/kg bwt; CMAE50—orally administered alkaloid extracts of C. papaya at 50 mg/kg bwt; CMAE500—orally administered alkaloid extracts of C. papaya at 500 mg/kg bwt; CMAE2000—orally administered alkaloid extracts of C. papaya at 2000 mg/kg bwt
Effects of CSAE, DSAE, NTAE, and CMAE on Nuclear Factor-Erythroid Factor 2-Related Factor 2 (Nrf2) mRNA expression. Results are expressed as mean ± SEM (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the normal control group. CSAE5—orally administered alkaloid extracts of C. sativa at 5 mg/kg bwt; CSAE50—orally administered alkaloid extracts of C. sativa at 50 mg/kg bwt; CSAE500—orally administered alkaloid extracts of C. sativa at 500 mg/kg bwt; CSAE2000—orally administered alkaloid extracts of C. sativa at 2000 mg/kg bwt; DSAE5—orally administered alkaloid extracts of D. stramonium at 5 mg/kg bwt; DSAE50—orally administered alkaloid extracts of D. stramonium at 50 mg/kg bwt; DSAE500—orally administered alkaloid extracts of D. stramonium at 500 mg/kg bwt; DSAE2000—orally administered alkaloid extracts of D. stramonium at 2000 mg/kg bwt; NTAE5—orally administered alkaloid extracts of N. tabacum at 5 mg/kg bwt; NTAE50—orally administered alkaloid extracts of N. tabacum at 50 mg/kg bwt; NTAE500—orally administered alkaloid extracts of N. tabacum at 500 mg/kg bwt; NTAE2000—orally administered alkaloid extracts of N. tabacum at 2000 mg/kg bwt; CMAE5—orally administered alkaloid extracts of C. papaya at 5 mg/kg bwt; CMAE50—orally administered alkaloid extracts of C. papaya at 50 mg/kg bwt; CMAE500—orally administered alkaloid extracts of C. papaya at 500 mg/kg bwt; CMAE2000—orally administered alkaloid extracts of C. papaya at 2000 mg/kg bwt
Effects of CSAE, DSAE, NTAE, and CMAE on Nuclear Factor-kappa B (NF-κB) mRNA expression. Results are expressed as mean ± SEM (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the normal control group. CSAE5—orally administered alkaloid extracts of C. sativa at 5 mg/kg bwt; CSAE50—orally administered alkaloid extracts of C. sativa at 50 mg/kg bwt; CSAE500—orally administered alkaloid extracts of C. sativa at 500 mg/kg bwt; CSAE2000—orally administered alkaloid extracts of C. sativa at 2000 mg/kg bwt; DSAE5—orally administered alkaloid extracts of D. stramonium at 5 mg/kg bwt; DSAE50—orally administered alkaloid extracts of D. stramonium at 50 mg/kg bwt; DSAE500—orally administered alkaloid extracts of D. stramonium at 500 mg/kg bwt; DSAE2000—orally administered alkaloid extracts of D. stramonium at 2000 mg/kg bwt; NTAE5—orally administered alkaloid extracts of N. tabacum at 5 mg/kg bwt; NTAE50—orally administered alkaloid extracts of N. tabacum at 50 mg/kg bwt; NTAE500—orally administered alkaloid extracts of N. tabacum at 500 mg/kg bwt; NTAE2000—orally administered alkaloid extracts of N. tabacum at 2000 mg/kg bwt; CMAE5—orally administered alkaloid extracts of C. papaya at 5 mg/kg bwt; CMAE50—orally administered alkaloid extracts of C. papaya at 50 mg/kg bwt; CMAE500—orally administered alkaloid extracts of C. papaya at 500 mg/kg bwt; CMAE2000—orally administered alkaloid extracts of C. papaya at 2000 mg/kg bwt
Effects of CSAE, DSAE, NTAE, and CMAE on Glycogen synthase kinase 3β (GSK3β) mRNA expression. Results are expressed as mean ± SEM (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the normal control group. CSAE5—orally administered alkaloid extracts of C. sativa at 5 mg/kg bwt; CSAE50—orally administered alkaloid extracts of C. sativa at 50 mg/kg bwt; CSAE500—orally administered alkaloid extracts of C. sativa at 500 mg/kg bwt; CSAE2000—orally administered alkaloid extracts of C. sativa at 2000 mg/kg bwt; DSAE5—orally administered alkaloid extracts of D. stramonium at 5 mg/kg bwt; DSAE50—orally administered alkaloid extracts of D. stramonium at 50 mg/kg bwt; DSAE500—orally administered alkaloid extracts of D. stramonium at 500 mg/kg bwt; DSAE2000—orally administered alkaloid extracts of D. stramonium at 2000 mg/kg bwt; NTAE5—orally administered alkaloid extracts of N. tabacum at 5 mg/kg bwt; NTAE50—orally administered alkaloid extracts of N. tabacum at 50 mg/kg bwt; NTAE500—orally administered alkaloid extracts of N. tabacum at 500 mg/kg bwt; NTAE2000—orally administered alkaloid extracts of N. tabacum at 2000 mg/kg bwt; CMAE5—orally administered alkaloid extracts of C. papaya at 5 mg/kg bwt; CMAE50—orally administered alkaloid extracts of C. papaya at 50 mg/kg bwt; CMAE500—orally administered alkaloid extracts of C. papaya at 500 mg/kg bwt; CMAE2000—orally administered alkaloid extracts of C. papaya at 2000 mg/kg bwt
Effects of CSAE, DSAE, NTAE, and CMAE on c-jun N-terminal kinase (JNK) mRNA expression. Results are expressed as mean ± SEM (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the normal control group. CSAE5—orally administered alkaloid extracts of C. sativa at 5 mg/kg bwt; CSAE50—orally administered alkaloid extracts of C. sativa at 50 mg/kg bwt; CSAE500—orally administered alkaloid extracts of C. sativa at 500 mg/kg bwt; CSAE2000—orally administered alkaloid extracts of C. sativa at 2000 mg/kg bwt; DSAE5—orally administered alkaloid extracts of D. stramonium at 5 mg/kg bwt; DSAE50—orally administered alkaloid extracts of D. stramonium at 50 mg/kg bwt; DSAE500—orally administered alkaloid extracts of D. stramonium at 500 mg/kg bwt; DSAE2000—orally administered alkaloid extracts of D. stramonium at 2000 mg/kg bwt; NTAE5—orally administered alkaloid extracts of N. tabacum at 5 mg/kg bwt; NTAE50—orally administered alkaloid extracts of N. tabacum at 50 mg/kg bwt; NTAE500—orally administered alkaloid extracts of N. tabacum at 500 mg/kg bwt; NTAE2000—orally administered alkaloid extracts of N. tabacum at 2000 mg/kg bwt; CMAE5—orally administered alkaloid extracts of C. papaya at 5 mg/kg bwt; CMAE50—orally administered alkaloid extracts of C. papaya at 50 mg/kg bwt; CMAE500—orally administered alkaloid extracts of C. papaya at 500 mg/kg bwt; CMAE2000—orally administered alkaloid extracts of C. papaya at 2000 mg/kg bwt
