Abstract
Background
Annona crassiflora Mart., popularly known as “Araticum”, is a native tree of the Brazilian Cerrado used in folk medicine for treatment of pain and inflammatory diseases. We proposed to analyze analgesic and anti-inflammatory properties of the filtrate (F1) and the precipitate (F2) of the hydroalcoholic fraction from the leaves of Annona crassiflora Mart. in mice.
Materials and methods
Swiss mice were submitted to formalin-induced nociception test and tail-flick reflex test, to assess antinociceptive properties, and to the rota-rod test, for motor performance analyses. To evaluate anti-inflammatory properties, F1 and F2 were orally administered 1 h prior to the intrathoracic injection of carrageenan, zymosan, LPS, CXCL8, or vehicle in Balb/c mice and neutrophil infiltration was evaluated 4 h after injection.
Results
F1 and F2 reduced the licking time in the second phase of formalin-induced nociception test, but only F2 showed a dose-dependent response. Neither F1 nor F2 reduced the latency time in the tail-flick reflex test. In addition, motor performance alteration was not observed in F1- or F2-treated mice. F2 treatment significantly inhibited the neutrophilia induced by carrageenan, LPS, or CXCL8, but not zymosan.
Conclusions
The experimental data demonstrated that hydroalcoholic fractions of Annona crassiflora Mart. leaves have remarkable anti-inflammatory and antinociceptive activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pain is the most commonly reported symptom in medical consultations, being associated with a myriad of pathological conditions (Van Hecke et al. 2013). In 2012, nearly 40 million adults (17.6% of all adults) were classified as having category 3 or 4 pain in the United States (Nahin 2015). In general, pain is usually well controlled through the use of relatively safe non-steroidal anti-inflammatory drugs, such as aspirin, as well as with the use of analgesic opioids, including morphine (Elsesser and Cegla 2017). In spite of the efficacy provided by analgesic opioids, these drugs have a low therapeutic index and they are usually accompanied by side effects, such as respiratory depression and dependency (Romanelli 2017). Thus, the development of new pharmacological approaches with less risk of dependency, improved safety, and efficacy could be useful regarding a more safe pain management in several diseases. In this context, natural products appear as a promising source of alternative pharmacological tools regarding the treatment of the pain (Petrovska 2012).
Annona crassiflora Mart., 1841, popularly known as “Araticum”, is a member of the Annonaceae family, found in the Brazilian Cerrado, with a widespread use in folk medicine (Ratter et al. 1997). Oral administration of an infusion of A. crassiflora Mart. leaves is used in the treatment of inflammatory and painful ailments such as wounds, snakebites, diarrheas, malaria, and rheumatism (Cruz 1979; De Mesquita et al. 2007; Lorenzi and Matos 2008). In addition, in vitro and in vivo studies have indicated that the ethanol extract of Annona crassiflora Mart. leaves does not exhibit mutagenic or genotoxic potential, suggesting a safe use in this regard (Vilar et al. 2008, 2011). Therefore, based on the need to develop new treatments with less risk of dependency, more safety and improved efficacy for refractory pain to conventional pharmacotherapy and taking into account the therapeutic potential of the Annona crassiflora Mart., we decided to evaluate the pharmacological effects of this natural product in animal models of nociception and inflammation.
Materials and methods
Animal
Male Swiss (20–30 g) or Balb/c (18–25 g) mice were used throughout the experiments. The animals were obtained from the Bioterism Center of the Biological Sciences Institute of the Federal University of Minas Gerais (CEBIO-ICB/UFMG) and were housed in a temperature-controlled room (23 ± 1 °C) on an automatic 12-h light/dark cycle (06:00–18:00 h). All tests were conducted during the light phase. Food and water were freely available until the onset of the experiments. The animals were euthanized in a CO2 chamber. All testing procedures were in accordance with the ethical guidelines of the International Association for the Study of Pain (IASP) (Zimmermann 1983) and the experimental protocol was approved by the “Ethics Committee in Animal Experimentation at the Federal University of Minas Gerais” in 20/05/2014 (Protocol Number 51/2014).
Drugs and reagents
Formaldehyde, carrageenan λ type IV, zymosan, and lipopolysaccharide (LPS) were purchased from Sigma (St. Louis, MO, USA). Interleukin (IL)-8/chemokine C-X-C motif ligand (CXCL8) was obtained from Peprotech (Rock Hill, NJ, USA). Morphine and dexamethasone were purchased from Merck (Germany) and Aché (Brazil), respectively. Indomethacin and xylazine were obtained from Sigma (St. Louis, MO, USA). Formaldehyde was diluted in sterile aqueous solution of sodium chloride (NaCl) 0.9% (sterile saline solution), in the ratio 1:50, prepared immediately prior injection. Carrageenan, zymosan, LPS, CXCL8, and dexamethasone were diluted in phosphate-buffered solution (PBS; 1 mM Na2HPO4, 1.84 mM KH2PO4, 0.14 M NaCl, and KCl 2.68 mM; pH 7. 8). Indomethacin was solubilized in tween 20:ethanol:sterile saline solution at 1:4:45 ratio. Morphine and xylazine were diluted in sterile saline solution. Control mice received drug vehicle.
