Abstract
Rhinosinusitis is a common disorder related to inflammation of paranasal sinuses and nasal cavity mucosa. Herbal medicines could be an option in the treatment of rhinosinusitis due to their anti-inflammatory and anti-oxidative properties. The study aims to investigate the effect of intranasal Sambucus nigra L. subsp. nigra (SN) extract against inflammation, oxidative stress, and tissue remodeling in nasal and sinus mucosa, but also in serum, lungs, and brain, in Wistar rat model of subacute sinonasal inflammation induced by local administration of lipopolysaccharides (LPS), from Escherichia Coli. The cytokines (TNF-α, IL-1β, IL-6) and oxidative stress (malondialdehyde) in nasal mucosa, blood, lungs, and brain were analyzed. In addition, a histopathological examination was performed, and NF-kB, MMP2, MMP9, TIMP1 expressions were also evaluated in nasal mucosa. Both doses of LPS increased the production of cytokines in all the investigated tissues, especially in the nasal mucosa and blood (p < 0.01 and p < 0.05), and stimulated their secretion in the lungs, and partially in the brain. Malondialdehyde increased in all the investigated tissues (p < 0.01 and p < 0.05). In parallel, upregulation of NF-kB and MMP2 expressions with downregulation of TIMP1, particularly at high dose of LPS, was observed. SN extract reduced the local inflammatory response, maintained low levels of IL-6, TNF-α, and IL-1β. In lungs, SN reduced all cytokines levels while in the brain, the protective effect was noticed only on IL-6. Additionally, SN diminished lipid peroxidation and downregulated NF-kB in animals exposed to a low dose of LPS, with increased TIMP1 expression, while in animals treated with a high dose of LPS, SN increased NF-kB, MMP2, and MMP9 levels. In conclusion, SN extract diminished the inflammatory response, reduced generation of reactive oxygen species (ROS) and, influenced MMPs expressions, suggesting the benficial effect of SN extract on tissue remodeling in subacute rhinosinusitis and on systemic inflammatory response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Upper respiratory tract obstruction, characterized by a blockage of any part of the airway above the superior thoracic aperture, is mostly induced by acute infections which sometimes can lead to chronic rhinosinusitis with or without nasal polyposis formation (Cao et al. 2018; Magliulo et al. 2019).
In subacute and chronic rhinosinusitis, the inflammation of the nasal mucosa and paranasal sinuses is contributed to the impaired mucociliary transport system and retention of the secretions (Yoruk et al. 2010). This will create a favorable environment for bacterial growth and contribute to the accumulation of inflammatory cells with releasing of pro-inflammatory cytokines, such as interleukin (IL)-1α, IL-1β, IL-6, IL-8 (Eloy et al. 2011; Al-Sayed et al. 2017,), and the tumor necrosis factor (TNF-α) (Kim et al. 2011). The cytokines attract neutrophils which will migrate to the inflammatory area, amplify the inflammation, leading to local edema and swelling of the mucosa (Berger et al. 2000; Naclerio 2010) and will disturb the mucocilliary clearance (Ali et al. 2005).
In addition, persistent inflammation promotes the oxidative stress, a process defined as an imbalance between the production of ROS and antioxidant defenses, with generation in excess of free radicals, key factors for local tissue injury (Istratenco 2019). They initiate lipid peroxidation with degradation of polyunsaturated fatty acids to malondialdehyde, a useful marker for clinical evaluation of oxidative stress in correlation with inflammation (Uslu et al. 2003; Yoruk et al. 2010). The redox imbalance also amplifies inflammation via activation of transcription factor NF-kB (Lingappan 2018; Jung et al. 2019). NF-kB regulates the inflammatory immune response and after its activation, induces the expression of pro-inflammatory genes, increasing the production of cytokines and chemokines (Liu et al. 2017). Inflammation and oxidative stress activates in turn matrix metalloproteinases (MMPs), zinc-dependent and calcium-dependent tissue proteases, responsible for the degradation of the extracellular matrix (Van Bruaene and Bachert 2011; Klein and Bischoff 2011). Moreover, NF-kB also stimulates MMPs production (Chase et al. 2002) and alters the balance between the MMPs and their tissue inhibitors (TIMPs) leading to excessive proteolysis and different diseases (Van Bruaene and Bachert 2011). Activated MMPs increase vascular permeability, induce edema, stimulate cell migration at the inflammatory area, and modulate the immune responses, thus, contributing to irreversible structural changes of the local mucosa (Klein and Bischoff 2011). As a result of long-standing local inflammation, mucosal remodeling occurs, the membrane thickens and number of glands and cells in the epithelium, including goblet cells, lymphocytes, neutrophils, and eosinophils increase ( Sobol et al. 2003; Rehl et al. 2007).
The purposes of treatment in subacute and chronic rhinosinusitis are to reduce mucosal inflammation, to restore mucocilliary clearance and to control the local infection (Yoruk et al. 2010; Suh and Kennedy 2011). In this strategy, alternative therapy with medicinal plants plays a special role due to their anti-inflammatory and anti-oxidative properties (Yoruk et al. 2010; Passali et al. 2018). Polyphenols found in vegetables, fruits, and plants could act both as anti-inflammatory (Ulbricht et al. 2014) and antioxidants agents, due to radical scavengers properties (Ciocoiu et al. 2009). Moreover, polyphenols have also been demonstrated to possess modulatory properties on extracellular matrix (Crascì et al. 2018). Sambucus nigra L. (SN) is a shrub belonging to the Adoxaceae family, having three subspecies: nigra, canadensis and cerulea (NRCS 2016). The first one is native to Europe, and the two latter ones, are native to North America. S. nigra L. subsp. nigra has blue-black elderberries and cream-white elderflowers. Polyphenols are the most important group of bioactive compounds present in elderberry (Młynarczyk et al. 2018). Traditional medicine used the elderberries and elderflowers to treat respiratory infections due to their antibacterial and antiviral properties (Mahboubi 2020). Previous studies showed that elderberry exhibited inhibitory bacterial activity and effectively suppressed viral replication, acting against pathogens that cause infections of the upper respiratory tract. (Kong 2009; Kinoshita et al. 2012; Álvarez et al. 2018). Studies have also shown that both elderberry and elderflower extracts exerted diverse biological activities in different biological system, such as antioxidant, anti-inflammatory, antidiabetic and diuretic effects (Ulbricht et al. 2014; Sidor and Gramza-Michalowska 2014). The anti-inflammatory effect of SN was proved by several studies, by modulating the production of pro-inflammatory cytokines, such as IL-6 and TNF-α, and suppressing neutrophils activations (Młynarczyk et al. 2018; Bartak et al. 2020). The antioxidant properties of elderberries is attributed to the phenolic compounds, with scavenger role against free radicals (Sidor and Gramza-Michalowska 2014; Viapiana and Wesolowski 2017).
The present study investigates the effect of intranasal administration of SN extract on inflammation, oxidative stress, and tissue remodeling of the nasal and sinus mucosa in a rat model of subacute inflammation induced by local administration of LPS (from Escherichia coli). To evaluate if the nasosinusal inflammation could produce a systemic inflammatory response, cytokines levels and oxidative stress markers in blood, lungs and brain were also assessed. The brain inflammation was evaluated because of the potential complications that can occur, such as intracranial extension of the infection through the sinus wall (Ziegler et al. 2018).
