Abstract
Purpose
Oleuropein and hydroxytyrosol are polyphenols that are extracted from olives and are major biological active components of olives and olive oil. Oleuropein and hydroxytyrosol exhibit interesting pharmacological effects on cells, and have been shown to have many health benefits such as anti-inflammatory effects. These effects were mainly attributed to their ability to scavenge the reactive oxygen species (ROS) produced by phagocytes such as neutrophils. The aim of this study was to investigate the effect of oleuropein and hydroxytyrosol on other neutrophil functions.
Methods
Human neutrophils were isolated from healthy donors. ROS production was measured by luminol-amplified chemiluminescence. Degranulation was assessed by measuring myeloperoxidase activity and Western blots. Chemotaxis was assessed by the under-agarose chemotaxis assay. Phosphorylated proteins were assessed by gel electrophoresis and Western blots.
Results
We show that in addition to their ROS scavenging effect, oleuropein and hydroxytyrosol significantly inhibited the bacterial peptide N-formyl-methionyl-leucyl-phenylalanine (fMLF)-induced degranulation of azurophilic and specific granules as measured by myeloperoxidase and lactoferrin release, respectively. We also show that oleuropein and hydroxytyrosol reduced fMLF-induced neutrophil chemotaxis. Interestingly, both agents impaired the fMLF-induced AKT, p38MAPKinase, and ERK1/2 phosphorylation, signaling molecules that are involved in pathways regulating neutrophil functions.
Conclusion
Our data suggest that the anti-inflammatory properties of oleuropein and hydroxytyrosol are not only restricted to their ROS scavenging effect, but also involve the inhibition of two other major pro-inflammatory neutrophil functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polymorphonuclear neutrophils play a key role in host defense against pathogens, as they are the first cells to migrate out of the circulation to the infection site (Nauseef and Borregaard 2014; El-Benna et al. 2016). The process by which pathogens are eliminated begins with adherence of neutrophils to the pathogen and phagocytosis, followed by production of high quantity of reactive oxygen species (ROS) or respiratory burst, and the release of proteases and anti-bacterial peptides into the phagosome through degranulation (Nauseef 2007; Nordenfelt and Tapper 2011).
The oriented migration of neutrophils towards the infection site (also called chemotaxis) is the first necessary and essential step that ensures bactericidal activity. Neutrophil chemoattractants include the fMLF (N-formyl-methionyl-leucyl-phenylalanine) bacterial peptide, IL-8, C5a and leukotriene B4 (LTB4) (Tan and Weninger 2016; Brazil and Parkos 2016). Once at the infectious site, neutrophils recognize the pathogen via different receptors and ligands, and recognition is generally followed by engulfment of the particle, surrounding it with a membrane to form a vacuole called the phagosome. Engulfment of the bacteria initiates the bactericidal program that involves mainly the respiratory burst and degranulation. The respiratory burst is mediated by the phagocyte NADPH oxidase, NOX2, which produces superoxide anion, the precursor of other ROS. Degranulation is the release of granule’s contents. Indeed, neutrophils contain four types of granules or vesicles that have different composition and density (Faurschou and Borregaard 2003). Those are azurophilic granules containing myeloperoxidase (MPO), elastase, cathepsins, etc., specific granules containing lactoferrin, lipocalin, membrane receptors, cytochrome b558, etc., tertiary granules, essentially containing gelatinase, and the highly mobilizable secretory vesicles. The release of these granules, i.e., degranulation, is important for immunity and inflammation. While neutrophils are required for host defense, their excessive recruitment and activation (degranulation and respiratory burst) can induce tissue injury contributing to enhanced inflammatory reaction and diseases, such as rheumatoid arthritis, inflammatory bowel disease, septic shock, and more (Babior 2000; Paige 2006; El-Benna et al. 2016). Thus, finding new molecules that inhibit neutrophil functions could lead to new anti-inflammatory drugs.
