Abstract
Sea cucumber, Holothuria scabra, is an echinoderm marine animal that has long been used as a traditional therapeutic in various diseases due to its chemical composition and protein enrichment. Many researchers have extensively studied the efficacy of sea cucumber extracts for many health benefits in recent years. Inflammation is a complex process involved in pro-/anti-inflammatory cytokine products. However, the role of the H. scabra extracts in anti-inflammation and its molecular regulations has not been apparently elucidated yet. In this study, we investigated the anti-inflammatory effect of H. scabra extracts by using lipopolysaccharide (LPS) from E. coli to induce an inflammatory response in RAW264.7 macrophage. It was found that ethyl acetate fraction of H. scabra extracts (EAHS) inhibited pro-inflammatory cytokines synthesis at both the transcriptional and translational levels, notably nitric oxide (NO), inducible nitric oxide synthase (iNOS), interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and prostaglandin E2 (PGE2). In addition, EAHS was able to downregulate IκB/NF-κB, and JNK expressions. These effects may be influenced by high contents of phenolic compound and triterpene glycosides in EAHS. Therefore, EAHS might have the potential to be developed as a natural anti-inflammatory agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Inflammation is one of the most important biological processes which can be triggered by pathogen invasion, tissue damaged, and/or irritation. Lipopolysaccharide (LPS)-induced nitric oxide production in RAW264.7 macrophage cell line is a well-known model that used to investigate the effects of interest compounds in the inflammatory process (Stone et al. 2006). This is because monocytes/macrophages are the main players in mediating inflammatory response by releasing various types of secretion including reactive-oxygen species (ROS), reactive nitrogen species (i.e., nitric oxide-NO), as well as pro-inflammatory cytokines such as interleukin 1β (IL-1β), tumor necrosis factor α (TNF-α), prostaglandin E2 (PGE2), and so on. The continuous progression of inflammation and the release of cytokines lead to chronic inflammation which is the key step in developing various diseases. These include wound healing, rheumatoid arthritis, bowel diseases, central nervous system inflammation, cardiovascular disease, cancers, etc. (Aoki and Narumiya 2012; Sharma et al. 2007). Therefore, manipulation of the inflammation mechanism is one of the vital steps which is beneficial to be used as therapeutic targets.
Sea cucumbers, Holothuria scabra, have been found in the coastal of Indo-Pacific, including Thailand. Currently, the local market consumption is continuously increased because it is generally regarded that the sea cucumbers have high chemical compositions, nutritional qualities, and medicinal values. Extensive studies have indicated that extracts of sea cucumber contained varieties of bioactive compounds, especially triterpene glycosides (saponin), chondroitin sulfates, glycosaminoglycan (GAGs), sulfated polysaccharides, sterols (glycosides and sulfates), phenolic, glycosphingolipids, and proteins (Dyshlovoy et al. 2016; Yu et al. 2015). Moreover, these compositions are relevant with many therapeutic applications, containing anti-fungal, anti-cancer, anti-angiogenesis, anticoagulant, antioxidant, and so on (Aminin et al. 2015; Nguyen et al. 2017; Park et al. 2011). With regard to inflammation in the animal study, the previous report demonstrated that the oral sea cucumber food supplement in rat had some synergistic effect with the PGE1 analogue and could suppress the inflammation by Carrageenan-induced paw oedema assay (Whitehouse and Fairlie 1994). However, information of the anti-inflammatory properties and the active compounds has remained unclear.
In the present study, we aim to elucidate the effects of Holothuria scabra extracts on anti-inflammation process and its related molecular pathways by measuring the NO and pro-inflammatory cytokines (IL-1β and TNF-α) expressions at the level of either transcription or translation. Furthermore, the effects of the sea cucumber extracts on many inflammatory pathways, such as NF-κB, JNK, p38, and STAT3, were also investigated.
Materials and methods
Materials and reagents
Murine macrophages RAW264.7 were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). Roswell Park Memorial Institute medium (RPMI 1640), penicillin/streptomycin, fetal bovine serum (FBS), and phosphate-buffered saline (PBS) were purchased from Gibco BRL (Life Technologies Inc., Grand Island, NY, USA). Lipopolysaccharide from E. coli, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Folin–Ciocalteau and Griess reagents were purchased from Sigma Aldrich (St. Louis, MO, USA). TRIZol™ reagent was purchased from Invitrogen (Carlsbad, CA, USA). Mouse cytokine enzyme-linked immunoabsorbent assays (IL-1β, TNF-α, and PGE2) were purchased from R&D Systems Inc. (Minneapolis, MN, USA). PathScan® Inflammation Multi-target Sandwich ELISA kit and cell lysate buffer were purchased from Cell Signaling Technology (Beverly, MA, USA). Other chemicals used were of analytical grade.