Materials and Methods
Reagents
2-Thiobarbituric acid, Bradford reagent, methanol, ABTS chromophore, diammonium salt, Trolox and acetic acid of HPLC analytical-grade were obtained from Merck KGaA (Darmstadt, Germany). ELISA tests for cytokines (TNF-α, IL-1β, and IL-6) were purchased from Elabscience (Houston, Texas, USA). Antibodies against NF-kB p65 (Ser536) (93H1), MMP-2, MMP-9, TIMP-1, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) while Folin-Ciocalteu reagent, sodium carbonate, aluminium chloride, sodium nitrite, sodium hydroxide, catechin, chlorogenic acid, p-coumaric acid, caffeic acid, rutin, apigenin, quercitrin, isoquercitrin, hyperoside, kaempferol, quercetin, myricetol, gallic acid and LPS reagent were purchased from Sigma-Aldrich Chemicals GmbH (Germany) and caftaric acid from Dalton (United States). All chemicals and reagents were of high-grade purity.
Preparation and characterization of Sambucus nigra fruit extract
Fruits of Sambucus nigra L. subsp. nigra were collected from Stâna de Mureş (46°25′31″N, 23°59′50″E), Alba county, Romania in August 2019. The plant was authenticated by the Botanical Department of the Faculty of Pharmacy Cluj-Napoca and a voucher specimen was deposited in the Herbarium of the Faculty of Pharmacy, “Iuliu Hatieganu” University of Medicine and Pharmacy, Cluj-Napoca. The fully ripened SN fruits were harvested, washed with tap water to remove impurities, and kept frozen until use. To obtain the fruit extract, 100 g of fruits puree were mixed with 300 mL food grad acetone. The mixture was stirred for 2 h at ambient temperature, and then filtered under vacuum. The solvent (acetone) was completely removed by vacuum evaporation, using a Buchi R114 rotary evaporator (Buchi Labortechnik AG, Switzerland), until a concentrated extract was obtained. The total phenolic content (TPC) characterized the resulting fruit extract, for which purpose the Folin-Ciocalteu method (Singleton et al. 1999) with some minor modifications previously applied by authors (Moldovan et al. 2012, 2016), was used.
Briefly, to a mixture of 1 mL of diluted fruit extract (128 fold) and 3 mL Folin-Ciocalteu reagent, 4.8 mL sodium carbonate solution (0.7 M) was added, and the resulted solution was kept in the dark, at room temperature, for 2 h. The absorbance of the reaction mixture was read at 765 nm, using a spectrophotometer (Perkin Elmer Lambda 25). Using a calibration curve, the absorbance values were converted in grams of gallic acid equivalents (GAE)/liter, used as the unit measurement for the total phenolic content of the fruit extract.
The total flavonoid content of the SN fruit extract was spectrophotometrically determined following the previously reported aluminium chloride colorimetric method (Chang et al. 2002). Briefly, in a 10 mL volumetric flask, 1 mL of crude extract was diluted with 4 mL distilled water and then 0.3 mL of 5% NaNO2 solution was added. After 5 min of incubation in the dark, 0.3 mL of 10% AlCl3 solution was added and the mixture was kept in the dark for other 6 min, then 2 mL 1 M NaOH solution were added and the final volume was adjusted to 10 mL with distilled water. The samples were well mixed and after that allowed to equilibrate for 15 min. The absorbance of the reaction mixture was recorded at 510 nm and the total flavonoid content was calculated and expressed as g catechin equivalents/L extract using a calibration curve of the catechin standard.
The total anthocyanin content of the SN L. fruit extract was determined using the widely applied method of Giusti and co-workers (2001), the so known pH differential method. To this end, 3 mL of properly diluted fruit extract (64-fold) were added to 3 mL potassium chloride buffer solution (0.025 M, pH 1) or sodium acetate buffer solution (0.04 M, pH 4.5), respectively. The resulting mixtures were allowed to equilibrate in the dark for 15 min and the absorbencies were measured at 512 nm and 700 nm against a blank sample. The total anthocyanin content was calculated using the following equations:
where TA = total anthocyanin content (mg∙l−1), A = absorbance, calculated as:
MW = molecular weight; DF = dilution factor; l = path length; ε = molar extinction coefficient; 1000 = conversion factor from gram to milligram.
The results were expressed as g cyanidin-3-glucoside equivalents/L.
The antioxidant capacity of the fruit extract was evaluated using the 2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) assay, by the slightly modified method of Arnao et al. (Arnao et al. 2001; David et al. 2019). The ABTS solution obtained by dissolving 360 mg of ABTS in 100 mL distilled water was mixed with 100 mL 2.45 mM of potassium persulfate solution to activate the ABTS cation radical. After 24 h, 20 mL of this mixture was diluted with distilled water until the absorbance measured at 734 nm ranged between 0.6 and 0.8. 3 mL diluted ABTS solution was added to 0.1 mL fruit extract and the sample was allowed to react for 15 min in the dark. The absorbance of the mixture was red at 734 nm against a blank sample and the total antioxidant capacity was expressed as mM Trolox equivalents using a calibration curve of the Trolox standard.
Identification and quantification of polyphenolic compounds
The phenolic compounds from the SN extract were analyzed by high performance liquid chromatography (HPLC) with UV and MS detection, as previously described (Mocan et al. 2015; Pop et al. 2017), using an Agilent 1100 HPLC Series system (Agilent, SUA) equipped with degasser, binary gradient pump, column thermostat, autosampler and UV detector. The chromatographic separation was performed on a reverse-phase analytical column (Zorbax SB-C18, 100 mm × 3.0 mm, 3.5 μm particles) using a mobile phase of a methanol/acetic acid 0.1% (v/v) mixture, starting with 5% methanol and ending at 42% methanol at 35 min, followed by 42% methanol for the next 3 min, rebalancing with 5% methanol in the next 7 min. The flow rate was 1 mL/min, the injection volume was 5 μL, and the column temperature was 48 °C. The UV detector was set at 330 nm until 17 min (for the detection of polyphenolic acids), then at 370 nm until the end of the analysis time (for the detection of flavonoids and their aglycones). The MS system (Agilent Ion Trap SL mass spectrometer) was operated using an electrospray ion source in negative mode (capillary 3000 V, nebulizer 60 psi (nitrogen), dry gas temperature 360 °C). The MS of the compounds from analyzed samples were compared to spectra from library, which allows positive identification of each substance, based on spectral match.
The external standard method was used for the quantitative determination of the identified compounds. Calibration curves of phenolic standard compounds in the range 0.5–50 mg/mL were used to quantify the polyphenols from Sambucus nigra L. extract. The results were expressed as micrograms of phenolic compounds/ml extract.
Animals and experimental design
Ethical approval for this study was obtained from the Local Ethics Committee on Experimental Animal Studies of the University of Medicine and Pharmacy “Iuliu Hatieganu”, Cluj-Napoca, Romania, according to the Directive 2010/63/EU on the protection of animals used for scientific purposes (no. 68/2019).
