Abstract
Information on the health benefits of ethanolic extracts obtained from Blutaparon portulacoides stem (EEBP) hasn´t been consistently described in the literature until the present moment. This study investigated the antimycobacterial, anti-inflammatory and toxicological effects of EEBP in models of inflammation/infection, as well as its chemical composition. Chemical analysis of EEBP by electrospray ionization–mass spectrometry/HPLC–MS/MS identified 3,5,3′-Trihydroxy-4′-methoxy-6,7-methylenedioxy-flavone, gomphrenol, ferulic, vanillic, and caffeic acids. The minimum inhibitory concentration of EEBP and isoniazid in the presence of Mycobacterium tuberculosis was 123.4 and 0.030 µg/ml, respectively. EEBP oral administration (p.o.) (300–1000 mg/kg) or dexamethasone subcutaneous injection (s.c.) (1 mg/kg) significantly inhibited leukocytes and proteins resulting from carrageenan-induced pleurisy in Swiss mice. In the BCG-induced pleurisy model, the oral treatments performed once a day for 7 days, with EEBP (30 and 100 mg/kg) and isoniazid (25 mg/kg), inhibited the increase in plasmatic IL-1β levels and in pleural exudate from C57BL-6 mice, and reduced M. tuberculosis growth in organs (colony forming units assays). EEBP (30–300 mg/kg, p.o.) and dexamethasone (1 mg/s.c.) significantly prevented carrageenan-induced oedema and mechanical hyperalgesia in Swiss mice. The treatments (once a day for 22 days) with EEBP (30 mg/kg, p.o.) and dexamethasone (1 mg/s.c.) substantially inhibited oedema and mechanical- and cold-hyperalgesia at 11, 16 and 22 days after the administration of Freund's Complete Adjuvant in C57bL6 mice. No evidence of physio-pathologic was observed in Wistar rats acutely treated with EEBP (2000 mg/kg, p.o.). This study confirms the anti-inflammatory and antibiotic properties of EEBP, opening possibilities for the development of safe new drugs with dual anti-inflammatory/antimycobacterial activities which could be favorable from a pharmacoeconomic perspective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since ancient times, medicinal plants have been widely investigated to validate their health benefits or to develop new products. Some secondary metabolites are obtained from natural products such as plants, microbes, and animals; many of these compounds were first investigated due to their use in folk medicine (Mathur and Hoskins 2017).
The Brazilian National List of Medicinal Plants of Interest to the Unified Health System (RENISUS) was developed in 2009 with the main goal of making herbal remedies derived from plants more widely available (Brasil 2009; Marmitt et al. 2018). Therefore, the investigation of the biological properties of plants used in folk medicine is important not only for drug discovery but also for the public health care system.
Mammals respond with inflammation and pain to infection by pathogens or physical injury as a result of endogenous or exogenous stimuli, and a goal of treatments is to control cellular stress and the inflammatory process (Xiahou et al. 2017). Almost all of the medications currently used to treat chronic inflammation, including nonsteroidal anti-inflammatory drugs (NSAIDs) and glucocorticoids, have a number of adverse side effects. Glucocorticoid drugs are an extraordinarily potent class of medications that are widely used to control inflammation in humans and animals; however, no glucocorticoid analogs have been developed not to cause adverse effects when used for long periods of time (Cain and Cidlowski 2017). Therefore, the investigation of novel anti-inflammatory and antibiotic drugs derived from folk medicine continues to be an important challenge.
Blutaparon portulacoides, also known as “capotiraguá,” “pirrixiu” or “bredo-de-praia” (Siqueira 1987; Bertier et al. 2008) is found in Brazilian northern and southern coastal areas (Pio et al. 2019). B. portulacoides is commonly used in folk medicine to treat leukorrhea and vulvovaginitis. Methylenedioxyflavonol and a mixture of acyl steryl glycosides have been identified and isolated from extracts of B. portulacoides (Ferreira and Dias 2000), and they were evaluated for their activity against Trypanosoma cruzi, Leishmania amazonensis, gram-positive and gram-negative bacteria, and yeasts (Salvador et al. 2002). The ethanolic extract from the aerial parts of B. portulacoides was also tested for its activity against the venom of the snake Bothrops jararacussu, and it was shown to inhibit oedema and leukocyte influx resulting from the exposure to the venom (Pereira et al. 2009).
Leukorrhea and vulvovaginitis could be indicative of the presence of several pathological conditions that involve infection, leukocyte migration, and inflammation. Our groups studied the ability of an extract from B. portulacoides to kill several different types of microorganisms (Salvador et al. 2002) and investigated the use of a crude ethanolic extract from B. portulacoides (EEBP) stems in the treatment of infections caused by Mycobacterium sp. and the prevention of leukocyte migration, inflammation, and pain. In the present study, a model of inflammation was developed based on the investigation of the biological actions of EEBP in vivo.
Material and methods
Plant material, extraction and phytochemical analysis
B. portulacoides (St. Hil) Mears (stem) was collected at Restinga de Marica, Rio de Janeiro (RJ, Brazil, in January 2015), and identified by Prof. J.C. de Siqueira (Pontifical Catholic University of Rio de Janeiro). The registration number in the National System for the Management of Genetic Heritage (SisGen) is A86487C.