Effects of CSAE, DSAE, NTAE, and CMAE on Nesfatin-1 mRNA expression. Results are expressed as mean ± SEM (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the normal control group. CSAE5—orally administered alkaloid extracts of C. sativa at 5 mg/kg bwt; CSAE50—orally administered alkaloid extracts of C. sativa at 50 mg/kg bwt; CSAE500—orally administered alkaloid extracts of C. sativa at 500 mg/kg bwt; CSAE2000—orally administered alkaloid extracts of C. sativa at 2000 mg/kg bwt; DSAE5—orally administered alkaloid extracts of D. stramonium at 5 mg/kg bwt; DSAE50—orally administered alkaloid extracts of D. stramonium at 50 mg/kg bwt; DSAE500—orally administered alkaloid extracts of D. stramonium at 500 mg/kg bwt; DSAE2000—orally administered alkaloid extracts of D. stramonium at 2000 mg/kg bwt; NTAE5—orally administered alkaloid extracts of N. tabacum at 5 mg/kg bwt; NTAE50—orally administered alkaloid extracts of N. tabacum at 50 mg/kg bwt; NTAE500—orally administered alkaloid extracts of N. tabacum at 500 mg/kg bwt; NTAE2000—orally administered alkaloid extracts of N. tabacum at 2000 mg/kg bwt; CMAE5—orally administered alkaloid extracts of C. papaya at 5 mg/kg bwt; CMAE50—orally administered alkaloid extracts of C. papaya at 50 mg/kg bwt; CMAE500—orally administered alkaloid extracts of C. papaya at 500 mg/kg bwt; CMAE2000—orally administered alkaloid extracts of C. papaya at 2000 mg/kg bwt
Discussion
Behavioral responses fashioned by antidepressant agents in experimental animals serve as suitable endpoints for evaluating possible neural mechanisms related to their therapeutic or detrimental effects. As revealed by elevated plus maze test, administration of CSAE, NTAE, and CMAE at 5, 50 and 500 mg/kg bwt significantly increased the frequency of entries and time spent in open arms by experimental animals, which is consistent with previous studies that evaluated anxiolytic ability using an elevated plus maze test (Leem and Oh 2015). Meanwhile, a decrease in the frequency of entries and time spent in open arms by experimental animals after sub-chronic exposure to DSAE thus suggests that exposure to the D. stramonium alkaloid may result in anxiety disorder. Interestingly, anxiety and exposure to D. stramonium lead to the development of sexual dysfunction (Olabiyi et al. 2016; Fasakin et al. 2022), which will ultimately result in depression.
Tail suspension test (TST) and forced swim test (FST) are proven despair models of depression, as antidepressants decrease immobility time in rats’ FST and TST (Dhingra and Valecha, 2014; Grizzell et al. 2014). The results of FST and TST showed that CSAE, NTAE, and CMAE decreased immobility time at 5, 50, and 500 mg/kg bwt in a dose-dependent pattern in comparison to the control group. However, 2000 mg/kg bwt CSAE, NTAE, and CMAE increased the immobility period of FST and TST, which indicates the extracts may induce depressive-like behavior at higher doses. This supports the elevated plus maze tests as well as confirms previous studies that observed depressive-like behavior in patients pre-exposed to psychoactive substances at high doses (Wheatley 2005). Furthermore, the ability of CSAE, NTAE, and CMAE to decrease immobility time but increase swimming time may indicate that the alkaloids are selective serotonin reuptake inhibitors (SSRIs) rather than noradrenergic reuptake inhibitors (NERIs) (Detke and Lucki 1995). Considering the effects of the plant alkaloids during elevated plus maze, FST and TST models, we opined that our study model of sub-chronic exposure to CSAE, DSAE, NTAE, and CMAE may be used to study anxiety and depression in a rat model as the changes observed were absent in the control group of the same experiment.
The major neurochemical process in depressive disorder is the impairment of the monoaminergic neurotransmission, with a concomitant depleted serotonin and dopamine concentration (Guzmán-Gutiérrez et al. 2015). The ability of CSAE, DSAE, NTAE, and CMAE to reduce MAO activity in the present study is consistent with a previous study where the alkaloid extracts inhibited brain MAO activities in a concentration-dependent manner during an ex vivo study (Fasakin et al. 2021). This ability to inhibit MAO activity is implicated as the major factor underlining the elevation of serotonin, dopamine, and noradrenaline levels and the upregulation of BDNF levels in the hippocampal region of experimental animal brains (Yao et al. 2009). Interestingly, previous research has shown that conventional antidepressant medications act via elevation of synaptic availability of monoamines and improvement of neurogenesis via BDNF induction (Duman and Monteggia 2006). However, DSAE had no significant effect on dopamine level despite significantly inhibiting MAO activity, while its depletion at 500 and 2000 mg/kg exposure may indicate potentiation of depression, as the inability to experience pleasure or stimulate the reward system is consistent in depressed patients. Furthermore, the relationship between dopaminergic system dysfunction and suicide prevalence have been established (Vargas-Medrano et al. 2020).