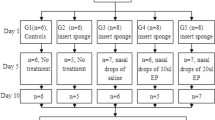
The study was conducted with 50 female Wister rats, age 20 days, weighing 100 ± 10 g. Before treatment, the rats were housed for 10 days in the Animal Facility of the Physiology Department, “Iuliu Hatieganu” University of Medicine and Pharmacy, Cluj-Napoca, for acclimatization. During the experiment, the animals were maintained under standard conventional conditions of a 12 h light/dark cycle, temperature of 18–21 °C, and humidity of 60–65%. Food and water were available ad libitum.
Rats were randomly divided into five groups of ten animals each. The medication used for the study was dropped into the nasal cavities using a micro-pipette, according to the following scheme: three times a week, for 3 consecutive weeks. Rats in group 1, the control group, were administered 20 µl of sterile normal saline solution. Rats in groups 2 and 3 received low-dose (5 µg) and high-dose (10 µg) of LPS. Rats in groups 4 and 5 received low-dose (5 µg) and high-dose (10 µg) of LPS, and after 2 h, 40 mg/kg BW of SN extract was administered intranasally once/day. This dose was chosen based on the dose used in the literature. Before and during the experiment, the nasal cavity was examined. After the first week of repeated intranasal LPS administration, nasal congestion with local erythema was detected in all rats from 2 to 5 groups.
At 24 h after the last treatment, under anesthesia with 10% ketamine and 2% xylasine, blood samples were collected to assess malondialdehyde and inflammatory markers (TNF-α, IL-1β, and IL-6). Nasal mucosa, lungs and brain tissue were removed by en bloc dissection. Nasal mucosa was used for histopathological analysis and for evaluation of inflammation and oxidative stress. NF-kB, MMP2, MMP9, TIMP1, and GAPDH were also evaluated in nasal mucosa by western blot. From lungs and brain tissues, oxidative stress and inflammatory markers were also performed. The harvested tissues were homogenized with a Polytron homogenizer (Brinkman Kinematica, Switzerland) as previously published (Filip et al. 2011), and the protein level was measured according to the Bradford protocol (Noble and Bailey 2009).
Oxidative stress assesment
Malondialdehyde, the most frequently used marker for lipid peroxidation, was determined in the blood, nasal mucosa, lungs, and brain, using the fluorimetric method with 2-thiobarbituric acid, described by Conti et al. (1991). The values are expressed either as pg/ml in blood or pg/mg protein in tissue homogenates.
Pro-inflammatory markers
Inflammation was quantified by measurement of TNF-α, IL-1β, and IL-6 in the blood, nasal mucosa, lungs, and brain homogenates, by ELISA assays according to the manufacturer’s protocol. Results are expressed as pg/ml in serum or pg/mg protein in tissue homogenates.
Matrix metalloproteinases and transcription factors assessment
Transcription factor NF-kB, metalloproteinases MMP2, MMP9, their inhibitor TIMP1, and GAPDH expressions were assessed by western blot analysis. For this purpose, the lysates (20 mg protein/lane) were separated by electrophoresis on SDS PAGE gels and then transferred to polyvinylidene difluoride membranes as previously described (Baldea, 2013). Blots were incubated with antibodies against NF-kB 65 (Ser536) (93H1), MMP2, MMP9, TIMP1, and GAPDH and secondary peroxidase-coupled antibody (1:1000). GAPDH was used as the protein loading control. The visualization and detection of proteins were made using Supersignal West Femto Chemiluminescent substrate (Thermo Fisher Scientific) and a Gel Doc Imaging system equipped with an XRS camera.
Histopathological analysis
For the histopathological analysis, the heads were harvested and fixed in 10% neutral-buffered formalin. After the complete fixation, the heads were cleaned of skin, subcutaneous connective tissue and muscle, and decalcified with Richard-Allan scientific decalcifying solution (Thermo Fisher Scientific). When decalcification was completed, the tissues were transversely trimmed in four planes following the previously described technique by Kittel et al. (2004), and embedded with the rostral faces down in paraffin wax. The samples were sectioned to 4 mm thickness with a rotary microtome and stained with hematoxylin–eosin (H&E). The histological slides were examined using an Olympus BX41 microscope, and the histological images were obtained with an Olympus UC30 digital camera and further processed with the stream basic program.
To evaluate the outcome of the different doses of LPS, in association with or without SN extract at the nasal and paranasal mucosa, various features were assessed including the exudate, inflammatory cell infiltration, goblet cell development, the aspect of the nasal and sinus epithelium, as well as signs of tissue remodeling, such as mucosal hypertrophy. The histopathologic grading scale, as described by Khalid et al. (2008) was used. The sections were examined and graded. Inflammation of the lamina propria, nasal and sinusal epithelium were graded from 0 to 4 (severe change), nasal and sinusal exudate was also graded from 0 to 4 (severe change). Mucosal hypertrophy was classified from 0 to 4 (severe change). Control sections were used to define the grade of 0. The histological grades of the treated animals were compared to the untreated group.
Statistical analysis
Experimental data were analyzed by one-way ANOVA test and for comparison between groups, the Tukey posttest was applied using the GraphPad Prism 8 software. All data are shown as mean value and standard deviation. A p value < 0.05 was considered statistically significant.
Results
Characterization of Sambucus nigra L. fruit extract
The chemical composition of SN fruits is rich, these berries being known as a valuable source of dietary phytochemicals among which polyphenols and flavonoids are the main bioactive compounds. The known elderberries curative properties are due to the presence of these secondary metabolites which are present in relatively high amounts compared to other fruits (Domingues et al. 2020). The total phenolic content of the SN fruit extract as evaluated by the Folin-Ciocalteu method was 15.6 g GAE/l. This high amount of the phenolic compounds is primarily due to the presence of flavonols but also phenolic acids and anthocyanins. The total flavonoid content of the investigated fruit extract was 6.43 catechin equivalents/l while the total anthocyanin content was 4.83 g cy-3-glu equivalents/l. These compounds are plant secondary metabolites well known for their free radical scavenging (antioxidant) capacity. The high antioxidant activity of the extract as determined by the ABTS radical cation discoloration method was 50.92 mM Trolox and can be associated with the therapeutic effects of these fruits.
The identification and quantification of the main polyphenols from SN fruits was achieved by HPLC (Fig. 1).
The analysis revealed the presence of seven phenolic compounds (Table 1), among these three phenolic acids (i.e., gentisic acid, chlorogenic acid, 4-O-caffeoylquinic acid) and four flavonols (i.e., quercetin-3-O-rutinoside (rutin), isoquercitrin, quercitrin and quercetin).
Quercetin-3-O-rutinoside, also known as rutin, was the major compound found in a significant quantity. Rutin is a flavonoid glycoside with multiple pharmacological activities, such as, antioxidant, anti-inflamatory, cardiovascular, neuroprotective, antidiabetic, and anticancer activities (Ganeshpurkar and Saluja 2017; Erkan et al. 2020). Among these effects, rutin also exerts antibacterial and antiviral activity (Ganeshpurkar and Saluja 2017). The exact role of rutin on immune cells nasal inflammation is not well understood. Study performed by Kim et al. (2015) investigated the effect of rutin on allergic rhinitis and reported that beside the anti-inflammatory effect, rutin inhibited intercellular adhesion molecules and the infiltrations of mast cells and eosinophils. In addition, rutin acts as a scavenger of free reactive oxygen species protecting against redox misbalance (Enogieru et al. 2018).
