Abstract
Autoimmune uveitis is an inflammatory disease of the eye and is one of the major causes of blindness worldwide. Experimental autoimmune uveoretinitis (EAU) constitutes an animal disease model of human endogenous uveitis. In our study, we investigated the immunomodulatory effect of dimethyl fumarate (DMF) using bovine retinal extract-induced uveitis in a Female Wistar rats. To evaluate the in vivo efficacy, Female Wistar rats were divided into seven experimental groups: control group (n = 5), consisting of non-immunized animals; Uveoretinitis (n = 5), and DMF/Uveoretinitis groups (n = 15), which received a subcutaneous injection of bovine retinal extract emulsified in complete Freund’s adjuvant; MC group (n = 5), treated by daily intragastric administration of methylcellulose 0.08% in tap water; DMF group, consisting of control positive group, rats received daily oral gavage administration of 500 μL of dimethyl fumarate at 100 mg/Kg dissolved in 0.08% methylcellulose in tap water (n = 5). On day 14 post immunization, the rats were then euthanized and associated indications were investigated to evaluate the therapeutic efficacy. Nitric oxide (NO) and TNF-α were assessed in plasma. Meanwhile, eyes were collected for histological and immunohistochemical studies. The retinal expression of iNOS, CD68, CD20, CD25, CD4, and CD8 was examined. Interestingly, DMF enhanced a significant reduction of NO and TNF-α production in the treated group. This effect was strongly related to the histological structure of eyes improvement. In the same context, a significant decrease of iNOS, CD68, and CD20 expression and CD25 increase expression were reported in retinal tissue of DMF/Uveoretinitis group in comparison to the immunized group. Collectively, our results indicate that DMF treatment has a beneficial effect in experimental autoimmune uveoretinitis and could constitute a good candidate for monitoring an ocular inflammatory diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uveitis is a general term used for the inflammation of the uveal tissue (iris, ciliary body, and choroid). Anatomically it has been classified as anterior, intermediate and posterior or as panuveitis. Noninfectious uveitis is believed to be autoimmune or immune-mediated (Gery et al. 2002). Patients with autoimmune uveitis frequently showed the presence of immune responses against retinal soluble antigen (AgS), inter-photoreceptor retinoid-binding protein (IRBP), and recoverin (Bansal et al. 2015).
The delicate nature of ocular tissues makes it difficult to obtain tissue specimens in the study of the pathogenicity of uveitis diseases. However, over the past several decades, animal models of autoimmune uveitis have been developed (Bansal et al. 2015; Agarwal and Caspi 2004).
Experimental autoimmune uveoretinitis (EAU) is T cell-mediated autoimmune disease that targets the neural retina and related tissues (Lee et al. 2014). It shares many common features in clinical and histological aspects with human uveitis (Agarwal et al. 2012). EAU is a Th1-dominant response. It was shown that the up-regulation of the Th2 cytokines production or down-regulation of the Th1 responses prevents inflammatory responses and protects against the EAU development (Caspi 2002; Nikoopour et al. 2019). In addition, many authors have reported the involvement of nitric oxide (NO) in mechanisms of pathogenesis of experimental autoimmune uveitis induced by IRBP, AgS and tropomyosin (Djeraba et al. 2010; Arroul-Lammali et al. 2012; Touri et al. 2019).
Currently, the treatment of uveitis is based on the use of corticosteroids and immunosuppressive agents. However, long-term application of these drugs frequently may have several intraocular and systemic side effects (Jonas et al. 2003; Moshfeghi et al. 2003). Furthermore, there are still a number of patients who do not respond to immunosuppressive treatment (Tian et al. 2011).
Oral dimethyl fumarate (DMF) is approved since 2014 in Europe for the treatment of multiple sclerosis based on a wide spectrum of immunomodulatory and neuroprotective effects with a favourable safety profile (Gold et al. 2012, b; Fox et al. 2012). DMF is rapidly hydrolyzed by esterases to its metabolite, monomethyl fumarate (MMF), after absorption in the small intestine (Litjens et al. 2004).