A voucher specimen (SPFR-2962) was deposited in the herbarium of the Faculty of Philosophy, Sciences and Letters of Ribeirão Preto, University of São Paulo (FFCLRP/USP). The stems of the plant (1800 g) were dried at 40 °C for 72 h, powdered and exhaustively extracted (maceration, at room temperature), with hexane and ethanol, successively, in the proportion plant powder/solvent 1:2 (w/v). The resulting biomass was filtered, and the solvents were removed in a rotatory evaporator under reduced pressure and temperature below 40ºC, obtaining 4 g of hexanic extract and 88 g of EEBP. The chemical composition of EEBP was analyzed by ESI–MS and HPLC–MS. For ESI–MS fingerprints the extract (1 mg/mL) was diluted in a solution containing 50% (v/v) chromatographic grade methanol, 50% (v/v) deionized water, and 0.5% of ammonium hydroxide (Merck, Darmstadt, Germany). The fingerprinting ESI-MS analyses were performed using UPLC-MS equipment, model ACQUITY TQD (Waters Corporation, Milford, MA, USA). The general conditions were: a source temperature of 100 °C, the capillary voltage of 3.0 kV, and cone voltage of 30 V. ESI-MS was performed by direct infusion using a syringe pump, with a flow rate of 10 μL. min/mL. Structural analysis of single ions in the mass spectra from the EEBP was performed by ESI-MS/MS in negative ion mode. The ion with the m/z of interest was selected and submitted to 15–45 eV collisions with argon in the collision quadrupole. The collision gas pressure was optimized to produce extensive fragmentation of the ion under investigation. The compounds were identified by comparing their ESI-MS/MS fragmentation spectra to fragmentation spectra of authentic standard samples and literature data (Ferreira and Dias 2000; Salvador et al. 2002). The HPLC–MS chromatographic analysis was conducted using a C-18 column (Kinetex, 1.7 μ, 100A, 50 × 2.1 mm). The mobile phase consisted of a linear gradient combining solvent A (water/formic acid, 99:1, v/v) and solvent B (methanol) as follows: 5–100% B (1–9 min), 100% B (9.1–10 min), and 5% B (10.1–12 min). The analyses were carried out in triplicate at a flow rate of 0.2 mL/min with the MS detector (ESI source) and an injection volume of 5 μL. The samples (1 mg/mL) were dissolved in methanol/water (1:1, v/v) and analyzed using the same chromatographic conditions as those for the standards. The general conditions for ESI-MS analysis were: temperature 130 °C, capillary voltage 3.0 kV and cone voltage 30 V. Identification of the chromatographic peak was based on the retention times of the single compound and confirmed by co-injection with an authentic standard, comparing their ESI-MS/MS fragmentation spectra to fragmentation spectra of authentic standard samples and literature data (Ferreira and Dias 2000; Salvador et al. 2002, 2012; Oliveira et al. 2003; Ferreres et al. 2011).
In vitro antibacterial activity
The minimum inhibitory concentration (MIC) values for EEBP (0.98–250 μg/ml) and isoniazid (0.004–1 μg/ml) in the presence of the M. tuberculosis strain H37Rv (ATCC27294) were determined according to the method described by Palomino et al. (2002).
MIC values were determined in the presence of Enterobacter aerogenes (ATCC13048), Salmonella typhimurium (ATCC14028), Staphylococcus saprophyticus (ATCC15305), and Burkholderia cepacia (ATCC25416). Each bacterial strain was incubated in brain–heart infusion broth at 37 °C for 24 h. Subsequently, the cultures were incubated on Mueller–Hinton agar plates 24 h. Each strain was then standardized in a solution of sterile saline (0.9%) according to the McFarland turbidity scale and diluted 1:10. Then, 10 µL was pipetted into a microplate well containing a variable concentration of EEBP in 100 µL and incubated at 37 °C for 24 h, and the optical density was measured at 580 nm (National Committee for Clinical Laboratory Standards 2002; Campos et al. 2014).
Animals
Female and male Swiss and C57BL/6 mice (20–30 g) and female Wistar rats (200–230 g) were provided by the central vivarium at the Federal University of Grande Dourados (UFGD). Animals were kept in polypropylene boxes in a room at the UFGD vivarium, at 22 °C, in the presence of a 12 h light/dark cycle, with food and water provided ad libitum. Approval of the animal protocol was granted by the UFGD ethics committee (32/2015).
For in vivo assays, the EEBP was dissolved in a saline solution containing Tween 80 (1% Tween 80 in 0.9% saline). This solution (1.5 mL) was made by mixing (biomixer QL-901 – Biomex Technology) 50 µL of Tween 80 with 150 mg of EEBP (for 1000 mg/kg) and subsequently the saline solution was added. The animals in the control groups received the vehicle (1% Tween 80 in 0.9% saline).
Firstly, to study dose–response effects, the EEBP was tested in four doses (30, 100, and 300, 1000 mg/kg) in carrageenan-induced pleurisy and three doses (30, 100, and 300) in carrageenan-induced paw inflammation. Since the doses of 30 and 100 mg/kg were effective in reducing some inflammatory parameters, we decided to evaluate these doses in BCG (Bacillus Calmette Guerin – Mycobacterium bovis) induced-pleurisy and CFA-induced paw inflammation.
The dexamethasone was used in the dose of 1 mg/kg (by subcutaneous route (s.c.)) since the literature showed its efficacy as a positive control in reducing inflammatory parameters in carrageenan and CFA models in mice (Kuraoka-Oliveira et al. 2020).
The isoniazid was tested in vivo in the dose of 25 mg/kg by oral route in the BCG-induced pleurisy model, since it was shown that isoniazid reduces M. bovis growth in organs (Nikonenko et al. 2017).
Carrageenan-induced pleurisy
One hour prior to the administration of carrageenan, 48 female Swiss mice were divided into seven groups of six animals; the first group received vehicle (saline 0.9%) orally (p.o.) (control group), while the second, third, fourth and fifth groups received EEBP p.o. at a dose of 30, 100, 300, and 1000 mg/kg, respectively. The positive control group received dexamethasone (1 mg/kg) via s.c. The naive group received 0.9% saline p.o. but did not receive carrageenan via intrathoracic (i.t) injection. Next, 100 μL of either 1% carrageenan or sterile saline (naïve mice only) (Vinegar et al. 1973) was injected i.t.; 4 h later, the animals were euthanized, and the thorax was opened to determine the total leukocyte number and the level of protein in the pleural exudate.
BCG-induced pleurisy
One hour prior to the induction of pleurisy, groups of six female C57BL/6 mice were treated orally with either EEBP (30 or 100 mg/kg), isoniazid (25 mg/kg) or saline solution (0.9%; control group). Pleurisy was induced with 0.1 ml of a suspension of BCG (4 × 105 colony forming units (CFU); Fundação Ataulpho de Paiva, Rio de Janeiro-Brazil) that was injected into the right pleural cavity. The animals were treated once daily for 7 days. The animals were then euthanized via an overdose of sodium thiopental, and the pleural cavity was washed with 1 ml of sterile phosphate-buffered saline. A 50 µL aliquot of the wash buffer was diluted with Evans blue to determine the total number of leukocytes. The sample was then centrifuged, and the supernatant was stored for the later determination of the cytokine levels (including Il-1β) using ELISA. The precipitate was resuspended in 0.5 mL of sterile ultrapure water and 0.1 mL of Ogawa Kudu, and 0.1 mL of the suspension was plated onto 7H11 agar. The spleen and liver were macerated with 1 mL of sterile saline, and 0.1 mL of the suspension was plated on 7H11 agar. The cells were cultured for 60 days at 37 °C in 5% CO2.