The mediterranean diet is known to be rich in bioactive compounds, such as polyphenols, flavonoids, beneficial unsaturated fatty acids, and numerous vitamins. In this context, olives and virgin olive oil, which are greatly consumed in this region, are potential sources of bioactive molecules (Owen et al. 2000; Tuck and Hayball 2002; Waterman and Lockwood 2007; Visioli and Bernardini 2011). Oleuropein and hydroxytyrosol are major polyphenols that are found in olives, olive leaves, and olive oil. Oleuropein is the most abundant compound and its enzymatic degradation generates hydroxytyrosol. The health benefits of olive polyphenols are well known in several diseases, such as inflammatory diseases, atherosclerosis, hypertension, degenerative diseases, and cancer (Barbaro et al. 2014; Andreadou et al. 2015; Giner et al. 2016). Oleuropein and hydroxytyrosol are mainly known for their antioxidant effect (Visioli et al. 1998, 2002; O’Dowd et al. 2004; Bedouhene et al. 2014). In particular, they have been shown to scavenge neutrophil ROS production, but their effects on other key inflammatory neutrophil functions have been less studied. The aim of this work was to investigate the effect of oleuropein and hydroxytyrosol on neutrophil degranulation and chemotaxis and the signaling pathways regulating these functions.
Methods
Reagents
Hydroxytyrosol, oleuropein, ortho-dianisidine dihydrochloride, fMLF, luminol, Phosphate Buffered Saline (PBS), Hanks’ Balanced Salt Solution (HBSS), protease and phosphatase inhibitors, mouse monoclonal anti-β-Actin antibody, protease and phosphatase inhibitors, and salt solutions were from Sigma Aldrich (Saint Quentin Fallavier, France). Dextran T500 was from Pharmacosmos (Holbaek, Denmark), Ficoll was from GE Healthcare Bio-Sciences AB (Uppsala, Sweden), SDS–PAGE and Western blotting reagents were purchased from Bio-Rad Laboratories (Hercules, CA, USA). Anti-MPO and anti-Lactoferrin antibodies were from Abcam (Cambridge, UK), anti-phospho-AKT, p-p38 and p-ERK1/2 and anti-AKT, p38 and ERK1/2 were from cell Signaling Technology (Boston, MA, USA). HRP-conjugated goat anti-rabbit, HRP-conjugated goat anti-mouse, AP-conjugated goat anti-rabbit antibodies and ECL (enhanced chemiluminescence) reagent were from Santa Cruz Biotechnology Inc. (Heidelberg, Germany).
Human neutrophil preparation
Venous heparinized blood obtained from healthy adult volunteers was used to isolate fresh neutrophils by Dextran (T500) sedimentation to remove red blood cells followed by Ficoll density gradient centrifugation (El-Benna and Dang 2007). After hypotonic lysis of red blood cells, the neutrophil pellet was collected and washed in PBS before counting cells. Neutrophils were 96% pure and 99% viable.
Luminol-amplified chemiluminescence assay
Isolated neutrophils (500,000 cells) were resuspended in 500 µl of HBSS and pre-incubated for 10 min at 37 °C in the presence of luminol (10 µM) and increasing concentration of oleuropein and hydroxytyrosol. Neutrophils were then stimulated with fMLF (10−6 M) and luminol-enhanced chemiluminescence was measured using a luminometer (Biolumat LB937; Berthold, France SAS, Thoiry, France) (O’Dowd et al. 2004).
Cell viability assay
Isolated neutrophils (1 × 106 cells) were resuspended in 1 ml of HBSS and incubated in the presence of increasing concentrations of oleuropein or hydroxytyrosol (10–100 µM) at 37 °C for 120 min. Cell viability was assessed by trypan-blue exclusion method and results are presented as the percentage of viable cells compared to untreated cells.
Neutrophil degranulation
Neutrophils (5 × 106/ml in 500 µl HBSS) were pre-incubated at 37 °C with or without increasing concentrations of oleuropein and hydroxytyrosol for 60 min prior to adding cytochalasin B (5 µg/ml) for 5 min. Neutrophils were then stimulated with fMLF (10−6 M) for 2 min at 37 °C. The reaction was stopped by centrifugation for 30 s at 12,000×g (Eppendorf Centrifuge 5415D, Hamburg, Germany), supernatants were collected, centrifuged again at 15,000×g for 10 min at 4 °C, and used for MPO and lactoferrin assays.
Measurement of myeloperoxidase activity
The MPO activity was measured using a modified spectroscopic method described by Bradley et al. (1982). Briefly, 50 µl of the centrifuged supernatant was mixed with phosphate buffer (350 µl) and an equal volume of a solution containing 1 mg/ml ortho-dianisidine dihydrochloride plus 0.0005% hydrogen peroxide. The change in absorbance at 460 nm was recorded by UVIKON 860 spectrophotometer at 22 °C for 10 min. The results were expressed as percent of control.
