Abstract
This study aimed to investigate the antioxidant and protective effect of E. persicus leaf hydroalcoholic extract (EPE) in preventing gastric ulcers induced by ethanol in rats. Wistar rats weighing 180–220 g were randomly divided into five groups. These groups included negative control (normal) group, positive control (ethanolic) group, comparative control (ranitidine recipient) group, group recipient the dose of 250 mg/kg plant extract, and group recipient the dose of 500 mg/kg plant extract. One hour after gavage of the drug and extract, the gastric ulcer was induced by feeding 1 ml of 96% ethanol to each animal except the rats of the negative control group. After one hour, the rats were killed, and their stomachs were separated. Then, the gastric Ulcer index (UI), pH, oxidative stress parameters, and histopathological changes in the stomach of all groups were measured. Pre-treatment of ethanol-induced rats with the EPE reduced (P < 0.05) the ulcer index and gastric juice pH, compared to ethanolic group rats. Furthermore, pre-treatment with EPE at a dose-dependent manner, alleviated the gastric oxidative stress injury in rats through increase the activity of CAT, tissue NO· and GSH levels. EPE also was able to decrease the levels of ROS, MDA, PCO and serum NO·. According to the results, it can be concluded that pre-treatment with EPE prevents the formation of gastric ulcers caused by ethanol, which can be attributed to the antioxidant activity of plant polyphenols compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A peptic ulcer is a lesion of the gastrointestinal mucosa that spreads to the mucosal muscle layer. It is usually characterized by various necrosis stages, neutrophil infiltration, decreased blood flow, increased oxidative stress, and other inflammatory reactions. (Sharifi-Rad et al. 2018). This disease does not have a high mortality rate in Iran, but statistics show more than 40% prevalent in the country (Sayehmiri and Tavan 2015). Pathophysiology of peptic ulcer disease is a multifactorial process caused by an imbalance between aggressive agents (especially acid and pepsin) and mucosal defence agents (especially blood flow and prostaglandins). Stress, alcohol consumption, smoking, Helicobacter pylori, and nonsteroidal anti-inflammatory drugs (NSAIDs) (Sistani Karampour et al. 2019) are factors that may increase the incidence of peptic ulcer disease. After all excessive alcohol consumption is one of the causes of gastric mucosal damage. Therefore, the ethanol-induced gastric ulcer model is often employed to study the anti-ulcer compounds (Adinortey et al. 2013). The most common drugs used to treat and prevent peptic ulcers include H2 receptor blockers (such as cimetidine, ranitidine, etc.) and proton pump inhibitors (such as omeprazole, lansoprazole, etc.), antacids, and cell protective factors. Because these drugs cause numerous adverse effects such as impotence, arrhythmia, hematopoietic alterations, hypersensitivity, and gynecomastia. For that reason, use of natural anti-ulcer drug with fewer side effects is inevitable (Kuna et al. 2019).
Since very old times, herbal medications have been used for relief of symptoms of disease. Despite the great advances observed in modern medicine in recent decades, plants still make an important contribution to health care. Much interest, in medicinal plants, however, emanates from their long use in folk medicines as well as their prophylactic properties, especially in developing countries. Large number of medicinal plants have been investigated for their antioxidant properties. Natural antioxidants either in the form of raw extracts or their chemical constituents are very effective to prevent the destructive processes caused by oxidative stress. Although the toxicity profiles of most medicinal plants have not been thoroughly evaluated, it is generally accepted that medicines derived from plant products are safer than their synthetic counterparts (Bari et al. 2021).
The species Eremurus persicus (Jaub & Spach) Boiss, known as serish, belongs to Xanthorrhoeaceae and the genus Eremurus. This genus is one of the most important genera of the Xanthorrhoeaceae family, with more than 40 species reported in the world and 7 species in Iran. Natural habitats of E. persicus are widely distributed in arid and semi-arid regions, mountain slopes, especially in central and middle Asia countries such as Afghanistan, Iran, Iraq, Tajikistan, Lebanon and Turkey. The slender and elongated leaves of this species are used as a vegetable in Central Asia. In earlier times, the roots of the serish have been used for bookbinding due to their glazed materials (Beiranvand and Beiranvand 2021).
Serish is used in traditional medicine to treat liver, stomach, constipation, and diabetes disorders. Decoction and root Poultice of this plant have been used in the past to treat ulcers and scabies. Its cooked leaves, along with rice, are currently used as traditional Iranian food (Batooli et al. 2015). In a research on E. persicus extract, antiglycation (Asgarpanah et al. 2011), antibacterial and cytotoxic (Vala et al. 2011), antimalarial and anticancer activities of this plant have been positively evaluated (Khan et al. 2011). On the other hand, the presence of terpenes and oxygenated terpene derivatives such as limonene, geranylgeraniol, n-nonanal, geranyl acetone, benzene acetaldehyde, linalool, α-pinene and 1,8-cineol have been confirmed in the essential oil of this plant. All studies of these abundant volatile compounds have shown that these compounds could have significant antioxidant activity, antimicrobial, antifungal, anticancer and acetylcholinesterase inhibitory (Salehi et al. 2017). Also, the presence of branched carbohydrates in the roots of this plant is confirmed (Jahanbin and Beigi 2016).
A recent study on the anti-leishmaniasis properties of E. persicus root extract has shown that the compound aloesaponol III 8-methyl ether isolated from the plant extract can have a good therapeutic potential against leishmaniasis infections (Rossi et al. 2017). However, to date, there is no evidence of biological activity of E. persicus in vivo. Therefore, in the present study, phytochemical constituents of the aqueous ethanolic extract of E. persicus aerial parts and its gastroprotective effect against ethanol-induced gastric ulcer rats have been investigated.
Materials and methods
Chemicals and drugs
Ranitidine was obtained by Kharazmi Pharmaceutical Company (Khorramabad, Iran). 2,2-Diphenyl-1-picrylhydrazyl (DPPH), Gallic Acid, Catechin, 5,5′-dithiobisnitro benzoic acid (DTNB), 2′,7′-dichlorofluorescein diacetate (DCFH-DA), 2,4-dinitrophenylhydrazine (DNPH), and guanidine hydrochloride from Sigma-Aldrich (St. Louis, MO, USA) were provided. Ethanol, Folin–Ciocalteu’s reagent (FCR), hydrogen peroxide (H2O2), 5-sulfosalicylic acid (C7H6O6S·2H2O), trichloroacetic acid (TCA), thiobarbituric acid (TBA), 1-naphtylethylenediamine, and Sulfanilamide were purchased from Merck Co (Germany). All other chemicals used were analytical grade.
Plant material and preparation of extract
The leaves of E. persicus plant were collected from Zagheh section, located 35 km from Khorramabad city (with geographical coordinates: 33° 29′ 56″ N 48° 42′ 31″ E) in mid-May 2019, and then registered by Dr. Hamed Khodayari with the herbarium code Hlukh 25,031,398 in the Biology Department of Lorestan University. Then, the plant was dried in the shadow, crushed and exhaustively extracted with 80% (v/v) aqueous EtOH under reflux. The obtained eluent was dried under vacuum (using a rotary device {RV 06ML/German}) at 40 °C. Then, the dried extract was weighed and stored for future use.
Total phenolic contents assay
The plant's total phenolic content was determined using the Folin–Ciocalteu’s reagent (FCR) according to the method described (Slinkard and Singleton 1977). At the first step, 0.5 ml of the extract with a concentration of 1 mg/ml was added with 2.5 ml of diluted FCR solution (with a proportion of 1–10). Subsequently, 2 ml of 7.5% Sodium carbonate (Na2CO3) solution was injected. Ninety minutes later, kept in the dark media, and then the samples' absorption at a wavelength of 765 nm was interpreted in the laboratory (Ardestani and Yazdanparast 2007a). The phenolic compounds extracted amount was determined using the standard curve of gallic acid. It was expressed as milligrams equivalent gallic acid per gram of dry weight (mg GA/g dry).