Carrageenan paw inflammation model
EEBP was given orally to male Swiss mice at a dose of 30, 100, or 300 mg/kg 60 min prior to the administration of carrageenan (300 µg /paw) subcutaneously in the right paw. The negative control group was administered a 0.9% saline solution p.o., while the positive control group was treated with subcutaneous (s.c.) dexamethasone (1 mg/kg). The left paw of each of the mice was injected with 100 µL of 0.9% saline. The measurement of oedema was made with a plethysmometer (Panlab) at 1, 2, and 4 h after the injection of carrageenan while the mechanical hyperalgesia (Vivancos et al. 2004) and cold sensitivity (Decosterd and Woolf 2000) were assessed at 3 and 4 h after carrageenan injection using a digital analgesimeter (Insight) and an acetone test, respectively. The basal value of the mechanical response from each animal was measured before the carrageenan injection.
CFA test
The experiments were conducted over a 22 day-period using male C57BL/6 mice that received 30 μL of Freund's complete adjuvant (CFA) in an oil suspension containing inactive Mycobacterium tuberculosis via intraplantar injection into the right paw. The mice were distributed into a control group (saline 0.9%, p.o.), EEBP treatment groups (30 and 100 mg/kg, p.o.), and a positive control group (dexamethasone 1 mg/kg, s.c.) every day, once a day. Mechanical sensitivity and cold sensitivity and paw oedema were measured 6, 11, 16 and 22 days after the injection of CFA.
Acute oral physio-pathologic study
This experiment was performed with female Wistar rats and according to OECD 425 guideline. The EEBP was administered by gavage (2000 mg/kg) in a single dose, in 1 animal, fasting for 12 h, which was observed after 30 min, 1, 2, 4, 8 and 24 h. Since there was no sign of physio-pathologic or death, the same administration was repeated in 4 animals which were observed for the same period, subsequently all animals remained under observation for 14 days (OECD 2008; Saleem et al. 2017). The five hippocratic parameters were observed daily: consciousness, motor coordination, reflexes (auditory and corneal), central (ataxia, tremors, sedation, seizure) and autonomic nervous system (piloerection, sialorrhea, cyanosis, ptosis and tearing), as well as body weight, water and food consumption. The animals were euthanized by ketamine and xilasin overdose. Organs such as heart, spleen, lungs, liver and kidneys were removed, macroscopically observed and weighed.
Statistical analysis
The data are presented as the mean ± standard error (SEM). The determination of significant differences between groups was made via an analysis of variance (one-way ANOVA) followed by a post hoc Newman–Keuls test (GraphPad Prism Software). The percentage of inhibition was calculated from the control group. Differences were considered to be significant when P < 0.05.
Results
Phytochemical analysis
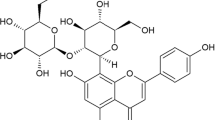
Phenolic acids and flavonoids (aglycones and glycosides) were identified in EEBP by HPLC–DAD/ESI–MS analysis (Fig. 1), which were characterized based on the fragmentation patterns. The identified phenolics include caffeic, ferulic and vanillic acids, and 6,7-methylenedioxyflavone derivatives reported before in the Amaranthaceae species (Ferreira and Dias 2000; Salvador et al. 2002, 2012; Oliveira et al. 2003; Ferreres et al. 2011).
HPLC–MS chromatogram (B) of EEBP. (1) caffeic acid – RT = 0.63 min, ESI–MS: m/z = 179 [M-H]−; (2) ferulic acid – RT = 1.87 min, ESI–MS: m/z = 195 [M-H]−; (3) vanillic acid – RT = 2.50 min, ESI–MS: m/z = 167 [M-H]−; (4) Gomphrenol – RT = 9.60 min, ESI–MS: m/z = 313 [M-H]−; (5) 3,5,3′-Trihydroxy-4′-methoxy-6,7-methylenedioxy-flavone – RT = 7.80 min, ESI–MS: m/z = 343 [M-H]−; (6) Gomphrenol—3- glucoside – RT = 6.23 min, ESI–MS: m/z = 475 [M-H]−; (7) 3,5,3′-Trihydroxy-4′-methoxy-6,7-methylenedioxy-flavone-glucosilated – RT 7.49 min, ESI–MS: m/z = 505 [M-H]−
EEBP antibacterial activity
The MIC value of EEBP in the presence of the M. tuberculosis strain was 123.4 µg/mL, while the MIC value of isoniazid was 0.030 µg/mL. The EEBP was effective against S. typhimurium (MIC = 1000 µg/mL) and B. cepacia (MIC = 1000 µg/mL). However, EEBP was not effective against S. saprophyticus and E. aerogenes.
EEBP inhibited pleurisy induced by carrageenan and inflammation induced by BCG
At a dose of 1000 mg/kg, EEBP significantly reduced the invasion of pleural spaces by leukocytes, with inhibition at 55%, while dexamethasone (1 mg/kg, s.c.) led to 87% inhibition. Doses of 30, 100, and 300 mg/kg of EEBP did not decrease the total leukocytes in pleural exudate (Fig. 2a). The protein exudation was significantly decreased at EEBP doses of 300 (41% inhibition), 1000 mg/kg (54% inhibition) and dexamethasone (58% inhibition) (Fig. 2b) while doses of 30 and 100 mg/kg of EEBP did not decrease the protein exudation. As expected, the naive group was statistically different from the control group (Fig. 2a and b).