DMF exerts an immunomodulatory and a neuroprotective effects in the experimental animal models of acute and chronic autoimmune, demyelinating diseases of the peripheral nervous system, and in the autoimmune neuritis (Kiefer et al. 2001; Pitarokoili et al. 2019). Studies in experimental autoimmune encephalomyelitis, the mouse model for multiple sclerosis, and the experimental autoimmune neuritis, revealed a neuroprotective mechanism of action through the activation of the transcription factor Nrf2 (nuclear factor (erythroid derived 2)-related factor 2) via MMF (Stoof et al. 2001).
In our current study, we investigated the ability of oral treatment with DMF to suppress EAU induced in rat by immunization with bovine retinal extract. The experiment was conducted to evaluate the immunomodulatory properties of DMF. In this sense, we analysed the systemic levels of NO and TNF-α secretion during the experimental autoimmune uveoretinitis. Histological changes and the expression of iNOS, CD68, CD20, CD25, CD4 and CD8 levels in retinal tissue from all experimental groups were assessed by semi-quantitative analysis using FIJI image processing software.
Materials and methods
Animals
A total of 35 female Wistar rats (4–6 weeks old) were purchased from the Pasteur Institute (Algiers, Algeria). These rats were acclimated for 1 week before the start of experiments and kept under normal conditions with a 12-h dark/light cycle with ad libitum access to food and water.
Disease induction and in vivo treatment with dimethyl fumarate
Animals ranging in weight from 160 to 180 g were divided into seven groups (n = 5 rat per group) as per Fig. 1. The control group (Ctrl) received no treatment. The Uveoretinitis and DMF/Uveoretinitis groups received a subcutaneous injection of bovine retinal extract emulsified in complete Freund’s adjuvant. The DMF (25 mg/Kg)/Uveoretinitis, DMF (45 mg/Kg)/Uveoretinitis, and DMF (100 mg/Kg)/Uveoretinitis groups treated by daily oral gavage administration of 500 μL of dimethyl fumarate (Biogen Idec, Cambridge, USA) at 25, 45 and 100 mg/Kg, respectively, dissolved in 0.08% methylcellulose in tap water starting after 2 days of immunization to day 12 p.i. The MC group received 500 µL of methylcellulose 0.08% orally twice daily. The DMF group treated by daily oral gavage administration of 500 μL of dimethyl fumarate at 100 mg/Kg dissolved in 0.08% methylcellulose in tap water. All our experiments were approved by Ethics Committee of the Thematic Research Agency in Health Sciences (N°58/DFPR/ATRSS).
All animals were euthanized 14 days p.i. using inhalation of anesthesia gas.
Plasma collection
Wistar rats were anesthetized, and blood was collected via cardiac puncture. Blood was centrifuged to isolate plasma, which was kept at− 20 °C until use.
Nitrite measurement
The Griess reaction was used to determine nitrite levels in plasma as an indicator of NO production, as described previously (Touil-Boukoffa et al. 1998). Briefly, 100 mL of each sample was mixed with 50 mL of Griess reagent (5% sulfanilamide, 0.5% napthylethylenediamine dihydrochloride, 20% HCl). Samples were incubated at room temperature for 20 min and the absorbance read at 543 nm by spectrophotometer. The nitrite concentration was determined using a standard curve constructed with sodium nitrite [NaNO2; (0–200) mmoL/mL].
Measurement of systemic TNF-α level
The systemic level of TNF-α was determined in the plasma of rats using commercial enzyme-linked immunosorbent assay kits, according to the manufacturer's instructions (Invitrogen, Camarillo, USA).