Plant material
Annona crassiflora Mart. was collected by Professor Lúcia Pinheiro Santos Pimenta in the town of Itatiaiuçu, Minas Gerais state, Brazil. The identification was made by the botanist João Renato Stehmann from the Department of Botany at the Federal University of Minas Gerais (UFMG). The gathering was held on July 17, 2007. A voucher specimen (number 22,988) is deposited in the herbarium of the Biological Sciences Institute at UFMG.
Obtention of hydroalcoholic fractions upon Annona crassiflora Mart. leaves
Filtered and precipitated hydroalcoholic fractions of Annona crassiflora Mart. leaves were obtained as described previously (Lage et al. 2014). Briefly, the extraction of the dried and crushed leaves (610.2 g) was performed by percolation with hexane followed by aqueous ethanol 80% (v/v), resulting in 13.5 and 268.6 g of hexane and EtOH extracts, respectively. Subsequently, a portion (157.5 g) of the EtOH extract was dissolved in 1 L of a mixture of methanol/water (8:2) and extracted, at room temperature, with chloroform (2 × 1000 mL), providing 121.3 g of hydroalcoholic fraction and 13.05 g of the chloroform fraction after solvent removal. During the solvent removal, spontaneous precipitation occurred in the hydroalcoholic fraction. The precipitate was filtered vacuum (10.92 g). The filtrate and the precipitate from hydroalcoholic fraction were named, respectively, F1 and F2.
TLC phytochemical screening and chromatographic separation of the chemical constituents from A. crassiflora Mart. leaf fractions
The following chemical constituents in the extracts were screened: alkaloids, flavonoids, and terpenoids. F1 and F2 were assessed by thin layer chromatography (TLC). F2 was also analyzed by polyamide and cellulose TLC. The screening was performed on the extracts using specific chemical reagents: (1) dragendorff reagent for alkaloid detection; (2) difenilboriloxietilamina/polyethylene glycol (NP/PEG) reagent to detect the presence of flavonoids; and (3) and vanillin/H2SO4 to detect other substances, for example, terpenoids (Zweig and Sherma 1972; Wagner and Bladt 1996; Cannell 1998). Chromatographic separation was performed on reverse phase polyamide column eluted with water, methanol, and ethyl acetate in gradient strength. Four compounds were obtained with their purity grade determined by HPLC analysis and then submitted to spectroscopic study.
HPLC analysis
High-performance liquid chromatography (HPLC) analyses were performed on Liquid Chromatography UFLC Shimadzu, consisting of LC-6AD binary pumps, a diode array SPD-M20A detector, and a CBM-20A integrator. The solubilized samples (20 mL) were injected on a semi-preparative reverse phase Supelco SPLC-18 column (250 mm × 10 mm × 5 μm). The flow rate was 800 μL/min, and data acquisition was performed in the range of 200–800 nm. The eluent used was an isocratic system consisting of acetonitrile–water (70:30).
Spectroscopy analysis
The 1D and 2D NMR spectra were recorded on Bruker Avance DPX200 and Avance DRX400 spectrometers in CD3OD or DMSO-d6. The spectra were calibrated to the solvent signal and the coupling constants (J values) were given in Hertz. Electrospray Ionization Mass Spectrometry (ESIMS) was performed using a Waters MICROMAS Q-TOF. Optical rotations were measured in MeOH on a Perkin–Elmer 241 polarimeter.
Formalin-induced nociception
Formalin solution (formaldehyde 2% in sterile saline solution [0.9% NaCl]) was injected at a volume of 30 µL/paw into the right hind paw plantar surface (intraplantar injection, i.pl.) of Swiss mice and, immediately after, the animals were individually placed in observation chambers, as previously described (Hunskaar et al. 1985). The time (s) spent licking the right paw was recorded during two time intervals after the formalin injection: 0–5 min (first phase or neurogenic pain) and 15–30 min (second phase or inflammatory pain). The F1-filtered and F2-precipitated hydroalcoholic fractions were solubilized in sterile saline solution with dimethylsulphoxide (DMSO) 4%. F1 was administered at 10, 30, 100, 300, and 1000 mg/kg doses and F2 at 53, 159, and 530 mg/kg doses per gavage (per os, p.o.) 60 min prior to formalin injection. Control group received sterile saline solution with 4% DMSO at a volume of 10 mL/kg, p.o. Mice were also pretreated with morphine subcutaneously (5 mg/kg, s.c.), solubilized in sterile saline solution, or with indomethacin (10 mg/kg, p.o.), solubilized in tween 20:ethanol:sterile saline solution (1:4:45), 30 and 60 min prior to formalin injection, respectively. F1 and F2 doses were determined as previously described (Ferreira et al. 2006), based on the 50% inhibitory dose (ID50) obtained from the effects of F1 treatment for the second phase of the formalin test, using the Graph Pad Prism 5.0 software to plot the log dose–response curve for the maximum effect percentage versus the F1 doses. ID50 was calculated by the graphic interpolation of this dose–effect curve. To maintain proportionality between F1 and F2 doses, since F2 was originated from F1, the reference dose (RD) of the F2-precipitated fraction was calculated by multiplying of ID50 obtained from the second phase effects of F1 to the formalin test (587 mg/kg) by the percentage yield of F2 in the obtaining process (9%). To the formalin-induced nociception test, the doses of F2 used were the equivalent of 1, 3, or 10 times the RD value (53, 159, and 530 mg/kg, respectively).