Total flavonoid contents assay
The total flavonoids content from plant was measured by aluminium chloride (AlCl3) Colorimetric method (Zhishen et al. 1999). 0.5 ml of a plant extract with a 1 mg/ml concentration was mixed to 2 ml of distilled water and 0.15 ml of 15% sodium nitrite (NaNO2) solution. After 6 min, 0.15 ml of 10% AlCl3 solution and 2 ml of 4% Sodium hydroxide were injected into the sample, and eventually, its volume with distilled water reached 5 ml. The absorbance was read after 15 min against the freshly prepared sample blank at a wavelength setting of 510 nm. The catechin compound was used to draw the standard curve, and the results were expressed as equivalent to catechin (mg catechin/g dry) (Ardestani and Yazdanparast 2007b).
Determination of radical scavenging capacity
Antioxidant activity of extract was measured using DPPH free radical based on the Blois (1958) method. In summary, 0.2 mM DPPH solution was prepared in ethanol, and 4925 ml of it was added to 1075 ml of various extract concentrations (400–25 μg/mL). The samples were then shaken vigorously and placed at Laboratory temperature for 30 min away from light and following this, their absorption at a wavelength of 517 nm was read by the Epoch microplate reader (Biotek model/USA). In this test, ascorbic acid (vitamin C) was used as a positive control (Yazdanparast et al. 2008). The percentage of inhibition of DPPH free radicals was calculated using the following formula:
In this formula, Ao absorption of DPPH and A1, absorption samples in the presence of DPPH. The results were compared with ascorbic acid as a positive control. Sample ability to inhibit (or scavenge) 50% of free DPPH radicals was determined as IC50.
FTIR spectrum analysis
Fourier-transform infrared (FTIR) spectroscopy analysis, to determine organic compounds and identification of functional groups in chemical compounds of plant extract for antioxidant activity, was used. Combine the powder of the plant’s completely dry extract and the potassium bromide seeds (KBr) in a ratio of 1–100, and after grinding them, pour some of their mixture into a special metal mold and press it with a hydraulic press machine until it became a transparent pill. The resulting tablet was analyzed by FTIR spectrophotometer (Shimadzu 8400S model/Japan) in the range of 4000–400 cm−1 wavelength with a resolution of 4 cm−1 (Packialakshmi and Sowndriya 2019).
Animals
Thirty adult albino male Wistar rats weighing 180–220 ± 20 g were purchased from the Animal Sciences Department of Kermanshah University of Medical Sciences. The rats were caged for 3 days in the animal house of the School of Veterinary Medicine of Lorestan University with controlled conditions for laboratory animals, including polypropylene cages with wood shaving and temperature of 22–24 °C with 12 h dark/light cycles and open access to pellet and water. Distilled water was used for the oral administration of the standard drug and plant extracts in all animals. All protocols were conducted in accordance with the ethical guidelines for lab animals' research with approval number LU.ECRA.2020.32 which, confirmed by ethical board of the School Veterinary Medicine of Lorestan University (at date of Ethical Approval: 11 April 2020).
Experimental design
Rats were weighed on day 1 and remained under 12 h day-night cycles for environmental adaptation with supply water and food. The next day, rats were incidentally divided into five groups, except the negative control stayed hungry for 24 h (food deprivation). During 24 h, to prevent dehydration, drinking water was free for all groups. On the third day, all rats were weighed again, and according to the designed model groups, all, except the first, received the extract and the drug with a gavage needle (Beiranvand and Bahramikia 2020). The rats were divided into five groups, as follows:
-
Negative control or normal group (normal healthy rats that did not receive extract, drug, or ethanol during the research)
-
Positive control or ethanolic group (received only 1 ml of ethanol per rat)
-
Comparative control group (recipient ranitidine 50 mg/kg + 1 ml ethanol per rat)
-
Experimental group 1 (received plant extract at a dose of 250 mg/kg + 1 ml ethanol per rat)
-
Experimental group 2 (received plant extract at a dose of 500 mg/kg + 1 ml ethanol per rat)
Ranitidine and extract pre-treatments were given orally only once/day for 1-day prior to ulcer induction. During the gavage of the extract and drug from third to fifth groups, to create the same stress conditions caused by the gavage, first and second groups, 1 ml of gavage distilled water were given.
With the exception of the first group (negative control), gastric ulcers were induced by 1 ml of 96% ethanol per gavage and followed with administration of drug or extract gavage in three other treatments 1 h after alcohol ingestion. Thereafter, animals were anesthetized for 2–5 min and euthanized by cervical dislocation. Blood collection tubes were centrifuged (3000g/10 min), allowed serum separated from cellular contents, and stored at − 20 °C for further investigation. At the same time, animal stomachs were pulled out, opened along the greater curvature, and collected for volume and pH determination. After that, gastric tissue specimens were rinsed gently with a physiology saline solution to remove blood clots and gastric contents, then for gross examination, scanned to calculate peptic ulcer index (Beiranvand and Bahramikia 2020). Subsequently, the stomach was incised longitudinally and shared by dividing it in half. One section was reserved for a sterile tissue container containing 10% neutral-buffered formalin for histopathological examination, and the rest was stored at − 20 °C for determination of antioxidant and stress oxidative parameters.
Measurement of stomach ulcer index (UI) and inhibition percentage (%I)
The gastric ulcer index (UI, mm2) and ulcer inhibition percentage (%I) were determined according to the Takagi and Okabe (1968) methods. In this method, the ulcerated surface area was initially measured with a ruler, and the ulcer's degree was assessed based on ulcer intensity using Table 1. Then the UI and %I were calculated with the following formulas (Bhattamisra et al. 2019):
Measurement of stomach juice volume and pH
The volume and pH of gastric acid juice were measured by the published observational studies (Dashputre and Naikwade 2011). The stomach juice of each rat for 10 min at 2000g was centrifuged. Then, the pellet was removed, and the volume of digestive fluid was measured, and in the following to 1 ml of supernatant, 1 ml of distilled water was added, and the pH was measured by a pH meter (Hanna pH meter model 211/USA).
Histopathological analysis
A section of the stomach tissue was stabilized at 10% formalin and was partially separated after longitudinal incision and dewatered by ethanol solutions from dilute to concentrated (70%, 80%, 90%, and 100%). Following that, the clarification step was applied to the sample with xylene and then the sample was molded by paraffin. After the paraffin penetrated into the tissue, the paraffin block containing the sample was cut into layers with a thickness of 5 µm by a microtome machine; the incisions were then placed on a glass slide and stained with haematoxylin and eosin (H&E). When the slides were prepared, the pathological changes were examined using an optical microscope (Karampour et al. 2019).
Biochemical analysis
To measure biochemical parameters, gastric tissue was homogenized (Jambi and Khattab 2019). The gastric tissue was first weighed using a calibrated scale, then 9 ml of PBS buffer was added to 1 g of it, and the resulting mixture was homogenized on ice with the help of a homogenizer. At this stage, ice was used to prevent damage to proteins and reduce activity and inactivation of enzymes within tissue during homogenization. Next, the homogeneous mixture was transferred to the falcon tube and centrifuged by refrigerated centrifuge for 20 min at a rate of 12,000g at 4 °C. The supernatant (surface liquid) was collected, and the resulting sediment was discarded. Supernatants were divided into separate micro-tubes and kept at − 20 °C until evaluating the oxidative stress biomarkers.