Histopathological analysis
Eyes were enucleated on day 14 following bovine retinal extract immunization and rinsed with phosphate-bufered saline (PBS), then fixed in 10% phosphate-buffered formaldehyde. Tissues were embedded in paraffin. Serial 5 μm sections were cut through the papillary-optic nerve axis and stained by haematoxylin and eosin. At least four sections of each eye cut at different levels were prepared and evaluated histologically. The intensity of EAU was graded in a masked fashion on a scale of 0–4, as previously described (Caspi et al. 1988). In brief, focal nongranulomatous, monocytic infiltrations in the choroids, ciliary body, and retina were scored as 0.5. Retinal perivascular infiltration and monocytic infiltration in the vitreous were scored as 1. Granuloma formation in the uvea and retina, the presence of occluded retinal vasculitis, along with photoreceptor folds, serous detachment, and loss of photoreceptor were graded as 2. In addition, the formation of granuloma at the level of the retinal pigmented epithelium and the development of subretinal neovascularization were scored as 3 and 4, according to the number and the size of the lesions. Histopathological diagnoses were performed in double blinded fashion by anatomopathologists.
Images were captured from each slide with a digital camera (Casio) on a light microscope (Motic) with 40 × objectives.
Immunohistochemical procedures
The technical procedures for immunohistochemical study of iNOS, CD68, CD20, CD25, CD4 and CD8 expression included, in succession, the steps previously described (Labsi et al. 2016). The adopted protocol for immunohistochemical exam was adapted to local laboratory conditions (Labsi et al. 2016; 2018; 2019). Slides were submitted to immunohistochemistry for incubation with rabbit monoclonal antibodies against mouse CD68, CD20, CD25, CD4, CD8, and iNOS (Santa Cruz Biotechnology, Dallas, TX, USA; diluted 1:500). Indirect immunoperoxidase staining was performed according to the manufacturer’s instructions (DAKO, Denmark A/S). The sections were counterstained with hematoxylin. Slides were covered with Faramount Mounting Medium (S3025, DAKO, Denmark A/S) and observed using a standard microscope (Motic). Pictures were taken using a digital camera (Casio) at 40 × magnification. The percentage of cells positively stained for iNOS, CD68, CD20, CD25, CD4 and CD8 was scored semiquantitatively. The quantification of positive cells was performed using FIJI image processing software after calibration with a graduated slide. The quantification was carried by two observers. iNOS expression was evaluated on the basis of a four-point scale: ʻ − ʼ: negative staining; ʻ + ʼ: low expression, less than 10% positive cells; ʻ + + ʼ: moderate expression, 10–30% positive cells; ʻ + + + ʼ: intense expression, more than 30% positive cells.
Statistical analysis
An ANOVA or Kruskal–Wallis one-way analysis of variance tests were used to compare the treatment effects between all groups. Probability values of P ≤ 0.05 were considered to be statistically significant. We have used the statistical software Past version 4.03 and GraphPad Prism 6.
Results
Dimethyl fumarate treatment reduced systemic levels of nitric oxide and TNF-α
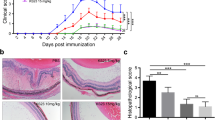
We investigated the effect of DMF on NO production in EAU. Nitrite measurement in plasma of rats showed a significant increase in the Uveoretinitis group compared to the Ctrl group (16.2 ± 0.3 vs. 3.4 ± 1.0 μM, ***P < 0.001; Fig. 2A). Oral administration of DMF at doses of 45 and 100 mg/Kg to immunized rats with bovine retinal extract caused a significant decrease in systemic NO level in comparison with the untreated-Uveoretinitis group (8.3 ± 5.2 vs. 16.2 ± 0.6 μM, *P < 0.05 and 6.5 ± 3.7 vs. 16.2 ± 0.6 μM, **P < 0.005, respectively; Fig. 2A).
To analyze the effect of DMF on proinflammatory cytokine production in vivo, we measured the level of TNF-α in plasma (Fig. 2B). The systemic TNF-α level was increased in the Uveoretinitis group in comparison with the Ctrl, MC and DMF groups (Fig. 2B). DMF treatment at a dose of 45 mg/Kg attenuates the systemic TNF-α level (5.0 ± 0.3 vs. 6.0 ± 0.4 pg/mL; Fig. 2B). There is an important correlation between the Nitrite and TNF-α systemic production during EAU development (r = 0.61, P < 0.005; Fig. 2C).