Additionally, the ability of an agent to improve brain neuronal serotonin bioavailability indicates its antidepressant potential as serotonin metabolism is severely depleted in depressed suicide patients (Åsberg 1997; Shulman et al. 2013). Therefore, the ability of CSAE, NTAE, and CMAE to inhibit MAO and increase serotonin expression may be a mechanism employed by the plants’ alkaloids to manage anxiety and depression as observed during FST in this present study. The present results are consistent with that observed by Müller et al. (2021), who observed inhibition of the kynurenine pathway and the activation of the serotonin synthesis pathway, resulting in higher serotonin bioactivity. Interestingly, major classes of antidepressant drugs are selective serotonin reuptake inhibitors (SSRIs) that bind to serotonin 1A receptors to inhibit the re-uptake of serotonin into neurons and thereby increase serotonin concentrations (Chollet et al. 2018). Aside from the direct effect of serotonin on depression and anxiety, its elevated concentrations may also help deplete the levels of GSK3β, a key pharmacological target in neuropsychiatric and mood disorders, via the 5-HT1A receptor-mediated pathway (Polter et al. 2012). However, the switching of tryptophan (the precursor of serotonin) catabolism from the serotonin synthesis pathway to the kynurenine synthesis pathway is strongly linked to neuro-inflammation as indole-amine 2,3-dioxygenase is inducible by pro-inflammatory cytokines (O’Connor et al. 2009), an indication that serotonin metabolism and neuro-anti-inflammation are interrelated factors in the management of depression. Meanwhile, the depletion in serotonin expression by DSAE may indicate it activated the kynurenine pathway at the expense of the serotonergic pathway despite inhibiting MAO activity. The activation of kynurenine has been postulated to activate microglia which converts kynurenine to quinolinic acid which leads to excess glutamate (an excitatory neurotransmitter) synthesis and release, and the inhibition of glutamate dehydrogenase activity (glutamate removing enzyme). More so, excess glutamate availability and the inhibition of its removing enzymes are directly linked to depleted BDNF expressions (Miller 2018). Furthermore, depleted BDNF is linked to hippocampal damage as well as the dysregulation of neuronal survival and plasticity that ultimately result in depression and even resistance to treatment in depression (Maes et al. 2012), which may help understand the trend observed during behavioral studies after exposure to DSAE.
Angiotensin 1-converting enzyme (ACE) catalyzes the biosynthesis of angiotensin 2 from angiotensin 1, the major step in renin cascade (Fasakin et al. 2022). The resulting angiotensin 2 then activates NF-κB, a ubiquitous transcriptional factor, via stimulation of both AT1 and AT2 receptors, which also degrades IκB, an inhibitor of NF-κB expression (Ferrari et al. 2002). This cascade results in production of the two major pro-inflammatory cytokines associated with depression (tumor necrosis factor-α and interleukins 1β), which stimulate microglia action and intensify cytokine activities that result in the disorder of neuroendocrine function, behaviors, and neurotransmitter metabolism (Paolucci et al. 2018). Interestingly, the present study observed a significant elevation in the levels of ACE, NF-κB, tumor necrosis factor-α and interleukins 1β after sub-chronic exposure of experimental rats to all doses of DSAE, and the high dose of CSAE, NTAE, and CMAE. The biphasic cytokine pattern of CSAE and NTAE administered groups may be the underlying factor behind the same pattern observed in the expression of serotonin, as pro-inflammatory cytokines levels and serotonergic expression are interrelated. Pro-inflammatory cytokines deplete serotonergic bioavailability by elevating the function and expression of presynaptic reuptake pumps (transporters) for the monoamine via the activation of p38 mitogen-activated protein kinase (MAPK) (Zhu et al. 2010). DSAE may have also depleted serotonin synthesis and release by mediating reactive nitrogen and oxygen species production which in turn inhibit enzymatic co-factors such as tetrahydrobiopterin, required for serotonin synthesis and release (Capuron et al. 2012; Raison et al. 2013).
Meanwhile, the generated tumor necrosis factor-α in the DSAE-administered group can bind to either 75 kDa TNF-R2 or 55 kDa TNF-R1 (Palin et al. 2008). Its binding to 55 kDa TNF-R1 results in a plethora of signaling cascades, such as stimulation of NF-κB, p38 MAPK, and c-jun N-terminal kinase (JNK) caspases (McCusker et al. 2006; O’Connor et al. 2008). Stimulation of these cascades results in neuro-inflammation, bodyweight loss, increase in immobility, a decline in food consumption and social interaction as well as altered memory and learning indexes (Bluthé et al. 2000; Palin et al. 2008), which have been implicated in the progression of depression. Interestingly, CSAE and NTAE significantly inhibited JNK and NF-κB expression which may indicate their anti-depressive abilities (Galeotti and Ghelardini 2012). More so, the inhibition of JNK during the inflammatory response was observed to occur via NF-κB inactivation or COX-2 inhibition (Park et al. 2008; Ki et al. 2013). Also, CSAE, NTAE, and CMAE exposure elevated the levels of interleukin 10, an anti-inflammatory cytokine that has been implicated to alleviate depression (Swann et al. 2019). The presence of this anti-inflammatory cytokine may have inhibited the progression of elevated cortisol levels and overactive hypothalamic–pituitary–adrenal axis, thereby potentiating improving moods as observed during the behavioral tests. Therefore, the ability of CSAE and NTAE to inhibit NF-κB, TNF-α, IL 1β, and JNK caspases while elevating IL-10 concentrations at 5, 50 and 500 mg/kg bwt may suggest that the plants’ alkaloid also exerts their anti-depressive and anxiolytic abilities via the inhibition of NF-κB mediated pathway (Lawal et al. 2019).
Glycogen synthase kinase 3β (GSK3β) plays a key role in the regulation of these anti– and pro-inflammatory cytokines production via both the adaptive and innate adaptive immune systems (Jope et al. 2007; Wang et al. 2011). Meanwhile, GSK3β inhibitors are potent in the reduction of neuro-inflammation and are implicated in the downstream and/or direct mechanism of action of most antidepressants and mood stabilizers (Pláteník et al. 2014). Therefore, the depletion of proinflammatory cytokines and concomitant elevation of anti-inflammatory cytokines observed with CSAE, NTAE, and CMAE oral administration may be dependent on their inhibitory effect on GSK3β. Furthermore, the regulatory effect of GSK3β on inflammatory transcription factors plays a key role in the modulation of immune responses of NF-κB, STAT3, and CREB via the PI3K signaling pathway or Toll-like receptors (TLRs) activation (Maes et al. 2012). Especially, the modulation of CREB results in increased expression of neurotrophic factors such as BDNF and its receptor (tropomyosin receptor kinase B (TrkB)) which are essential for neurogenesis and neuron development (Pláteník et al. 2014). The mechanism of action employed is the phosphorylation of CREB at critical serine residue 133 (a point where several major signaling pathways congregate) by protein kinases which leads to transcriptional activation that results in the production of BDNF required for cognitive function and synaptic plasticity (Pláteník et al. 2005). Interestingly, the present result correlates with the effects of antidepressants in relation to the neurotrophic hypothesis of depression states (Ghasemi et al. 2015). Therefore, the ability of CSAE and NTAE to significantly increase BDNF may also be due to their ability to elevate CREB phosphorylation and thereby prevent the deleterious role of its signaling pathway.