Sea cucumber extraction
Sea cucumbers, H. scabra, were collected from Coastal Fisheries Research and Development Centre, Prachuap Khiri Khan Province, Thailand. The sea cucumbers were cut into small pieces. Samples were then lyophilized and fractionated by sequential liquid–liquid extraction to obtain the three fractions of the H. scabra which were hexanes (HEHS), ethyl acetate (EAHS), and n-butanol (BUHS). These extracts were concentrated by rotary evaporator to dryness and kept at − 20 °C until further analysis. To elucidate the chemical compositions of H. scabra extracts, proton nuclear magnetic resonance (1H-NMR) was performed. Concisely, each extracted fraction was diluted with deuterated NMR solvent in the NMR tube. The 1H-NMR was recorded on a Bruker Ascend™ 400 MHz, or Bruker AVANCE 500 MHz in CDCl3 using tetramethylsilane (TMS) as an internal standard, unless otherwise instructed.
Total phenolic content analysis
The total phenolic content of all the three extracts of the sea cucumbers were evaluated by Folin–Ciocalteau’s method (Himaya et al. 2010). Briefly, 100 μl of 10 mL of each fraction was mixed in 0.5 mL Folin–Ciocalteau reagent, followed by the addition of 2 mL of 20% (W/V) sodium carbonate. The mixtures were incubated in the dark condition for 90 min. At indicated time, 200 μl of each mixture was then transferred to 96-well plates. The absorbance of the mixture was measured at 750 nm using a microplate reader (ELx808™, Biotek). Analysis of all samples was performed in triplicate and compared with the gallic acid standard curve (0.001–10 mg/mL).
Cell culture
Murine macrophage cell lines, RAW264.7, were cultured in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin. Cells were maintained at 37 °C, 5% CO2 atmosphere incubator. The cells were then subcultured twice a week.
Cell viability assay
Cell viability was investigated by MTT assay (van Meerloo et al. 2011). In brief, RAW264.7 cells were pre-incubated overnight in 96-well plates at a density of 1 × 105 cells/well. After 24 h of incubation, the adherent cells were treated with each of the sea cucumber extracts (HEHS, EAHS, BUHS) in various concentrations (1–100 µg/mL) for 24 h. A 10-µl aliquot of MTT was added and incubated at 37 °C for 3 h. Supernatants were then removed. The formazan crystal was dissolved in acidified isopropanol (0.1 M hydrochloric in isopropanol). The absorbance was measured at 540 nm using a microplate reader (ELx808™, Biotek). The viability of cell was calculated and compared with the untreated control group. With this result, the fraction that showed the highest potential (cell viability > 85%) was selected for subsequent nitric oxide measurement experiment.
Nitric oxide determination
Macrophages were seeded as 1 × 105 cells/well and incubated overnight. Nitric oxide (NO) productions were stimulated with 1 µg/mL of LPS and followed by treatment of the sea cucumber extracts with the indicated concentration for 24 h. About 100 µl of supernatants were used to measure NO level with 100 µl Griess reagent (1% sulfanilamide in 5% phosphoric acid dissolved with 0.1% N-1-naphthylenediamine dihydrochloride) (Stone et al. 2006). The absorbance value was read at 540 nm using a microplate reader (ELx808™, Biotek). Nitrite concentrations were calculated from standard concentration of sodium nitrite dissolved in RPMI 1640.
Cytokines enzyme-linked immunosorbent assay (ELISA)
Effects of the sea cucumber extract on pro-inflammatory cytokines (IL-1β, TNF-α, and PGE2) were determined using ELISA kits from R&D Systems as describes in manufacturer’s protocol. Briefly, RAW264.7 cells were treated with various concentrations of the extracts, and then with or without LPS for 24 h. Each culture supernatant (200 μl) was collected and incubated with the coated plate at room temperature for 2 h. After washing the plate 3 times, 100 μl of desired antibodies were added in each well and incubated at room temperature for 2 h. Then, the treated plate was incubated with peroxidase-conjugated secondary antibody for 2 h. After treatment, the substrate solution was added and incubated for 30 min, followed by adding stop reagent. Ultimately, the absorbance was monitored at 450 nm using a microplate reader (ELx808™, Biotek).
