Abstract
Nicotine mediates some of the injurious effects caused by consuming tobacco products. The aim of this work is to investigate the protective effects of Mentha spicata extract (ME) supplementation on the testis and brain of nicotine-induced oxidative damage rats. ME extract showed interesting hydrogen peroxide-scavenging activity. HPLC–DAD analysis of ME revealed the presence of nine compounds among them gallic acid was the major one (165.44 µg/g ME). Thirty-two rats were randomly divided into four groups: control, a nicotine-treated group (1 mg/kg i.p.), a group receiving ME (100 mg/kg), and a group receiving both ME (100 mg/kg) and nicotine (1 mg/kg). After 2 months of treatment, the in vivo results showed that nicotine exhibited an increase in the body, brain, testis and accessory sex organ weights, sperm count and sperm motility. In addition, exposure to nicotine significantly (p < 0.01) increased acetylcholinesterase level (AChE) in brain, lipid peroxidation level in brain and testis as compared to control group. The antioxidant enzymes results showed that nicotine treatment induced a significant decrease (p < 0.01) in brain and testis antioxidant enzymes such as catalase, superoxide dismutase and glutathione peroxidase as compared to control group. Interestingly, pretreatment with ME significantly (p < 0.01) restored the majority of these biological parameters to normal levels, as well as a histological improvement. Obtained results suggest that ME contains promising substances that counteract the nicotine-intoxication and can be efficient in the prevention of brain and testis toxicity complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nicotine, the primary addictive component of cigarette smoke, is rapidly absorbed by the mucosal lining of the respiratory tract and the lungs and it can rapidly reach peak levels in the bloodstream and brain (Motaghinejad et al. 2017; Mosbah et al. 2015). Besides its pharmacodynamics activities, nicotine is the main toxic component of cigarette smoking, with genotoxic, immunotoxic as well as reproductive effects in both sexes; it plays a detrimental role in the development of cardiovascular diseases, and respiratory and digestive tract cancers (Polyzos et al. 2009; Nacerai et al. 2017). Previous in vivo studies have shown that chronic use of nicotine disrupts the balance between the prooxidants and antioxidants in the circulation of experimental rats (Motaghinejad et al. 2017). Nicotine triggers the production of free radicals and reactive oxygen species (ROS), which overwhelm the antioxidative defence system and eventually generate oxidative stress (Mosbah et al. 2015; Ben Saad et al. 2017).
Recently, antioxidants and especially natural ones have attracted much interest with respect to their protective effect against free radical damage that may be the cause of many diseases including cancer (Vaqar and Hasan 2007). Several results from human experiments and animal reveal that some natural antioxidants such as polyphenols, flavonoids, lipoic acid and ascorbic acid could act as potent antioxidants in vivo (Yamamura et al. 2008). Recent studies have determined these secondary metabolites of plants that are well known for their beneficial properties by acting as protective agents against oxidative stress in liver (Akdogan et al. 2004; Ben Saad et al. 2017), kidney (Akdogan et al. 2003), erythrocytes and brain disorders (Avram et al. 2017; Ben Saad et al. 2017).
Mentha spicata L., also known as spearmint that belongs to the Lamiaceae family is distributed across the Europe, Africa, Australia, and North America. It is commonly used in culinary preparations to add flavor and aroma (Choudhury et al. 2006). Mint oil is widely used in pharmaceutical, cosmetic, food, confectionary and beverage industries (Kanatt et al. 2007).
Its leaves are well known as source of bioactive compounds and have been used since ancient times and for centuries in folk medicine. The plant is widely used in traditional medicine for treatment of many gastrointestinal disorders such as diarrhea, abdominal pain, gut spasm (El-Badry et al. 2010; Shah et al. 2010) and respiratory disorders such as cough, cold, and influenza (Naghibi et al. 2009). In addition, M. spicata is also known for its insecticidal, antinociceptive, antimicrobial, anti-inflammatory and antiplatelet properties (Ozgen et al. 2006; Mogosan et al. 2017).
The objective of the present study was to evaluate the in vitro and the in vivo antioxidant properties of M. spicata extract against nicotine-induced oxidative damage in brain and kidney of rats.
Materials and methods
Plant materials
The aerial parts of M. spicata L. were collected in March from Gafsa (Tunisia). The plant was identified and authenticated by a taxonomist and a voucher specimen is deposited at the Faculty of Sciences of Gafsa (Gafsa, Tunisia) under the number MS 0315.