Another flavonoid glycoside compound which was found and quantified in SN extracts was isoquercitrin, which is known to have good anti-inflammatory properties (Rogerio et al. 2007). The quantities obtained are increased, but significantly lower than those obtained for rutin.
Evaluation of oxidative stress in blood, nasal mucosa, lungs and brain
To quantify the presence of oxidative stress, malondialdehyde levels were evaluated in blood, nasal mucosa, lungs, and brain homogenates. Intranasal administration of LPS increased malondialdehyde level in all the investigated tissues, as compared to the control group, especially after high dose of LPS (p < 0.05 and p < 0.01), (Fig. 2). Also, a significant rise of malondialdehyde level was detected in nasal mucoasa after low dose of LPS (p < 0.05). Intranasal administration of SN extract diminished malondialdehyde formation compared with rats that received LPS, but without statistically significance (p > 0.05).
Malondialdehyde levels in blood a nasal mucosa b lungs c and brain d homogenates in rats treated with LPS and SN extract. The rats were intranasal treated for 3 consecutive weeks, three times a week, with two different doses of LPS, with or without SN extract. Malondialdehyde levels were quantified in blood, nasal mucosa, lungs, and brain homogenates at 24 h after the last treatment. Data are means ± standard deviation. Statistical analysis was performed by a one-way ANOVA, with Tukey's multiple comparisons posttest. *p < 0.05; **p < 0.01 as compared to the control group
Inflammatory markers and transcription factors assessment in blood, nasal mucosa, lungs and brain
To evaluate the local and systemic inflammation, the levels of IL-6, IL-1β and TNF-α were quantified by ELISA assay in blood, nasal mucosa, lungs, and brain homogenates.
High levels of IL-6 were found in blood, nasal mucosa, and brain in rats treated with low dose of LPS (p < 0.05 for brain levels and p < 0.01 for blood and nasal mucosa) (Fig. 3a, b, d), as compared to untreated animals. Administration of a high dose of LPS also significantly increased the IL-6 levels in serum, nasal mucosa, and brain, as well as in the lungs (p < 0.05 and p < 0.01) (Fig. 3). The local administration of SN extract significantly diminished the IL-6 level in the nasal mucosa and blood, compared with groups treated only with both doses of LPS (p < 0.05 and p < 0.01), and in lungs compared to a high dose of LPS (p < 0.05) (Fig. 3a, b, c). In the lungs, the IL-6 secretion increased in rats treated with LPS low dose and SN extract, compared to those that received only LPS low dose, but without statistical significance (Fig. 3c).
IL-6 levels in blood a nasal mucosa b lungs c and brain d in rats treated with LPS and SN extract. The rats were intranasal treated for 3 consecutive weeks, three times a week, with two different doses of LPS, with or without SN extract. IL-6 secretion was quantified in serum, nasal mucosa, lungs and brain homogenates at 24 h after the last treatment. Data are means ± standard deviation. Statistical analysis was performed by a one-way ANOVA, with Tukey´s multiple comparisons posttest. *p < 0.05; **p < 0.01 as compared to control group and #p < 0.05, ##p < 0.01 compared to groups treated with LPS
After a low dose of LPS, IL-1β secretion increased in all the investigated tissues, the difference being statistically significant in the nasal mucosa (p < 0.05) (Fig. 4b). High dose of LPS significantly enhanced IL-1β levels in serum, nasal mucosa, and lungs (p < 0.05), as compared to the control group (Fig. 4a, b, c). The administration of SN extract, diminished IL-1β level in the nasal mucosa (p < 0.05) (Fig. 4b), compared with a LPS high dose, but without significant changes in the blood, lungs, and brain.
IL-1β levels in blood a nasal mucosa b lungs c and brain d in rats treated with LPS and SN extract. The rats were intranasal treated for 3 consecutive weeks, three times a week, with two different doses of LPS, with or without SN extract. IL-1β secretion was quantified in serum, nasal mucosa, lungs and brain homogenates at 24 h after the last treatment. Data are means ± standard deviation. Statistical analysis was performed by a one-way ANOVA, with Tukey’s multiple comparisons posttest. **p < 0.01 as compared to control group, and #p < 0.05 compared to groups treated with LPS
TNF-α quantified by ELISA displayed that repeated intranasal administration of LPS stimulated TNF-α secretion in all the investigated tissues. Significantly high levels of TNF-α were found in blood, nasal mucosa, and lungs in rats treated with high dose of LPS (p < 0.05 and p < 0.01) (Fig. 5a, b, c), as compared to untreated animals. Administration of a low dose of LPS also significantly increased the protein level in the lungs (p < 0.05) (Fig. 5c). SN extract reduced TNF-α levels in the blood compared with rats treated with a LPS high dose (p < 0.05), but without significant changes in the nasal mucosa, and lungs. The protein level slightly increased after administration of SN extract in brain in rats treated with a low dose of LPS without statistical significance (Fig. 5d).
TNF-α levels in blood a nasal mucosa b lungs c and brain d in rats treated with LPS and SN extract. The rats were intranasal treated for 3 consecutive weeks, three times a week, with two different doses of LPS, with or without SN extract. The TNF-α levels were quantified in serum, nasal mucosa, lungs and brain homogenates at 24 h after the last treatment. Data are means ± standard deviation. Statistical analysis was performed by a one-way ANOVA, with Tukey’s multiple comparisons posttest. *p < 0.05 as compared to control group. #p < 0.05 compared to groups treated with LPS
Besides the inflammatory cytokines, the evaluation of NF-kB, metalloproteinases (MMP2, MMP9), and TIMP1expressions were also performed in nasal mucosa (Fig. 6).
NF-kB, MMP2, MMP9 and TIMP1 expressions in nasal mucosa homogenates in rats treated with LPS with or without SN extract. Image analysis of the intensity of the Western blot bands was performed through densitometry and the data, normalized to GAPDH, are shown as graphs in a NF-kB b MMP2, c MMP9 and d TIMP1. Each bar represents mean ± standard deviation; *p < 0.05, ** p < 0.01***, p < 0.001 as compared to control group, ##p < 0.01, ###p < 0.05 compared to groups treated with LPS
The investigation of NF-kB expressed as ratios with GAPDH, showed significant differences between all the experimental groups (p < 0.001). So, both doses of LPS induced a significant increase of NF-kB expression (Fig. 6a), while the intranasal administration of SN extract amplified NF-kB, especially after a high dose (Fig. 6a). Western blot analysis of MMPs bands exhibited upregulation of MMP2 after the high dose of LPS compared to the untreated group and downregulation of TIMP1 in rats which received both doses of LPS. MMP9 expression decreased in rats treated with a low dose of LPS and SN extract compared to rats treated only with LPS (p < 0.001), as seen in Fig. 6c. SN extract upregulated MMP2 expression in rats treated with both doses of LPS and MMP9 expression in association with a high dose of LPS. TIMP1 expression increased significantly in rats treated with SN extract and both doses of LPS (Fig. 6d).
Histopathological analysis
To quantify the inflammation produced by intranasal administration of LPS and the impact of local administration of SN extract, a conventional histopathology analysis of the nasal mucosa and the paranasal sinuses in hematoxylin–eosin was performed. The analysis evaluated the exudate, inflammation of the lamina propria, the aspect of the nasal and sinus epithelium, as well as the existence of signs of tissue remodeling.