RNA preparations and reverse transcription-polymerase chain reaction (RT-PCR) analysis
The expressions of IL-1β, TNF-α, and iNOS transcripts were measured by RT-PCR. RAW264.7 macrophage cell line was plated onto a 6-well plate prior to reaching confluence of 1 × 106 cells/well. The cell was consequently induced with LPS and treated with indicated extracts for 24 h. Total RNA was extracted by using TRIZol™ reagent. The RNA product was quantified by measuring the absorbance at 260 nm. Then, 1 μg of RNA was reversed into complementary DNA (cDNA). GADPH was served as an internal control. Primer sequences were used as shown in Table 1. The mRNA products were analysed on a 1.5% agarose gel electrophoresis and stained in ethidium bromide prior to visualization using a UV transilluminator. The intensity of the band was quantitated by using ImageJ software (National Institute of Health, Bethesda, MA, USA) and normalized to the mean of GAPDH signal intensity.
Screening inflammatory pathway by ELISA
After treated with the sea cucumber extracts, the cells were washed with cold PBS and lysate with 1 mM phenylmethylsulfonyl fluoride (PMSF) in 1X cell lysate buffer. Lysates were centrifuged for 10 min at 4 °C. The supernatants were transferred and stored at − 80 °C until analysis. To study the inflammatory pathways that involved with the inhibitory effect of the sea cucumber extracts, PathScan® Inflammation Multi-target Sandwich ELISA Kit was utilized. According to the manufacturer's recommendations, cell lysates were incubated in ELISA-coated plate overnight. After washing the plate with PBS, the treated plate was incubated with detecting primary antibody at 37 °C for 1 h, followed by horseradish peroxidase (HRP)-linked secondary antibody at 37 °C for 30 min. Then, colour development was detected by the addition of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate. The absorbance value at 450 nm was read using a microplate reader (ELx808™, Biotek). The expressions of indicated molecular cascades were evaluated by comparing with untreated control.
Statistical analysis
All experiments were repeated at least three times. Data were analysed using GraphPad Prism5® software. The results were presented as means ± S.D. One-way ANOVA followed by Bonferroni tests were performed to examine the difference between each group. A p < 0.05 was considered as statistically significant.
Results
Cytotoxicity effects of the sea cucumber extracts
MTT assay was performed to determine the cytotoxicity effects of H. scabra extracts, using hexane (HEHS), ethyl-acetate (EAHS), and n-butanol (BUHS) fractions, at various concentrations (1, 10, 50 and 100 µg/mL). As shown in Fig. 1, all three fractions showed no significant differences cytotoxicity effects to RAW264.7 macrophage cell line (p < 0.05) as compared with the untreated group. Obviously, EAHS and BUHS depicted markedly effect to promote cell proliferation. Based on our preliminary results of cell viability assay, all sample concentrations were chosen for further studies.
Effects of H. scabra extracts on cell viability. Cell were induced with LPS and treated with each of the extracts, which were hexane (HEHS), ethyl-acetate (EAHS), and n-butanol (BUHS), using various concentrations as indicated for 24 h. MTT assay was performed to examine the cell viability. Values present as the mean ± S.E.M. of three independent experiments. *p < 0.05 vs. control group
Effects of EAHS on anti-inflammatory mediators in LPS-induced murine macrophage model
To examine the effects of H. scabra extracts on inflammatory mediators, macrophages were induced with LPS. The NO assay indicated that EAHS and BUHS fractions played a significant role in the inhibition of nitric oxide production in a dose-dependent manner when compared with the positive control (Fig. 2a). In contrast, HEHS did not clearly reduce nitric oxide production even at the high dosage. Moreover, the IC50 of EAHS and BUHS based on percentage of NO inhibition were shown as 9.44 ± 0.21 and 14.11 ± 0.46 µg/mL, respectively (Fig. 2b). Thus, EAHS was selected to examine further the effect on anti-inflammation activity.