Neutrophil activation, sample preparation and Western blotting analysis
Neutrophils (10 × 106/400 µl HBSS) were pretreated with oleuropein, hydroxytyrosol, or buffer, for 60 min, and stimulated with fMLF (10−6 M for 30 s) at 37 °C with mild shaking. The reaction was stopped by adding 100 µl of 5× concentrated modified-Laemmli sample buffer (Laemmli 1970; Belambri et al. 2014). Samples were vigorously vortexed, and then denatured for 3 min at 100 °C. Solubilized proteins were stored at −80 °C until use. Neutrophil supernatants and lysates were sonicated prior to being subjected to 10% SDS–PAGE (Eq. 1 × 106 cells/well) using standard techniques. The separated proteins were transferred to nitrocellulose, and then blocked with 5% milk in Tris-buffered saline containing Tween 20 (TBS-T) for 1 h. After blocking, the membranes were probed with the appropriate antibody overnight at 4 °C. After several washes (3 × 5 min) with TBS-Tween 0.1%, the membranes were incubated with specific antibodies, e.g., anti-MPO (1:10,000); anti-lactoferrin (1:10,000), phospho-Akt, phospho-p38, phospho-Erk, HRP-labeled goat anti-mouse antibody (1:10,000) for actin, and HRP-labeled goat anti-rabbit antibody (1:10,000). The protein bands were revealed by enhanced chemiluminescence (Santa Cruz, Heidelberg, Germany) (Belambri et al. 2014).
Under-agarose neutrophil chemotaxis assay
The migration of neutrophils was assessed using the under-agarose chemotaxis assay (Nelson et al. 1975). Agarose (0.7%) was dissolved in HBSS by heating for 1 min in a microwave. After cooling to 48 °C, 10% of decomplemented SVF was added to the agarose, and 5 ml of the mixture was added to each culture plate (Falcon plastics, 5 cm diameter). Once the agarose was solidified, four series of three aligned wells (3 mm internal diameter, 3 mm interspace) were punched in the agarose gel in each plate. The agarose cores were removed with a pipet using vacuum. For the chemotaxis assay, cells were resuspended to 100 × 106 cell/ml. Cells treated with increasing concentrations of oleuropein, hydroxytyrosol or control cells were added in the central well (5 µl), and 5 µl of the chemoattractant fMLF (10−7 M) or buffer PBS was placed in the other wells. Finally, the plates were incubated for 2 h at 37 °C. Cells were viewed by light microscopy (ZEISS) using 40× magnification. The chemotaxis index was determined as the ratio of chemotaxis/spontaneous migration.
Statistical analysis
All the experimental data were expressed as mean ± SEM. The analysis was performed using Graph-Pad Prism version 4.0 for Windows, and the averages for the different groups were compared by one-way ANOVA test. *P < 0.05 was considered significant.
Results
Oleuropein and hydroxytyrosol strongly inhibit fMLF-induced ROS production and are not toxic for human neutrophils
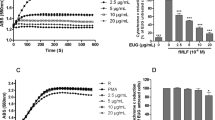
Oleuropein and hydroxytyrosol are polyphenols that have different structure (Fig. 1). They are well known for their potent antioxidant effect. As expected, oleuropein and hydroxytyrosol dose-dependently (2.5–80 µM) inhibited the fMLF-induced ROS production as assessed by the luminol-amplified chemiluminescence assay (Fig. 1). We thus used these oleuropein and hydroxytyrosol concentrations to study the effect on other neutrophil functions. Furthermore, incubation of neutrophils at 37 °C with different concentrations of oleuropein and hydroxytyrosol for 120 min did not reduce neutrophil viability, even at 100 µM (Fig. 2) showing that these molecules are not toxic for human neutrophils in these conditions.