Effects of oral administration of EEBP on leukocyte migration a and protein leakage b in the pleurisy induced by carrageenan (cg). The animals received EEBP (30, 100, 300 or 1000 mg/kg, p.o.), vehicle (control) or dexamethasone (DEX, 1 mg/kg, s.c.), and 1h later, an intrathoracic injection of Cg was administered. The naïve group received an intrapleural injection of sterile saline instead of Cg and was also treated with saline solution. Each bar represents the mean ± SEM of 6 animals. The letters a, b and c indicate significant differences between groups according to One-way ANOVA followed by the Newman–Keuls test
Doses of 30 and 100 mg/kg of EEBP did not decrease the invasion of leukocytes into the pleural spaces after intrathoracic injection of BCG, therefore, isoniazid reduced significantly the leukocyte migration to pleura (Fig. 3a). Administration of EEBP (30 mg/kg) led to a significant reduction of 47% of IL-1β levels in pleural exudate compared to control group, while administration of EEBP (100 mg/kg, p.o.) or isoniazid (25 mg/kg, p.o.) reduced IL-1β levels by 70% or 83%, respectively (Fig. 3b). In serum, compared to the values of the control group, the reduction of IL-1β levels was observed to be 60%, 61% and 67% for the administration of 30 and 100 mg/kg EEBP (p.o.) and isoniazid (25 mg/kg, p.o.), respectively (Fig. 3c). The statistical comparison between groups showed that isoniazid group, and 30 and 100 mg/kg of the EEBP groups were different on pleural IL-1β analysis (Fig. 3b) while the statistical comparison of same groups did not differ on plasmatic IL-1β dosage (Fig. 3c). As expected, the naive group was statistically different from the control group (Fig. 3a, b, and c).
Effects of oral administration of EEBP on leukocyte a in the pleurisy, Il-1β levels in blood b, and pleural exudate c induced by BCG. The animals received EEBP (30, or 100, p.o.), vehicle (control) or isoniazid (ISO, 25 mg/kg, p.o.) for 7 days and an intrathoracic injection of BCG was administered since the first day. The naive group received an intrapleural injection of sterile saline instead of BCG and was also treated with saline solution. Each bar represents the mean ± SEM of 6 animals. The letters a, b, c, and d indicate significant differences between groups according to One-way ANOVA followed by the Newman–Keuls test
During cell culture of the pleural lavage samples, there was a decrease in the development of CFUs from those taken from the groups treated with EEBP, with few or no colonies observed after 60 days (Table 1). For samples from the 100 mg/kg EEBP group, only 1 plate had 3 CFU, and for the 30 mg/kg EEBP group, 3 plates had a mean of 5.2 CFU. The samples from the negative control group presented mycobacterial growth in all cultivated plaques, with a mean of 54 CFU after 60 days of culture, while the isoniazid group samples did not show any growth after 60 days, and all plaques were found to be positive for mycobacteria in the presence of Ziehl–Neelsen stain and Ogawa Kudu culture medium (Table 1). Samples of macerated liver and spleen presented similar levels of mycobacterial growth in both the negative control and EEBP treatment groups (data not shown).
EEBP inhibited carrageenan- and CFA-induced paw inflammation in a mouse model
Oedema was reduced at all doses of EEBP at 2 h (with 33%, 33%, and 42% reduction at doses of 30, 100 and 300 mg/kg, respectively) and 4 h (50%, 58%, and 67% reduction at 30, 100 and 300 mg/kg, respectively) after the injection of carrageenan (Fig. 4a–b); however, no oedema reduction was observed 1 h after carrageenan administration (data not shown). All the groups treated with EEBP did not differ statistically among themselves when oedema was analyzed 2 and 4 h after the carrageenan injection (Fig. 4a and b). All the groups treated with EEBP were statistically different from dexamethasone group on the oedema observed at 2 h (Fig. 4a) from the carrageenan injection, however, at 4 h, the dexamethasone group did not exhibit a significative difference in relation to All the groups treated with EEBP (Fig. 4b).
Effect of oral administration of EEBP on the carrageenan (Cg)-induced paw oedema and mechanical hyperalgesia in mice. The animals received EEBP (Naïve, 30, 100, and 300 mg/kg, p.o.), vehicle (control) or dexamethasone (DEX, 1 mg/kg, s.c.), and 1h later, an intraplantar injection of Cg (300μg/paw) was administered. Graphs (a), and b represent the evaluation of the paw oedema at 2, and 4h, respectively, after carrageenan injection while graphs (c), and d represent the evaluation of the mechanical hyperalgesia. The letters a, b, and c indicate significant differences between groups according to One-way ANOVA followed by the Newman–Keuls test
Additionally, after the intraplantar injection of carrageenan, 30 mg/kg EEBP decreased the sensitivity to mechanical stimuli 3 h after administration, and a sensitivity decrease was observed at all doses after 4 h. All doses of EEBP reduced the ability of carrageenan to induce mechanical hyperalgesia (Fig. 4c–d). No significant effect of EEBP in the reduction in cold hyperalgesia was detected (data not shown). As expected, carrageenan-induced inflammation was significantly reduced by the treatment with dexamethasone, as shown by the measurement of some inflammatory parameters (Fig. 4a, b, and d). Therefore, the response to mechanical hyperalgesia from the dexamethasone group did not differ from the control group (Fig. 4c). All the groups treated with EEBP or with dexamethasone did not differ statistically among themselves when mechanical hyperalgesia was analyzed 3 and 4 h after the carrageenan injection. As expected, the basal group was statistically different from the control group (Fig. 4c and d).
Six days after the administration of CFA, sensitivity to mechanical stimuli was inhibited by approximately 60, 63 and 90% by the treatment with EEBP at doses of 30 and 100 mg/kg and dexamethasone (1 mg/kg, s.c.), respectively. After 11 days, the inhibition observed with 30 mg/kg EEBP was 76%; after 16 days, the inhibition observed with 30 mg/kg EEBP was 90%; and 22 days after CFA, the inhibition observed with 30 mg/kg EEBP was 77% (Fig. 5). The the groups treated with EEBP (30 and 100 mg/kg) did not differ statistically among themselves however they were different from dexamethasone group on six days from CFA injection (Fig. 5a). The group treated with 30 mg/kg of EEBP was statistically different from other groups on CFA induced mechanical hyperalgesia at 11, 16 and 22 days (Fig. 5b–d).