Effects of EAHS on inflammatory mediators. a After incubation of the cells with the sea cucumber extracts, the concentration of nitrite was determined using NO assay compared with standard curve of sodium nitrite (NaNO2) from 0–100 μM. b NO assay was evaluated in term of % inhibiton which indicated the ability of the extracts on nitric oxide inhibition by comparing with control group. c The mRNA level of iNOS expression after treated with EAHS for 24 h. d ELISA technique manifested the expression of PGE2 after treated the LPS-induced macrophage cell line with EAHS. The values correspond with mean ± S.E.M. of three dependent experiments. Statistically significant was *p < 0.05 when compared with the control group
The inflammatory mediators such as iNOS and PGE2 were then further investigated. Upon treatment with EAHS (10 µg/mL), cells showed a significant reduction of iNOS expression (p < 0.05) as evidenced in Fig. 2c. Level of PGE2 expression was remarkably Decreased during LPS stimulation (p < 0.05) (Fig. 2d). Therefore, EAHS has potential to downregulate pro-inflammatory mediators of related enzymes, in this case NO/iNOS, and PGE2.
Effects of EAHS on TNF-α and IL-1β mRNA expressions and productions
To further investigate the anti-inflammatory effects of EAHS in LPS-induced RAW264.7 cells, the mRNA expression and secretion levels of pro-inflammatory cytokine were determined by RT-PCR and ELISA kit, respectively. As presented in Fig. 3a–c, the mRNA levels of TNF-α and IL-1β were increased after LPS stimulation. EAHS significantly inhibited the mRNA expressions of IL-1β and TNF-α. Likewise, the EAHS fraction also exhibited in the same manner that could suppress IL-1β and TNF-α at translational level as compared with control (Fig. 3d, e). The IL-1β secretion was slightly decreased in the presence of 5 and 10 µg/mL EAHS (p < 0.05). Distinctly, EAHS at the concentrations of 1, 5 and 10 µg/mL obviously decreased the expression of TNF-α (p < 0.05) (Fig. 3e). Therefore, EAHS may play a role in anti-inflammation via the suppression of IL-1β and TNF-α at the transcription and translation levels.
The effects of EAHS on the LPS-induced pro-inflammatory cytokines expression. a–c mRNA expressions of IL-1β and TNF-α detected by PCR. d and (e) protein expressions of IL-1β and TNF-α after treated with EAHS detected by ELISA. The reported values are the mean ± S.E.M. (n = 3). *p < 0.05 indicates a significant difference from the control group
Effect of EAHS on the inflammatory pathway
There are several molecular pathways that mediate inflammatory response, e.g. NF-κB, MAPK, and STAT pathways (Kaminska 2009; Lawrence 2009). The nuclear factor NF-κB is considered as a pro-inflammatory regulator which contributes substantially to the production of pro-inflammatory cytokines. In this study, we used the ELISA kit to examine whether EAHS plays a role in NF-κB pathway. As presented in Fig. 4a–c, EAHS was able to reduce phosphorylation of NF-κB (pNF-κB) and IκB (pIκB) significantly (p < 0.05) but did not alter the total level of NF-κB. Similarly, EAHS significantly inhibited the phosphorylation of JNK (pJNK) which is another stress response pathway (Fig. 4d). However, there were no significant suppressions on phosphorylation of p38 and STAT3 (Fig. 4e–f). Hence, EAHS mediates inhibition of inflammatory response via IκB/NF-κB and JNK pathways.
Effects of EAHS on inflammatory pathways in LPS-induced RAW264.7 cells. The ELISA assays were performed by using culture media after treated the cells with EAHS for 24 h. The inflammatory pathways which are IκB/NF-κB, p38, JNK, and STAT3 were detected. All data are representative of three independent experiments. The reported values are the mean ± S.E.M. (n = 3). *p < 0.05 indicates a significant difference from the control group
Terpene, triterpene glycoside, and glycoside are the major components in ethyl-acetate fraction of the sea cucumber extracts
To elucidate the chemical components related to EAHS fraction, the unique value components of EAHS were evaluated by using Folin–Ciocalteau’s method and 1H-NMR. Generally, Folin–Ciocalteau’s phenol reagent is used for the determination of total phenolic content. The results showed that EAHS fraction was composed of phenolic compound (23.00 ± 2.64 mg gallic acid/g of extract), but not greater than that of BUHS (46.54 ± 1.51 mg gallic acid/g of extract) (Fig. 5a). Interestingly, analysing and interpretation of 1H NMR spectra of EAHS fraction reflected very promising and considering potential signals. The high-field shifted region of 1H NMR showed 4 broad singlet signals at 0.87, 1.22, 1.60, and 2.01 ppm, which indicated a hybridization of sp3 carbon. The low-field shifted region of 1H-NMR signals at δ 2.2–4.5 ppm displayed the sp3 carbon connected to the set of heteroatomic functional groups. Additionally, 1H-NMR spectrum at 5.2–5.3 ppm demonstrated the sp2 hybridization of unsaturated carbon. These evidences suggested that EAHS was composed of terpene, triterpene glycoside, and steroids (Fig. 5b). Thus, the consolidated consequences implied that phenolic and terpenoid may be the considerable components which play a vital role in inflammatory regulation.