Retina architecture was improved in rats treated with dimethyl fumarate
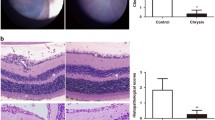
In our study, histological changes were evaluated using Hematoxylin and eosin staining. Histological analysis of eye isolated from EAU, showed fragmentation and alteration of the outer nuclear layer (ONL), structural disorganization of the inner nuclear layer (INL), and destruction of photoreceptors cells layer (PCL), in retina (Fig. 3C).
Representative photomicrographs (× 40) of H and E stained rat’s retinal tissue. The bovine retina extract-induced morphological changes in the architecture of retina of Wistar rats. square fragmentation and alteration of the ONL; (): structural disorganization of the INL; square destruction of photoreceptors cells layer. GCL ganglion cells layer, INL inner nuclear layer, ONL outer nuclear layer, PCL photoreceptors cells layer, C choroid
Interestingly, the oral administration of DMF at doses of 45 and 100 mg/Kg to rats with autoimmune uveitis caused an improvement in the histological structure in retina sections relative to untreated immunized-rats (Fig. 3E, F). We showed a significant important histology damage score (HAI) in Uveoretinitis and DMF (25 mg/Kg)/Uveoretinitis groups in comparison with the Ctrl and MC groups (Fig. 4). Histopathological evaluation revealed that 25 mg/Kg of DMF is too low and have no effect on bovine retinal extract-induced uveitis (Fig. 3D). Indeed, A decrease in HAI was shown in the DMF (45 mg/Kg)/Uveoretinitis and DMF (100 mg/Kg)/Uveoretinitis groups compared with the Uveoretinitis group [0.5(35.9) vs. 3.0(57.5) and 0.25(33.7) vs. 3.0(57.5), respectively; Fig. 4].
Improvement of the histological structure by administration of dimethylfumarate in rats immunized with bovine retinal extract. The histological activity index (HAI) was calculated as described in Material and Methods. Data are presented as median (range). P values from Kruskal–Wallis test are indicated (**P < 0.005, ***P < 0.001, ****P < 0.0001)
Wistar rats from control, MC and DMF groups exhibited normal retinal tissue (Fig. 3A, B and G).
Anti-inflammatory and immune-modulatory effects of dimethyl fumarate therapy
To evaluate the effect of DMF on inflammatory reaction in our model of autoimmune uveitis, we examined the retinal expression of iNOS, CD68, CD20, CD25, CD4 and CD8.
The expression of iNOS, CD68, CD20, CD25 and CD4 was shown in retina (Fig. 5). We observed a little expression of CD8 in retinal tissue in some cases (Fig. 5). Importantly, the expression of pro-inflammatory markers (iNOS, CD68, CD20) was significantly increased in the Uveoretinitis group compared with the control groups (P < 0.005; Table 1). This increase in expression in retina was significantly attenuated by DMF treatment (P < 0.05; Table 1, Fig. 5D, I, N). In retinal tissue of the DMF (45 mg/Kg)/Uveoretinitis, Ctrl, MC and DMF groups, there was, strikingly, no significant difference in iNOS, CD68, CD20, CD4 and CD8 expression. In the same way, we observed with interest a significant expression of Treg marker (CD25) in retinal tissue of DMF(45 mg/Kg)/Uveoretinitis group compared with Ctrl and MC groups (P < 0.005; Table 1, Fig. 5P, Q, S).