M. spicata extract preparation
The air-dried plant material was extracted by maceration in water (10 g/100 ml) at ambient temperature for 24 h with continuous stirring. After that, the extract was filtrated with Whatmann Millipore filter paper and concentrated to dryness with a rotary evaporator at 40 °C. Finally, the M. spicata aqueous extract (ME) was kept in the dark at 4 °C until further analysis.
Antioxidant activity of ME in vitro
Hydrogen peroxide (H2O2) scavenging assay
Hydrogen peroxide scavenging activity of ME was determined according to the method reported by Liu et al. (2010). Butylated hydroxytoluene (BHT) was used as positive control. The H2O2 scavenging activity was calculated according to the formula below:
where, A1 is the absorbance of the sample, A2 is the absorbance of the sample only (water instead of H2O2 solution) and A0 is the absorbance of the control (water instead of sample).
Reducing power determination
The reducing power of ME was determined by the method of Yildirim et al. (2001). Sample solutions (0.5 ml) containing different concentrations of dried extract (0.1–1.1 mg/ml) were mixed with 1.25 ml of 0.2 M phosphate buffer (pH 6.6) and 1.25 ml of 10 g/l potassium ferricyanide solution. The mixtures were incubated for 30 min at 50 °C. After incubation, 1.25 ml of 100 g/l TCA was added and the reaction mixtures were centrifuged for 10 min at 3000g. A 1.25 ml aliquot of the supernatant from each sample mixture was mixed with 1.25 ml of distilled water and 0.25 ml of 1.0 g/l ferric chloride solution in a test tube. After a 10 min reaction time, the absorbance was measured at 700 nm. Higher absorbance of the reaction mixture indicated higher reducing power. The control was conducted in the same manner, except that distilled water was used instead of sample. Values presented are the mean of triplicate analyses.
High-performance liquid chromatography (HPLC) analysis
Mentha spicata extract analyses were performed in HPLC–DAD with a Varian ProStar HPLC System (Varian 330/Vis Detector and Varian 230 SDM). A reverse phase chromatography performed under gradient conditions with C18 column (4.6 mm × 250 mm) was used. Solvent A: acetic acid at 2% in water and solvent B: 40% acetonitrile, 2% acetic acid, and 58% water. The gradient was composed of 0–80% B for 25 min, 80–100% B for 10 min and 100–0% B for 5 min. The extract was utilized in the concentration of 1 mg/ml. The flow rate was 0.8 ml/min and the volume injected was 40 μl. The detected compounds were identified by comparison with authentic standards injected under the same conditions and the use of DAD spectra (200–600 nm).
Rat sources
Adult male Wistar rats weighing 140–150 g were obtained from Central Pharmacy of Tunisia (SIPHAT, Tunisia).The animals were handled under standard laboratory conditions of a 12-h light/dark cycle in a temperature- and humidity-controlled room. The rats were fed with a commercial balanced diet (SICO, Sfax, Tunisia) and drinking water was offered ad libitum. The handling of the animals was approved by the Medical Ethics Committee for the Care and Use of Laboratory Animals of the Pasteur Institute of Tunis (approval number: FST/LNFP/Pro 152012) and carried out according to the European convention for the protection of living animals used in scientific investigations (Council of European Communities 1986).
Experimental protocols
1 week after acclimatization to laboratory conditions, the rats were randomly divided into four groups of eight animals each.
Group C Control rats were injected intraperitoneally (i.p.) with distilled water (0.5 ml/100 g of b.w./day).
Group NT Injected i.p. with nicotine in aqueous solution with 1 mg/kg b.w./day for 30 days.
Group ME Rats administrated by gastric gavage the M. spicata extract (ME) at 100 mg/kg b.w./day for 60 days and then injected i.p. with distilled water (0.5 ml/100 g b.w./day) during the last 30 days of ME treatment (Ben Saad et al. 2018).
Group NT + ME Rats given by gastric gavage the ME at 100 mg/kg b.w./day for 60 days and then injected i.p. with nicotine at a dose 1 mg/kg b.w./day during the last 30 days of ME treatment.
The experimental protocol was approved by the Ethics Committee in Research and all efforts were made to minimize animal suffering and reduce the number of animals used. After treatment, the animals were sacrificed by decapitation under ether inhalation anesthesia to minimize the handling stress. The blood serum was obtained by centrifugation (1500 rpm, 15 min, 4 °C) and stored at − 80 °C until use for biochemical determination.