Major compositions of EAHS. a The total phenolic contents of all three extracts of H. scabra, hexane (HEHS), ethyl-acetate (EAHS), and n-butanol (BUHS), detected by Folin–Ciocalteau’s method. b The study of 1H NMR spectrum of EAHS indicated that EAHS consists of terpene, triterpene glycoside, and steroids
Discussion
Inflammation is one of the most important defensive processes that responses to tissue injury, infection and/or radiation (White and Mantovani 2013). The imbalance of excessive and persistent pro-inflammatory cytokines and mediators can initiate the severity of diseases, for example, atherosclerosis, diabetes mellitus, and cancer (Prasad et al. 2014). So far, many researchers have targeted inhibition of the releasing factors, including nitric oxide (NO), and pro-inflammatory cytokines (IL-1β, TNF-α, PGE2), those of which can be employed for novel anti-inflammatory therapeutics (Aoki and Narumiya 2012; Tripathi et al. 2007; Wei et al. 2015; Zhou et al. 2017). In the present study, H. scabra was sequentially fractionated by hexanes (HEHS), ethyl acetate (EAHS), and n-butanol (BUHS), and then the efficacy comparison of each extract was performed. It was found that BUHS and EAHS did not show any cytotoxicity to the macrophages based on mitochondrial reduction of MTT assay. Interestingly, EAHS was proved valuable in suppressing NO production using a model of LPS-stimulated RAW264.7 macrophage cells. Moreover, it strongly inhibited the productions of inflammatory mediators and pro-inflammatory cytokines, containing PGE2, iNOS, IL-1β, and TNF-α, through the inhibiting NF-κB and JNK pathways.
Macrophages act as an essential cellular component that play a vital role in the inflammation process by releasing either inflammatory mediators or suppressors (Li et al. 2017). The outer membrane component of Gram-negative bacteria represents the core LPS region can induce macrophages to produce two major inflammatory mediators, like NO and PGE2. Those of which are generated by the inducible enzymes iNOS and COX-2, respectively. The upregulation of PGE2 is correlated with an increased nitric oxide expression, thus triggering the basic signs of inflammation, including redness, pain, and swelling (Wei et al. 2015). In the present study, EAHS significantly inhibited NO production as well as PGE2 in LPS-induced inflammatory responses in a concentration-dependent manner. Moreover, EAHS dose-dependently reduced the transcription of iNOS. Apparently, these findings have indicated that EAHS can suppress anti-inflammatory activity as a result of inhibiting the expression of LPS-induced pro-inflammatory mediators, including NO and iNOS. Furthermore, the generations of pro-inflammatory cytokines, such as IL-1β and TNF-α, were markedly increased in LPS-treated RAW264.7 cells. These pro-inflammatory mediators are responsible for pathology defence and tissue reconstruction in inflammation (Ma et al. 2015). Our study demonstrated that the expression of TNF-α and IL-1β at the transcription and secretion levels were attenuated by EAHS. Remarkably, a low dose of EAHS dramatically inhibited TNF-α production when compared with the control.
NF-κB, MAPK, and Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) pathways are involved with the inflammatory response by regulating the expression of cytokines and other mediators (Calay and Hotamisligil 2013; Yu et al. 2009). After LPS binding to Toll-like receptor 4 (TLR4) on the transmembrane of the activated macrophages, the consequences of phosphorylation of cytoplasmic proteins, i.e. NF-κB and MAPK, mediate activation of downstream target genes. NF-κB is located in the cytoplasm and binds to IκB as an inactive form. By induction of LPS, phosphorylation of IκB released from the complex structure activates translocation of NF-κB from the cytoplasm to the nucleus of a cell leading to arousal of the genes encoding pro-inflammatory cytokines, such as iNOS, COX-2, TNF-α, and IL-1β expressions (Hussein et al. 2013; Intayoung et al. 2016; Lawrence 2009; Vo et al. 2014). Thereby, this study is provided that EAHS is likely to reduce pIκB and pNF-κB expression levels in macrophage cell lines.