Representative images (× 40) of immunohistochemical analysis for iNOS, CD68, CD20, CD25, CD4, CD8 expression in retina. A, B, E retina sections of Ctrl, MC and DMF groups with low expression of iNOS, scored as “ + ”. C retina section of Uveoretinitis group showed diffuse expression of iNOS, scored as “ + + ”.D retina section of DMF (45 mg/Kg)/Uveoretinitis group showed low expression of iNOS, graded as “ + ”. F, G, J, K, L, O retina sections of Ctrl, MC and DMF groups with low expression of CD68 and CD20. H, M an intense expression of CD68 and CD20 was observed in Uveoretinitis group. I, N Treatment with DMF reduced the intensity of CD68 and CD20 expression observed in DMF (45 mg/Kg)/Uveoretinitis group. P, Q, R, T The expression of CD25 was lower in retina sections of Ctrl, MC, Uveoretinitis and DMF groups. (S) Treatment with DMF increased the intensity of CD25 expression observed in DMF (45 mg/Kg)/Uveoretinitis group. U, V, W, X, Y) an important expression of CD4 was shown in all experimental groups. Z, Z1, Z4) no expression of CD8 in retina sections of Ctrl, MC and DMF groups. Z2, Z3 a little expression of CD8 in retina sections of Uveoretinitis and DMF (45 mg/Kg)/Uveoretinitis groups. (►): Arrows indicate positive staining
Discussion
Uveitis is comprised of a variety of a heterogeneous group of disorders with uncertain etiology and pathogenesis. Because of the limit in obtaining samples at ocular level, various EAU animal models have been developed for studying and understanding this pathology. Numerous therapeutic agents have been discovered, which have been put into successful clinical use (Bansal et al. 2015).
Dimethyl fumarate is commonly prescribed as a first-line agent with favourable safety and efficacy profiles. The potential benefits of DMF against other diseases appears pathogenically different (Kourakis et al. 2020). In the study outlined here, we examined the potential anti-inflammatory and immunomodulatory effect of DMF on the experimental model of autoimmune uveitis with regards to systemic levels of NO and TNF-α production, retina histological changes, and local expression of iNOS, CD68, CD20, CD25, CD4 and CD8.
Dimethyl fumarate is a methyl ester of fumaric acid registered for the treatment of relapsing forms of Multiple Sclerosis (Kappos et al. 2008; Gold et al. 2012; Michell-Robinson et al. 2015) and psoriasis (Litjens et al. 2004; Reszke and Szepietowski 2020). DMF influences a variety of cell types and molecules pathways involved in inflammatory mechanisms. Indeed, Wilms et al. (2010) demonstrated that DMF exerts an inhibitory effect on the proinflammatory response characterised by increased microglial and astrocytic synthesis of NO, IL-1, IL-6 and TNF-α in an in vitro model of brain inflammation. In our conditions, we reported with interest a significant decrease of systemic NO and TNF-α level after DMF treatment. In the same line, we showed a positive correlation between the NO and the TNF-α systemic production (Fig. 2C).
As previously reported by our team, during EAU activity, a significant proportion of damages in ocular structures was observed and attributed mainly to nitric oxide (Djeraba et al. 2010; Arroul-Lammali et al. 2012; Touri et al. 2019). In addition, TNF-α, has been shown to be an important cytokine involved in alteration of ocular tissue (Dick et al. 2004). The histological structure of retina showed an alteration and disorganization of different layers (Fig. 3C). Interestingly, the damage observed in retina architecture with significant expression of iNOS, CD68 and CD20, was related to establishment of inflammatory reaction (P < 0.005, Table 1, Fig. 5C, H and M). Heiligenhaus et al. (2011) have used an anti-CD20 monoclonal antibody (rituximab) for the treatment of severe uveitis associated with juvenile idiopathic arthritis.
Intragastric administration of DMF in Wistar rats caused an amelioration of tissue architecture and an important decrease of inflammatory cells infiltration (CD68, CD20). These observations correlate with down-regulation of iNOS expression and up regulation of the Treg markers (CD4 and CD25) in DMF/Uveoretinitis group. Pitarokoili et al. (2019) demonstrated that DMF exhibited an immunomodulatory effect by an increase of regulatory T cells (CD4+ CD25+), exerting a protective role in experimental neuritis.
We noted a little expression of CD8 in Uveoretinitis and DMF/Uveoretinitis groups. Calder et al. (1993) suggest that depleting CD8 + cells had no effect on the course of disease and that CD8 + cells do not play a crucial role in the immunoregulation of EAU.