Tail-flick nociceptive reflex
Based on published data (D’Amour and Smith 1941), a constant focused heat was delivered to the Swiss mouse tail. The tail-flick reflex latency was evaluated immediately prior (baseline), 30, 60, and 120 min after the treatment of animals with vehicle (sterile saline solution in 4% DMSO, 10 mL/kg, p.o.), F1 (587 mg/kg, p.o.), F2 (53 mg/kg, p.o.), or morphine (5 mg/kg, s.c.). Three measurements were performed per animal and the average of these values was considered the final measurement. A cut-off time of 9 s was maintained throughout the experiment.
Motor performance test
Motor performance was evaluated by time spent walking on a rotating rod (16 rpm) trials (Rota-rod, Ugo Basile mod 7600) during 2 min (cut-off time), as previously described (Dunham and Miya 1957). Swiss male mice underwent three training sessions 24 h prior testing. Motor performance was analyzed immediately prior (basal), 30, 60, and 120 min after treatment with vehicle (sterile saline solution in 4% DMSO, 10 mL/kg, p.o.), F1 (587 mg/kg, p.o.), F2 (53 mg/kg, p.o.), or xylazine (2 mg/kg, s.c.).
Leukocyte migration into the thoracic cavity
Balb/c male mice were pretreated with F1 (587 mg/kg, p.o.), F2 (0.053, 0.53, 5.3, 53, 159 and 530 mg/kg, p.o.), vehicle (sterile saline solution in 4% DMSO, 10 mL/kg, p.o.), or dexamethasone intraperitoneally (0.5 mg/kg, i.p.) 1 h prior intrathoracic (i.t.) injection of vehicle (phosphate buffered solution [PBS], 100 µL), carrageenan (200 µg/100 µL), zymosan (200 µg/100 µL), LPS (250 ng/100 µL), or CXCL8 (60 ng/100 µL). Doses and volumes of inflammatory agents were according to literature data (Ferreira et al. 2012; Henriques et al. 2017). Animals were sacrificed in a CO2 chamber 4 h after inflammatory stimuli i.t. injection and the cells in the thoracic cavity were harvested after i.t. injection of 2 mL of PBS. Total cell counts were performed in a modified Neubauer chamber using Turk’s stain and differential cell counts were performed on cytospin preparations stained with May–Grunwald and Giemsa using the standard morphologic criteria to identify cell types, as previously described (Klein et al. 2001). The results were presented as the number of cells per cavity.
Statistical analysis
The results were analyzed using the Graph Pad Prism 5.0 and expressed as mean ± SEM. Statistically significant differences among groups were calculated by the application of analysis of variance (ANOVA) followed by the Bonferroni’s post test, with the level of significance set at p < 0.05.
Results
Phytochemical constituents present in F1-filtered and F2-precipitated hydroalcoholic fractions from A. crassiflora Mart. leaves following RP–HPLC–DAD analysis
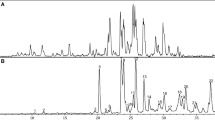
The phytochemical screening showed the presence of alkaloids in the F1-filtered, but not in the F2-precipitated, hydroalcoholic fractions from A. crassiflora Mart. leaves. On the other hand, both fractions present flavonoids (Table 1). F1 fraction was submitted to RP–HPLC–DAD analysis and four compounds were characterized by their retention time and UV spectra after comparison with authentic samples. Figure 1 presents the chromatogram obtained at 254 nm and the UV spectra of the assigned peaks. Compounds 1, 2, and 3 were identified as the flavonoids (−)-epicatechin, quercetin-3-O-β-d-glucopyranosyl (1 → 6)-O-α-L-arabinoside and quercetin-3-O-β-l-arabinopiranoside. Compound 4 was identified as the noraporphine alkaloid norstephalagine and it is described for the first time in this species (Fig. 1). TLC analysis of F1 and F2 fractions permitted to identify 2 as the main compound in F2 by comparison with standard isolated from the F1 fraction. The four compounds were isolated and unambiguously characterized by 1D and 2D-NMR, ESI–MS, UV/Vis spectroscopy, and optical rotation analysis.
HPLC chromatogram of filtered hydroalcoholic fraction (F1) from Annona crassiflora Mart. Leaves at 254 nm and the UV–Vis absorbance spectra of the assigned peaks. Peaks: 1, (−)-epicatechin; 2, quercetin-3-O-β-d-glucopyranosil (1 → 6)-O-α-l-arabinoside; 3, quercetin-3-O-β-l-arabinopiranoside; 4, norstephalagine
Effects of F1-filtered and F2-precipitated hydroalcoholic fractions from A. crassiflora Mart. leaves in nociceptive tests in Swiss mice
In the first phase of the formalin-induced nociception test, neither F1-filtered nor F2-precipitated, from hydroalcoholic fraction, reduced the paw licking time when compared to the vehicle (Fig. 2a, c). However, in the second phase, F1-filtered (1000 mg/kg, p.o.) and F2-precipitated (53, 159, and 530 mg/kg) fractions induced a significant reduction of paw licking time when compared to the saline group, as indomethacin. The morphine, in turn, induced antinociception in both phases (Fig. 2b, d).
Effects of filtered (F1) and precipitated (F2) hydroalcoholic fractions from Annona crassiflora Mart. leaves in the first (a, c) and the second (b, d) phases of formalin-induced nociception test in mice. 1 h after F1 or F2 oral administration, formalin was intraplantar injected into the right hind paw and the licking time (s) measured on the first and second stages. Each column represents the mean ± SEM (6 mice/group). Asterisk indicates significant differences compared to vehicle (Veh, 4% DMSO in sterile saline solution, p.o.) group (p < 0.05, ANOVA + Bonferroni’s test). I (Indomethacin, 10 mg/kg, p.o.); M (Morphine, 5 mg/kg, s.c.)