Reactive oxygen species assay
The rate of reactive oxygen species (ROS) formation in the reaction mixture was measured by following the oxidation of 2,7-dichlorofluorescein diacetate (DCFH-DA) to a highly fluorescent 2,7-dichlorofluorescein (DCF) compound, according to the documented literature as modified (Bahramikia et al. 2009). In summary, each sample consisted of 1.7 ml of phosphate buffer solution (50 mM, pH 7.4), 0.2 ml of gastric homogenate, and 100 μl of DCFH-DA solution (10 μM). The samples were incubated in a warmed water bath at 37 °C temperature for 15 min. ROS production was measured by DCF formation using a Cary-Eclipse fluorescence spectrophotometer (Agilent/USA) with excitation wavelength and emission at 488 and 521 nm.
Measurement of catalase enzyme activity
The activity of Catalase (CAT) in all study groups was measured with a slight modification by Aebi (1984) method. 100 μl of stomach homogenate supernatant was added to the cuvette containing 1900 μl of phosphate buffer (50 mM, pH 7). The reaction mixture was prepared by adding 1 ml of hydrogen peroxide (H2O2) and absorbance changes were measured at 15-s intervals within 60 s. H2O2 decomposition rate was measured by a UV/VIS spectrophotometer (T80 + /England) at 240 nm. The expression of the CAT activity was calculated based on the following formula.
\(\Delta A\) is the absorption difference; \(\Delta t\) is the time difference; ε is the molar absorption coefficient H2O2 = 43.6 cm−1 M−1.
Reduced glutathione assay
Glutathione (GSH) level, to calculate its differences among all groups was measured by Jollow et al. (1974) with a little change. To the 0.5 ml of stomach homogenate, 1 ml of sulfosalicylic acid (4%) was added and then placed at 4 °C. After1 h, the samples were centrifuged for 15 min at 3000 rpm at 4 °C. To 1 ml of each sample’s supernatant, 0.1 ml of DTNB (4 mg/ml) and 0.9 ml of phosphate buffer (0.1 M, pH 7.4) were added. With yellow colour formation, the absorption of samples was read at a wavelength of 412 nm. Reduced glutathione was expressed as μg/mg of protein.
Measurement of lipid peroxidation
Malondialdehyde (MDA) levels were measured to determine the evidence of membrane lipid peroxidation probably increased after tissue damages by double heating method with a slight change (Draper and Hadley 1990). In short, to 0.5 ml of each homogenized sample of the stomach, 2.5 ml of trichloroacetic acid (TCA, 10%) was added, and for 15 min placed in a boiling water bath. With gradual cooling, at room temperature, and 4 °C, the test tubes were centrifuged at 3000 rpm for 10 min. The following 0.3 ml of supernatant per tube to new tubes containing 0.3 ml were transferred from the TBA solution (0.67%). Each tube was placed in a boiling water bath (95 °C) for 20 min in the next step. Ultimately, the absorption of samples was measured at 532 nm against the blank solution after cooling tubes were placed at room temperature and at 4 °C. MDA absorption was calculated based on the molecular absorption coefficient of MDA–TBA complex (ε = 1/56 × 105 cm−1.M−1) and was expressed based on nmol/mg Protein.
Protein carbonyl content assay
Protein carbonyl (PCO) content as an indicator of protein oxidation was measured using Reznick and Packer (1994) methods. One ml of DNPH (10 mM in HCl) was added to the reaction mixture (2 mg protein). The samples were incubated at room temperature for 1 h and were vortexed every 15 min and added 1 ml of trichloroacetic acid (TCA) (10%, w/v) to each reaction mixture centrifuged at 3000g for 10 min. Consequently, the protein precipitate was washed three times with 2 ml of ethanol/ethyl acetate solution (v/v, 1:1) and dissolved in 1 ml of guanidine hydrochloride solution (6 M, pH 2.3). After 10 min of incubation of the samples at room temperature, their absorbance at a 370 nm wavelength was read against the blank solution. The PCO content was calculated based on the molar extinction coefficient of DNPH (ε = 2.2 × 104 cm−1 M−1). The data were expressed as nmol/mg protein (Bahramikia et al. 2009).
Determination of total nitric oxide levels in serum and tissue
Nitric oxide (NO·) is one of the reactive nitrogen species that acts as an important radical molecule in the intracellular signalling pathway and has a protective role with the effect of vascular dilation. However, its elevation, which principally induced after inflammatory cells activation, can become destructive. Therefore, concerning to the importance of NO·, the level of this molecule was determined in the serum and gastric tissue of rats by measuring the accumulation of its stable degradation products, namely nitrite and nitrate. The serum and tissue nitrite levels were bio-assayed using the Griess reagent, according to Hortelano et al. (1995). Griess reagent is a mixture (1:1) of sulfanilamide (SA) 1% in phosphoric acid 5% and 1-naphthyl ethylene diamine (NED) 0.1%, which in the presence of nitrite produce a red diazo complex. The reaction products of colour intensity were measured at 540 nm, and the results were expressed as μmol/L using the standard NaNO2 curve (Bahramikia and Yazdanparast 2012).
Statistical analysis
The results were expressed as the means ± SD. The Student's t test detected statistical differences between the two groups. When more than two groups are compared (or analyzed), a one-way analysis of variance (ANOVA) was used, followed by the Dunnett post hoc test. Values of P < 0.05 were considered statistically significant. The data were analyzed using GraphPad Prism 8.0 software (San Diego, CA, USA).
Results
Phytochemical examination
The phytochemical screening of aqueous ethanolic extract of E. persicus plant revealed polyphenols and flavonoids. The quantitative phytochemical investigation of total phenolic and flavonoid contents in the extract is shown in Table 2.
Radical scavenging activity of EPE
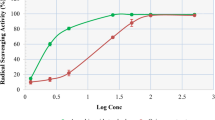
As indicated in Fig. 1, with increasing concentration, the percentage of free radical scavenging (Inhibitory) was increased. The 50% inhibition concentration (IC50) of free radicals by the aqueous ethanolic extract was estimated to be 79.80 ± 0.03 μg/ml.
Investigation of FTIR spectrum of E. persicus extract (analysis of isolated components)
As shown in Fig. 2, the main peaks of the aqueous ethanolic extract can be seen in the range of 3340, 2937, 1625, 1409, that with the stretching vibration of alcohol O–H, phenols or N–H amines, the stretching vibration C–H in CH3, stretching vibration C=O in carboxyl or amide group, strong stretching vibration C–C at aromatic compounds correspond. The range peaks of 1076 cm−1 and 1267 cm−1 are related to the vibrational tension of C–O ethers and esters or C–N amines (Oliveira et al. 2016; Sangeetha et al. 2016; Pavia et al. 2014). According to the observed peaks, as well as a recent study by our colleague that described all the compounds isolated from EPE, the presence of about 11 compounds in the plant extract can be confirmed (Beiranvand and Beiranvand 2021). These previously isolated compounds are shown in Fig. 3 and Table 3 describe some of their biological and medicinal properties. On the other hand, according to some reports, the main compound of EPE is Isoorientin. This compound has antinociceptive, anti-inflammatory, anti-proliferative and gastroprotective effects (Mottaghipisheh et al. 2020). Therefore, our opinion is that the anti-ulcer property of EPE, which was observed here, is also related to this compound.