Tissue preparation
During the treatment period, the body weight of the animals was monitored daily. On the day of sacrifice, testes and accessory sex organs (seminal vesicle and epididymis) were dissected out, trimmed off the attached tissues, and weighed. Brain and testis were cut into small pieces and immersed into a 2-ml ice-cold lysis buffer (TBS, pH 7.4); the mixtures were homogenized on ice using an ultra-turraks homogenizer for 15 min and then filtered and centrifuged (5000 rpm, 30 min, 4 °C). Supernatants were collected and stored at − 80 °C until use.
Sperm count and motility
Assessment of sperm count and motility estimation was performed according to Freund and Carol (1964). The two cauda epididymis from each rat was placed in 2 ml of Earles buffer at 37 °C. Sperm count and motility were examined using Malassez cells (CML, Nemours, France) and a light microscope (Olympus BX51, Tokyo, Japan). The count was repeated six times for each sample to minimize error.
Determination of acetylcholinesterase activity in brain
Acetylcholinesterase (AChE) activity was measured immediately in brain according to the method of Ellman et al. (1961) using acetylthiocholine iodide as a substrate. The reaction mixture was composed as follows: phosphate buffer (0.1 M; pH 8) and 0.01 M DTNB. The hydrolysis rate of acetylthiocholine iodide is measured at 412 nm through the release of the thiol compound which, when reacted with DTNB, produced the colour-forming compound DTNB. The reaction was initiated by adding 0.075 M acetylthiocholine iodide. Activities were expressed as micromole of substrate/min/mg protein.
Lipid peroxidation determination
Lipid peroxidation in the tissue was estimated colorimetrically by measuring thiobarbituric acid reactive substances (TBARS) which were expressed in terms of malondialdehyde content according to the method of Buege and Aust (1978). Briefly, aliquots of kidney homogenates were mixed with 1 ml of 5% TCA and centrifuged at 4000×g for 10 min. One ml of thiobarbituric acid reagent (TBA 0.67%) was added to 500 ml of supernatant and heated at 95 °C for 15 min. The mixture was then cooled and was measured for absorbance at 532 nm. TBARS values were expressed as nanomoles of MDA per millilitre.
Assay of antioxidant enzymes
Catalase (CAT) was assayed by the decomposition of hydrogen peroxide according to the method of Aebi (1984). The decrease in absorbance due to H2O2 degradations was monitored at 240 nm for 1 min and the enzyme activity was expressed as µmol H2O2 consumed/min/mg protein.
Superoxide dismutase (SOD) activity was determined by spectrophotometer absorbance at 580 nm following the technique published by Beyer and Fridovich (1987) which involves inhibition of nitrobluetetrazolium reduction. SOD activity was expressed as units per milligram of protein. One unit of activity was considered as the amount of protein that gives half-maximal inhibition. SOD activity as expressed as U/mg of protein.
The glutathione peroxidase (GPx) activity was measured according to Flohé and Gunzler (1984). The enzyme activity was expressed as nmoles of GSH oxidized/min/mg protein.
Protein quantification
Protein content was evaluated as described by Lowry and Rosenbrough (1951) using bovine serum albumin as standard.
Histopathological studies
Testis and brain sections fixed in formalin solution were washed with distilled water and treated by a series of alcohol baths and embedded in paraffin. Next, they were cut at 4–6 μm thickness, stained with hematoxylin–eosin and observed under a microscope.
Statistical analysis
Data are expressed as mean ± standard error of mean (SEM). Data were compared using one way analysis of variance followed by Student’s t test to compare means between the different treatment groups. Differences were considered statistically significant at p < 0.05.
Results
HPLC analysis of phenolic compounds and biological properties
The identification and quantification of the bioactive compounds are summarized in Table 1. Six flavonoids and three phenolic acids were found in the extract of M. spicata (Figs. 1, 2). Gallic acid (165.44 μg/g) was the major phenolic acid in the ME followed by vanillic acid (120.40 μg/g) and coumaric acid (23.14 μg/g). The highest levels of flavonoids were obtained for quercetin (145.03 μg/g), rutin (102.43 μg/g), catechin (99.23 μg/g), Apigenin (87.91 μg/g).