The pretreatment with F1 (587 mg/kg) or F2 (53 mg/kg) did not induce significant changes in the latency of the tail withdrawal in the tail-flick nociceptive reflex test, differently of the morphine pretreatment which significantly increased the latency of tail withdrawal (Fig. 3). In the rota-rod test, F1 (587 mg/kg) or F2 (53 mg/kg) fraction treated animals did not show significant changes in motor performance, differently of xylazine pretreatment, which significantly reduced the permanence time of the animals on the rotating rod (Fig. 4).
Effects of filtered (F1) and precipitated (F2) hydroalcoholic fractions from Annona crassiflora Mart. leaves in the tail-flick reflex test in mice. 0, 30, 60, and 120 min after F1 or F2 oral administration, tail-flick reflex latency was measured (s). Each column represents the mean ± SEM (6 mice/group). Asterisk indicates significant differences compared to vehicle (Veh, 4% DMSO in sterile saline solution, p.o.) group (p < 0.05, ANOVA + Bonferroni’s test). M (morphine, 5 mg/kg, s.c.); F1 (587 mg/kg, p.o.); F2 (53 mg/kg, p.o.)
Effects of filtered (F1) and precipitated (F2) hydroalcoholic fractions from Annona crassiflora Mart. leaves in the rota-rod test in mice. 0, 30, 60 and 120 min after F1 or F2 oral administration, the permanence time on the rotating rod was measured (s). Each column represents the mean ± SEM (6 mice/group). Asterisk indicates significant differences compared to vehicle (Veh, 4% DMSO in sterile saline solution, p.o.) group (p < 0.05, ANOVA + Bonferroni’s test). Xyl (Xylazine; 2 mg/kg, s.c); F1 (587 mg/kg, p.o.); F2 (53 mg/kg, p.o.)
Effects of F1-filtered and F2-precipitated hydroalcoholic fractions from A. crassiflora Mart. leaves on carrageenan-, zymosan-, LPS-, or CXCL8-induced neutrophil recruitment in the pleural cavity of Balb/c mice
F1-filtered and F2-precipitated fractions inhibited the leukocyte recruitment to the pleural cavity. F1 inhibited carrageenan-induced neutrophil recruitment (Veh 1 + Veh 2, 0.00 ± 0.00; Veh 1 + Cg, 1.95 ± 0.23; Dexa + Cg, 0.24 ± 0.01; F1 587 mg/kg + Cg, 0.43 ± 0.01; F2 53 mg/kg + Cg, 0.63 ± 0.10; F2 159 mg/kg + Cg, 0.75 ± 0.07; F2 530 mg/kg + Cg, 0.26 ± 0.07 neutrophils × 105/cavity, Fig. 5a). In addition, different doses of F2 also inhibited the recruitment of neutrophils in response to carrageenan intrapleural injection (Veh 1 + Veh 2, 0.02 ± 0.01; Veh 1 + Cg, 8.70 ± 0.86; F2 0.053 mg/kg + Cg, 9.60 ± 0.32; F2 0.53 mg/kg + Cg, 5.54 ± 0.42; F2 5.3 mg/kg + Cg, 4.45 ± 0.35; F2 53.0 mg/kg + Cg, 2.26 ± 0.25 neutrophils × 105/cavity; Fig. 5b). Interestingly, F2 did not inhibit the recruitment of neutrophils into the thoracic cavity induced by the zymosan (Veh 1 + Veh 2, 0.04 ± 0.01; Veh 1 + Zym, 11.55 ± 0.34; Dexa + Zym, 6.27 ± 0.47;F2 0.053 mg/kg + Zym, 11.85 ± 0.59; F2 0.53 mg/kg + Zym, 12.68 ± 0.58; F2 5.3 mg/kg + Zym, 13.66 ± 0.64; F2 53.0 mg/kg + Zym, 13.24 ± 0.59 neutrophils × 105/cavity; Fig. 6). Indeed, the mononuclear cells and leukocyte total number were also not reduced by F2-pretreatment against zymosan (data not shown). The F2-precipitated fraction pretreatment also significantly inhibited the lipopolysaccharide (LPS)-induced neutrophil recruitment (Veh 1 + Veh 2, 0.02 ± 0.01; Veh 1 + LPS, 1.18 ± 0.24; Dexa + LPS, 0.20 ± 0.30; F2 53 mg/kg + LPS, 0.47 ± 0.06 neutrophils × 105/cavity, Fig. 7a). Although the number of mononuclear cells was not altered significantly, the leukocyte total number was also reduced by F2-precipitated fraction pretreatment against LPS (Table 2). F2-precipitated fraction, orally administered, also reduced the CXCL8-induced neutrophilia (Veh 1 + Veh 2, 0.01 ± 0.01; Veh 1 + CXCL8, 4.26 ± 0.33; Dexa + CXCL8, 0.68 ± 0.04; F2 53 mg/kg + CXCL8, 0.88 ± 0.08 neutrophils × 105/cavity, Fig. 7a), besides that the total number of leukocytes and mononuclear cells were reduced significantly after treatment with F2-precipitated fraction or dexamethasone (Table 3).