The chemical structures of the isolated phytochemicals from E. persicus extracts (Beiranvand and Beiranvand 2021)
Effect of EPE on ulcer index, percentage of Inhibition of ulceration and gastric juice pH
According to Table 4, the stomach juice pH of the ethanolic group rats was significantly reduced compared to negative control animals. This (pH 2.6) is one reason for the greater value of the ulcer index in ethanolic rats. Meanwhile, rats of the pre-treated with ranitidine, as a standard group, had a significant increase in stomach juice pH, indicating drug effectiveness in reducing acidity (secretion of acid) caused by ethanol gavage. Also, the ulcer index of this group was mild and the percentage of ulcer inhibition by this drug was at the highest level. Rats of experimental groups 1 and 2 that received doses of 250 and 500 mg/kg of EPE had a pH of 4.5 and 6.4 gastric juice, and inhibition percentage of ulcers by 37 and 66.
The dose of 500 mg/kg of EPE was better than the 250 mg/kg, that this indicates inhibition of ulceration at higher doses of by EPE.
Pathological findings on the gastric mucosa
Macroscopic findings
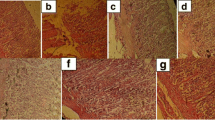
Acute stomach ulcers were induced by intra-gastric administration of ethanol. As depicted in Fig. 4, the peptic ulcer surface was more extended significantly in the ethanol-stimulated rats than in normal rats. Pre-treatment with EPE or RAN could effectively be prevented the acute form of ethanol caused diffuse mucosal damage.
Histopathologic findings
Results of observation of microscopic lesions in various groups are as follows:
-
Ethanolic group: extensive destruction and necrosis of mucosal tissue, severe haemorrhage, edema, and infiltration of leukocyte cells (Fig. 5B).
-
Ranitidine 50 mg/kg recipient group: Mild degeneration and necrosis at the apex of mucosa tissue, very small amounts of edema, haemorrhage, and infiltration of inflammatory cells (Fig. 5C).
-
Group 250 mg/kg plant extract: moderate destruction and necrosis of mucosal tissue (one-third to one-fourth the mucosa), low edema values, haemorrhage, and leukocyte infiltration (Fig. 5D).
-
Group 500 mg/kg plant extract: Mild degeneration and necrosis at the apex of mucosal tissue, very small amounts of edema, haemorrhage, and infiltration of inflammatory cells (Fig. 5E).
microscopic view of the stomach tissue in rats of normal group (A), ethanolic group (B), ranitidine-pre-treated group (C), plant extract group at a dose of 250 mg/kg (D) and plant extract group at a dose of 500 mg/kg (E). Yellow arrow: necrosis and destruction of the mucosa at the apex of the mucosa; Red arrow: necrosis and destruction of mucosa in the depth of the mucosa; White arrow: Healthy appearance or with minimal changes in the coating structure and mucosal layer; (E: edema, L: infiltration of inflammatory cells, H: Haemorrhage)
Effect of EPE on biochemical analysis results
Level of ROS
According to Fig. 6, induction of gastric ulcer by ethanol increased the ROS formation rate from 53.09 (a.m.u) in the normal group to 288.67 (a.m.u) in the ethanol intoxicated rats. DCF fluorescence intensity in the group receiving ranitidine decreased from 288.67 to 90.12 (a.m.u) compared to the ethanolic group. Also, fluorescence intensity decreased in the pre-treatment groups with doses of 250 and 500 mg/kg of EPE, to 142.19 and 107.92 (a.m.u), respectively.
The effects of EPE and ranitidine drug on the level of ROS formation in studied groups. The results were expressed as means ± SD for six rats in each group. (Eremurus persicus (Jaub & Spach) Boiss extract = EPE; Ranitidine = RAN). *Significant difference with P < 0.01 compared to normal group, **Significant difference with P < 0.01 compared to ethanolic group, *** Significant difference with P < 0.05 compared to ethanolic group
CAT enzyme activity
Changes in CAT enzyme activity in this study are shown in Fig. 7. Induction of gastric ulcer by ethanol reduced the activity of this enzyme in the ethanol intoxicated rats to the amount 0.266 (IU/mg Pr). On the other hand, administration of ranitidine increased the activity of this enzyme to the amount 0.673 (IU/mg Pr). Moreover, administration of the 250 and 500 mg/kg doses of EPE to rats increased the activity of this enzyme by 0.362 and 0.529 (IU/mg Pr), respectively.
Changes in the activity of the CAT enzyme in gastric tissue of different groups. The results are shown based on the means ± SD of the data for 6 rats in each group. (Eremurus persicus (Jaub & Spach) Boiss extract = EPE; Ranitidine = RAN). *Significant difference with P < 0.05 compared to normal group, **Significant difference with P < 0.05 compared with ethanolic group, ***Significant difference with P < 0.001 compared with ethanolic group
Level of GSH
As depicted in Fig. 8, GSH levels in the gastric tissue of ethanol intoxicated rats decreased to the amount 4.22 (μg/mg Pr). However, administration of ranitidine and administration of 250 and 500 mg/kg doses of plant extract increased the levels of this non-enzymatic antioxidant to 5.01, 4.83 and 5.22 (μg/mg Pr), respectively.
Changes of GSH level in gastric tissue of different groups. The results are shown based on the means ± SD of the data for 6 rats in each group. (Eremurus persicus (Jaub & Spach) Boiss extract = EPE; Ranitidine = RAN). *Significant difference with P < 0.001 compared to normal group, **Significant difference with P < 0.05 compared to ethanolic group, ***Significant difference with P < 0.01 compared to ethanolic group
Lipid peroxidation measurement
As be displayed in Table 5, the amount of MDA in the ethanolic group was increased to the amount of 0.55 ± 0.02 (nmol/mg Pr) compared to the negative control group. While pre-treatment with ranitidine and plant extract at doses of 250 and 500 mg/kg reduced MDA levels to the amount of 0.41 ± 0.01, 0.45 ± 0.01 and 0.4 ± 0.01(nmol/mg Pr), respectively.
Measurement of PCO
According to Table 5, ethanol-induced oxidative damage increased PCO levels in the ethanolic group to the amount 14.98 ± 0.4 (nmol/mg Pr). However, pre-treatment with ranitidine and doses of 250 mg/kg and 500 mg/kg of plant extract inhibited the level of PCO formation by 12.99 ± 0.2, 13.58 ± 0.43 and 12.88 ± 0.24 (nmol/mg Pr), respectively.
Level of serum NO·
As be exhibited in Table 5, the serum NO· level in the ethanol intoxicated group was 43.1 ± 2.1 (μmol/L) compared to the normal group. However, administration of ranitidine reduced serum NO· levels to the amount 25.7 ± 2.7 (μmol/L). Pre-treatment with doses of 250 mg/kg and 500 mg/kg of plant extract also reduced the level of this factor to amount 33 ± 2 and 29 ± 1.8 (μmol/L), respectively.
Level of tissue NO·
With reference to Table 5, induction of gastric ulcer by ethanol in the ethanolic group led NO· tissue level to reach 0.94 ± 0.04 (μmol/mg Pr). Whereas, pre-treatment with ranitidine and doses of 250 mg/kg and 500 mg/kg of plant extract increased the level of NO· tissue in these groups to the amount 1.39 ± 0.08, 1.02 ± 0.08 and 1.37 ± 0.06 (μmol/mg Pr).