Furthermore, GSK3β directly phosphorylates Nuclear Factor-Erythroid Factor 2-Related Factor 2 (Nrf2) at the ser338 and ser335 of its Neh6 domain, resulting in the destabilization of the Nrf2 and the binding of β-transducin repeat-containing protein (β-TrCP) to the Nrf2 protein, which encrypts the complex for proteasomal degradation thereby inhibiting its function against cellular stress as a cytoprotective factor (McCallum and Perreault, 2021). Nrf2 is the protein responsible for the regulation of glutamate/cysteine exchange transporter and induction of detoxifying enzymes (e.g., HO-1 (heme oxygenase-1) and NADPH (nicotinamide adenine dinucleotide phosphate) expressions as well as several other antioxidant genes that have been implicated in anti-inflammatory responses that are characterized by IL-RA and IL-10 upregulation, and CD4 + CD25 regulatory T cells’ promotion (Maes et al. 2012; Ahmed et al. 2017). Interestingly, this protein was significantly elevated in CSAE, NTAE and CMAE administered rats which indicates that the ability of the alkaloid extracts to inhibit GSK3β activity transcends to elevated Nrf2 expression. Furthermore, it also substantiates the anti-inflammatory ability of the alkaloid extracts at 5, 50 and 500 mg/kg bwt as Nrf2 enthusiastically inhibits proinflammatory effects of NF-κB and thereby reduces neuroinflammation through the augmentation of apoptotic cells’ phagocytic removals and various pro-inflammatory cytokines downregulation (McCallum and Perreault, 2021).
Oxidative stress leads to the stimulation of hypothalamus–pituitary–adrenal axis that results in high glucocorticoids levels, impairment of neuronal survival, neurogenesis and macromolecules (proteins, DNA, and lipids) which cause neuronal dysfunction and depression (Dhingra and Valecha, 2014; Keller et al. 2017). Interestingly, ROS production was elevated in 2000 mg/kg bwt administration of CSAE, DSAE, NTAE, and CMAE. This trend of ROS may be the underlying factor behind the modulation of cytokines levels, as increased levels of proinflammatory cytokines have been shown in adolescents predisposed to early-life stress, which has been observed to result in elevated levels of circulating cytokines and cortisol in adult depressive patients (Horowitz and Zunszain 2015; Miller and Chen 2010). Furthermore, excess ROS production induces region-specific changes in BDNF expression, a mechanism that has also been observed to mediate depressive-like behaviors (Leem and Oh 2015). Aside from excess ROS production depleting BDNF expression, it can also result in elevated nesfatin-1 mRNA expression. Interestingly, upregulation of nesfatin-1 has been implicated to result in HPA axis hyperactivity, a dysfunction that is consistent in depressive pathophysiology (Xiao et al. 2018). Therefore, the significant reduction in hippocampal nesfatin-1 expression after exposure to CSAE and NTAE may further establish their potential to act as anti-depressive agents as nesfatin-1 has been implicated in altered energy expenditure, appetite, and food intake, lipogenesis and elevated blood pressure, as well as glucose homeostasis enhancing glucose-stimulated insulin sensitivity and secretion (Weibert et al. 2019).
Although acetylcholine release may be also induced by elevated oxidative stress, it is the inhibition of hippocampal acetylcholinesterase activity that is the key factor underlining homeostatic acetylcholine levels and upregulated cholinergic neurotransmission implicated in induced depressive- and anxiety-like behaviors (Mineur et al. 2013; Picciotto et al. 2015). Meanwhile, both the pharmacological inhibition of acetylcholinesterase and elevated oxidative stress has been proven to be consistent with anxiety and long-term depression (Martinowich et al. 2012). An indication that the ability of the alkaloid extracts to significantly inhibit the enzyme acetylcholinesterase and elevate ROS levels at 2000 mg/kg bwt may explain why the alkaloid extracts at that dose had negative results during behavioral tests. Inhibition of acetylcholinesterase and elevated ROS levels were also observed to deplete resilience to repeated oxidative stress in a social defeat paradigm alongside an increase in depressive-like and anxiety-like behaviors which were all reversed after administration of SSRIs (Mineur et al. 2013).
The roles of CSAE, DSAE, NTAE, and CMAE in modulating depression was established by assessing their alkaloid constituents using GCMS. Amid the various compounds screened, cannabidiol, Δ-9-tetrahydrocannabinol, hyoscyamine, scopolamine, nicotine, anabasine, anatabine, cotinine and carpaine were linked to the modulatory effects of the plants in depressive states. Network pharmacology have identified the ability of C. sativa and its constituents to target key genes of depression (Ma et al. 2021; Shi et al. 2021). The ability of Δ-9-tetrahydrocannabinol administration to mediate a network-wide shift from a bias for negative emotional content to a bias for positive emotional content has been ascertained in a pharmacological functional magnetic resonance imaging (fMRI) study (Bossong et al. 2013). While the anxiolytic and antidepressant effects of cannabidiol was observed via stimulation of 5-HT1A receptor and hippocampal neurogenesis in a biphasic dose–response curve with low dosages more effective than the higher dosages (Zlebnik and Cher 2016). The ability of nicotine to mediate MAO-inhibiting medication and nicotinic acetylcholine receptors (nAChRs) inhibition in depressed smokers, and enhance stimulation, mood modulation and pleasure in anxiety and stressed individuals have also been established (Benowitz 2010; Picciotto et al. 2015; Prochaska and Benowitz 2019). Also, cotinine has been documented to act as antidepressant via its anti-inflammatory effects and agonist acts on nAChR subtypes that activates dopaminergic and serotonergic systems (Grizzell et al. 2014; Iarkov et al. 2016). However, anabasine and anatabine was documented to show no significant anxiolytic effects during a sub-chronic anxiety-like behavior study using Zebrafish (Hawkey et al. 2021), an indication that the anxiolytic ability of NTAE is dependent on nicotine and cotinine. This further substantiates the use of N. tabacum as traditional herbs in the management of anxiety and depression (Hall et al. 1993; Berlowitz et al. 2020). Scopolamine has been observed to elicit antidepressant effects by reducing cholinergic tone via its inhibition of acetylcholine muscarinic 1 (AChM1) receptor on γ-aminobutyric acid (GABA) interneurons and the subsequent increase of glutamate function and transmission by the spine synapse while exerting nominal effects on nicotinic receptors (Jaffe et al. 2013; Li et al. 2020; Fasakin et al. 2021). However, its antidepressant use has been limited to as few as 7–14 days due to its downstream mechanism of action, which may explain the negative results obtained after sub-chronic exposure of experimental animals to DSAE (Jaffe et al. 2013). Furthermore, hyoscyamine have been associated with adverse effects on cornu ammonis 1 (CA1) regions and diminished novel object recognition (Abdu et al. 2020). Finally, C. papaya and its constituents, choline and carpaine have been documented to modulate depression of the central nervous system (Olley 2007; Oyewole and Owoyele 2012; Riley and Renshaw 2018).