Similarly, the phosphorylation of MAPK pathways (p38, JNK, and ERK) is simulated by TLR4 activation, promoting the production of pro-inflammatory cytokines and mediators. Several studies have reported that the activations of p38 and JNK are mostly observed in pro-inflammatory reactions and obviously related to IL-1β and TNF-α activations (Hommes et al. 2003). LPS-induced inflammation in macrophage cell line upregulates p-p38 and JNK expressions. In contrast, EAHS significantly decreased the expression level of JNK. This finding is in agreement with the study of Li and colleagues (Li et al. 2015), thus confirming that NF-κB and JNK are essential pathways for NO and iNOS expressions rather than p38 and ERK (Caivano 1998; Paul et al. 1999). Meanwhile, JAK/STAT3 has been reported as another pathway that is related to the inflammatory signals. Activation of STAT3 also turns on the function of genes that play a role in pro-inflammatory mediators (Zhou et al. 2017). However, EAHS could not alter the STAT3 activity. The obtained results verified that EAHS was capable of suppressing the synthesis and activity of pro-inflammatory mediators and transcription factors (e.g., NF-κB and JNK). Therefore, NF-κB and JNK are the key pathways that regulate inflammatory response in EAHS treatment.
Terpene, triterpene glycoside, and steroids are the major compounds that have been found in echinoderms, particularly in sea cucumbers (Kim and Himaya 2012; Mitu et al. 2017). The proton NMR spectrum exhibited the signals that shifted in range of 5 ppm downfield and 2 ppm upfield are the characteristic of triterpene glycoside (Mondol et al. 2017). This was correlated with our previous research that LC–MS/MS of H. scabra ethyl-acetate fraction is enriched in triterpene glycosides (Mitu et al. 2017; Sangpairoj et al. 2016). All of these secondary metabolites mediate anti-inflammatory effects through various pathways, thus resulting in inhibition of pro-inflammatory cytokines and mediators during inflammation (Salih et al. 2014). Similar result has also been found in Stichopus japonicus. This sea cucumber contains high phenolic content (20.38 ± 0.69 mg gallic acid/ g of extract) in ethyl-acetate extract which appears to play an important role in quenching ROS generation during inflammatory responses via MAPK pathway (Himaya et al. 2010). In comparison with the study of Himaya and colleague, EAHS also effectively suppressed inflammation by NF-κB and JNK pathways. It was indicated that EAHS contains mainly terpene, triterpene glycoside, steroid, and phenolic compounds that could reduce pro-inflammatory cytokines production. As a consequence, EAHS may be one of the potential candidates to be used as a natural alternative anti-inflammatory therapeutic agent. Moreover, the enrichment of these components and the high potential to inhibit the pro-inflammatory cytokines of EAHS may describe the early in vivo study of the anti-inflammatory effect of the supplementary food from the sea cucumber (Whitehouse and Fairlie 1994).
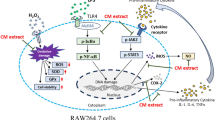
Altogether data revealed the potential of the extracts from H. scabra, in particular ethyl-acetate fraction (EAHS), on anti-inflammation in LPS-induced RAW264.7 macrophages (Fig. 6). EAHS significantly inhibited either inflammatory mediators (NO/iNOS and PGE2) or pro-inflammatory cytokines (IL-1β and TNF-α) in translation and transcription levels. These inhibitory effects were attributed to the suppression of the NF-κB and JNK pathways. These results suggest that EAHS possesses high potential to be a marine anti-inflammatory agent or as a functional food for prevention inflammation-associated disorders.