Discussion
Peptic ulcer disease (gastric and duodenal ulcers) is a serious and growing health problem in around the world that is estimated to affect 10% of the world's population with increase in annual of 4 million people. Furthermore, it is the most common gastrointestinal disease, involving 40% of developed countries and 80% of developing countries because of overuse of NSAIDs and Helicobacter pylori infection (Ajeigbe et al. 2014). Current clinical treatment of gastric ulcer indicates a high recurrence rate and low recovery. Therefore, gastric ulcers treatment is still a major challenge that has made the development of new drugs and alternative therapies as a necessary and urgent need. Previous studies have reported the anti-ulcer properties of many medicinal plants as an excellent potential source for drugs production (Srinivas et al. 2013). In recent years, many attempts have been made to detect gastroprotective and anti-ulcer drugs (Jambi and Khattab 2019). In this regard, several studies have been widely performed using ethanol-induced gastric ulcer model in animals to investigate the gastroprotective activity of plants and drugs. The effect of ethanol generated gastric ulcer begins with its rapid penetration into the gastric mucosa, thereby increasing mucosal permeability and secretion of vasoactive mediators such as endothelin-1, leukotrienes C4, and histamine. These vasoactive agents increase gastric mucosal lesions by reducing blood flow to the mucosal membranes.
Moreover, ethanol could damage blood vessels by reducing mucus production (by damaging its constituent components) and increasing the production of ROS, which leads to bleeding, tissue necrosis, and ultimately disruption of the protective barrier. Previous studies suggest that ethanol-induced gastric mucosal lesions are significantly associated with increased oxidative stress following oxygen-derived free radicals’ production and also, reducing antioxidant enzymes activity. In this context, the infiltration and activation of neutrophils directly contribute to the increased ROS production. Therefore, it is likely that the infiltration and accumulation of neutrophils in the gastric mucosa result in free radical formation, which damages cellular components, including lipids and proteins (Birdane et al. 2007; Mshelia et al. 2017). PCO is involved in many diseases, and it can be used as a diagnostic marker for oxidative stress. Therefore, the detection and reduction of PCO plays a pivotal role in recovering such diseases (Dalle-Donne et al. 2003; Elliott and Wallace 1998). Importantly, in our study, the pre-treatment of the ethanol-induced rats with EPE and ranitidine ameliorate the ROS levels and PCO production. These effects are likely through the strengthening the antioxidant defence system of gastric cells.
Several studies have been reported that ethanol can stimulate lipid peroxidation in cell membranes through ROS production, resulting in cell damage. Many studies have shown that ethanol can stimulate lipid peroxidation in cell membranes via ROS (Cederbaum 2001). The increased lipid peroxidation by ethanol accelerates gastric ulcers by destroying membrane integrity and increasing cell permeability of gastric epithelial cells. These events lead to MDA production as the end product of the oxidation of unsaturated fatty acids in cell membranes. MDA is commonly used as a reliable indicator of lipid peroxidation, which its level can be used to estimate the extent of ethanol-induced gastric tissue damage. In agreement with previous studies, our data showed that ethanol gavage significantly increases MDA production in gastric tissue (Chen et al. 2019; Rocha et al. 2011; Antonisamy et al. 2015). Significantly, pre-treatment of ethanol-induced rats by EPE at doses of 250 mg/kg and 500 mg/kg reduced MDA levels. Different studies have shown that the generation of hypochlorous acid (HOCL), as one of the dangerous species resulting from the enzyme myeloperoxidase (MPO), increased following the oxidation of chloride ions by hydrogen peroxide in neutrophils. At this time, antioxidant defences of gastric cells are activated and destroy H2O2, with the purpose to suppress oxidative process. The antioxidant enzyme CAT mediates the conversion of H2O2 into water and oxygen. GSH can also neutralize H2O2 by functioning as a cofactor for the glutathione peroxidase (GPx) enzyme. Many evidences show the reduction of CAT activity and GSH content in gastric tissue resulted from ethanol-induced gastric mucosal damage. This reduction leads to elevated ROS levels, resulting in increased lipid peroxidation and PCO formation (Antonisamy et al. 2014; Das and Vasudevan 2007). In this context, Amaral et al. (2013) have demonstrated that the ethanol-induced mucosal damage increases the production and accumulation of ROS and MDA. The authors further showed that this occurs by enhancing the MPO enzyme activity that resulted from decreased CAT activity and GSH levels in gastric tissue. As well, Liu et al. (2016) have reported that ethanol administration, could damage gastric tissue by increasing MDA and PCO levels. The results of our study showed that EPE has high antioxidant properties by reason of its relatively high levels of phenolic and flavonoid compounds, glycosides, coumarin derivatives, and naphthoquinones (Gaggeri et al. 2013, 2015). Polyphenols, as one of the plant antioxidants, can terminate the chain reaction by reacting with free radicals so that their phenolic hydroxyl group reduces this radical formation by giving an electron to free radicals. As a result, the formation of aromatic free radicals will be stable owing to the resonance effect. On the other hand, polyphenols can enter the lipid bilayer of the gastric mucosa, protecting the entire lipid layer from oxidation (Zhu et al. 2020; Bors et al. 1990). Our results indicated that EPE has a gastroprotective effect against ethanol-induced gastric ulcers. Because phenolic compounds play a major role in antioxidant activity of plants, the EPE's anti-ulcer activity may be related to these compounds. With their synergistic effect with antioxidants such as GSH, CAT, etc., these compounds can inhibit the chain reaction of free radicals and increase the antioxidant level of gastric tissue to protect it against ethanol-induced damage.
Here, we used ranitidine, which inhibits gastric acid secretion by blocking H2 receptors. Furthermore, ranitidine has antioxidant properties (Ahmadi et al. 2011), and therefore, its functions more efficiently than EPE. We could show the effects of ranitidine by biochemical, macroscopic, and microscopic analysis. Additionally, ranitidine increased the pH of gastric juice, which led to a decrease in the ulcer index. Many evidences show that even after gastric mucosal injury, the stomach is stimulated; thus, gastric juice does not affect gastric mucosa. It secretes a large amount of stomach acid, which increases the volume of gastric juice and reduces its pH. These events worsen gastric mucosal damage and, as a result, intensify gastric injury (Liu et al. 2019). It is manifested in our results that EPE effectively reduces gastric juice volume and increases gastric juice pH in rats that receive alcohol, thereby could protect the gastric tissues and preventing ethanol-induced gastric injuries. Yet, herbal extracts might act as H2-receptor blockers and prevent histamine from binding to its receptor (Adefisayo et al. 2017). Therefore, a mechanism of action of EPE is related to a further reduction of gastric juice pH. In addition, macroscopic and microscopic evaluations and the inhibition percentage of ulcers also confirmed this effect.
NO·, as a gaseous free radical, plays a role in physiological processes and in pathophysiological conditions. The level of NO· is increased due to the activity of inducible nitric oxide synthase (iNOS), anion superoxide (O2·−) resulting from ethanol metabolism and neutrophils (Tamura et al. 2013; Kwiecien et al. 2002). Thus, NO· is prone to react with O2·− that results in proxy nitrite (ONOO−). This later free radical is a species with cellular toxicity that can oxidize various cellular components, disrupt cellular processes, disrupt cellular signaling pathways, and induce cell death. Interestingly, ethanol consumption is associated with increased expression of iNOS, which leads to increase NO· levels.
Along with this evidence, we also found increased NO· production in response to ethanol-induced gastric ulcer. Therefore, this could be attributable to the increased induction of iNOS expression, which in this context can be noted in the study of Yu et al. (2014). Li et al. (2015) also reported that ethanol gavage significantly increased serum NO· levels and iNOS expression in the ethanolic group compared to the normal group.