Effects of oral administration of EEBP on mechanical hyperalgesia induced by CFA. The animals received EEBP (30, or 100, p.o., everyday, once a day), vehicle (control) or dexamethasone (DEXA, 1 mg/kg, s.c., everyday, once a day) for 6 (a), 11(b), 16 (c), and 22 (d) days. The CFA injection was performed on the first day. Each bar represents the mean ± SEM of 6 animals. The letters a, b, and c indicate significant differences between groups according to One-way ANOVA followed by the Newman–Keuls test
Six days after the administration of CFA, the oedema in the injected paw was not significantly reduced by the treatment with EEBP or treatment with dexamethasone (1 mg/kg, s.c.). However, after 11 days, a reduction of 63% was observed at a dose of 30 mg/kg EEBP compared with the control group while a reduction of 70% was observed with 100 mg/kg of EEBP and the treatment with dexamethasone (1 mg/kg, s.c.) inhibited 62% of paw oedema. After 16 and 22 days, the reduction in oedema was 65% and 80% with 30 mg/kg EEBP treatment, 69 and 80% for treatment with 100 mg/kg of EEBP, and for dexamethasone group the reduction in oedema was 69% and 89%, respectively (Fig. 6). When the statistical analysis was performed, the groups treated with 30 or 100 mg/kg of EEBP as well as the dexamethasone group were not different in relation to the oedema analysis on days 6, 11, 16 and 22 after CFA (Fig. 6a–d).
Effects of oral administration of EEBP on oedema induced by CFA. The animals received EEBP (30, or 100, p.o., everyday, once a day), vehicle (control) or dexamethasone (DEXA, 1 mg/kg, s.c., everyday, once a day) for 6 (a), 11(b), 16 (c), and 22 (d) days. The CFA injection was performed on the first day. Each bar represents the mean ± SEM of 6 animals. The letters a and b indicate significant differences between groups according to One-way ANOVA followed by the Newman–Keuls test
Six days after the intraplantar administration of CFA, cold sensitivity was observed to be inhibited by 80 and 51% at a dose of 30 and 100 mg/kg EEBP while dexamethasone inhibited 63%. After 11, 16 and 22 days, cold sensitivity was observed to be inhibited by 85, 60 and 50%, respectively, at an EEBP dose of 30 mg/kg while a reduction of 45, 57 and 55% was observed at 100 mg/kg EEBP, and a reduction of 29, 62 and 56% was observed at 1 mg/kg (s.c.) dexamethasone (Fig. 7). When the statistical analysis was performed, the groups treated with 30 or 100 mg/kg of EEBP and dexamethasone group were not different in relation to cold hyperalgesia on days 6, 11, 16 and 22 after CFA (Fig. 7a–d).
Effects of oral administration of EEBP on cold hyperalgesia induced by CFA. The animals received EEBP (30, or 100, p.o., everyday, once a day), vehicle (control) or dexamethasone (DEXA, 1 mg/kg, s.c., everyday, once a day) for 6 (a), 11(b), 16 (c), and 22 (d) days. The CFA injection was performed on the first day. Each bar represents the mean ± SEM of 6 animals. The letters a and b indicate significant differences between groups according to One-way ANOVA followed by the Newman–Keuls test
EEBP in acute physio-pathologic test
Although medicinal plants have been widely used by the population, toxicological analysis is essential to ensure safe use. In this experiment, no signs or symptoms of acute and clinical oral physio-pathologic were observed with the use of EEBP.
Discussion
B. portulacoides is a species commonly used in folk medicine in the treatment of leukorrhea and other infections; however, its use for these types of infections has not been previously subjected to scientific investigation. The activities of B. portulacoides against trypanosomiasis, leishmaniasis, B. jararacussu snake venom-induced oedema, and bacteria have been studied in the past (Salvador et al. 2002; Pereira et al. 2009). These infections stimulate a number of inflammatory and pain-related processes, and the present study may support the medicinal use of B. portulacoides in their treatment by demonstrating its anti-inflammatory activity, including its inhibition of leukocyte migration and protein extravasation and its antihyperalgesic and antibacterial effects in vivo and in vitro. The use of B. portulacoides against infection is supported by the fact that NSAIDs and glucocorticoids decrease inflammation and fight infection in manner similar to B. portulacoides.
The folk medicinal use of B. portulacoides extracts in the treatment of vaginitis spurred us to test its effects against other pathogens. Salvador et al. (2002) demonstrated the efficacy of ethanolic extracts derived from both the aerial parts and roots of B. portulacoides, as well as the compounds contained in these extracts, against several microorganisms, especially S. aureus, S. mutans, and S. sobrinus. In the present study, EEBP was effective in inhibiting the growth of S. typhimurium, B. cepacia, and M. tuberculosis. The fact that EEBP was not effective against S. saprophyticus and E. aerogenes showed that EEBP has nonspecific action for all the cells. These three pathogens are of great medical importance and are, therefore, relevant to the testing of EEBP in the treatment of infections. The results reported by Salvador et al. (2002) corroborate the fact that EEBP has antibacterial activity.
EEBP has the most potent effects against M. tuberculosis; however, the development of a treatment model using this agent is difficult since working with M. tuberculosis in laboratory requires high levels of security. BCG was chosen for this study since it is also a component of the Mycobacterium complex that causes tuberculosis (Spargo et al. 1993), however, with a lower counting when compared to M. tuberculosis. Using the BCG model, we observed the expected systemic inflammatory reaction (acute-phase) that resulted from infection with mycobacteria. The EEBP was tested in carrageenan-induced pleurisy with doses of 30 and 100 mg/kg. Since these doses did not inhibit the leukocyte or protein in pleural exudate, the doses of 300 and 1000 mg/kg of EEBP were also tested in this model and these doses induced inhibition of protein accumulation in pleural exsudation while only the dose of 1000 mg/kg inhibited the leukocyte migration to the pleura. The inhibitions induced by EEBP (1000 mg/kg, p.o.) and dexamethasone (1 mg/kg, s.c.) did not differ, showing the same efficacy in reduction of leukocyte and protein in pleural exudate.
In BCG pleurisy model, leukocyte migration to the pleural exudate was not altered with oral doses of 30 and 100 mg/kg of EEBP, therefore, the IL-1β levels in serum and pleural exudate, and CFU were altered with both doses, showing that low doses of EEBP controlled the inflammation and M. Bovis infection. The IL-1β levels in the negative control group (infected) significantly differed from those of the naïve group (uninfected) and isoniazid treatment group after 7 days. Indeed, there was a statistically significant increase in serum IL-1β levels during the course of the infection, and this increase was highly inhibited in the EEBP (30 and 100 mg/kg) treatment groups, demonstrating that EEBP has anti-inflammatory properties. The efficacy of isoniazid in inhibiting IL-1β in the pleural exudate is superior to EEBP (Fig. 3a) when related to the plasmatic levels of IL-1β, in which the EEBP and isoniazid showed the same inhibitory level (Fig. 3b). The reduction in IL-1β levels may be correlated with mycobacterial growth, as previous studies have indicated that mice deficient in either IL-1α or IL-1β showed some reduction in bacterial load 35 days postinfection, with a limited increase in bacterial load 2–3 months postinfection (Bourigault et al. 2013). According to Krishnan et al. (2013), IL-1β secretion is dependent on the presence of viable mycobacteria, which was demonstrated using ELISA and by the growth of CFU in plaques. In addition to its effects on BCG growth in pleural, liver, and spleen tissues ex vivo, EEBP was able to inhibit the formation of CFUs, demonstrating its antibacterial and/or bacteriostatic effects (Table 1 and Fig. 3). However, EEBP was not able to reduce the leukocyte infiltration that was induced by BCG.