Fumarates are potent therapeutic compounds that exert pleiotropic immunomodulatory effects. Multiple pathways have been implicated in mediating these effects, the cytoprotective and anti-oxidant responses through the strong potentiation of the Nrf2 pathway, and the anti-inflammatory effect by inhibition of NF-κβ pathway (Gillard et al. 2015). DMF attenuate colon injury, pro-inflammatory cytokine and adhesion molecule production, and NF-κβ signalling, in experimental rodent models of colitis (Casili et al. 2015). In mouse models of Parkinson’s disease, DMF reduced damage and degeneration to preserve neuronal populations by upregulating Nrf2-dependent antioxidant genes and suppressing NF-κβ-mediated inflammation (Lastres-Becker et al. 2016; Campolo et al. 2017). In the same context of inflammatory conditions, it was reported that COX-2 plays an important role in inflammation during experimental autoimmune uveitis (Shiratori et al. 2004). Lal et al (2021) have reported that DMF treatment reduced the COX-2 levels. The anti-arthritic activity of DMF may be mediated by the activation of the Nrf2/HO-1 pathway, which reduced oxidative damage and inflammation.
NF-κβ is recognized as a central mediator in the pathophysiology of many chronic inflammatory diseases, which lead to an increased risk of ocular inflammation. Moreover, the expression of TNF-α and iNOS are transcriptionally regulated by the transcription factor NF-κβ. DMF showed inhibition of NF-κβ activity in an Nrf2-independent manner (Gillard et al. 2015).
In conclusion, our analysis of local retinal inflammatory responses using an experimental autoimmune uveitis could be in part related to the increased expression of inflammatory markers such as iNOS, CD68, CD20 and CD4. Dimethyl fumarate represents a promising approach for the treatment of EAU. Our results suggest that DMF appears to be more effective in reducing inflammatory effects in the retina and could constitute a good candidate for monitoring an acute ocular inflammatory diseases.
References
Agarwal RK, Caspi RR (2004) Rodent models of experimental autoimmune uveitis. Methods Mol Med 102:395–419
Agarwal RK, Silver PB, Caspi RR (2012) Rodent models of experimental autoimmune uveitis. Methods Mol Biol 900:443–469
Arroul-Lammali A, Djeraba Z, Belkhelfa M, Belguendouz H, Hartani D, Lahlou-Boukoffa OS, Touil-Boukoffa C (2012) Early involvement of nitric oxide in mechanisms of pathogenesis of experimental autoimmune uveitis induced by interphotoreceptor retinoid-binding protein (IRBP). J Fr Ophtalmol 35(4):251–259
Bansal S, Barathi AV, Iwata D, Agrawal R (2015) Experimental autoimmune uveitis and other animal models of uveitis: an update. Indian J Ophthalmol 63(3):211–218. https://doi.org/10.4103/0301-4738.156914
Calder VL, Zhao ZS, Wang Y, Barton K, Lightman SL (1993) Effects of CD8 depletion on retinal soluble antigen induced experimental autoimmune uveoretinitis. Immunology 79(2):255–262
Campolo M, Casili G, Biundo F, Crupi R, Cordaro M, Cuzzocrea S, Esposito E (2017) The neuroprotective effect of dimethyl fumarate in an MPTP-mouse model of parkinson’s disease: involvement of reactive oxygen species/nuclear factor-κB/nuclear transcription factor related to NF-E2. Antioxid Redox Signal 27:453–471
Casili G, Cordaro M, Impellizzeri D, Bruschetta G, Paterniti I, Cuzzocrea S, Esposito E (2015) Dimethyl fumarate reduces inflammatory responses in experimental colitis. J Crohn’s Colitis 10:472–483
Caspi RR (2002) Th1 and Th2 responses in pathogenesis and regulation of experimental autoimmune uveoretinitis. Int Rev Immunol 21:197–208