On the other hand, NO· as a vasodilator factor with a short half-life regulates vascular and nutrient blood flow. It maintains epithelial integrity in the stomach by regulation of mucus barrier. Also, most importantly, it has a pivotal role in the angiogenesis, tissue regeneration, and ulcer healing (Moawad et al. 2019). As a result, in the present study, its reduction in the gastric tissue of the ethanol group may be caused by increased ONOO− and oxidative damage and the decreased endothelial nitric oxide synthase (eNOS) expression as a protective factor. Besides, direct-ethanol damage on epithelial cells and sub-mucosal endothelial vessels might also contribute to the reduction in the gastric tissue. According to Zhang et al. (2020), this event leads to the rupture of blood capillaries and reduced blood flow in these vessels (Elliott and Wallace 1998). Numerous studies have also confirmed that NO· level of gastric mucosa in rats under ethanol gavage was significantly decreased (Sidahmed et al. 2013; Arab et al. 2015, 2019; Rouhollahi et al. 2014). In this study, EPE improved the antioxidant defence system (by participating in free radical scavenging). It probably leads to a change in the nitric oxide system (by reducing iNOS expression and increasing eNOS expression) during ethanol-induced oxidative damage.
Conclusion
Based on the results, it can be concluded that the mechanisms which are involved in damaging the gastric mucosa after ethanol administration, include the overproduction of free radicals, reduction of internal antioxidants, and increase of acid secretion. In this study, EPE's protective effect on the prevention of ethanol-induced gastric ulcers depends on the antioxidant effect of phenolic, flavonoid compounds, and possibly glycosides and coumarin derivatives, which are available in the plant. With an increasing dose of EPE, the amount of these compounds in the extract are elevated; thus, the protective effect of EPE is subsequently increased. Therefore, EPE's main mechanism in preventing gastric ulcers is due to the scavenging of free radicals caused by ethanol and its synergistic effect, which causes increased levels of CAT, GSH, and tissue nitric oxide and decreased levels of ROS, lipid peroxidation, and PCO.
Availability of data and materials
This study's data are included in the article, and the corresponding author can provide the primary data.
Code availability
Not applicable.
Abbreviations
- EPE:
-
Eremurus persicus (Jaub & Spach) Boiss Extract
- RAN:
-
Ranitidine
- FCR:
-
Folin–Ciocalteu’s reagent
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- FTIR:
-
Fourier-transform infra-red spectroscopy
- PBS:
-
Phosphate-buffered saline
- DCF:
-
2,7-Dichlorofluorescein
- NO· :
-
Nitric oxide
- MDA:
-
Malondialdehyde
- PCO:
-
Protein carbonyl
- GSH:
-
Glutathione
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- ROS:
-
Reactive oxygen species
- CAT:
-
Catalase
- H2O2 :
-
Hydrogen peroxide
- iNOS:
-
Inducible nitric oxide synthase
- eNOS:
-
Endothelial nitric oxide synthase
References
Adefisayo MA, Akomolafe RO, Akinsomisoye SO, Alabi QK, Ogundipe OL, Omole JG, Olamilosoye KP (2017) Gastro-protective effect of methanol extract of Vernonia amygdalina (del.) leaf on aspirin-induced gastric ulcer in Wistar rats. Toxicol Rep 4:625–633. https://doi.org/10.1016/j.toxrep.2017.11.004
Adinortey MB, Ansah C, Galyuon I, Nyarko A (2013) In vivo models used for evaluation of potential antigastroduodenal ulcer agents. Ulcers. https://doi.org/10.1155/2013/796405
Aebi H (1984) [13] Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Ahmadi A, Ebrahimzadeh MA, Ahmad-Ashrafi S, Karami M, Mahdavi MR, Saravi SSS (2011) Hepatoprotective, antinociceptive and antioxidant activities of cimetidine, ranitidine and famotidine as histamine H2 receptor antagonists. Fundam Clin Pharmacol 25:72–79. https://doi.org/10.1111/j.1472-8206.2009.00810.x
Ajeigbe K, Onifade A, Omotoso D, Enitan S, Olaleye S (2014) Anti-ulcerogenic activity of Aspilia africana leaf extract: roles of gastric acid, oxidative stress and neutrophil infiltration. Afr J Biomed Res 17:193–201
Amaral GP, de Carvalho NR, Barcelos RP, Dobrachinski F, de Lima PR, da Silva MH, Lugokenski TH, Dias GRM, da Luz SCA, Boligon AA (2013) Protective action of ethanolic extract of Rosmarinus officinalis L. in gastric ulcer prevention induced by ethanol in rats. Food Chem Toxicol 55:48–55. https://doi.org/10.1016/j.fct.2012.12.038
Antonisamy P, Subash-Babu P, Alshatwi AA, Aravinthan A, Ignacimuthu S, Choi KC, Kim J-H (2014) Gastroprotective effect of nymphayol isolated from Nymphaea stellata (Willd.) flowers: contribution of antioxidant, anti-inflammatory and anti-apoptotic activities. Chem Biol Interact 224:157–163. https://doi.org/10.1016/j.cbi.2014.09.020
Antonisamy P, Duraipandiyan V, Aravinthan A, Al-Dhabi NA, Ignacimuthu S, Choi KC, Kim J-H (2015) Protective effects of friedelin isolated from Azima tetracantha Lam. against ethanol-induced gastric ulcer in rats and possible underlying mechanisms. Eur J Pharmacol 750:167–175. https://doi.org/10.1016/j.ejphar.2015.01.015
Arab HH, Salama SA, Omar HA, Arafa E-SA, Maghrabi IA (2015) Diosmin protects against ethanol-induced gastric injury in rats: novel anti-ulcer actions. PLoS ONE 10:e0122417. https://doi.org/10.1371/journal.pone.0122417
Arab HH, Salama SA, Eid AH, Kabel AM, Shahin NN (2019) Targeting MAPKs, NF-κB, and PI3K/AKT pathways by methyl palmitate ameliorates ethanol-induced gastric mucosal injury in rats. J Cell Physiol 234:22424–22438. https://doi.org/10.1002/jcp.28807
Ardestani A, Yazdanparast R (2007a) Cyperus rotundus suppresses AGE formation and protein oxidation in a model of fructose-mediated protein glycoxidation. Int J Biol Macromol 41:572–578. https://doi.org/10.1016/j.ijbiomac.2007.07.014
Ardestani A, Yazdanparast R (2007b) Inhibitory effects of ethyl acetate extract of Teucrium polium on in vitro protein glycoxidation. Food Chem Toxicol 45:2402–2411. https://doi.org/10.1016/j.fct.2007.06.020
Asgarpanah J, Amin G, Parviz M (2011) In vitro antiglycation activity of Eremurus persicus (Jaub. ET Sp.) Boiss. Afr J Biotechnol 10:11287–11289. https://doi.org/10.5897/AJB11.672
Bahramikia S, Yazdanparast R (2012) EUK-8 and EUK-134 reduce serum glucose and lipids and ameliorate streptozotocin-induced oxidative damage in the pancreas, liver, kidneys, and brain tissues of diabetic rats. Med Chem Res 21:3224–3232. https://doi.org/10.1007/s00044-011-9855-2
Bahramikia S, Ardestani A, Yazdanparast R (2009) Protective effects of four Iranian medicinal plants against free radical-mediated protein oxidation. Food Chem 115:37–42. https://doi.org/10.1016/j.foodchem.2008.11.054