Effects of filtered (F1) and precipitated (F2) hydroalcoholic fractions from Annona crassiflora Mart. leaves on neutrophil recruitment induced by intrathoracic injection of carrageenan (200 µg/cavity). Each column represents the mean ± SEM (6 mice/group). Asterisk and hash symbol indicates significant differences compared to Veh 1/Veh 2 (4% DMSO/PBS) and Veh 1/Carrageenan groups, respectively (p < 0.05, ANOVA + Bonferroni’s test). Dexa (dexamethasone, 0.5 mg/kg, intrathoracic)
Effects of the precipitated hydroalcoholic fraction (F2) from Annona crassiflora Mart. leaves on neutrophil recruitment induced by zymosan (200 µg/cavity). Each column represents the mean ± SEM (6 mice/group). Asterisk indicates significant differences compared to vehicle (Veh, 4% DMSO in sterile saline solution, p.o.) group (p < 0.05, ANOVA + Bonferroni’s test). Asterisk and hash symbol indicates significant differences compared to Veh 1/Veh 2 (4% DMSO/PBS) and Veh 1/Zymosan groups, respectively (p < 0.05, ANOVA + Bonferroni’s test). Dexa (dexamethasone, 0.5 mg/kg, intrathoracic)
Effects of the precipitated hydroalcoholic fraction (F2) from Annona crassiflora Mart. Leaves on neutrophil recruitment induced by intrathoracic injection of LPS 250 ng/cavity (a) or CXCL8 60 ng/cavity (b). Each column represents the mean ± SEM (6 mice/group). Asterisk and hash symbol indicates significant differences compared to Veh 1/Veh 2 (4% DMSO/PBS) and Veh 1/LPS or CXCL8 groups, respectively (p < 0.05, ANOVA + Bonferroni’s test). Dexa (dexamethasone, 0.5 mg/kg, intrathoracic)
Discussion
The Brazilian flora has a great biological diversity with a large number of plant species with medicinal potential. Like the Cerrado biome, there are more than 600 medicinal species (Neto and De Morais 2003), among which we can mention the Annona crassiflora Mart. Earlier studies have reported the antimalarial activity in vivo of flavonoids and alkaloids enriched fractions from Annona crassiflora Mart. leaf extract against P. berghei infected mice model (Pimenta et al. 2014), although the authors were not able to identify any compounds in those fractions. In the present work, we observed antinociceptive and anti-inflammatory effects of filtered (F1) and precipitated (F2) hydroalcoholic fractions from Annona crassiflora Mart. leaves. Phytochemical studies demonstrated that both F1 and F2 are enriched in flavonoids. Four pure compounds were isolated and identified in the crude fractions by HPLC–DAD analysis: (−)-epicatechin, two quercetin glycosides and one noraporphine alkaloid. Flavonoids have been described in this species (Lage et al. 2014). However, this is the first report of norstephalagine in Annona crassiflora Mart. leaves. Once flavonoids and alkaloids are substances with therapeutic effects, including anti-inflammatory and analgesic activities (Di Carlo et al. 1999; Barbosa-Filho et al. 2006; Shoaib et al. 2016), our results support prospective assays regarding the potential anti-inflammatory activity from Annona crassiflora Mart. leaves constituents.
The formalin-induced nociception test is a method commonly employed as acute pain model, characterized by the presence of a distinct biphasic nociceptive response. The initial phase (first phase) corresponds to a direct activation by formalin of primary afferent sensory neurons (C fibers) with the involvement of compounds as substance P and bradykinin (Shibata et al. 1989). The late phase (second phase) is characterized by an inflammatory reaction in the periphery and central sensitization in the spinal dorsal horn (Tjølsen et al. 1992; Mcnamara et al. 2007), involving several hyperalgesic and inflammatory mediators such as histamine, serotonin, bradykinin, and prostaglandin (Shibata et al. 1989). Pharmacologically, the first phase is sensitive to centrally acting opioids, such as morphine, while the second phase is inhibited by steroidal or non-steroidal anti-inflammatory drugs such as indomethacin, besides opioids with peripheral action (Hunskaar and Hole 1987; Oluyomi et al. 1992; Corrêa and Calixto 1993). Oral administration of F1-filtered or F2-precipitated hydroalcoholic fractions from A. crassiflora Mart. leaves induced significant antinociception only in the second phase of the test, corresponding to inflammatory pain. Therefore, we suggest the antinociceptive effect may be due to an anti-inflammatory action by inhibiting the synthesis or action of inflammatory mediators.
To evaluate a possible central antinociceptive effect, both F1-filtered and F2-precipitated fractions were conducted to the tail-flick reflex test in which the animal removes the tail of a gradually heated resistance through a reflex spinal integration and pharmacologically sensitive centrally acting opioids (Le Bars et al. 2001). F1-filtered and F2-precipitated fraction treatment, unlike morphine, were not able to increase the response latency to thermal stimulus, suggesting that activation of central opioid receptors is not involved in the antinociceptive effects of F1 or F2 fractions.
Motor performance alterations may be erroneously evaluated as analgesic action (false-positive result), since most of the experimental models used in pain evaluation require motor skills (Millan 1999, 2002). Supportively, in the rota-rod test, no F1- or F2-induced motor alteration was observed, validating the results from formalin-induced nociception and tail-flick tests.