Determination of antioxidant activity of M. spicata extract
Based on the reducing power assay, it was observed that ME was able to reduce the Fe3+ to Fe2+ by donating an electron. Figure 3a shows that the extract concentration (EC50) providing 0.5 of an absorbance at 700 nm was 1.1 ± 0.9 mg/ml. ME also presents an interesting hydrogen peroxide-scavenging activity. In fact, the IC50 value, defined as the extract concentration needed to scavenge 50% of hydrogen peroxide, was found to be 0.36 ± 0.1 mg/ml (Fig. 3b).
Body, reproductive organs and brain weights
The results presented in Table 2 showed that after 60 days of nicotine treatment, the body weight of rats decreased (p < 0.05) in comparison to normal rats. However, the administration of ME significantly (p < 0.01) increased the body weight of rats compared with the nicotine-treated group. In addition, treatment with ME alone increased the net body weight of rats compared to control. Moreover, brain, testis and accessory sex organ (seminal vesicle and epididymis) weights decreased significantly (p < 0.01) in nicotine-treated animals. On the other hand, ME supplementation in nicotine-treated group (ME + NT) resulted in a significant increase (p < 0.01) in brain, testis and accessory sex organ weights in comparison to the nicotine-treated rats (NT).
Epididymal sperm count and motility
Nicotine treatment significantly decreased (p < 0.01) the epididymal sperm number in the lumen of the seminars tubule of the nicotine-treated (NT) group (Table 2). The motility test showed that the number of dead spermatozoa was higher in NT group in comparison to normal control rats (C). In contrast, most of the sperm remained alive in nicotine-intoxicated rats pretreated with M. spicata extract.
Acetylcholinesterase assay
Figure 4 revealed that acetylcholinesterase (AChE) was significantly increased (p < 0.01) in brain of nicotine-treated group (NT) compared to those of normal group. The rats treated with nicotine and M. spicata extract revealed no significant changes in brain AChE as compared to normal rats. M. spicata extract alone did not affect AChE activity.
Lipid peroxidation product (MDA levels)
The effect of nicotine treatment on the MDA levels of brain and testis is presented in Table 3. In the brain and testis, MDA levels were significantly (p < 0.01) higher in rats treated only with nicotine when compared with control group. Concurrent administration of M. spicata extract in the nicotine-treated rats resulted in significant decrease (p < 0.01) in the MDA levels.
Antioxidant enzyme analysis
Changes in the antioxidant enzyme activities of the brain and testis samples of all the treated groups were evaluated (Table 3). The results revealed significant decreases in superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) activities in nicotine-treated rats as compared to control rats. Interestingly, administration of M. spicata extract in nicotine-treated group was markedly noted to restore these antioxidant enzymes activities to normal levels.
Histopathological studies
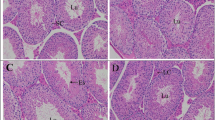
The histopathological examination of testis of control and ME groups (Fig. 5a, c), showed a normal tissue structure and architecture of the spermatogenic cycle. However, NT rats (Fig. 5b) exhibited evident signs of testicular injury, such as atrophy and degeneration of the seminiferous epithelium with increased number of mature spermatozoa in the tubular lumen, as well as widening of the interstitial spaces with few number of Leydig cells. No such changes or any other appreciable abnormal findings were present in the (NT + ME) group (Fig. 5d).
Light microscopic sections of testes from various examined groups, control (a), nicotine-treated animals (b), M. spicata extract-treated group (c), and combination M. spicata extract plus nicotine (d). Sections (5 μm thickness) were stained with haematoxylin–eosin (× 200). ST seminiferous tubule, IT interstitial tissue, L lumen, SG spermatogonia, SZ spermatozoa, arrows increased number of mature spermatozoa in the tubular lumen Star widened interstitial spaces with few number of Leydig cells
Upon histological examination of control rats, cerebral cortex tissue presented normal histoarchitecture (Fig. 6a). In the NT rats, histological sections showed abnormalities (Fig. 6b) when compared to controls. In fact, cerebral cortex exhibited hemorrhage, vacuolated spaces in the affected area and the network of finely branching small blood vessels. Pretreatment with ME ameliorated cerebral cortex histological pictures and histological damages that significantly decreased (Fig. 6d). The histological pattern was normal in rats treated only with ME (Fig. 2c).