Bari MS, Khandokar L, Haque E, Romano B, Capasso R, Seidel V, Haque MA, Rashid MA (2021) Ethnomedicinal uses, phytochemistry, and biological activity of plants of the genus Gynura. J Ethnopharmacol. https://doi.org/10.1016/j.jep.2021.113834
Batooli H, Haghir EA, Nourouzi M, Mazoochi A (2015) Essential oil composition in different parts of Eremurus persicus (joub. & spach) boiss. From Azaran of Kashan. EJMP 3:1–11
Beiranvand M, Bahramikia S (2020) Ameliorating and protective effects mesalazine on ethanol-induced gastric ulcers in experimental rats. Eur J Pharmacol 888:173573. https://doi.org/10.1016/j.ejphar.2020.173573
Beiranvand M, Beiranvand F (2021) Iranian plant Eremurus persicus: an overview of botany, traditional uses, phytochemistry and pharmacology. Nat Prod Res 35:1–15. https://doi.org/10.1080/14786419.2021.1916744
Bhattamisra SK, Yan VLY, Lee CK, Kuean CH, Candasamy M, Liew YK, Sahu PS (2019) Protective activity of geraniol against acetic acid and Helicobacter pylori-induced gastric ulcers in rats. J Tradit Complement Med 9:206–214. https://doi.org/10.1016/j.jtcme.2018.05.001
Birdane FM, Cemek M, Birdane YO, Gülçin İ, Büyükokuroğlu ME (2007) Beneficial effects of Foeniculum vulgare on ethanol-induced acute gastric mucosal injury in rats. World J Gastroenterol 13:607. https://doi.org/10.3748/wjg.v13.i4.607
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200. https://doi.org/10.1038/1811199a0
Bors W, Heller W, Michel C, Saran M (1990) [36] Flavonoids as antioxidants: determination of radical-scavenging efficiencies. Methods Enzymol 186:343–355. https://doi.org/10.1016/0076-6879(90)86128-I
Cederbaum AI (2001) Introduction-serial review: alcohol, oxidative stress and cell injury. Free Radic Biol Med 31:1524–1526
Chen H, Nie Q, Xie M, Yao H, Zhang K, Yin J, Nie S (2019) Protective effects of β-glucan isolated from highland barley on ethanol-induced gastric damage in rats and its benefits to mice gut conditions. Food Res Int 122:157–166. https://doi.org/10.1016/j.foodres.2019.04.011
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329:23–38. https://doi.org/10.1016/S0009-8981(03)00003-2
Das SK, Vasudevan D (2007) Alcohol-induced oxidative stress. Life Sci 81:177–187. https://doi.org/10.1016/j.lfs.2007.05.005
Dashputre N, Naikwade N (2011) Evaluation of anti-ulcer activity of methanolic extract of Abutilon indicum Linn leaves in experimental rats. IJPSDR 3:97–100
Draper HH, Hadley M (1990) [43] Malondialdehyde determination as index of lipid Peroxidation. Methods Enzymol 186:421–431. https://doi.org/10.1016/0076-6879(90)86135-I
Elliott SN, Wallace JL (1998) Neutrophil-mediated gastrointestinal injury. Can J Gastroenterol 12:559–568. https://doi.org/10.1155/1998/398384
Gaggeri R, Rossi D, Azzolina O, Ahmed KM, Avanzini A, Mantelli M, Gozzini D, Paolillo M, Collina S (2013) Anti-inflammatory properties of ethanolic root extract of Eremurus persicus (Jaub and Spach) Boiss, a Kurdish herbal remedy. J Med Plant Res 7:1730–1735
Gaggeri R, Rossi D, Mahmood K, Gozzini D, Mannucci B, Corana F, Daglia M, Avanzini A, Mantelli M, Martino E (2015) Towards elucidating Eremurus root remedy: chemical profiling and preliminary biological investigations of Eremurus persicus and Eremurus spectabilis root ethanolic extracts. J Med Plant Res 9:1038–1048
Genovese S, Epifano F (2011) Auraptene: a natural biologically active compound with multiple targets. Curr Drug Targets 12(3):381–386. https://doi.org/10.2174/138945011794815248
Hortelano S, Dewez B, Genaro AM, Diaz-Guerra MJ, Boscá L (1995) Nitric oxide is released in regenerating liver after partial hepatectomy. Hepatology 21:776–786. https://doi.org/10.1016/0270-9139(95)90532-4
Jahanbin K, Beigi M (2016) Isolation and structural characterization of a glucomannan from Eremurus persicus roots. Food Sci Technol 14:288–277
Jambi HA, Khattab HAE-RH (2019) Potential antioxidant, anti-inflammatory and gastroprotective effect of grape seed extract in indomethacin-induced gastric ulcer in rats. Int J Pharmacol 15:209–218
Jollow D, Mitchell J, Zampaglione N, Gillette J (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacol 11:151–169. https://doi.org/10.1159/000136485
Karampour NS, Arzi A, Rezaie A, Pashmforosh M, Rad H (2019) Gastroprotective effects of betanin against ethanol-induced gastric ulcer in rats. Jundishapur J Nat Pharm Prod. https://doi.org/10.5812/jjnpp.14473
Keithley JK, Swanson B (2005) Glucomannan and obesity: a critical review. Altern Ther Health Med 11(6):30–35
Khan SS, Ahmad VU, Saba N, Tareen RB (2011) Eremurus persicus, a new source of medicinally important compounds. Pak J Bot 43:2311–2313
Kozioł E, Skalicka-Woźniak K (2016) Imperatorin–pharmacological meaning and analytical clues: profound investigation. Phytochem Rev 15(4):627–649. https://doi.org/10.1007/s11101-016-9456-2
Kuna L, Jakab J, Smolic R, Raguz-Lucic N, Vcev A, Smolic M (2019) Peptic ulcer disease: a brief review of conventional therapy and herbal treatment options. J Clin Med 8:179. https://doi.org/10.3390/jcm8020179
Kwiecien S, Brzozowski T, Konturek S (2002) Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J Physiol Pharmacol 53:39–50
Li W, Yao H, Niu X, Wang Y, Zhang H, Li H, Mu Q (2015) Protective effect of δ-amyrone against ethanol-induced gastric ulcer in mice. Immunobiology 220:798–806. https://doi.org/10.1016/j.imbio.2014.12.014
Li J, Sun F, Fan H, Zhou H, Huang C, Yang J (2019) A systematic review exploring the anticancer activity and mechanisms of glucomannan. Front Pharmacol 10:930. https://doi.org/10.3389/fphar.2019.00930
Liu J, Wang F, Luo H, Liu A, Li K, Li C, Jiang Y (2016) Protective effect of butyrate against ethanol-induced gastric ulcers in mice by promoting the anti-inflammatory, anti-oxidant and mucosal defense mechanisms. Int Immunopharmacol 30:179–187. https://doi.org/10.1016/j.intimp.2015.11.018
Liu B, Feng X, Zhang J, Wei Y, Zhao X (2019) Preventive effect of Anji White tea flavonoids on alcohol-induced gastric injury through their antioxidant effects in Kunming mice. Biomolecules 9:137. https://doi.org/10.3390/biom9040137
Moawad H, El Awdan SA, Sallam NA, El-Eraky WI, Alkhawlani MA (2019) Gastroprotective effect of cilostazol against ethanol-and pylorus ligation–induced gastric lesions in rats. Naunyn Schmiedebergs Arch Pharmacol 392:1605–1616. https://doi.org/10.1007/s00210-019-01699-y
Mottaghipisheh J, Kúsz N, Hohmann J, Tsai Y-C, Csupor D (2020) Isolation of secondary metabolites from the Iranian medicinal plant Eremurus persicus. Z Naturforsch C J Biosci. https://doi.org/10.1515/znc-2020-0059
Mshelia HS, Karumi Y, Dibal NI (2017) Therapeutic effect of Momordica balsamina leaf extract on ethanol-induced gastric ulcer in Wistar rats. Ann Res Hosp 1:1–5. https://doi.org/10.21037/arh.2017.04.03