The intrathoracic injection of inflammatory stimuli in mice is an acute inflammation model well established useful to evaluate important hallmarks of inflammation, such as the formation of exudates, protein extravasation or migration of leukocytes into inflamed tissues (Ackerman et al. 1980). The intrathoracic administration of carrageenan induces an inflammatory process characterized by the formation of exudate with releasing of lipid mediators such as prostaglandins and leukotrienes (Raychaudhuri et al. 1997; Yuhki et al. 2004), besides of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)α, IL-1, IL-6, and the chemokine CXCL8 (Frode et al. 2001). These mediators promote the recruitment of neutrophils and mononuclear cells from the blood vessels (Kolaczkowska and Kubes 2013). Consistent with the results in the second phase of formalin test, both F1-filtered and F2-precipitated fractions significantly reduced the neutrophil recruitment into the thoracic cavity induced by carrageenan. Furthermore, our data showed that the F2-precipitated fraction partially inhibited carrageenan-induced neutrophil recruitment in a dose-dependent manner, in addition to abolish LPS-induced neutrophil migration. Recently, it was demonstrated that the methanolic extract of Annona crassiflora Mart. leaves was also able to attenuate the carrageenan-induced pleurisy process (Rocha et al. 2016). However, different isolated compounds were considered as majorities, suggesting distinct anti-inflammatory mechanisms between hydroalcoholic fraction and methanolic extract. Given the more potent anti-inflammatory action of F2 than F1 and once compound 2 was taken as the main compound found in F2, we hypothesized that this compound may have a relevant action in this process. In addition, our research group previously suggested that compound 2 has antinociceptive properties at the peripheral level (Oliveira et al. 2017).
Different from carrageenan and LPS challenging, the pretreatment of mice with F2-precipitated fraction did not reduce the influx of leukocytes into the pleural cavity of the animals challenged with zymosan. Zymosan is a glucan present on the cell wall of fungi, such as Saccharomyces cerevisiae (Voluiskaia et al. 1959), which promotes the activation of the complement system commited to the innate immune response, leading to the release of inflammatory mediators involved in distinct pathways of those evoked by carrageenan and others inflammatory stimuli (Imai et al. 1991). We speculate that inhibitory effects of the F2-precipitated fraction on the neutrophil recruitment are not due to a blockade on the complement activation. Interestingly, although both LPS and zymosan activate the complement system (Mizuno et al. 2009), there are differences between their mechanisms of activation. These stimuli interact with toll-like receptors (TLR), promoting the activation of the nuclear factor kappa B (NF-κB) inducing the underlying transcription of several genes related to the acute inflammatory response, zymosan-induced inflammation is primordially TLR-2 dependent, while LPS-induced inflammation is more dependent of TLR-4 activation (Chow et al. 1999; Raetz and Whitfield 2002; Underhill 2004; Ikeda et al. 2008). Based on this, we speculate that F2-precipitated fraction inhibits downstream signaling mediated by TLR-4, but not TLR-2 to regulate neutrophil recruitment in the experimental pleurisy. More experiments are necessary to clarify the underlying mechanisms involved in this inhibitory action on TLR-4 in comparison with zymosan.
In addition, because carrageenan and LPS promote the releasing of the chemokine CXCL8 chemoattractant for neutrophils, and the F2-precipitated fraction was able to inhibit the neutrophil migration induced by both of these stimuli, we decided to investigate the effect of F2-precipitated treatment on CXCL8-induced neutrophil recruitment to the pleural cavity. F2-precipitated oral pretreatment abolished leukocyte recruitment in CXCL8-treated mice. CXCL8 induces neutrophil recruitment directly, acting on CXCR8 receptors on the neutrophil surface, or indirectly, through the releasing of other neutrophil chemo attractants such as leukotriene (LT)B4 (Kobayashi 2008). Phytocompounds can block the pathways of lipoxygenase and cyclooxygenase, inhibiting the production of inflammatory mediators, including LTB4 (Landolfi et al. 1984), and phytocompounds, such as flavonoids, are components of F2-precipitated hydroalcoholic fraction of Annona crassiflora Mart. leaves as demonstrated in the phytochemical screening performed, and we hypothesized that these chemical constituents may act on the pathways of lipoxygenase and cyclooxygenase, inhibiting the production of inflammatory mediators, including LTB4.
In summary, we demonstrate anti-inflammatory and antinociceptive effects present in filtered and precipitated hydroalcoholic fractions obtained from A. crassiflora Mart. leaves, exhibiting a potential contribution in the treatment of painful inflammatory diseases, where leukocyte accumulation is crucial to the pathophysiology.