An analysis of the composition of EEBP revealed the presence of caffeic acid, ferulic acid, vanillic acid, gomphrenol, gomphrenol-3-glucoside, and 3,5,3′-trihydroxy-4′-methoxy-6,7-methylenedioxyflavone (as well as its glycosylated form). Caffeic acid (Maresca et al. 2013; Dey et al. 2015) and ferulic acid (Maresca et al. 2013) are effective against M. tuberculosis. To study the general effects of EEBP on inflammation, carrageenan was injected in the pleural cavity of the lung, and it was shown that EEBP inhibited the infiltration of total leukocytes and the extravasations of protein (Fig. 2). This result demonstrated the anti-inflammatory effects of EEBP and revealed a possible effect on vasodilation. Studies performed by Pereira et al. (2009) showed that the essential oil derived from B. portulacoides significantly reduced oedema induced by a myotoxin. Other studies conducted by our group demonstrated the antiedematogenic activity of topically applied EEBP in response to inflammation induced in mice using croton oil (Marson et al. 2009). In vitro, The MIC result for isoniazid (0.03 ug/mL) is similar to the one described previously observed by Genestet et al. (2017) however the concentration of isoniazid is 0.125 ug/mL to inhibit M. tuberculosis found intracellularly in macrophages (granulomas) (Teskey et al. 2018). For in vivo studies, the antimicobacterial dose used of isoniazid is 25 mg/kg (Cynamon et al. 1999; Nikonenko et al. 2017) which is not statistically different from the antimycobacterial dose of EEBP that was 30 mg/kg. Zhou et al. (2013) showed that with or without Pyridoxine, the absorption of isoniazid is up to 95% in rats however the use of high doses of isoniazid could be explained by the low lipophylicity of isoniazid. To treat tuberculosis patients high doses of isoniazid must penetrate the waxy granulomas and also the latent M. tuberculosis which lost the typical cell wall (Khan et al. 2019). These results are of interest, as they demonstrate that EEBP has anti-inflammatory effects and is able to inhibit the growth of BCG or M. tuberculosis in vitro and ex vivo in the lung pleura, liver, and spleen, with results similar to those of isoniazid.
Using a model of acute inflammation induced by the injection of carrageenan into the paw, EEBP was shown to inhibit oedema and mechanical hyperalgesia at doses of 30, 100 and 300 mg/kg by a single oral administration. The EEBP treated groups with 30, 100, and 300 mg/kg did not differ statistically in relation to oedema and mechanical hyperalgesia on carrageenan paw inflammation (Fig. 4a–d). And oedema is characterized by the abnormal accumulation of interstitial fluid in the extracellular compartment and can be analyzed as an inflammatory parameter. EEBP is more potent in its inhibition of oedema formation than it is for inhibiting leukocyte accumulation at the sites of inflammation. EEBP also inhibited mechanical hyperalgesia at all doses tested (30, 100, and 300 mg/kg), and its maximal efficacy was achieved (Fig. 4). The EEBP treated groups with 30, 100, and 300 mg/kg and also treated with dexamethasone did not differ statistically in relation to oedema and cold hyperalgesia on days 6, 11, 16 and 22 days after CFA paw injection (Figs. 6a–d and 7a–d). Greater efficacy of 30 versus 100 mg/kg of EEBP is observed in cold hyperalgesia induced by CFA, mechanical hyperalgesia induced by carrageenan and by CFA, showing that EEBP did not act in a dose-dependent manner. It is uncommon since the extract effects are usually dose dependent.
Mechanical hyperalgesia is an indicator of inflammation-induced pain arising from peripheral sensitization (Andrew and Greenspan 1999). After tissue injury, the process of peripheral sensitization is triggered by mediators that include cytokines TNF and IL-1β, which promotes the synthesis of other cytokines such as NO, chemokines, and kinins.
EEBP contains caffeic acid (Choudhary et al. 2016; Choi et al. 2017) and ferulic acid (Liu et al. 2017; Lampiasi and Montana 2018), both of which have effects on the interruption of IKKβ-inducible NF-κB activation and the NF-κB-regulated expression of TNF-α and IL-1α (Choi et al. 2017). Yrbas et al. (2015) showed that vanillic acid, another component of EEBP, may play a role in controlling pain by interfering with the activity of ASICs (acid-sensing ion channels) and TPRV1, TRPA1, and TRPM8 receptors. Carrageenan induces the typical symptoms of inflammation (Morris 2003) by increasing the levels of several inflammatory mediators, such as histamine, serotonin, kinins, and prostaglandins, and by increasing NO release (Di Rosa et al. 1971; Posadas et al. 2004). According to the results of this study, EEBP reduced the formation of oedema after 4 h, which could suggest that the treatment acts on the components involved in the production of prostaglandins. Therefore, the existing studies of the mechanisms underlying the control of inflammation or pain by compounds found in EEBP are difficult to interpret, however, it is possible to conclude that EEBP is effective against oedema and peripheral sensitization induced by inflammation.
The development of safe new drugs with dual anti-inflammatory and antimycobacterial activities could favour the patient compliance and to bring a new perspective to the pharmacoeconomics (Cholo et al. 2012; Tseng et al. 2017). One example is the anti-leprosy drug clofazimine that is a dual anti-inflammatory and antimycobacterial drug (Cholo et al. 2012). The candidates for dual activities observed by EEBP are caffeic and ferrulic acid because they are the most studied compounds showing anti-inflammatory (Choi et al. 2017) and antimycobacterial properties (Maresca et al. 2013; Dey et al. 2015). Caffeic acid and ferrulic acid are very similar compounds and their mechanism of action was investigated trying to indicate the pharmacophore group (Andrade et al. 2015). The results indicate the EEBP as a new dual anti-inflammatory or/and anti-mycobacterial agent, therefore, the compound(s) responsible(s) for the dual action must be studied.