Photographs showing histological changes in cerebral cortex in different groups; control group (a); nicotine-treated group (b); M. spicata extract alone treated group (c); M. spicata extract plus nicotine-treated group (d). Hematoxylin and eosin staining × 400. 1: Glial cells; 2: neurons; 3: a network of finely branching small blood vessels; 4: hemorrhage; 5: vacuolated cytoplasm
Discussion
Flavonoids have been reported to have several pharmacological effects (Romani et al. 2002; Kim et al. 2008). Generally, the ethanol extracts of M. spicata extract are known for their richness in phenolic compounds, such as phenolic acids and flavonoids (Iqbal et al. 2013). In this context, it is important to note that M. spicata flavonoids are known for their numerous pharmacological properties, including antispasmodic, antimicrobial, and antioxidant activities, in addition to its ability to inhibit human platelet aggregation. Likewise, several studies showed that phenolic compounds such as gallic, vanillic, coumaric acids have various biological effects including anti-inflammatory, hepatoprotective, antiviral and anticarcinogenic (Sharma et al. 2014; Mogosan et al. 2017). The chemical composition of M. spicata extract was described through HPLC analyses. This phytochemical investigation allowed us to identify three phenolic acids (gallic acid, vanillic acid, and coumaric acid) and six flavonoids (rutin, catechin, quercetin, apigenin, naringenin and kaempferol), which have been previously reported in other Mentha species and well-known for their efficient antioxidant properties (She et al. 2010; Krzyzanowska et al. 2011; Rita et al. 2016). Antioxidant activity is associated with a polyphenol ability to scavenge free radicals. In the present study, the antioxidant activity of M. spicata extract was assessed as content of phenols and flavonoids and their ferric reducing power and hydrogen peroxide-scavenging activity. Antioxidants were believed to play a very important role in the body defence system against ROS or free radicals. Similar results have been reported by Snoussi et al. (2015) and Fitsiou et al. (2016) emphasizing that antioxidant capacity of M. spicata plants was due to their richness in antioxidant components. Based on these results, the M. spicata extract was selected to explore the eventual protective effect against nicotine-induced oxidative damage of testis and brain. To the best of our knowledge, the approach has not been addressed so far. Reduction in body weight is used as an indicator for the deterioration of rat general health status. It has been reported that nicotine could induce toxicological effects and biochemical dysfunctions representing serious health hazards (Mosbah et al. 2015). The findings from the present study indicate that excessive nicotine treatment has changed body weight and negatively affects the function of the reproductive system and brain of adult male rats. M. spicata extract prevented the nicotine-induced reduction in body weight. Mosbah et al. (2015) reported that nicotine administration to rats decreased body weight gain by both affecting metabolism (decreased fat stores) and reducing appetite. Indeed, nicotine can increase the metabolic rate and energy expenditure by sympatho-adrenal activation (Nunn 1993). Nicotine exhibited a marked testicular toxicity as evidenced by the most tested parameters, such as the reduced weight of testis, seminal vesicle and epididymis, overall impaired semen quality and testicular histopathology. Cigarette smoking and nicotine administration are recognized to reduce the male sexual function in animals and humans. In fact, many epidemiological and experimental studies show similar effects to those observed in nicotine-treated rats of our study (Jana et al. 2010; Oyeyipo et al. 2011; Sankako et al. 2013). Moreover, sperm count and motility were also reduced in nicotine-treated animals. Semen data in humans and animals exposed to nicotine clearly indicate a drastic depletion in sperm counts, which may lead to male infertility (Jalili et al. 2014; Kolawole et al. 2015). The concurrent exposure to M. spicata extract fully protected the reproductive toxicity of nicotine. Overall, the findings on body weight and reproductive effects may suggest that substances, like phenolic compounds, contained in M. spicata extract might exert a protective action also towards oxidative stress and neuroendocrine disruption (Ben Saad et al. 2018). AChE is an adequate enzymatic