Conclusion
Communally, this study established the relationship between depression, neurotransmitter, neurotrophic and inflammatory systems, and the neuro-modulatory effects of CSAE, DSAE, NTAE, and CMAE on the systems. The difference between the effects of CSAE, CMAE, and NTAE at low and high doses may be the underlying factor in the discrepancies between the results of studies that have evaluated depression and psychoactive substance use. This study established the antidepressant and anxiolytic potentials of CSAE, CMAE, and NTAE via improving monoaminergic bioavailability, activation of neurotrophic signaling cascades and de-hyper-activation of hypothalamic–pituitary–adrenal axis. However, their use is still a risk as it is associated with dependence and addiction which may result in more detrimental effects than any therapeutic potential they may proffer. Therefore, the present study recommends isolating the bioactive constituents of CSAE, CMAE, and NTAE to evaluate their anxiolytic and anti-depressive effects while monitoring the resulting prevalence of dependence and addiction.
Data availability
Enquiries about data availability should be directed to the authors.
References
Abdel-Zaher AO, Abdel-Rahman MS, Elwasei FM (2011) Protective effect of Nigella sativa oil against tramadol-induced tolerance and dependence in mice: role of nitric oxide and oxidative stress. Neurotoxicology 32:725–733. https://doi.org/10.1016/j.neuro.2011.08.001
Abdu IT, Musa SA, Iliya IA, Nzalak JO (2020) Evaluation of the effects of lactational exposure to hyoscyamine fraction of Datura stramonium L. seeds on learning and memory in Wistar rats (Rattus norvegicus). J Neurobehav Sci 7(3):109–117
Ademiluyi AO, Ogunsuyi OB, Oboh G, Agbebi OJ (2016) Jimson weed (Datura stramonium L.) alkaloid extracts modulate cholinesterase and monoamine oxidase activities in vitro: possible modulatory effect on neuronal function. Compar Clin Pathol 25(4):733–741
Adeniyi PA, Ishola AO, Laoye BJ, Olatunji BP, Bankole OO, Shallie PD, Ogundele OM (2016) Neural and behavioural changes in male periadolescent mice after prolonged nicotine-MDMA treatment. Metabol Brain Dis 31(1):93–107
Ahmed SMU, Luo L, Namani A, Wang XJ (1863) Tang X (2017) Nrf2 signaling pathway: pivotal roles in inflammation. BBA Mol Basis Dis 2:585–597
Åsberg M (1997) Neurotransmitters and suicidal behavior: the evidence from cerebrospinal fluid studiesa. Ann New York Acad Sci 836(1):158–181
Benowitz NL (2010) Nicotine addiction. N Engl J Med 362(24):2295–2303
Berlowitz I, Torres EG, Walt H, Wolf U, Maake C, Martin-Soelch C (2020) “Tobacco is the chief medicinal plant in my work”: therapeutic uses of tobacco in peruvian amazonian medicine exemplified by the work of a Maestro Tabaquero. Front Pharmacol 11:1600
Bitner RS (2012) Cyclic AMP response element-binding protein (CREB) phosphorylation: a mechanistic marker in the development of memory enhancing Alzheimer’s disease therapeutics. Biochem Pharmacol 83(6):705–714
Bluthé RM, Layé S, Michaud B, Combe C, Dantzer R, Parnet P (2000) Role of interleukin-1β and tumour necrosis factor-α in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur J Neurosci 12(12):4447–4456
Bossong MG, van Hell HH, Jager G, Kahn RS, Ramsey NF, Jansma JM (2013) The endocannabinoid system and emotional processing: a pharmacological fMRI study with∆ 9-tetrahydrocannabinol. Eur Neuropsychopharmacol 23(12):1687–1697
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Ann Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Calvó-Perxas L, Vilalta-Franch J, Turró-Garriga O, López-Pousa S, Garre-Olmo J (2016) Gender differences in depression and pain: a two-year follow-up study of the Survey of Health, Ageing and Retirement in Europe. J Affect Disord 193:157–164. https://doi.org/10.1016/j.jad.2015.12.034
Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Votaw JR, Goodman MM, Miller AH (2012) Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry 69(10):1044–1053
Chollet F, Rigal J, Marque P, Barbieux-Guillot M, Raposo N, Fabry V, Albucher JF, Pariente J, Loubinoux I (2018) Serotonin selective reuptake inhibitors (SSRIs) and stroke. Curr Neurol Neurosci Rep 18(12):1–11
Cryan JF, Mombereau C, Vassout A (2005) The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 29:571–625
Cushman DW, Cheung HS (1971) Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol 20(7):1637–1648
Detke MJ, Lucki I (1995) Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: the effects of water depth. Behav Brain Res 73(1–2):43–46
Dhingra D, Valecha R (2014) Punarnavine, an alkaloid isolated from ethanolic extract of Boerhaavia diffusa Linn. reverses depression-like behaviour in mice subjected to chronic unpredictable mild stress. Indian J Exp Biol 52:799–807
Duman RS, Monteggia LM (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59:1116–1127
Fasakin OW, Oboh G, Ademosun AO (2021) Neuromodulatory evaluation of commonly abused plants ex vivo: a comparative study. Comp Clin Pathol 30:671–680. https://doi.org/10.1007/s00580-021-03259-4
Fasakin OW, Oboh G, Ademosun AO (2022) Alkaloids-rich extracts from Cannabis sativa, Datura stramonium, and Nicotiana tabacum modulate sexual behavior and key enzymes relevant to sexual function in rats. Comp Clin Pathol. https://doi.org/10.1007/s00580-022-03339-z
Fedotova J, Kubatka P, Büsselberg D, Shleikin AG, Caprnda M, Dragasek J, Rodrigo L, Pohanka M, Gasparova I, Nosal V, Opatrilova R (2017) Therapeutical strategies for anxiety and anxiety-like disorders using plant-derived natural compounds and plant extracts. Biomed Pharmacother 95:437–446