No evidence of physio-pathologic was observed in the Wistar rats treated with only one dose of EEBP (2000 mg/kg, p.o.). Thus, we can imply that the EEBP has low physio-pathologic and the Lethal Dose 50 (LD50) is above 2000 mg/kg.
To verify the effects of the EEBP treatment after 21 days, persistent paw inflammation was induced by the injection of an oil suspension with killed M. tuberculosis (CFA model). Using this model, it was possible to evaluate the effects of the EEBP on oedema, mechanical sensitivity, and hyperalgesia. The persistent oral administration of EEBP inhibited oedema, mechanical hyperalgesia and cold sensitivity induced by CFA. These observations showed that EEBP exhibited anti-inflammatory and antihyperalgesic effects against inflammation induced by M. tuberculosis throughout the course of the treatment.
Conclusion
EEBP showed antiinflammatory/antimycobacterial properties in experimental animal models and in vitro tests contributing, at least in part, to show a potential candidate to develop new antibiotic/antiinflammatory drugs. EEBP chemical composition showed the presence of several compounds such as caffeic and ferrulic acid which are the most studied compounds showing anti-inflammatory and antimycobacterial properties. Therefore, the compound(s) responsible(s) for the dual EEBP action must be studied. Low doses of EEBP controlled the inflammation and M. Bovis infection since it reduced the IL-1β levels in serum and pleural exudate, and also inhibited the M. bovis growth (CFU) in cultures obtained from the pleural wash. Low doses of EEBP also reduced cold hyperalgesia, mechanical hyperalgesia, and oedema induced by carrageenan or killed M. tuberculosis (CFA). Therefore, the EEBP did not act in a classical dose-dependent manner.
References
Andrew D, Greenspan JD (1999) Mechanical and heat sensitization of cutaneous nociceptors after peripheral inflammation in the rat. J Neurop 82:2649–2656
Andrade M, Benfeito S, Soares P et al (2015) Fine-tuning of the hydrophobicity of caffeic acid: studies on the antimicrobial activity against Staphylococcus aureus and Escherichia coli. Roy Soc Chem Advanc 5:53915–53925
Bertier RM, Dias RVC, Batista JS, Soto-Blanco B (2008) Intoxicação por pirrixiu (Blutaparon portulacoides) em ovinos. Arq Inst Biol 99–102
Bourigault M-L, Segueni N, Rose S et al (2013) Relative contribution of IL-1α, IL-1β and TNF to the host response to Mycobacterium tuberculosis and attenuated M. bovis BCG. Immun Inflamm Dis 1:47–62
Brasil. Ministério da Saúde (2009) Portal da Saúde. Programa Nacional de Plantas Medicinais e Fitoterápicos. https://portalsaude.saude.gov.br/images/pdf/2015/janeiro/05/ programa-nacional-plantas-medicinais-fitoter--picos-pnpmf.pdf
Cain DW, Cidlowski JA (2017) Immune regulation by glucocorticoids. Nat Rev Immunol 17:233–247
Campos F, Santos UP, Macorini LFB et al (2014) Antimicrobial, antioxidant and cytotoxic activities of propolis from Melipona orbignyi (Hymenoptera, Apidae). Food Chem Toxicol 65:374–380
Choi JH, Park SH, Jung JK et al (2017) Caffeic acid cyclohexylamide rescues lethal inflammation in septic mice through inhibition of IκB kinase in innate immune process. Sci Report 41180
Cholo MC, Steel HC, Fourie PB et al (2012) Clofazimine: Current status and future prospects. J Antimicrob Chemotherap 67:290–298
Choudhary S, Mourya A, Ahuja S et al (2016) Plausible anti-inflammatory mechanism of resveratrol and caffeic acid against chronic stress-induced insulin resistance in mice. Inflammopharmacol 24:347–361
Curfs JH, Meis JFJ, Hoogkamp-Korstange A (1997) A primer on cytokines: sources, receptors, effects, and inducers. Clinic Microb Rev 10:742–780
Cynamon MH, Zhang Y, Harpster T, Cheng S, DeStefano MS (1999) High-dose isoniazid therapy for isoniazid-resistant murine Mycobacterium tuberculosis infection. Antimicrob Agents Chemother 43(12):2922–2924
Decosterd I, Woolf CJ (2000) Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87(2):149–158
Dey D, Ray R, Hazra B (2015) Antimicrobial activity of pomegranate fruit constituents against drug-resistant Mycobacterium tuberculosis and β-lactamase producing Klebsiella pneumoniae. Pharm Biol 53(10):1474–1480
Di Rosa ML, Giroud JP, Willoughby DA (1971) Studies of the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol 104(1):15–29
Ferreira EO, Dias DA (2000) A methylenedioxyflavonol from aerial parts of Blutaparon portulacoides. Phytochemistry 53(1):145–147
Ferreres F, Gil-Izquierdo A, Valentão P, Andrade PB (2011) Structural characterization of phenolics and betacyanins in Gomphrena globosa by high-performance liquid chromatography-diode array detection/electrospray ionization multi-stage mass spectrometry. Rapid Commun Mass Spectrom 25(22):3441–3446
Genestet C, Ader F, Pichat C et al (2017) Assessing the combined antibacterial effect of isoniazid and rifampin on four Mycobacterium tuberculosis strains using In Vitro experiments and response-surface modeling. Antimicrob Agents Chemother 62(1):e01413–e1417
Khan SR, Manialawy Y, Siraki AG (2019) Isoniazid and host immune system interactions: A proposal for a novel comprehensive mode of action. Br J Pharmacol 176(24):4599–4608
Krishnan N, Robertson BD, Thwaites G (2013) Pathways of IL-1β secretion by macrophages infected with clinical Mycobacterium tuberculosis strains. Tuberc (Edinb) 93(5):538–547
Kuraoka-Oliveira AM, Radai JAS, Leitão MM, Lima Cardoso CA, Silva-Filho SE, Leite Kassuya CA (2020) Anti-inflammatory and anti-arthritic activity in extract from the leaves of Eriobotrya japonica. J Ethnopharmacol 249:112418
Lampiasi N, Montana G (2018) An in vitro inflammation model to study the Nrf2 and NF-κB crosstalk in presence of ferulic acid as modulator. Immunobiology 223:349–355