biomarker of neurotoxicity, as it was inhibited by the presence of anticholinesterase chemicals. However, in our study, nicotine administration caused significant increase in brain AChE activity and conversely with several studies, which showed that nicotine inhibits the acetyl cholinesterase activity (Ashare et al. 2017; Hasan et al. 2018). AChE activity decreased after treatment with M. spicata extract at a dose of 100 mg/kg b.w. Bone and Mills (2013) found that the polyphenols, tannins and flavonoids present in biological material have shown a profound analgesic and cholinesterase inhibitory properties. In addition, several studies indicated that plant extracts used to treat cognitive disorders were found to act as AChE inhibitors (Mogosan et al. 2017). The effect of nicotine was detected also at the level of specific biomarkers such as MDA. This is consistent with previous studies on nicotine oxidative stress in testis and brain (Mosbah et al. 2015; Budin et al. 2017). The observed reduction in the TBARS level in testis and brain of rats of (NT + ME) group after treatment with the ME suggests the potential protection of M. spicata extract. Such protective effect of Mentha extract on lipid membranes or proteins would proceed through ROS scavenging (or neutralising), which can be explained by the presence of phenolic compounds such as rutin, quercetin and kaempferol in the extract. Some previous studies have indicated that phenolic compounds can prevent lipid peroxidation and cells damage (Snoussi et al. 2015; Rita et al. 2016). In the present investigation, nicotine-induced oxidative damage by producing reactive oxygen species and decreasing the biological activities of testis and brain antioxidant enzymes, like SOD, CAT and GPx. The decreased activities of these enzymes in nicotine-treated animals substantiate the occurrence of an oxidative stress in brain tissue. Nicotine was shown to accumulate in the brain up to concentrations high enough to explain its neurotoxic effects. Pretreatment of M. Spicata extract markedly reversed these perturbations, confirming its antioxidant potential. The current findings corroborate with previous studies of Murad et al. (2016) who suggested that natural antioxidants are effective in reducing and repairing the damage caused by nicotine intoxication. Histological study confirms the antioxidant properties of M. spicata extract. In the present study, exposure to nicotine caused histopathological changes in the testes including evident signs of testicular injury (Fig. 5). In fact, an atrophy and degeneration of the seminiferous epithelium with increased number of mature spermatozoa in the tubular lumen, as well as widening of the interstitial spaces with few number of Leydig cells were observed. Brain tissue of rat treated with nicotine showed in cerebral cortex hemorrhage, vacuolated spaces in the affected area and the network of finely branching small blood vessel (Fig. 6). Contrary to this, histological architecture of the testis tissue of control and M. spicata extract groups was normal. Pretreatment with ME in nicotine-treated rats group preserved the brain histological architecture. From this study, it can be concluded that the obtained protective activities of M. spicata extract, may be at least in part, explained by the presence of the potent antioxidant compounds.
Conclusion
In summary, our results showed that oxidative stress induced by nicotine causes testis and brain structural and functional injury. The consequences were drastic alterations in various cellular/physiological processes. Conversely, pretreatment with M. spicata extract mitigated nicotine-induced tissue damages, in correlation with direct antioxidant effects. Therefore, M. spicata extract may potentially, through its phenolic compounds, play an important role in the protection of populations chronically exposed to nicotine. However, further studies are needed to elucidate the exact mechanism of protective effect of M. spicata extract on nicotine-induced testis and brain malfunction.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Akdogan M, Kilinç I, Oncu M, Karaoz E, Delibas N (2003) Investigation of biochemical and histopathological effects of Mentha piperita L. and Mentha spicata L. on kidney tissue in rats. Hum Exp Toxicol 22:213–219
Akdogan M, Ozguner M, Aydin G, Gokalp O (2004) Investigation of biochemical and histopathological effects of Mentha piperita Labiatae and Mentha spicata Labiatae on liver tissue in rats. Hum Exp Toxicol 23:21–28