Caspi RR, Roberge FG, Chan CC, Wiggert B, Chader GJ, Rozenszajn LA, Lando Z, Nussenblatt RB (1988) A new model of autoimmune disease: experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J Immunol 140:1490–1495
Dick AD, Forrester JV, Liversidge J, Cope AP (2004) The role of tumour necrosis factor (TNF-alpha) in experimental autoimmune uveoretinitis (EAU). Prog Retin Eye Res 23:617–637
Djeraba Z, Arroul-Lammali A, Medjber O, Belguendouz H, Hartani D, Lahlou-Boukoffa OS, Touil-Boukoffa C (2010) Nitric oxide, biomarker of experimental autoimmune uveitis induced by S antigen. J Fr Ophtalmol 33:693–700
Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M et al (2012) Placebo-controlled phase 3 study of oral BG12 or glatiramer in multiple sclerosis. N Engl J Med 367:1087–1097. https://doi.org/10.1056/NEJMoa1206328
Gery I, Nussenblatt RB, Chan CC, Caspi RR (2002) Autoimmune diseases of the eye. In: Theofilopoulos AN, Bona CA (eds) The molecular pathology of autoimmune diseases. Taylor and Francis, New York, pp 978–998
Gillard GO, Collette B, Anderson J, Chao J, Scannevin RH, Huss DJ, Fontenot JD (2015) DMF, but not other fumarates, inhibits NF-κB activity in vitro in an Nrf2-independent manner. J Neuroimmunol 283:74–85. https://doi.org/10.1016/j.jneuroim.2015.04.006
Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj KW, Tornatore C, Sweetser MT, Yang M, Sheikh SI et al (2012) Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. New Engl J Med 367:1098–1107. https://doi.org/10.1056/NEJMoa1114287
Heiligenhaus A, Miserocchi E, Heinz C, Gerloni V, Kotaniemi K (2011) Treatment of severe uveitis associated with juvenile idiopathic arthritis with anti-CD20 monoclonal antibody (rituximab). Rheumatology 50(8):1390–1394. https://doi.org/10.1093/rheumatology/ker107
Jonas JB, Kreissig I, Degenring R (2003) Intraocular pressure after intravitreal injection of triamcinolone acetonide. Br J Ophthalmol 87:24–27
Kappos L, Gold R, Miller DH, MacManus DG, Havrdova E, Limmroth V, Polman CH, Schmierer K, Yousry AT, Yang M et al (2008) Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 372:1463–1472
Kiefer R, Kieseier BC, Stoll G, Hartung HP (2001) The role of macrophages in immune-mediated damage to the peripheral nervous system. Prog Neurobiol 64:109–127. https://doi.org/10.1016/S0301-0082(00)00060-5
Kourakis S, Timpani CA, de Haan JB, Gueven N, Fischer D, Rybalka E (2020) Dimethyl fumarate and its esters: a drug with broad clinical utility? Pharmaceuticals 13:306. https://doi.org/10.3390/ph13100306
Labsi M, Khelifi L, Mezioug D, Soufli I, Touil-Boukoffa C (2016) Antihydatic and immunomodulatory effects of Punica granatum peel aqueous extract in a murine model of echinococcosis. Asian Pac J Trop Med 9(3):211–220
Labsi M, Soufli I, Khelifi L, Amir Z-C, Touil-Boukoffa C (2018) In vivo treatment with IL-17A attenuates hydatid cyst growth and liver fibrogenesis in an experimental model of echinococcosis. Acta Trop 181:6–10
Labsi M, Soufli I, Khelifi L, Amir Z-C, Touil-Boukoffa C (2019) A preventive effect of the combination of albendazole and pomegranate peel aqueous extract treatment in cystic echinococcosis mice model: an alternative approach. Acta Trop 197:105050
Lal R, Dhaliwal J, Dhaliwal N, Dharavath RN, Chopra K (2021) Activation of the Nrf2/HO-1 signaling pathway by dimethyl fumarate ameliorates complete Freund’s adjuvant-induced arthritis in rats. Eur J Pharmacol 899:174044
Lastres-Becker I, García-Yagüe AJ, Scannevin RH, Casarejos MJ, Kügler S, Rábano A, Cuadrado A (2016) Repurposing the NRF2 activator dimethyl fumarate as therapy against synucleinopathy in parkinson’s disease. Antioxid Redox Signal 25:61–77