Ferrari R, Guardigli G, Cicchitelli G, Valgimigli M, Merli E, Soukhomorskaia O, Ceconi C (2002) Angiotensin II overproduction: enemy of the vessel wall. Eur Heart J Suppl 4(Supplement A):A26–A30
File SE, Kenny PJ, Cheeta S (2000) The role of the dorsal hippocampal serotonergic and cholinergic systems in the modulation of anxiety. Pharmacol Biochem Behav 66(1):65–72
Gadassi R, Mor N (2016) Confusing acceptance and mere politeness: depression and sensitivity to Duchenne smiles. J Behav Ther Exp Psychiatry 50:8–14. https://doi.org/10.1016/j.jbtep.2015.04.007
Galeotti N, Ghelardini C (2012) Regionally selective activation and differential regulation of ERK, JNK and p38 MAP kinase signalling pathway by protein kinase C in mood modulation. Int J Neuropsychopharmacol 15:781–793
Ghasemi T, Abnous K, Vahdati F, Mehri S, Razavi BM, Hosseinzadeh H (2015) Antidepressant effect of Crocus sativus aqueous extract and its effect on CREB, BDNF, and VGF transcript and protein levels in rat hippocampus. Drug Res 65(07):337–343
Green AL, Haughton TM (1961) Colorimetric method for the estimation of monoamine oxidase. Biochem J 78:172
Grizzell JA, Iarkov A, Holmes R, Mori T, Echeverria V (2014) Cotinine reduces depressive-like behavior, working memory deficits, and synaptic loss associated with chronic stress in mice. Behav Brain Res 268:55–65. https://doi.org/10.1016/j.bbr.2014.03.047
Guo L, Zhang Y, Li Q (2009) Spectrophotometric determination of dopamine hydrochloride in pharmaceutical, banana, urine and serum samples by potassium ferricyanide-Fe (III). Anal Sci 25(12):1451–1455. https://doi.org/10.2116/analsci.25.1451
Guzmán-Gutiérrez SL, Bonilla-Jaime H, Gómez-Cansino R, Reyes-Chilpa R (2015) Linalool and β-pinene exert their antidepressant-like activity through the monoaminergic pathway. Life Sci 128:24–29
Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M (2015) Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun 49:206–215
Hall SM, Muñoz RF, Reus VI, Sees KL (1993) Nicotine, negative affect, and depression. J Consult Clin Psychol 61(5):761–767
Hawkey AB, Hoeng J, Peitsch MC, Levin ED, Koshibu K (2021) Subchronic effects of plant alkaloids on anxiety-like behavior in zebrafish. Pharmacol Biochem Behav 207:173223. https://doi.org/10.1016/j.pbb.2021.173223
Hayashi I, Morishita Y, Imai K, Nakamura M, Nakachi K, Hayashi T (2007) High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat Res Genet Toxicol Environ Mutagenes 631(1):55–61
Hennessy MB, Chun K, Capitanio JP (2017) Depressive-like behavior, its sensitization, social buffering, and altered cytokine responses in rhesus macaques moved from outdoor social groups to indoor housing. Soc Neurosci 12(1):65–75. https://doi.org/10.1080/17470919.2016.1145595
Horowitz MA, Zunszain PA (2015) Neuroimmune and neuroendocrine abnormalities in depression: two sides of the same coin. Ann New York Acad Sci 1351(1):68–79
Houle J, Gascon-Depatie M, Bélanger-Dumontier G, Cardinal C (2013) Depression self-management support: a systematic review. Patient Educ Counsel 91(3):271–279
Iarkov A, Appunn D, Echeverria V (2016) Post-treatment with cotinine improved memory and decreased depressive-like behavior after chemotherapy in rats. Cancer Chemother Pharmacol 78(5):1033–1039
Jaffe RJ, Novakovic V, Peselow ED (2013) Scopolamine as an antidepressant: a systematic review. Clin Neuropharm 36:24–26
Jope RS, Yuskaitis CJ, Beurel E (2007) Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res 32(4):577–595
Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM, Schatzberg AF (2017) HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry 22(4):527–536
Ki YW, Park JH, Lee JE, Shin IC, Koh HC (2013) JNK and p38 MAPK regulate oxidative stress and the inflammatory response in chlorpyrifos-induced apoptosis. Toxicol Lett 218:235–245
Lawal AO, Oluyede DM, Adebimpe MO, Olumegbon LT, Awolaja OO, Elekofehinti OO, Crown OO (2019) The cardiovascular protective effects of rooibos (Aspalathus linearis) extract on diesel exhaust particles induced inflammation and oxidative stress involve NF-kB- and Nrf2-dependent pathways modulation. Heliyon 5:e01426
Leem Y-H, Oh S (2015) 3,4,5-Trimethoxycinnamin acid ameliorates restraint stress-induced anxiety and depression. Neurosci Lett 585:54–59
Li J-M, Liu L-L, Su W-J, Wang B, Zhang T, Zhang Y, Jiang C-L (2019) Ketamine may exert antidepressant effects via suppressing NLRP3 inflammasome to upregulate AMPA receptors. Neuropharmacology 146:149–153
Li C, Huang J, Cheng Y-C, Zhang Y-W (2020) Traditional Chinese medicine in depression treatment: from molecules to systems. Front Pharmacol 11:586
Lipton RB, Schwedt TJ, Friedman BW (2016) GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1545–1602
Lister RG (1987) The use of a plus-maze to measure anxiety in mouse. Psychopharmacology 92:180–185
Ma H, Xu F, Liu C, Seeram NP (2021) A network pharmacology approach to identify potential molecular targets for cannabidiol’s anti-inflammatory activity. Cannab Cannab Res 6(4):288–299
Maes M, Fišar Z, Medina M, Scapagnini G, Nowak G, Berk M (2012) New drug targets in depression: inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. And new drug candidates—Nrf2 activators and GSK-3 inhibitors. Inflammopharmacology 20:127–150. https://doi.org/10.1007/s10787-011-0111-7