Ashare RL, Kimmey BA, Rupprecht LE, Bowers ME, Hayes MR, Schmidt HD (2017) Repeated administration of an acetylcholinesterase inhibitor attenuates nicotine taking in rats and smoking behavior in human smokers. Transl Psychiatry 28:7
Avram S, Mernea M, Bagci E, Hritcu L, Borcan LC, Mihailescu DF (2017) Advanced structure-activity relationships applied to Mentha spicata L. Subsp. spicata essential oil compounds as AChE and NMDA ligands, in comparison with donepezil, galantamine and memantine—new approach in brain disorders pharmacology. CNS Neurol Disord Drug Targets 16:800–811
Ben Saad A, Rjeibi I, Alimi H, Ncib S, Bouhamda T, Zouari N (2017) Protective effects of Mentha spicata against nicotine induced toxicity in liver and erythrocytes. Appl Physiol Nutr Metab 11:1–7
Ben Saad A, Rjeibi I, Alimi H, Ncib S, Bouhamda T, Zouari N (2018) Protective effects of Mentha spicata against nicotine-induced toxicity in liver and erythrocytes of Wistar rats. Appl Physiol Nutr Metab 43:77–83
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Bone K, Mills S (2013) Principles and practice of phytotherapy: modern herbal medicine. Elsevier Health Sciences, London
Budin SB, Kho JH, Lee JH, Ramalingam A, Jubaidi FF, Latif ES, Zainalabidin S, Taib IS, Mohamed J (2017) Low-dose nicotine exposure induced the oxidative damage of reproductive organs and altered the sperm characteristics of adolescent male rats. Malays J Med Sci 24:50–57
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Choudhury RP, Kumar A, Garg AN (2006) Analysis of Indian mint (Mentha spicata) of essential, trace and toxic elements and its antioxidant behaviour. J Pharm Biomed Anal 41:825–832
Council of European Communities (1986) Council instructions about the protection of living animals used in scientific investigations. Off J Eur Communities (JO 86/609/CEE) 358:1–18
El-Badry AA, Al-Ali KH, El-Badry YA (2010) Activity of Mentha longifolia and Ocimum basilicum against Entamoeba histolytica and Giardia duodenalis. Sci Parasitol 11:109–117
Ellman GL, Courtney KD, Andres V, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Fitsiou E, Mitropoulou G, Spyridopoulou K, Tiptiri-Kourpeti A, Vamvakias M, Bardouki H, Panayiotidis MΙ, Galanis A, Kourkoutas Y, Chlichlia K, Pappa A (2016) Phytochemical profile and evaluation of the biological activities of essential oils derived from the greek aromatic plant species Ocimum basilicum, Mentha spicata, Pimpinella anisum and Fortunella margarita. Molecules 21(8):1069
Flohé L, Gunzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–120
Freund M, Carol B (1964) Factors affecting haemocytometer counts of sperm concentration in human semen. J Reprod Fertil 42:149–155
Hasan MK, Friedman TC, Sims C, Lee DL, Espinoza-Derout J, Ume A, Chalfant V, Lee ML, Sinha-Hikim I, Lutfy K, Liu Y, Mahata SK, Sinha-Hikim AP (2018) α7-Nicotinic acetylcholine receptor agonist ameliorates nicotine plus high-fat diet-induced hepatic steatosis in male mice by inhibiting oxidative stress and stimulating AMPK signaling. Endocrinology 159(2):931–944
Iqbal T, Hussain AI, Chatha SAS, Naqvi SAR, Bokhari TH (2013) Antioxidant activity and volatile and phenolic profiles of essential oil and different extracts of wild mint (Mentha longifolia) from the Pakistani Flora. J Anal Methods Chem 2013:536490
Jalili C, Salahshoor MR, Naseri A (2014) Protective effect of Urtica dioica L. against nicotine-induced damage on sperm parameters, testosterone and testis tissue in mice. Iran J Reprod Med 12:401–418
Jana K, Samanta PK, De Kumar D (2010) Nicotine diminishes testicular gametogenesis, steroidogenesis, and steroidogenic acute regulatory protein expression in adult albino rats: possible influence on pituitary gonadotropins and alteration of testicular antioxidant status. Toxicol Sci 116:647–659
Kanatt SR, Chander R, Sharma A (2007) Antioxidant potential of mint (Mentha spicata L.) in radiation-processed lamb meat. Food Chem 100:451–458
Kim HP, Park H, Son KH, Chang HW, Kang SS (2008) Biochemical pharmacology of biflavonoids: implications for anti-inflammatory action. Arch Pharm Res 31:265–273
Kolawole TA, Oyeyemi WA, Adigwe C, Leko B, Udeh C, Dapper DV (2015) Honey attenuates the detrimental effects of nicotine on testicular functions in nicotine treated wistar rats. Niger J Physiol Sci 30:11–16
Krzyzanowska J, Janda B, Pecio L, Stochmal A, Oleszek W, Czubacka A (2011) Determination of polyphenols in Mentha longifolia and M. piperita field-grown and in vitro plant samples using UPLC-TQ-MS. J AOAC Int 94:43–50