References
Aminin DL, Menchinskaya ES, Pisliagin EA, Silchenko AS, Avilov SA, Kalinin VI (2015) Anticancer activity of sea cucumber triterpene glycosides. Mar Drugs 13:1202–1223. https://doi.org/10.3390/md13031202
Aoki T, Narumiya S (2012) Prostaglandins and chronic inflammation. Trends Pharmacol Sci 33:304–311. https://doi.org/10.1016/j.tips.2012.02.004
Caivano M (1998) Role of MAP kinase cascades in inducing arginine transporters and nitric oxide synthetase in RAW264 macrophages. FEBS Lett 429:249–253. https://doi.org/10.1016/s0014-5793(98)00578-x
Calay ES, Hotamisligil GS (2013) Turning off the inflammatory, but not the metabolic, flames. Nat Med 19:265–267
Dyshlovoy SA et al (2016) The marine triterpene glycoside frondoside A exhibits activity in vitro and in vivo in prostate cancer. Int J Cancer 138:2450–2465. https://doi.org/10.1002/ijc.29977
Himaya SW, Ryu B, Qian ZJ, Kim SK (2010) Sea cucumber, Stichopus japonicus ethyl acetate fraction modulates the lipopolysaccharide induced iNOS and COX-2 via MAPK signaling pathway in murine macrophages. Environ Toxicol Pharmacol 30:68–75. https://doi.org/10.1016/j.etap.2010.03.019
Hommes DW, Peppelenbosch MP, van Deventer SJ (2003) Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut 52:144–151
Hussein SZ, Mohd Yusoff K, Makpol S, Mohd Yusof YA (2013) Gelam honey attenuates carrageenan-induced rat paw inflammation via NF-kappaB pathway. PLoS ONE 8:e72365. https://doi.org/10.1371/journal.pone.0072365
Intayoung P, Limtrakul P, Yodkeeree S (2016) Antiinflammatory activities of crebanine by inhibition of NF-κB and AP-1 activation through suppressing MAPKs and Akt signaling in LPS-induced RAW264.7 macrophages. Biol Pharm Bull 39:54–61. https://doi.org/10.1248/bpb.b15-00479
Kaminska B (2009) Molecular characterization of inflammation-induced JNK/c-Jun signaling pathway in connection with tumorigenesis. In: Kozlov SV (ed) Inflammation and cancer: methods and protocols: volume 2: molecular analysis and pathways, Humana Press, Totowa, pp. 249–264. doi: 10.1007/978-1-60327-530-9_13.
Kim SK, Himaya SW (2012) Triterpene glycosides from sea cucumbers and their biological activities. Adv Food Nutr Res 65:297–319. https://doi.org/10.1016/b978-0-12-416003-3.00020-2
Lawrence T (2009) The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol 1:a001651. https://doi.org/10.1101/cshperspect.a001651
Li Y et al (2017) Immune regulation mechanism of astragaloside IV on RAW2647 cells through activating the NF-κB/MAPK signaling pathway. Int Immunopharmacol 49:38–49. https://doi.org/10.1016/j.intimp.2017.05.017
Li L, Sapkota M, Kim S-w, Soh Y (2015) Herbacetin inhibits inducible nitric oxide synthase via JNK and nuclear factor-κB in LPS-stimulated RAW2647 cells. Eur J Pharmacol 765:115–123. https://doi.org/10.1016/j.ejphar.2015.08.032
Ma C, Wang Y, Dong L, Li M, Cai W (2015) Anti-inflammatory effect of resveratrol through the suppression of NF-kappaB and JAK/STAT signaling pathways. Acta Biochim Biophys Sin 47:207–213. https://doi.org/10.1093/abbs/gmu135
Mitu SA et al (2017) Evidence for a saponin biosynthesis pathway in the body wall of the commercially significant sea cucumber Holothuria scabra. Mar Drugs 15:349. https://doi.org/10.3390/md15110349
Mondol MAM, Shin HJ, Rahman MA, Islam MT (2017) Sea cucumber glycosides: chemical structures, producing species and important biological properties. Mar Drugs 15:317. https://doi.org/10.3390/md15100317
Nguyen BCQ, Yoshimura K, Kumazawa S, Tawata S, Maruta H (2017) Frondoside a from sea cucumber and nymphaeols from okinawa propolis: natural anti-cancer agents that selectively inhibit PAK1 in vitro. Drug Discoveries Therapeutics 11:110–114. https://doi.org/10.5582/ddt.2017.01011