In the control group, the histopathological analysis revealed no specific findings, with minimal inflammatory infiltrate of neutrophils only in nasal mucosa, the severity of the lesions being between 0 and 1 (Fig. 7a and 8a).
Histopathological images of nasal mucosa of Wistar rats after three times a week, for 3 consecutive weeks of intranasal LPS administration with or without SN extract. All panels represent nasal mucosa sampling areas in H&E-stained sections. In each panel, representative pictures from control a LPS 5 µg b LPS 10 µg c LPS 5 µg + SN d and LPS 10 µg + SN e are shown. The nasal septum is marked by an asterisk, nasal turbinates by black arrowheads, lamina propria with arrows, and nasal-liminal exudates by red arrowheads
Histopathological images of sinusal mucosa of Wistar rats after three times a week, for 3 consecutive weeks of intranasal LPS administration with or without SN extract. All panels represent sinusal mucosa sampling areas in H&E-stained sections. In each panel, representative pictures from control a LPS 5 µg b LPS 10 µg c LPS 5 µg + SN d and LPS 10 µg + SN e are shown. Nasal sinus mucosa is marked by the black arrowhead, the sinusal lamina propria by black arrows, and the nasal-liminal exudates by red arrowheads
In animals treated with a low dose of LPS, the local changes were more evident in nasal mucosa (Fig. 7b). An exudate rich in neutrophils, moderate infiltration consisting of lymphocytes, neutrophils, and eosinophils, congestion of the lamina propria, with focal intraepithelial neutrophils, lymphocytes, and eosinophils were found. The semi-quantitative evaluation of lesions severity showed an average of 3 + in all aspects analyzed. Instead, in sinusal mucosa, only a minimal focal lymphocytic and neutrophilic inflammation was noticed (Fig. 8b).
Animals treated with a high dose of LPS showed more evident changes in the nasal mucosa (Fig. 7c), with an important exudate rich in neutrophils and significant congestion of the lamina propria extensively infiltrated by plasma cells, lymphocytes, eosinophils, neutrophils and few macrophages. In the nasal epithelium, mixed inflammatory infiltrate, goblet cells and squamous metaplasia were present. The severity of the nasal mucosa lesion showed an average of 3 + compared to paranasal sinuses, where the inflammatory changes were minimal (1 +), (Fig. 8c).
In both groups treated with LPS, the histopathological analysis showed no signs of mucosal hypertrophy suggesting a negligible progression to a tissue remodeling process. SN extract administered in rats associated with low dose of LPS reduced the exudate. However, a slight degree of inflammation in lamina propria, with lymphocytic, neutrophilic and eosinophilic infiltrate was present (Fig. 7d). In animals treated with high dose of LPS and SN extract, the exudate in nasal mucosa was slightly reduced, with focal neutrophils and lymphocytes in the lamina propria and the epithelium (Fig. 7e).
Discussion
This study aimed to explore the anti-inflammatory and antioxidant potential of SN extract, and its effect against mucosal remodeling in subacute rhinosinusitis in rats, induced by intranasal administration of LPS. The results demonstrated that SN extract diminished the local inflammation and inhibited the secretion of pro-inflammatory cytokines, attenuated histopathological changes, and decreased the generation of ROS. Additionally, SN extract influenced MMPs expressions and upregulated TIMP1, suggesting a possible protective role of SN on tissue remodeling.
The experimental model was chosen for several reasons: firstly, subacute rhinosinusitis is a frequent condition characterized by persistent inflammation in the mucous membranes from the nasal cavity and the paranasal sinuses (Meltzer et al. 2004). Cytokines, chemokines and ROS generated through NF-kB pathway activation are key factors that amplify the secretion of cytokines and aggravate the inflammation (Opris et al. 2017). Secondly, local inflammation and oxidative stress lead to activation of matrix metalloproteinases (MMPs), especially 2 and 9, findings noticed in patients with chronic rhinosinusitis (Van Bruaene and Bachert 2011). Activated MMPs lead to degradation of the extracellular matrix and contribute to irreversible structural changes of nasal and sinus mucosa (Van Bruaene and Bachert 2011; Klein and Bischoff 2011). Thirdly, therapeutic strategy in subacute and chronic rhinosinusitis aims to reduce the inflammation and restore mucociliary clearance to prevent mucosal remodeling. Although medical and surgical treatment is often effective, there are still patients with subacute and chronic rhinosinusitis who do not respond favorably to standard therapy, probably due to the local changes in the nasal mucosa. Therefore, the alternative therapies, such as herbal medicines with anti-inflammatory properties, can be a good option, especially for the prevention of local changes progression to severe disease (Yoruk et al. 2010; Passali et al. 2018).
Several studies have attributed the anti-inflammatory properties of SN extract due to the inhibition of pro-inflammatory cytokines releasing from macrophages and the suppression of neutrophils activation (Harokopakis et al. 2006; Bartak et al. 2020). These effects are related to the inhibition by SN extract of NF-kB and phosphatidylinositol (PI) 3 kinase activation (Harokopakis et al. 2006). Moreover, several studies have shown that SN extract could inhibit MMPs activity and inflammation in different pathological conditions including periodontal (Oberbaum et al. 2016) and liver disease (Opris et al. 2017). As far as we know, there are no reported studies in the literature regarding the effect of SN extract on the MMPs expression in rhinosinusitis.
To induce a subacute rhinosinusitis in rats, the intranasal administration of LPS in two different doses (5 μg and 10 μg), three times a week, for 3 consecutive weeks, was used. To quantify the effects of LPS administration, the cytokines IL-6, IL-1β, TNF-α and oxidative stress marker (malondialdehyde) in blood, nasal mucosa, lungs and brain, were evaluated. In addition, NF-kB, MMP2, MMP9 and TIMP1 expressions in the nasal mucosa, along with the histopathological analysis of the nasal mucosa, were assessed. The IL-6, IL-1β, and TNF-α levels increased in rats treated with both doses of LPS in all the investigated tissues, especially in the nasal mucosa, blood, and lungs. Moreover, both doses of LPS enhanced IL-6 secretion in the brain, suggesting that local administration of LPS may induce a systemic inflammation with the involvement of the lungs and brain. Our results are in agreement with other studies and confirmed the secretion of cytokines in chronic rhinosinusitis in parallel with the upregulation of NF-kB (Kim et al. 2011; Wang et al. 2017; Geng et al. 2019). Inflammation activates the NF-kB pathway which increases production of cytokines and ROS (Opris et al. 2017). Similar to other studies (Uslu et al. 2003; Yoruk et al. 2010; Istratenco 2019), the malondialdehyde formation enhanced in all investigated tissue, especially after the high dose of LPS, suggesting the important role of persistent inflammation in ROS production.
The local inflammation also stimulates MMPs in macrophages and inactivates their inhibitors TIMPs (Okamoto et al. 2004), both having an essential role in tissue remodeling. Thus, increased expression of MMPs or reduced TIMP-1 could induce destructions of the extracellular matrix, with tissue damage and remodeling process (Rehl et al. 2007; Lou et al. 2018).
Several studies investigated the role of MMPs in chronic rhinosinusitis. Can et al. (2008) demonstrated that MMPs activation is a key factor for progression to chronic rhinosinusitis or nasal polyps. Li et al. (2010) reported that MMP-9 is likely associated with airway remodeling in chronic rhinosinusitis with nasal polyps. Malinsky et al. (2013) also confirmed a significant increase of MMP-9 expression in chronic rhinosinusitis with nasal polyps.