Martinowich K, Schloesser RJ, Lu Y, Jimenez DV, Paredes D, Greene JS, Greig NH, Manji HK, Lu B (2012) Roles of p75NTR, Long-term depression, and cholinergic transmission in anxiety and acute stress coping. Biol Psychiat 71(1):75–83
McCallum RT, Perreault ML (2021) Glycogen synthase kinase-3: a focal point for advancing pathogenic inflammation in depression. Cells 10:2270. https://doi.org/10.3390/cells10092270
McCusker RH, Strle K, Broussard SR, Dantzer R, Bluthé RM, Kelley KW (2006) Crosstalk between insulin-like growth factors and pro-inflammatory cytokines. In: Adler R, Dantzer R, Glaser R, Heijnen C, Irwin M, Padgett D, Sheridan J (eds) Psychoneuroimmunology. Academic Press, New York, pp 171–191
Miller AH (2018) Five things to know about inflammation and depression. Psychiatr times 35(4):2018
Miller GE, Chen E (2010) Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol Sci 21(6):848–856
Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, Picciotto MR (2013) Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety-and depression-like behavior. Proc Natl Acad Sci 110(9):3573–3578
Muhammad N, Saeed M, Khan H, Haq I (2013) Evaluation of n-hexane extract of Viola betonicifolia for its neuropharmacological properties. J Nat Med 67:1–8. https://doi.org/10.1007/s11418-012-0636-0
Müller E, Hillemacher T, Müller CP (2020) Kratom instrumentalization for severe pain self-treatment resulting in addiction—a case report of acute and chronic subjective effects. Heliyon 6(7):e04507
Müller E, Hillemacher T, Müller CP (2021) Kratom use for depression/anxiety self-management: challenges during the COVID-19 pandemic—a case report. Heliyon 7:e07039
O’Connor JC, McCusker RH, Strle K, Johnson RW, Dantzer R, Kelley KW (2008) Regulation of IGF-I function by proinflammatory cytokines: at the interface of immunology and endocrinology. Cell Immunol 252(1–2):91–110
O’Connor JC, André C, Wang Y, Lawson MA, Szegedi SS, Lestage J, Castanon N, Kelley KW, Dantzer R (2009) Interferon-γ and tumor necrosis factor-α mediate the upregulation of indoleamine 2, 3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci 29(13):4200–4209
Olabiyi AA, Oboh G, Adefegha SA (2016) Effect of dietary supplementation of tiger nut (Cyperus esculentus L.) and walnut (Tetracarpidium conophorum mull. Arg.) on sexual behavior, hormonal level, and antioxidant status in male rats. J Food Biochem 00:e12351
Olley BO (2007) Is dried pawpaw leaf a psychoactive substance? IFE Psychol 15(1):25–39
Oyewole AL, Owoyele BV (2012) Neurobehavioural effects of exposure of Wistar rats to smoke from traditional Carica papaya (pawpaw) leaves. Cell Med 2(4):36–41
Palin K, McMcuster RH, Strle K, Moos F, Dantzer R, Kelley KW (2008) Tumor necrosis factor-α-induced sickness behavior is impaired by central administration of an inhibitor of c-jun N-terminal kinase. Psychopharmacology 197:629–635
Paolucci EM, Loukov D, Bowdish DM, Heisz JJ (2018) Exercise reduces depression and inflammation but intensity matters. Biol Psychol 133:79–84. https://doi.org/10.1016/j.biopsycho.2018.01.015
Park HJ, Lee HJ, Choi MS, Son DJ, Song HS, Song MJ, Lee JM, Han SB, Kim Y, Hong JT (2008) JNK pathway is involved in the inhibition of inflammatory target gene expression and NF-kappaB activation by melittin. J Inflamm 5(1):1–13
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open closed arm entries in an elevated plus maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167
Perry NS, Houghton PJ, Theobald A, Jenner P, Perry EK (2000) In-vitro inhibition of human erythrocyte acetylcholinesterase by Salvia lavandulaefolia essential oil and constituent terpenes. J Pharm Pharmacol 52(7):895–902
Perviz S, Khan H, Pervaiz A (2016) Plant alkaloids as an emerging therapeutic alternative for the treatment of depression. Front Pharmacol 7:28. https://doi.org/10.3389/fphar.2016.00028
Picciotto MR, Lewis AS, van Schalkwyk GI, Mineur YS (2015) Mood and anxiety regulation by nicotinic acetylcholine receptors: a potential pathway to modulate aggression and related behavioral states. Neuropharmacology 96(Pt B):235–243. https://doi.org/10.1016/j.neuropharm.2014.12.028
Pláteník J, Balcar VJ, Yoneda Y, Mioduszewska B, Buchal R, Hynek R, Kilianek L, Kuramoto N, Wilczynski G, Ogita K, Nakamura Y (2005) Apparent presence of Ser133-phosphorylated cyclic AMP response element binding protein (pCREB) in brain mitochondria is due to cross-reactivity of pCREB antibodies with pyruvate dehydrogenase. J Neurochem 95(5):1446–1460
Pláteník J, Fišar Z, Buchal R, Jirák R, Kitzlerová E, Zvěřová M, Raboch J (2014) GSK3β, CREB, and BDNF in peripheral blood of patients with Alzheimer’s disease and depression. Prog Neuropsychopharmacol Biol Psychiatry 50:83–93. https://doi.org/10.1016/j.pnpbp.2013.12.001
Polter AM, Yang S, Jope RS, Li X (2012) Functional significance of glycogen synthase kinase-3 regulation by serotonin. Cell Signal 24:265–271. https://doi.org/10.1016/j.cellsig.2011.09.009
Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266:730–732
Prochaska JJ, Benowitz NL (2019) Current advances in research in treatment and recovery: Nicotine addiction. Sci Adv 5:eaay9763
Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH (2013) A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiat 70(1):31–41
Riley CA, Renshaw PF (2018) Brain choline in major depression: a review of the literature. Psychiatry Res Neuroimag 271:142–153
Sarubin N, Nothdurfter C, Schmotz C, Wimmer AM, Trummer J, Lieb M, Uhr M, Baghai TC, Wetter TC, Bühner M, Rupprecht R (2014) Impact on cortisol and antidepressant efficacy of quetiapine and escitalopram in depression. Psychoneuroendocrinology 39:141–151