Park S-Y, Lim HK, Lee S, Cho SK, Park S, Cho M (2011) Biological effects of various solvent fractions derived from Jeju Island red sea cucumber (Stichopus japonicas). J Korean Soc Appl Biol Chem 54:718–724. https://doi.org/10.1007/BF03253150
Paul A et al (1999) Involvement of mitogen-activated protein kinase homologues in the regulation of lipopolysaccharide-mediated induction of cyclo-oxygenase-2 but not nitric oxide synthase in RAW 2647. Macrophages Cellular Signalling 11:491–497. https://doi.org/10.1016/S0898-6568(99)00018-2
Prasad S, Tyagi AK, Aggarwal BB (2014) Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat 46:2–18. https://doi.org/10.4143/crt.2014.46.1.2
Salih M, Ahmed W, Garelnabi E, Osman Z, Osman B, Khalid H, Mohamed M (2014) Secondary metabolites as anti-inflammatory agents. Eur J Med Chem 3:257
Sangpairoj K et al (2016) Extract of the sea cucumber holothuria scabra, induces apoptosis in human glioblastoma cell lines. Functional Foods Health Dis 6:452–468
Sharma JN, Al-Omran A, Parvathy SS (2007) Role of nitric oxide in inflammatory diseases. Inflammopharmacology 15:252–259. https://doi.org/10.1007/s10787-007-0013-x
Stone WL, Yang H, Qui M (2006) Assays for nitric oxide expression. Methods in molecular biology (Clifton, NJ) 315:245–256
Tripathi P, Tripathi P, Kashyap L, Singh V (2007) The role of nitric oxide in inflammatory reactions. FEMS Immunol Med Microbiol 51:443–452. https://doi.org/10.1111/j.1574-695X.2007.00329.x
van Meerloo J, Kaspers GJ, Cloos J (2011) Cell sensitivity assays: the MTT assay. Methods in molecular biology (Clifton, NJ) 731:237–245. https://doi.org/10.1007/978-1-61779-080-5_20
Vo VA et al (2014) N-(p-Coumaryol)-Tryptamine suppresses the activation of JNK/c-Jun signaling pathway in LPS-challenged RAW2647 cells. Biomol Ther (Seoul) 22:200–206. https://doi.org/10.4062/biomolther.2014.013
Wei J, Zhang X, Bi Y, Miao R, Zhang Z, Su H (2015) Anti-Inflammatory effects of cumin essential oil by blocking JNK, ERK, and NF-κB signaling pathways in LPS-stimulated RAW 264.7 cells. Evid Based Complement Alternat Med 2015:1–8. https://doi.org/10.1155/2015/474509
White ES, Mantovani AR (2013) Inflammation, wound repair, and fibrosis: reassessing the spectrum of tissue injury and resolution. J Pathol 229:141–144. https://doi.org/10.1002/path.4126
Whitehouse MW, Fairlie DP (1994) Anti–inflammatory activity of a holothurian (sea cucumber) food supplement in rats. InflammoPharmacol 2:411–417. https://doi.org/10.1007/BF02678607
Yu H, Pardoll D, Jove R (2009) STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9:798–809. https://doi.org/10.1038/nrc2734
Yu S, Ye X, Huang H, Peng R, Su Z, Lian XY, Zhang Z (2015) Bioactive sulfated saponins from sea cucumber Holothuria moebii. Planta Med 81:152–159. https://doi.org/10.1055/s-0034-1383404
Zhou Y, Wang J, Yang W, Qi X, Lan L, Luo L, Yin Z (2017) Bergapten prevents lipopolysaccharide-induced inflammation in RAW2647 cells through suppressing JAK/STAT activation and ROS production and increases the survival rate of mice after LPS challenge. Int Immunopharmacol 48:159–168. https://doi.org/10.1016/j.intimp.2017.04.026
Acknowledgements
This work was supported by Agriculture Research Development Agency (Public Organization), Thailand to Prof. Prasert Sobhon, Research Fund, and the Central Instrument Facility (CIF) grant, Faculty of Science, Mahidol University, Bangkok, Thailand.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pranweerapaiboon, K., Apisawetakan, S., Nobsathian, S. et al. An ethyl-acetate fraction of Holothuria scabra modulates inflammation in vitro through inhibiting the production of nitric oxide and pro-inflammatory cytokines via NF-κB and JNK pathways. Inflammopharmacol 28, 1027–1037 (2020). https://doi.org/10.1007/s10787-019-00677-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-019-00677-3