On the other hand, the role of MMP-2 in nasal polyps’ formation in chronic rhinosinusitis is quite controversial and unclear. Thus, studies performed by Bhandari et al. (2004), Can et al. (2008), and Malinsky et al. (2013), demonstrated a higher expression of MMP-2 in the nasal polyposis tissue, while other authors did not found significant values of MMP2 in this condition (Ozgul et al. 2008; Li et al. 2010). In our study, the MMP2 upregulation was demonstrated after the high dose of LPS in parallel with the low expression of TIMP1. To evaluate the sinonasal mucosal remodeling in rats, a histopathological examination was performed and suggestive changes for subacute inflammation were noticed after LPS administration. An exudate rich in neutrophils, substantial congestion of the lamina propria, inflammatory infiltrate with neutrophils, lymphocytes, and eosinophils, goblet cells and squamous metaplasia at the nasal epithelium were identified. Wang et al. (2017) found, in a mouse model of chronic rhinosinusitis induced by LPS, using the same doses administrated three times a week, for 3 consecutive months, high levels of inflammatory cytokines and neutrophilic nasal polyps in the nasal mucosa. In our study, LPS administration induced a local inflammatory response with secretion of cytokines, without signs of sinonasal mucosa thickening, or nasal polyp formation, although MMP2 expression increased significantly in rats treated with a high dose of LPS and TIMP1 was downregulated. Our results can be explained by a shorter time of treatment and evaluation, or probably by the young age of the animals.
Several in vivo studies demonstrated the anti-inflammatory and antioxidant propreties of SN extract in airways inflammation. In an in vivo study (Ismail 2005), different groups of mice were inoculated with Streptococcus pneumoniae and treated with antibiotic, corticosteroids and SN extract. A reduction in bacterial growth after 4 and 8 days of treatment was demonstrated in all the studied animals (Ismail 2005). Moreover, SN administered in mice with allergic asthma induced by alum-emulsified-ovalbumin, reduced cytokines levels and oxidative stress, as well as lung congestion and inflammation (Alrumaihi et al. 2020).
Our study demonstrated that SN extract diminished the severity of rhinosinusitis by the regulation of pro-inflammatory cytokines secretion. The present study showed a decrease in malondialdehyde levels compared to rats receiving only LPS, but without statistical significance. It is generally accepted that inflammation and oxidative stress are interdependent pathophysiological processes (Toiu et al. 2019). Inflammation promotes the oxidative stress with generation of ROS at the site of inflammation which will further exaggerate inflammation through activation of multiple pathways, especially activation of transcription factor NF-kB (Biswas 2016). Both, the anti-inflammatory and antioxidant propreties of SN extract were demonstrated in different studies. Sometimes the antioxidants do not inhibit oxidative stress and the associated inflammation at the same time and same proportion, or may even block some of the oxidative and/or inflammatory pathways and exaggerate the others (Biswas 2016; Toiu et al. 2019). This could be responsible for the failures of the antioxidants. Therefore, more and deeply studies are needed to clarify these aspects. Increased cytokines and malondialdehyde levels in the blood, lungs and brain highlighted that local inflammation progressed to a systemic one. The SN extract reduced the inflammatory cytokines, especially in the blood, with a slight decrease in the lungs and brain. Therefore, SN extract also proves its systemic anti-inflammatory properties.
Increased cytokines and malondialdehyde levels in the blood, lungs and brain highlighted that local inflammation progressed to a systemic one. The SN extract reduced the inflammatory cytokines, especially in the blood, with a slight decrease in the lungs and brain. Therefore, SN extract also proves its systemic anti-inflammatory properties. Additionally, SN extract downregulated MMP9 in rats treated with a low dose of LPS in parallel with the improvement of the TIMP1 expression. MMPs regulate the inflammatory reaction by processing the monocyte chemoattractant proteins to reduce the agonist activity of chemokine receptors and generate antagonist gradients (McQuibban et al. 2002; Manicone and McGuire 2008). This process depletes the cellular infiltrates and decreases the number of leukocytes that express these receptors (McQuibban et al. 2002). SN extract amplified MMP2 expression in rats treated with both doses of LPS and MMP9 expression in association with a high dose of LPS, suggesting its stimulatory role on MMPs. It is known that MMPs are highly expressed in different conditions involving inflammation due to their activation by local cytokines and chemokine. In our study, the expression of MMP2 and MMP9 was significantly higher in animals treated with a high dose of LPS and SN extract. Probably, LPS in a high dose generates an intense inflammatory reaction, and SN extract stimulates MMPs expression to regulate the inflammatory process and to prevent tissue damage and mucosal remodeling. In rats treated with LPS high dose and SN extract, NF-kB expressions increased and its activation could regulate the transcription and expression of MMPs. However, more studies are required to clarify the effect of SN extract on NF-kB activity and MMPs expression, particularly in severe inflammatory reaction.
The histopathological analysis of the nasal and sinus mucosa revealed that SN extract diminished the exudate, the congestion, and the inflammatory cells infiltration. These results demonstrated the protective role of SN extract on nasal and sinusal inflammation and also proved the protective effect on different tissues as response to inflammation.
Conclusions
The present study suggests that SN extract could attenuate the inflammatory response, including the production of cytokines, and the histopathological changes, as well as the generation of ROS in LPS-induced subacute rhinosinusitis in rats. The results also revealed that SN extract administration influenced MMPs expressions, the effect being dose dependent. Thus, at a low dose of LPS, SN extract reduced MMP9 expression while at high dose of LPS, SN extract upregulated these enzymes through the activation of NF-kB. These data could suggest that SN extract may have a protective role in vascular remodeling and the effect is different depending on the dose used. More in vivo studies are required to investigate the SN extract effects on the tissue remodeling process in subacute rhinosinusitis.