Shi Y, Chen D, Ma S, Xu H, Deng L (2021) Identification of potential biomarkers of depression and network pharmacology approach to investigate the mechanism of key genes and therapeutic traditional Chinese medicine in the treatment of depression. Evid Based Complement Alternat Med 2021:1–14. https://doi.org/10.1155/2021/2165632
Shulman KI, Herrmann N, Walker SE (2013) Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs 27(10):789–797
Singkhorn O, Apidechkul T, Pitchalard K, Moonpanane K, Hamtanon P, Sunsern R, Leaungsomnapa Y, Thepsaw J (2021) Prevalence of and factors associated with depression in the hill tribe population aged 40 years and older in northern Thailand. Int J Ment Heal Syst 15(62):1–9. https://doi.org/10.1186/s13033-021-00487-7
Song J, Hou X, Hu X, Lu C, Liu C, Wang J, Liu W, Teng L, Wang D (2015) Not only serotonergic system, but also dopaminergic system involved in albiflorin against chronic unpredictable mild stress-induced depression-like behavior in rats. Chem Biol Interact 242:211–217
Stein DJ, Naudé PJ, Berk M (2018) Stress, depression, and inflammation: molecular and microglial mechanisms. Biol Psychiat 83:5–6. https://doi.org/10.1016/j.biopsych.2017.10.025
Stelzer EM, Book S, Graessel E, Hofner B, Kornhuber J, Luttenberger K (2019) Bouldering psychotherapy reduces depressive symptoms even when general physical activity is controlled for: a randomized controlled trial. Heliyon 4(3):e00580
Swann OG, Kilpatrick M, Breslin M, Oddy WH (2019) Dietary fiber and its associations with depression and inflammation. Nutr Rev 78(5):394–411
Tondo L, Isacsson G, Baldessarini R (2003) Suicidal behaviour in bipolar disorder: risk and prevention. CNS Drugs 17:491–511. https://doi.org/10.2165/00023210-200317070-00003
Troubat R, Barone P, Leman S, Desmidt T, Cressant A, Atanasova B, Brizard B, El Hage W, Surget A, Belzung C, Camus V (2020) Neuroinflammation and depression: a review. Eur J Neurosci 00:1–21. https://doi.org/10.1111/ejn.14720
Vargas-Medrano J, Diaz-Pacheco V, Castaneda C, Miranda-Arango M, Longhurst MO, Martin SL, Ghumman U, Mangadu T, Chheda S, Thompson M, Gadad BS (2020) Psychological and neurobiological aspects of suicide in adolescents: current outlooks. Brain Behav Immun Health 7:100124. https://doi.org/10.1016/j.bbih.2020.100124
Wang H, Brown J, Martin M (2011) Glycogen synthase kinase 3: a point of convergence for the host inflammatory response. Cytokine 53(2):130–140
Weibert E, Hofmann T, Stengel A (2019) Role of nesfatin-1 in anxiety, depression and the response to stress. Psychoneuroendocrinology 100:58–66. https://doi.org/10.1016/j.psyneuen.2018.09.037
Wheatley D (2005) Medicinal plants for insomnia: a review of their pharmacology, efficacy and tolerability. J Psychopharmacol 19(4):414–421. https://doi.org/10.1177/0269881105053309
United Nations Office on Drugs and Crime (UNODC) (2018) World Drug Report 2018. Drug use in Nigeria. United Nations Office on Drugs and Crime. Vienna International Centre, 1400 Vienna, Austria. Retrieved from UNODC Website: www.unodc.org/wdr/2018/
World Health Organization (WHO) (2000) General guidelines for methodologies on research and evaluation of traditional medicine. World Health Organization, Geneva
World Health Organization (WHO) (2017) Depression and Other Common Mental Disorders: Global Health Estimates, World Health Organization, Geneva, 2017: 1–24. https://apps.who.int/iris/bitstream/handle/10665/254610/WHO-MSD-MER-2017.2-eng.pdf
Xiao MM, Li JB, Jiang LL, Shao H, Wang BL (2018) Plasma nesfatin-1 level is associated with severity of depression in Chinese depressive patients. BMC Psychiatry 18(1):1–7
Yao CY, Wang J, Dong D, Qian FG, Xie J, Pan SL (2009) Laetispicine, an amide alkaloid from Piper laetispicum, presents antidepressant and antinociceptive effects in mice. Phytomedicine 16:823–829
Yates D (2011) Tipping the cortical balance. Nat Rev Neurosci 12(9):487–487
Zhu CB, Lindler KM, Owens AW, Daws LC, Blakely RD, Hewlett WA (2010) Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioural despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology 35(13):2510–2520
Zlebnik NE, Cher JF (2016) Beyond the CB1 receptor: is cannabidiol the answer for disorders of motivation? Annu Rev Neurosci 39:1–17
Funding
No funding was recieved for the project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Informed consent
All the authors contributed substantially to the work, participated in the writing and have seen and approved the submitted version.
Ethical approval
The care and use of Laboratory Animals were approved by the Federal University of Technology Akure ethical committee, which was followed strictly and in compliance with the National Institute of Health guidelines. Ethical approval was obtained from the Centre for Research and Development (CERAD), Federal University of Technology, Akure with the number FUTA/ETH/2020/016.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fasakin, O.W., Oboh, G., Ademosun, A.O. et al. The modulatory effects of alkaloid extracts of Cannabis sativa, Datura stramonium, Nicotiana tabacum and male Carica papaya on neurotransmitter, neurotrophic and neuroinflammatory systems linked to anxiety and depression. Inflammopharmacol 30, 2447–2476 (2022). https://doi.org/10.1007/s10787-022-01006-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-022-01006-x