References
Ackerman N, Tomolonis A, Miram L et al (1980) Three day pleural inflammation: a new model to detect drug effects on macrophage accumulation. J Pharmacol Exp Ther 215:588–595
Barbosa-Filho JM, Piuvezam MR, Moura MD et al (2006) Anti-inflammatory activity of alkaloids: a twenty-century review. Braz J Pharmacol 16:109–139. https://doi.org/10.1590/S0102-695X2006000100020
Cannell R (1998) Natural products isolation, 1st edn. Humana Press, Totowa
Chow JC, Young DW, Golenbock DT et al (1999) Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem 274:10689–10692. https://doi.org/10.1074/jbc.274.16.10689
Corrêa CR, Calixto JB (1993) Evidence for participation of B1 and B2 kinin receptors in formalin-induced nociceptive response in the mouse. Br J Pharmacol 110:193–198. https://doi.org/10.1111/j.1476-5381.1993.tb13791.x
Cruz G (1979) Dicionário das plantas úteis do Brasil [Dictionary of useful plants from Brazil], 1st edn. Civilização Brasileira SA, Rio de Janeiro
D’Amour FE, Smith DLA (1941) A method for determining loss of pain sensation. J Pharmacol Exp Ther 72:74–79
De Mesquita ML, Grellier P, Mambu L et al (2007) In vitro antiplasmodial activity of Brazilian Cerrado plants used as traditional remedies. J Ethnopharmacol 110:165–170. https://doi.org/10.1016/j.jep.2006.09.015
Di Carlo G, Mascolo N, Izzo AA, Capasso F (1999) Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci 65:337–353. https://doi.org/10.1016/S0024-3205(99)00120-4
Dunham NW, Miya TS (1957) A note on a simple apparatus for detection of neurological deficit in rats and mice. J Am Pharm Assoc 46:208–209. https://doi.org/10.1002/jps.3030460322
Elsesser K, Cegla T (2017) Long-term treatment in chronic noncancer pain: results of an observational study comparing opioid and nonopioid therapy. Scand J Pain 17:87–98. https://doi.org/10.1016/j.sjpain.2017.07.005
Ferreira AA, Amaral FA, Duarte IDG et al (2006) Antinociceptive effect from Ipomoea cairica extract. J Ethnopharmacol 105:148–153. https://doi.org/10.1016/j.jep.2005.10.012
Ferreira RG, Matsui TC, Godin AM et al (2012) Neutrophil recruitment is inhibited by nicotinamide in experimental pleurisy in mice. Eur J Pharmacol 685:198–204. https://doi.org/10.1016/j.ejphar.2012.04.014
Frode TS, Souza GEP, Calixto JB (2001) The modulatory role played by TNF-alpha and IL-1 beta in the inflammatory responses induced by carrageenan in the mouse model of pleurisy. Cytokine 13:162–168. https://doi.org/10.1006/cyto.2000.0816
Henriques GM, Miotla JM, Cordeiro SB et al (1996) Selectins mediate eosinophil recruitment in vivo: a comparison with their role in neutrophil influx. Blood 87:5297–5304
Hunskaar S, Hole K (1987) The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain 30:103–114. https://doi.org/10.1016/0304-3959(87)90088-1
Hunskaar S, Fasmer OB, Hole K (1985) Formalin test in mice, a useful technique for evaluating mild analgesia. J Neurosci Methods 14:69–76
Ikeda Y, Adachi Y, Ishii T et al (2008) Dissociation of Toll-like receptor 2-mediated innate immune response to zymosan by organic solvent-treatment without loss of Dectin-1 reactivity. Biol Pharm Bull 31:13–18. https://doi.org/10.1248/bpb.31.13
Imai Y, Hayashi M, Oh-ishi S (1991) Key role of complement activation and platelet-activating factor in exudate formation in zymosan-induced rat pleurisy. Jpn J Pharmacol 57:225–232. https://doi.org/10.1254/jjp.57.225
Klein A, Talvani A, Silva PM et al (2001) Stem cell factor-induced leukotriene B4 production cooperates with eotaxin to mediate the recruitment of eosinophils during allergic pleurisy in mice. J Immunol 167:524–531. https://doi.org/10.4049/jimmunol.167.1.524
Kobayashi Y (2008) The role of chemokines in neutrophil biology. Front Biosci 13:2400–2407. https://doi.org/10.2741/2853
Kolaczkowska E, Kubes P (2013) Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13:159–175. https://doi.org/10.1038/nri3399
Lage GA, Medeiros FDS, Furtado WDL et al (2014) The first report on flavonoid isolation from Annona crassiflora Mart. Nat Prod Res 28:808–811
Landolfi R, Mower RL, Steiner M (1984) Modification of platelet function and arachidonic acid metabolism by bioflavonoids. Biochem Pharmacol 33:1525–1530. https://doi.org/10.1016/0006-2952(84)90423-4
Le Bars D, Gozariu M, Cadden SW (2001) Animal models of nociception. Pharmacol Rev 53:597–652. https://doi.org/10.1111/j.1476-5381.2011.01386.x
Lorenzi H, Matos F (2008) Plantas medicinais brasileiras: nativas e exóticas cultivadas [Brazilian medicinal plants: native and exotic cultivated], 2nd edn. Plantarum, São Paulo
Mcnamara CR, Mandel-brehm J, Bautista DM et al (2007) TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA 104:30. https://doi.org/10.1073/pnas.0705924104
Millan MJ (1999) The induction of pain: an integrative review. Prog Neurobiol 57:1–164. https://doi.org/10.1016/S0301-0082(98)00048-3
Millan MJ (2002) Descending control of pain. Prog Neurobiol 66:355–474. https://doi.org/10.1016/S0301-0082(02)00009-6