References
Ali MS, Hutton DA, Wilson JA, Pearson JP (2005) Major secretory mucin expression in chronic sinusitis. Otolaryngol Head Neck Surg 133:423–428
Alrumaihi F, Almatroudi A, Allemailem KS, Rahmani AH, Khan A, Khan MA (2020) Therapeutic effect of Bilsaan Sambucus nigra stem exudate, on the OVA-induced allergic asthma in mice. Oxid Med Cell Longev 2020:3620192
Al-Sayed AA, Agu RU, Massoud E (2017) Models for the study of nasal and sinus physiology in health and disease: a review of the literature. Laryngoscope Investig Otolaryngol 2:398–409
Álvarez CA, Barriga A, Albericio F, Romero MS, Guzmán F (2018) Identification of peptides in flowers of sambucus nigra with antimicrobial activity against aquaculture pathogens. Molecules 23:1033
Arnao MB, Cano A, Acosta M (2001) The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem 73:239–244
Baldea I, Costin GE, Shellman Y, Kechris K et al (2013) Biphasic pro-melanogenic and pro-apoptotic effects of all-trans-retinoic acid (ATRA) on human melanocytes: time-course study. J Dermatol Sci 72:168–176
Bartak M, Lange A, Słońska A, Cymerys J (2020) Antiviral and healing potential of Sambucus nigra extracts. Bionatura 5:1264–1670
Berger G, Kattan A, Bernheim J, Ophir D, Finkelstein Y (2000) Acute sinusitis: a histopathological and immunohistochemical study. Laryngoscope 110:2089–2094
Bhandari A, Takeuchi K, Suzuki S, Harada T, Hayashi S et al (2004) Increased expression of matrix metalloproteinase-2 in nasal polyps. Acta Otolaryngol 124:1165–1170
Biswas SK (2016) Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev. https://doi.org/10.1155/2016/5698931
Can IH, Ceylan K, Caydere M, Samim EE, Ustun H, Karasoy DS (2008) The expression of MMP-2, MMP-7, MMP-9, and TIMP-1 in chronic rhinosinusitis and nasal polyposis. Otolaryngol Head Neck Surg 139:211–215
Cao Y, Wu S, Zhang L, Yang Y, Cao S, Li Q (2018) Association of allergic rhinitis with obstructive sleep anea: a meta-analysis. Medicine (Baltimore) 97:e13783
Chang C, Yang M, Wen H, Cher J (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10:178–182
Chase AJ, Bond M, Crook MF, Newby AC (2002) Role of nuclear factor-kappa b activation in metalloproteinase-1, -3, and -9 secretion by human macrophages in vitro and rabbit foam cells produced in vivo. Arterioscler Thromb Vasc Biol 22:765–771
Ciocoiu M, Mirón A, Mares L, Tutunaru D, Pohaci C, Groza M, Badescu M (2009) The effects of Sambucus nigra polyphenols on oxidative stress and metabolic disorders in experimental diabetes mellitus. J Physiol Biochem 65:297–304
Conti M, Morand PC, Levillain P, Lemonnier A (1991) Improved fluorimetric determination of malondaldehyde. Clin Chem 37:1273–1275
Crascì L, Lauro MR, Puglisi G, Panico A (2018) Natural antioxidant polyphenols on inflammation management: anti-glycation activity vs metalloproteinases inhibition. Crit Rev Food Sci Nutr 58:893–904
David L, Danciu V, Moldovan B, Filip A (2019) Effects of in vitro gastrointestinal digestion on the antioxidant capacity and anthocyanin content of Cornelian cherry fruit extract. Antioxid Redox Signal 8:114
Domingues R, Zhang L, Rocchetti G, Lucini L, Pateiro M, Munekata PES, Lorenzo JM (2020) Elderberry Sambucus nigra L. as potential source of antioxidants. Characterization, optimization of extraction parameters and bioactive properties. Food Chem 330:127266
Eloy P, Poirrier AL, De Dorlodot C, Van Zele T, Watelet JB, Bertrand B (2011) Actual concepts in rhinosinusitis: a review of clinical presentations, inflammatory pathways, cytokine profiles, remodeling and management. Curr Allergy Asthma Rep 11:146–162
Enogieru AB, Haylett W, Hiss DC, Bardien S, Ekpo OE (2018) Rutin as a potent antioxidant: implications for neurodegenerative disorders. Oxid Med Cell Longev. https://doi.org/10.1155/2018/6241017
Erkan H, Aliseydi B, Keskin E, Abdullah E, Ali GM, Halis S, Zeynep S (2020) Effect of rutin on oxidative and proinflammatory damage induced by cisplatin in blood serum, ureter, bladder and urethra in rats. Biotechnol Biotechnol Equip 34:171–181
Filip A, Daicoviciu D, Clichici S, Bolfa P, Catoi C, Baldea I et al (2011) The effects of grape seeds polyphenols on SKH-1 mice skin irradiated with multiple doses of UV-B. J Photochem Photobiol B 105:133–142
Ganeshpurkar A, Saluja AK (2017) The pharmacological potential of rutin. Saudi Pharm J 25:149–164
Geng L, Wang S, Zhao Y, Hu H (2019) Gene expression profile in mouse bacterial chronic rhinosinusitis. Exp Ther Med 17:3451–3458
Giusti MM, Wrolstad RE (2001) Characterization and measurement of anthocyanins by UV-visible spectroscopy. In: Giusti MM, Wrolstad RE (eds) Current protocols in food analytical chemistry. John Wiley and Sons Inc, Hoboken, pp F1.2.1.–F1.2.13
Harokopakis E, Albzreh M, Haase E, Scannapieco F, Hajishengallis G (2006) Inhibition of proinflammatory activities of major periodontal pathogens by aqueous extracts from elder flower (Sambucus nigra). J Periodontol 77:271–279
Ismail C (2005) Pharmacology of sinupret. Recent results on the rational for the sinupret compound. HNO 53:S38–S42
Istratenco A (2019) Oxidative stress-related pathophysiology in chronic rhinosinusitis with nasal polyps: research challenges. Rom J Rhinol 9:71–77
Jung HJ, Zhang YL, Kim DK, Rhee CS, Kim DY (2019) The role of NF-κB in chronic rhinosinusitis with nasal polyps. Allergy Asthma Immunol Res 11:806–817
Khalid AN, Woodworth BA, Prince A, Quraishi SA, Antuneset MB et al (2008) Physiologic alterations in the murine model after nasal fungal antigenic exposure. Otolaryngol Head Neck Surg 139:695–701
Kim DH, Jeon E, Park SN, Park KH, Park YS, Yeo SW (2011) Effects of a tumor necrosis factor-α antagonist on experimentally induced rhinosinusitis. J Biomed Biotechnol. https://doi.org/10.1155/2011/360457
Kim HY, Nam SY, Hong SW, Kim MJ, Jeong HJ, Kim HM (2015) Protective effects of rutin through regulation of vascular endothelial growth factor in allergic rhinitis. Am J Rhinol Allergy 29:e87-94
Kinoshita E, Hayashi K, Katayama H, Hayashi T, Obata A (2012) Anti-influenza virus effects of elderberry juice and its fractions. Biosci Biotechnol Biochem 76:1633–1638
Kittel B, Ruehl-Fehlert C, Morawietz G, Klapwijk J, Elwell MR, Lenz B et al (2004) Revised guides for organ sampling and trimming in rats and mice–Part 2: a joint publication of the RITA and NACAD groups. Exp Toxicol Pathol 55:413–431
Klein T, Bischoff R (2011) Physiology and pathophysiology of matrix metalloproteases. Amino Acids 41:271–290
Kong F (2009) Pilot clinical study on a proprietary elderberry extract: efficacy in addressing influenza symptoms. Online J Pharmacol Pharmacokin 5:32–43
Li X, Meng J, Qiao X, Liu Y, Liu F et al (2010) Expression of TGF, matrix metalloproteinases, and tissue inhibitors in Chinese chronic rhinosinusitis. J Allergy Clin Immunol 125:1061–1068
Lingappan K (2018) NF-kB in oxidative stress. Curr Opin Toxicol 7:81–86
Liu T, Zhang L, Joo D, Sun SC (2017) NF-κB signaling in inflammation. Signal Transduct Target Ther 2:17023