Liu YM, Shen JD, Xu LP et al (2017) Ferulic acid inhibits neuro-inflammation in mice exposed to chronic unpredictable mild stress. Int Immunopharmacol 45:128–134
Maresca A, Vullo D, Scozzafava A et al (2013) Inhibition of the β-class carbonic anhydrases from Mycobacterium tuberculosis with carboxylic acids. J Enzyme Inhib Med Chem 28(2):392–396
Marmitt DJ, Bitencourt S, Silva ADCE et al (2018) The healing properties of medicinal plants used in the Brazilian public health system: a systematic review. J Wound Care 27(Sup6):S4–S13
Marson CV, Boas EV, Zamuner S, Salvador MJ (2009) Preparo de uma formulação tópica e avaliação antiinflamatória do extrato Blutaparon portulacoides. In: XIII Encontro Latino de Iniciação Científica, IX Encontro Latino Americano de Pós Graduação e III Encontro Latino de Iniciação Científica Junior
Mathur S, Hoskins C (2017) Drug development: Lessons from nature. Biomed Report 6:612–614
Morris CJ (2003) Carrageenan-induced paw edema in the rat and mouse. Methods Mol Biol 225:115–121
National Committee for Clinical Laboratory Standards (NCCLS) (2002) Performance standards for antimicrobial susceptibility testing. Inf Supplements. 100–112
Nikonenko BV, Bocharova IV, Korotetskaya MV, Averbakh MM, Apt AS (2017) Efficacy of isoniazid therapy in mice with different genetic susceptibility to infection. Mycobact Dis 7(4):1–4
OECD (2008) Organisation for Economic Co-operation and Development In: CHEMICALS, O. G. F. T. T. O. (Ed.). Guideline 425: Acute Oral Toxicity – Up-and-Down-Procedure (UDP), Paris: Head of Publications Service, 27
Oliveira DBD, de Almeida AP, Gabriela L et al (2003) First isolation of a symmetrical glycosylated methylene bis flavonoid. Planta Med 69(04):382–384
Palomino JC, Martin A, Camacho M et al (2002) Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 46(8):2720–2722
Pereira IC, Barbosa AM, Salvador MJ et al (2009) Anti-inflammatory activity of Blutaparon portulacoides ethanolic extract against the inflammatory reaction induced by Bothrops jararacussu venom and isolated myotoxins BTHTX-I and II. J Venom Anim Toxins incl Trop Dis 15(3):527–545
Pio IDSL, Lavor AL, Damasceno CMD et al (2019) Traditional knowledge and uses of medicinal plants by the inhabitants of the islands of the São Francisco river, Brazil and preliminary analysis of Rhaphiodon echinus (Lamiaceae). Braz J Biol 79:87–99
Posadas I, Bucci M, Roviezzo F et al (2004) Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Br J Pharmacol 142(2):331–338
Salvador MJ, Ferreira EO, Pral EM et al (2002) Bioactivity of crude extracts and some constituents of Blutaparon portulacoides (Amaranthaceae). Phytomedicine 9(6):566–571
Salvador MJ, Andreazza NL, Pascoal ACRF et al (2012) Bioactive chemical constituents and biotechnological production of secondary metabolites in Amaranthaceae Plants. Bentham Science Publishers Ltd, Gomphreneae Tribe. Benth
Saleem U, Amin S, Ahmad B et al (2017) Acute oral toxicity evaluation of aqueous ethanolic extract of Saccharum munja Roxb. roots in albino mice as per OECD 425 TG. Toxicol Reports 31:580–585
Siqueira JC (1987) Importância alimentícia e medicinal das amarantáceas do Brasil. Act Biologica Leopold 09:99
Spargo CA, Haaland PD, Jurgensen SR et al (1993) Chemiluminescent detection of strand displacement amplified DNA from species comprising the Mycobacterium tuberculosis complex. Mol Cell Probes 7(5):395–404
Teskey G, Cao R, Islamoglu H et al (2018) The synergistic effects of the glutathione precursor, NAC and first-line antibiotics in the granulomatous response against Mycobacterium tuberculosis. Front Immunol 9:2069
Tseng CH, Tung CW, Wu CH et al (2017) Discovery of Indeno[1,2-c]quinoline derivatives as potent dual antituberculosis and anti-inflammatory agents. Molecules 22:1–15
Vinegar R, Truax JF, Selph JL (1973) Some quantitative temporal characteristics of carrageenin-induced pleurisy in the rat. Proc Soc Exp Biol Med 143(3):711–714
Vivancos GG, Verri WA, Cunha TM et al (2004) An electronic pressure-meter nociception paw test for rats. Braz J Med Biol Res 37(3):391–399
Xiahou Z, Wang X, Shen J et al (2017) NMI and IFP35 serve as proinflammatory DAMPs during cellular infection and injury. Nat Commun 8(1):950
Yrbas ML, Morucci F, Alonso R, Gorzalczany S (2015) Pharmacological mechanism underlying the antinociceptive activity of vanillic acid. Pharmacol Biochem Behav 132:88–95
Zhang JM, An J (2007) Cytokines, inflammation and pain. Int Anesthesiol Clin 45:27–37
Zhou Y, Jiao Y, Wei YH et al (2013) Effects of pyridoxine on the intestinal absorption and pharmacokinetics of isoniazid in rats. Eur J Drug Metab Pharmacokinet 38(1):5–13
Acknowledgements
The authors would like to thank National Council for Scientific and Technological Development (CNPq), Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Mato Grosso do Sul (FUNDECT), and Coordination for the Improvement of Higher Education Personnel (CAPES), Fund for Support to Teaching, Research and Outreach Activities of University of Campinas (FAEPEX-UNICAMP).
Funding
This work was funded by FAPESP- Fundação de Amparo à Pesquisa do Estado de São Paulo (Proc. 15/03,726–8 and 06/06,079–4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) confirm that this article content has no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kassuya, R.M., Radai, J.A.S., Macorini, L.F.B. et al. Blutaparon portulacoides ethanolic extract reduced IL-1β and inflammatory parameters induced by the Mycobacterium complex and carrageenan in mice. Inflammopharmacol 29, 439–450 (2021). https://doi.org/10.1007/s10787-020-00752-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-020-00752-0