Lee RW, Nicholson LB, Sen HN, Chan CC, Wei L, Nussenblatt RB, Dick AD (2014) Autoimmune and autoinflammatory mechanisms in uveitis. Semin Immunopathol 36:581–594. https://doi.org/10.1007/s00281-014-0433-9
Litjens NH, van Strijen E, van Gulpen C, Mattie H, van Dissel JT, Thio HB et al (2004) In vitro pharmacokinetics of anti-psoriatic fumaric acid esters. BMC Pharmacol 4:22. https://doi.org/10.1186/1471-2210-4-22
Michell-Robinson MA, Moore CS, Healy LM, Osso LA, Zorko N, Grouza V, Touil H, Poliquin-Lasnier L, Trudelle A-M, Giacomini PS, Bar-Or A, Antel JP (2015) Effects of fumarates on circulating and CNS myeloid cells in multiple sclerosis. Ann Clin Transl Neurol 3(1):27–41. https://doi.org/10.1002/acn3.270
Moshfeghi DM, Kaiser PK, Scott IU, Sears JE, Benz M, Sinesterra JP, Kaiser RS, Bakri SJ, Maturi RK, Belmont J, Beer PM, Murray TG, Quiroz-Mercado H, Mieler WF (2003) Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol 136:791–796. https://doi.org/10.1016/s0002-9394(03)00483-5
Nikoopour E, Lin CM, Sheskey S, Heckenlively JR, Lundy SK (2019) Immune cell infiltration into the eye is controlled by IL-10 in Recoverin-induced autoimmune retinopathy. J Immunol 202:1057–1068. https://doi.org/10.4049/jimmunol.1800574
Pitarokoili K, Bachir H, Sgodzai M, Grüter T, Haupeltshofer S, Duscha A, Pedreiturria X, Motte J, Gold R (2019) Induction of regulatory properties in the intestinal immune system by dimethyl fumarate in lewis rat experimental autoimmune neuritis. Front Immunol 10:2132. https://doi.org/10.3389/fimmu.2019.02132
Reszke R, Szepietowski JC (2020) A safety evaluation of dimethyl fumarate in moderate-to-severe psoriasis. Expert Opin Drug Saf 19:373–380
Shiratori K, Ohgami K, Bozhidarova I, Koyama Y, Yoshida K, Ohno S (2004) Inhibition of endotoxin-induced uveitis and potentiation of cyclooxygenase-2 protein expression by α-melanocyte–stimulating hormone. Invest Ophthalmol vis Sci 45:159–164
Stoof TJ, Flier J, Sampat S, Nieboer C, Tensen CP, Boorsma BM (2001) The antipsoriatic drug dimethylfumarate strongly suppresses chemokine production in human keratinocytes and peripheral blood mononuclear cells. Br J Dermatol 144:1114–1120. https://doi.org/10.1046/j.1365-2133.2001.04220.x
Tian L, Yang P, Lei B, Shao J, Wang C, Xiang Q, Wei L, Peng Z, Kijlstra A (2011) AAV2-mediated subretinal gene transfer of hIFN-a attenuates experimental autoimmune Uveoretinitis in mice. PLoS ONE 6(5):19542
Touil-Boukoffa C, Bauvois B, Sanceau J, Hamrioui B, Weitzerbin J (1998) Production of nitric oxide (NO) in human hydatidosis: relationship between nitrite production and interferongamma levels. Biochemie 80:738–744
Touri K, Belguendouz H, Medjeber O, Djeraba Z, Lahmar K, Touil-Boukoffa C (2019) Propolis modulates NOS2/arginase-1 pathway in tropomyosin-induced experimental autoimmune uveitis. Inflammopharmacology 26:1293–1303
Wilms H, Sievers J, Rickert U, Rostami-Yazdi M, Mrowietz U, Lucius R (2010) Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1β, TNF-α and IL-6 in an in-vitro model of brain inflammation. J Neuroinflammation 7:30
Acknowledgements
The author would like to thank Leila Daiah and Soraya Houmel for their technical help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Labsi, M., Soufli, I., Belguendouz, H. et al. Beneficial effect of dimethyl fumarate on experimental autoimmune uveitis is dependent of pro-inflammatory markers immunomodulation. Inflammopharmacol 29, 1389–1398 (2021). https://doi.org/10.1007/s10787-021-00864-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-021-00864-1