Lou H, Zhang N, Bachert C, Zhang L (2018) Highlights of eosinophilic chronic rhinosinusitis with nasal polyps in definition, prognosis, and advancement. Int Forum Allergy Rhinol 8:1218–1225
Magliulo G, Iannella G, Ciofalo A, Polimeni A, De Vincentiis M, Pasquariello B et al (2019) Nasal pathologies in patients with obstructive sleep apnoea. Acta Otorhinolaryngol Ital 39:250–256
Mahboubi M (2020) Sambucus nigra (black elder) as alternative treatment for cold and flu. Adv Tradit Med (ADTM). https://doi.org/10.1007/s13596-020-00469-z
Malinsky RR, Valera FCP, Cavallari FE, Küpper DS et al (2013) Matrix metalloproteinases and their impact on sinusal extension in chronic rhinosinusitis with nasal polyps. Eur Arch Oto-Rhino-L 270:1345–1348
Manicone AM, McGuire JK (2008) Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol 19:34–41
McQuibban GA, Gong JH, Wong JP, Wallace JL et al (2002) Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood 100:1160–1167
Meltzer EO, Hamilos DL, Hadley JA et al (2004) Rhinisinusitis: establishing definition for clinical reseach and patient care. J Allergy Clin Immunol 114:155–212
Młynarczyk K, Walkowiak-Tomczak D, Łysiak GP (2018) Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J Funct Foods 40:377–390
Mocan A, Vlase L, Raita O, Hanganu D, Paltinean R, Dezsi S, Gheldiu AM, Oprean R, Crisan G (2015) Comparative studies on antioxidant activity and polyphenolic content of Lycium barbarum L. and Lycium chinense Mill. leaves. Pak J Pharm Sci 28:1511–1515
Moldovan B, Ghic O, David L, Chisbora C (2012) The influence of storage on the total phenols content and antioxidant activity of the cranberrybush (Viburnum opulus L.) fruits extract. Rev Chim 63:463–464
Moldovan B, Popa A, David L (2016) Effects of storage temperature on the total phenolic content of Cornelian cherry (Cornus mas L.) fruits extracts. J Appl Bot Food Qual 89:208–211
Naclerio RM, Bachert C, Baraniuk JN (2010) Pathophysiology of nasal congestion. Int J Gen Med 3:47–57
Noble JE, Bailey MJ (2009) Quantitation of protein. Meth Enzymol 463:73–95
NRCS, Natural Resources Conservation Service, United States Department Of Agriculture (2016) Classification for kingdom plantae down to genus sambucus L.http://plants.usda.gov/java/ClassificationServlet?source=display&classid=SAMBU 17 Noc 2016
Oberbaum M, Levine WZ, Saffer AJ, Koren N, Sarment D (2016) Inhibition of matrix metalloproteinase activity by a transmucosal patch containing botanical compounds. Dent Oral Craniofac Res 2:349–354
Okamoto T, Akuta T, Tamura F, Van Der Vliet A, Akaike T (2004) Molecular mechanism for activation and regulation of matrix metalloproteinases during bacterial infections and respiratory inflammation. Biol Chem 385:997–1006
Opris R, Tatomir C, Olteanu D, Moldovan R, Moldovan B, David L et al (2017) The effect of Sambucus nigra L. extract and phytosinthesized gold nanoparticles on diabetic rats. Colloids Surf B 150:192–200
Ozgul T, Selim SE, Halil K, Ozcan C (2008) Expression of metalloproteinases MMP-2 and MMP-9 in antrochoanal polyps. Am J Rhinol Allergy 22:339–342
Passali D, Passali GC, Bellussi LM, Sarafoleanu C, Loglisci M, Iosif C, Passalli FM (2018) Bromelain’s penetration into the blood and sinonasal mucosa in patients with chronic rhinosinusitis. Acta Otorhinolaryngol Ital 38:225–228
Pop CE, Pârvu M, Arsene AL, Pârvu AE, Vodnar DC, Tarcea M, Toiu AM, Vlase L (2017) Investigation of antioxidant and antimicrobial potential of some extracts from Hedera helix L. Farmacia 65:624–629
Rehl RM, Balla AA, Cabay RJ, Hearp ML, Pytynia KB, Joe SA (2007) Mucosal remodeling in chronic rhinosinusitis. Am J Rhinol 21:651–657
Rogerio AP, Kanashiro A, Fontanari C, Da Silva EV et al (2007) Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm Res 56:402–408
Sidor A, Gramza-Michałowska A (2014) Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food—a review. J Funct Foods 18B:941–958
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth Enzymol 299:152–178
Sobol SE, Fukakusa M, Christodoulopoulos P et al (2003) Inflammation and remodeling of the sinus mucosa in children and adults with chronic sinusitis. Laryngoscope 113:410–414
Suh JD, Kennedy DW (2011) Treatment options for chronic rhinosinusitis. Proc Am Thorac Soc 8:132–140
Toiu A, Mocan A, Vlase L, Pârvu AE, Vodnar DC, Gheldiu AM, Moldovan C, Oniga I (2019) Comparative phytochemical profile, antioxidant, antimicrobial and in vivo anti-inflammatory activity of different extracts of traditionally used Romanian Ajuga genevensis L. and A. reptans L. (Lamiaceae). Molecules 24:1597
Ulbricht C, Basch E, Cheung L, Goldberg H, Hammerness P, Isaac R et al (2014) An evidence-based systematic review of elderberry and elderflower (Sambucus nigra) by the natural standard research collaboration. J Diet Suppl 11:80–120
Uslu C, Taysi S, Bakan N (2003) Lipid peroxidation and antioxidant enzyme activity in experimental maxillary sinusitis. Ann Clin Lab Sci 33:18–22
Van Bruaene N, Bachert C (2011) Tissue remodeling in chronic rhinosinusitis. Curr Opin Allergy Clin Immunol 11:8–11
Viapiana A, Wesolowski M (2017) The Phenolic contents and antioxidant activities of infusions of Sambucus nigra L. Plant Foods Hum Nutr 72:82–87
Wang S, Zhang H, Xi Z, Huang J, Nie J, Zhou B et al (2017) Establishment of a mouse model of lipopolysaccharide-induced neutrophilic nasal polyps. Exp Ther Med 14:5275–5282
Yoruk O, Gur FO, Uyanik H, Yasar M, Mutlu V, Altas E et al (2010) Antioxidant effects of Nigella Sativa in the treatment of experimentally induced rhinosinusitis. Maced J Med Sci 3:132–137
Ziegler A, Patadia M, Stankiewicz J (2018) Neurological Complications of Acute and Chronic Sinusitis. Curr Neurol Neurosci Rep 18:5
Author information
Authors and Affiliations
Contributions
Conceptualization of the research was made by CNŢS and GAF. The present study was performed under the supervision of Professor GAF., who was also responsible for project administration, the analysis of the results and the writing-review of the manuscript; CNŢS was involved in the methodology of the research, analysis of the results, and writing of the original draft, with support from SCM ND and RM carried out the experiments and contributed to the sample preparation. DO and IB contributed to the study and the analysis of the data. LD and BM were responsible for the preparation and the characterization of the SN fruit extract. F. and RO performed the histopathological analysis. AM Gheldiu performed HPLC analysis and phytochemical evaluation of the extract. All authors approved the final article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tiboc Schnell, C.N., FILIP, G.A., Decea, N. et al. The impact of Sambucus nigra L. extract on inflammation, oxidative stress and tissue remodeling in a rat model of lipopolysaccharide-induced subacute rhinosinusitis. Inflammopharmacol 29, 753–769 (2021). https://doi.org/10.1007/s10787-021-00805-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-021-00805-y