Liu J, Luo J, Ye H, Sun Y, Lu Z, Zeng X (2010) In vitro and in vivo antioxidant activity of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbohydr Polym 82:1278–1283
Lowry OH, Rosenbrough NJ (1951) Randall, protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Mogosan C, Vostinaru O, Oprean R, Heghes C, Filip L, Balica G, Moldovan RI (2017) A Comparative analysis of the chemical composition, anti-inflammatory, and antinociceptive effects of the essential oils from three species of mentha cultivated in Romania. Molecules 10:22
Mosbah R, Yousef MI, Mantovani A (2015) Nicotine induced reproductive toxicity, oxidative damage, histological changes and haematotoxicity in male rats: the protective effects of green tea extract. Exp Toxicol Pathol 67:253–259
Motaghinejad M, Motevalian M, Fatima S, Faraji F, Mozaffari S (2017) The neuroprotective effect of curcumin against nicotine-induced neurotoxicity in mediated by CREB-BDNF signaling pathway. Neurochem Res 42:2921–2932
Murad HA, Abdallah HM, Ali SS (2016) Mentha longifolia protects against acetic acid induced colitis in rats. J Ethnopharmacol 190:354–361
Nacerai H, Gregory T, Sihem B, Salah A, Souhila AB (2017) green tea beverage and epigallocatechin gallate attenuate nicotine cardiotoxicity in rat. Acta Pol Pharm 74:277–287
Naghibi F, Mosaddegh M, Mohammadi Motamed S, Ghorbani A (2009) Labiatae family in folk medicine in Iran: from ethnobotany to pharmacology, Iran. J Pharm Res 4:63–79
Nunn JF (1993) Oxygen and smoking. In: Nunn’s applied respiratory physiology. Butterworth-Heinemann Ltd, Oxford
Oyeyipo IP, Raji Y, Emikpe BO, Bolarinwa AF (2011) Effects of nicotine on sperm character-istics and fertility profile in adult male rats: a possible role of cessation. J ReprodInfertil 12:201–207
Ozgen U, Mavi A, Terzi Z, Yildirim A, Coşkun M, Houghton PJ (2006) Antioxidant properties of some medicinal Lamiaceae (Labiatae) species. Pharm Biol 44:107–112
Polyzos A, Schmid TE, Piña-Guzmán B, Quintanilla-Vega B, Marchetti F (2009) Differential sensitivity of male germ cells to mainstream and sidestream tobacco smoke inthe mouse. Toxicol Appl Pharmacol 237:298–305
Rita I, Pereira C, Barros L, Santos-Buelga C, Ferreira IC (2016) Mentha spicata L. infusions as sources of antioxidant phenolic compounds: emerging reserve lots with special harvest requirements. Food Funct 7:4188–4192
Romani A, Galardi C, Pinelli P, Mulinacci N, Heimle D (2002) HPLC quantification of flavonoids and biflavonoids in Cupressaceae leaves. Chromatographia 56:469–474
Sankako MK, Garcia PC, Piffer RC, Pereira OM (2013) Semen and reproductive parameters during some abstinence periods after cigarette smoke exposure in male rats. Braz Arch Biol Technol 56:93–100
Shah AJ, Bhulani NN, Khan SH, Ur Rehman N, Gilani AH (2010) Calcium channel blocking activity of Mentha longifolia L. explains its medicinal use in diarrhoea and gut spasm. Phytother Res 24:1392–1397
Sharma V, Hussain S, Gupta M, Saxena AK (2014) In vitro anticancer activity of extracts of Mentha spp against human cancer cells. Indian J Biochem Biophys 51:416–419
She GM, Xu C, Liu B, Shi RB (2010) Polyphenolic acids from mint (the aerial of Mentha haplocalyx Briq.) with DPPH radical scavenging activity. J Food Sci 75:359–362
Snoussi M, Noumi E, Trabelsi N, Flamini G, Papetti A, De Feo V (2015) Mentha spicata essential oil: chemical composition, antioxidant and antibacterial activities against planktonic and biofilm cultures of vibrio spp. strains. Molecules 20:14402–14424
Vaqar MA, Hasan M (2007) Anti-oxidants from green tea and pomegranate for chemoprevention of prostate cancer. Mol Biotechnol 37:52–57
Yamamura S, Ozawa K, Ohatani K, Kasai R, Yamasaki K (2008) Antihistaminic flavones and aliphatic glycosides from Mentha spicata. Phytochemistry 48:131–136
Yildirim A, Mavi A, Kara AA (2001) Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem 49:4083–4089
Acknowledgements
This research was funded by the Tunisian Ministry of Higher Education. We would like to express our special thanks to Unit of common services, Faculty of Sciences Gafsa, Tunisia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saad, A.B., Rjeibi, I., Brahmi, N. et al. Nicotine-induced oxidative stress, testis injury, AChE inhibition and brain damage alleviated by Mentha spicata. Inflammopharmacol 28, 939–948 (2020). https://doi.org/10.1007/s10787-019-00650-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-019-00650-0