Mizuno M, Ito Y, Hepburn N et al (2009) Zymosan, but not lipopolysaccharide, triggers severe and progressive peritoneal injury accompanied by complement activation in a rat peritonitis model. J Immunol 183:1403–1412. https://doi.org/10.4049/jimmunol.0804245
Nahin RL (2015) Estimates of pain prevalence and severity in adults: United States, 2012. J Pain 16:769–780. https://doi.org/10.1016/j.jpain.2015.05.002
Neto GG, De Morais RG (2003) Recursos medicinais de espécies do Cerrado de Mato Grosso: um estudo bibliográfico. Acta Bot Brasilica 17:561–584. https://doi.org/10.1590/S0102-33062003000400009
Oliveira CC, Veloso CC, Ferreira RC et al (2017) Peltatoside isolated from Annona crassiflora induces peripheral antinociception by activation of the cannabinoid system. Planta Med 83:261–267. https://doi.org/10.1055/s-0042-113386
Oluyomi AO, Hart SL, Smith TW (1992) Differential antinociceptive effects of morphine and methylmorphine in the formalin test. Pain 49:415–418. https://doi.org/10.1016/0304-3959(92)90249-B
Petrovska BB (2012) Historical review of medicinal plants’ usage. Pharmacogn Rev 6:1–5. https://doi.org/10.4103/0973-7847.95849
Pimenta LPS, Garcia GM, do Vale Gonçalves SG et al (2014) In vivo antimalarial efficacy of acetogenins, alkaloids and flavonoids enriched fractions from Annona crassiflora Mart. Nat Prod Res 28:1254–1259. https://doi.org/10.1080/14786419.2014.900496
Raetz CR, Whitfield C (2002) Lipopolysaccharide endotoxins. Annu Rev Biochem 71:635–700. https://doi.org/10.1146/annurev.biochem.71.110601.135414
Ratter JA, Ribeiro JF, Bridgewater S (1997) The Brazilian cerrado vegetation and threats to its biodiversity. Ann Bot 80:223–230. https://doi.org/10.1006/anbo.1997.0469
Raychaudhuri A, Chertock H, Chovan J et al (1997) Inhibition of LTB4 biosynthesis in situ by CGS 23885, a potent 5-lipoxygenase inhibitor, correlates with its pleural fluid concentrations in an experimentally induced rat pleurisy model. Naunyn Schmiedebergs Arch Pharmacol 355:470–474. https://doi.org/10.1007/PL00004971
Rocha RS, Kassuya CAL, Formagio ASN et al (2016) Analysis of the anti-inflammatory and chemopreventive potential and description of the antimutagenic mode of action of the Annona crassiflora methanolic extract. Pharm Biol 54:35–47. https://doi.org/10.3109/13880209.2015.1014567
Romanelli R (2017) Opioid prescribing for chronic pain in a community-based healthcare system. Am J Manag Care 23:138–146
Shibata M, Ohkubo T, Takahashi H, Inoki R (1989) Modified formalin test: characteristic biphasic pain response. Pain 38:347–352. https://doi.org/10.1016/0304-3959(89)90222-4
Shoaib M, Shah SWA, Ali N et al (2016) Scientific investigation of crude alkaloids from medicinal plants for the management of pain. BMC Complement Altern Med 16:178. https://doi.org/10.1186/s12906-016-1157-2
Tjølsen A, Berge O-G, Hunskaar S et al (1992) The formalin test: an evaluation of the method. Pain 51:5–17. https://doi.org/10.1016/0304-3959(92)90003-T
Underhill DM (2004) Toll-like receptors and microbes take aim at each other. Curr Opin Immunol 16:483–487. https://doi.org/10.1016/j.coi.2004.05.012
Van Hecke O, Torrance N, Smith BH (2013) Chronic pain epidemiology and its clinical relevance. Br J Anaesth 111:13–18. https://doi.org/10.1093/bja/aet123
Vilar JB, Ferreira FL, Ferri PH et al (2008) Assessment of the mutagenic, antimutagenic and cytotoxic activities of ethanolic extract of araticum (Annona crassiflora Mart. 1841) by micronucleus test in mice. Braz J Biol 68:141–147. https://doi.org/10.1590/S1519-69842008000100020
Vilar J, Ferri Chen-Chen, Ferri P (2011) Genotoxicity investigation of araticum(Annona crassiflora Mart., 1841, Annonaceae) using SOS-Inductest and Ames test. Braz J Biol 71:197–202. https://doi.org/10.1590/S1519-69842011000100028
Voluiskaia EN, Cheburkina NV, Tovarnitskii VI, Nikol’skaia IN (1959) Isolation and chemical composition of zymosan. Vopr Med Khim 5:143–148
Wagner H, Bladt S (1996) Plant drug analysis, 1st edn. Springer, Munich
Yuhki K, Ueno A, Naraba H et al (2004) Prostaglandin receptors EP2, EP3, and IP mediate exudate formation in carrageenin-induced mouse pleurisy. J Pharmacol Exp Ther 311:1218–1224. https://doi.org/10.1124/jpet.104.071548
Zimmermann M (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109–110. https://doi.org/10.1016/0304-3959(83)90201-4
Zweig G, Sherma J (1972) Handbook of chromatography, Vol. II: General data and principles, 1st edn. CRC Press, Boca Raton
Acknowledgements
This work was supported by grants from Conselho Nacional de Pesquisa (CNPq/Brazil) and Fundação de Pesquisa do Estado de Minas Gerais (FAPEMIG/Brazil).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
da Costa Oliveira, C., de Matos, N.A., de Carvalho Veloso, C. et al. Anti-inflammatory and antinociceptive properties of the hydroalcoholic fractions from the leaves of Annona crassiflora Mart. in mice. Inflammopharmacol 27, 397–408 (2019). https://doi.org/10.1007/s10787-017-0426-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-017-0426-0