Oliveira RN, Mancini MC, Oliveira FCSd, Passos TM, Quilty B, Thiré RMdSM, McGuinness GB (2016) FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Matéria (rio j) 21:767–779. https://doi.org/10.1590/S1517-707620160003.0072
Packialakshmi B, Sowndriya SR (2019) Anti-cancer effect of Gymnema sylvestre Leaf Extract against MG63, Human Osteosarcoma cell line—an in vitro analysis. Int J Curr Res Rev 11:18–24. https://doi.org/10.31782/IJCRR.2019.11114
Pavia DL, Lampman GM, Kriz GS, Vyvyan JA (2014) Introduction to spectroscopy. Nelson Education, Toronto, pp 14–86
Pellavio G, Rui M, Caliogna L, Martino E, Gastaldi G, Collina S, Laforenza U (2017) Regulation of aquaporin functional properties mediated by the antioxidant effects of natural compounds. Int J Mol Sci 18(12):2665. https://doi.org/10.3390/ijms18122665
Reznick AZ, Packer L (1994) [38] Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363
Rocha NFM, de Oliveira GV, de Araújo FYR, Rios ERV, Carvalho AMR, Vasconcelos LF, Macêdo DS, Soares PMG, De Sousa DP, de Sousa FCF (2011) (−)-α-Bisabolol-induced gastroprotection is associated with reduction in lipid peroxidation, superoxide dismutase activity and neutrophil migration. Eur J Pharm Sci 44:455–461. https://doi.org/10.1016/j.ejps.2011.08.020
Rossi D, Ahmed KM, Gaggeri R, Della Volpe S, Maggi L, Mazzeo G, Longhi G, Abbate S, Corana F, Martino E (2017) (R)-(−)-Aloesaponol III 8-methyl ether from Eremurus persicus: a novel compound against leishmaniosis. Molecules 22:519. https://doi.org/10.3390/molecules22040519
Rouhollahi E, Moghadamtousi SZ, Hamdi OAA, Fadaeinasab M, Hajrezaie M, Awang K, Looi CY, Abdulla MA, Mohamed Z (2014) Evaluation of acute toxicity and gastroprotective activity of curcuma purpurascens BI. rhizome against ethanol-induced gastric mucosal injury in rats. BMC Complement Altern Med 14:1–10. https://doi.org/10.1186/1472-6882-14-378
Salehi B, Ayatollahi S, Segura-Carretero A, Kobarfard F, Contreras MdM, Faizi M, Sharifi-Rad M, Tabatabai S, Sharifi-Rad J (2017) Bioactive chemical compounds in Eremurus persicus (Joub. & Spach) Boiss. essential oil and their health implications. Cell Mol Biol 63:1–7. https://doi.org/10.14715/cmb/2017.63.9.1
Sangeetha R, Niranjan P, Dhanalakshmi N (2016) Characterization of silver nanoparticles synthesized using the extract of the Leaves of Tridax procumbens. Res J Med Plant 10:159–166
Sayehmiri K, Tavan H (2015) Systematic review and meta-analysis methods prevalence of peptic ulcer in IRAN. J Govaresh 20:250–258
Sharifi-Rad M, Fokou PVT, Sharopov F, Martorell M, Ademiluyi AO, Rajkovic J, Salehi B, Martins N, Iriti M, Sharifi-Rad J (2018) Antiulcer agents: from plant extracts to phytochemicals in healing promotion. Molecules 23:1751. https://doi.org/10.3390/molecules23071751
Sidahmed HM, Hashim NM, Amir J, Abdulla MA, Hadi AHA, Abdelwahab SI, Taha MME, Hassandarvish P, Teh X, Loke MF (2013) Pyranocycloartobiloxanthone A, a novel gastroprotective compound from Artocarpus obtusus Jarret, against ethanol-induced acute gastric ulcer in vivo. Phytomedicine 20:834–843. https://doi.org/10.1016/j.phymed.2013.03.002
Sistani Karampour N, Arzi A, Rezaie A, Pashmforoosh M, Kordi F (2019) Gastroprotective effect of zingerone on ethanol-induced gastric ulcers in rats. Medicina 55:64. https://doi.org/10.3390/medicina55030064
Slinkard K, Singleton VL (1977) Total phenol analysis: automation and comparison with manual methods. Am J Enol Vitic 28:49–55
Srinivas TL, Lakshmi SM, Shama SN, Reddy GK, Prasanna K (2013) Medicinal plants as anti-ulcer agents. J Pharmacogn Phytochem 2:91–97
Takagi K, Okabe S (1968) The effects of drugs on the production and recovery processes of the stress ulcer. Jpn J Pharmacol 18:9–18. https://doi.org/10.1254/jjp.18.9
Tamura M, Matsui H, Kaneko T, Hyodo I (2013) Alcohol is an oxidative stressor for gastric epithelial cells: detection of superoxide in living cells. J Clin Biochem Nutr 53:75–80. https://doi.org/10.3164/jcbn.13-32
Vala MH, Asgarpanah J, Hedayati MH, Shirali J, Bejestani FB (2011) Antibacterial and cytotoxic activity of Eremurus persicus (Jaub and Spach) Boiss. Afr J Microbiol Res 16:2349–2352
Yazdanparast R, Bahramikia S, Ardestani A (2008) Nasturtium officinale reduces oxidative stress and enhances antioxidant capacity in hypercholesterolaemic rats. Chem Biol Interact 172:176–184. https://doi.org/10.1016/j.cbi.2008.01.006
Yu C, Mei X-T, Zheng Y-P, Xu D-H (2014) Gastroprotective effect of taurine zinc solid dispersions against absolute ethanol-induced gastric lesions is mediated by enhancement of antioxidant activity and endogenous PGE2 production and attenuation of NO production. Eur J Pharmacol 740:329–336. https://doi.org/10.1016/j.ejphar.2014.07.014
Zhang J, Lu D-Y, Yuan Y, Chen J, Yi S, Chen B, Zhao X (2020) Liubao Insect tea polyphenols prevent HCl/ethanol induced gastric damage through its antioxidant ability in mice. RSC Adv 10:4984–4995. https://doi.org/10.1039/C9RA09641H
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559. https://doi.org/10.1016/S0308-8146(98)00102-2
Zhu K, Peng P, Wu N, Zhou X, Mu J, Zhao X (2020) Preventive effect of Liupao tea polyphenols on HCl/ethanol-induced gastric injury in mice. J Food Qual. https://doi.org/10.1155/2020/5462836
Acknowledgements
The authors appreciate the financial support of this investigation by Lorestan University.
Funding
This study was supported by a grant from the Faculty of Basic Sciences, Lorestan University. The founding sponsor had no role in the study design, performance, data collection and analysis, decision to publish, or preparation/writing of the manuscript.
Author information
Authors and Affiliations
Contributions
MB conducted the experiments and wrote the manuscript and all images were drawn. SB participates in the design and interpretation of the studies, and the review of the manuscript. OD participated in the preparation of tissue samples and pathological analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
All procedures performed in this study involving animals were in accordance with the ethical standards and the international regulations of the usage and welfare of laboratory animals and were approved by the clinical ethics committee of the Faculty of Veterinary Medicine of Lorestan University with (Ethical code: LU.ECRA.2020.32).
Consent for publication
All authors have read the final manuscript and express their consent to its publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Beiranvand, M., Bahramikia, S. & Dezfoulian, O. Evaluation of antioxidant and anti-ulcerogenic effects of Eremurus persicus (Jaub & Spach) Boiss leaf hydroalcoholic extract on ethanol-induced gastric ulcer in rats. Inflammopharmacol 29, 1503–1518 (2021). https://doi.org/10.1007/s10787-021-00868-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-021-00868-x