Chemical structure and effect of hydroxytyrosol and oleuropein on fMLF-induced ROS production by human neutrophils. Neutrophils were incubated without or with different concentrations of hydroxytyrosol (a) or oleuropein (b) for 10 min in the presence of luminol (10 µm). ROS production was measured after stimulation with fMLF (10−6 M) using the luminol-enhanced chemiluminescence assay for 30 min. Histograms represent ROS production as calculated by the mean of the total area under the curves. Data are mean ± SEM of three separate experiments (**P < 0.05, ***P < 0.001)
Effect of hydroxytyrosol and oleuropein on neutrophil viability. Neutrophils were incubated without or with different concentrations of hydroxytyrosol or oleuropein for 120 min at 37 °C. Cell viability was assessed by trypan blue exclusion and results expressed as % of control untreated cells. Data are mean ± SEM of three separate experiments
Oleuropein and hydroxytyrosol inhibit the release of azurophilic and specific granules from human neutrophils
To investigate the effect of oleuropein and hydroxytyrosol on neutrophil degranulation, cells were incubated with increasing concentrations of polyphenols for 60 min before treatment with cytochalasin B and fMLF, and MPO was assayed as described in the “Methods”. MPO release from fMLF-stimulated neutrophils was inhibited in a dose-dependent manner by oleuropein and hydroxytyrosol (Fig. 3a). This was confirmed by SDS–PAGE and immunoblotting with a specific antibody against MPO, as evidenced by the decrease in MPO levels in the cell-free supernatants (Fig. 3b). The release of lactoferrin, a specific marker for specific granules was also analyzed using SDS–PAGE/Western blotting and a specific antibody. Indeed, oleuropein and hydroxytyrosol also inhibited lactoferrin release in a dose-dependent manner (Fig. 3c). Thus, these results show that in addition to their ROS scavenging effect, oleuropein and hydroxytyrosol inhibit neutrophil degranulation.
Effect of hydroxytyrosol and oleuropein on the release of neutrophil azurophil granules. Neutrophils were incubated without or with different concentrations of hydroxytyrosol or oleuropein, then treated with cytochalasin B and stimulated with fMLF (10−6 M). After centrifugation, the supernatants were recovered to measure MPO activity using a spectrophotometric method and expressed as % of control (fMLF alone). Data are mean ± SEM of three separate experiments (*P < 0.05) (a). Equal amounts of supernatants (MPO- and Lact-upper blots) and total cell lysates (Total) were subjected to SDS–PAGE followed by immunoblot analysis with anti-MPO (b) or anti-lactoferrin (Lact) antibody (c). Representative of three different experiments
Oleuropein and hydroxytyrosol inhibit neutrophil migration
Chemotaxis is fundamental for neutrophil recruitment to the inflammation site. Using the agarose neutrophil chemotaxis assay, we showed that PBS did not attract neutrophils while fMLF induced chemotaxis (Fig. 4a, Upper panel). Interestingly, oleuropein and hydroxytyrosol inhibit neutrophil chemotaxis towards fMLF in a dose-dependent manner (Fig. 4a, b).
Effect of hydroxytyrosol and oleuropein on neutrophils chemotaxis. Neutrophils (100 × 106 cell/ml) were pretreated with increasing concentrations of hydroxytyrosol (Hy) or oleuropein (Ole). Treated cells (5 µl) with or without (control) agents were added in the central well, 5 µl of the chemoattractant fMLF (10−7 M) or buffer PBS were placed in the other wells, and the plates were incubated for 120 min at 37 °C. Migration was determined using a ZEISS microscope. A representative experiment showing neutrophil migration towards fMLF (a). The chemotaxis index was determined as the ratio of the migrated distance of cells with hydroxytyrosol or oleuropein towards FMLF over the migration to fMLF alone. Data are mean ± SEM of four separate experiments (**P < 0.05, ***P < 0.001) (b)
Oleuropein and hydroxytyrosol inhibit fMLF-induced AKT, p38MAPK, and ERK phosphorylation in human neutrophils
Several signaling pathways such as the AKT, p38MAPKinase, and ERK1/2 pathways are involved in neutrophil degranulation and chemotaxis (Heit et al. 2002; Futosi and Mócsai 2016). We thus studied the impact of oleuropein and hydroxytyrosol on the phosphorylation status of these molecules as it reflects their activation. Both molecules reduced the phosphorylation of AKT, p38MAPKinase and ERK1/2 induced by fMLF without affecting the amount of these kinases (Fig. 5). Thus, the inhibition of the neutrophil functions by oleuropein and hydroxytyrosol could be due to the inhibition of AKT, p38MAPKinase and ERK1/2 activation.
Effect of hydroxytyrosol and oleuropein on fMLF-induced phosphorylation of AKT, p38MAPK, and ERK1/2 in human neutrophils. Neutrophils (5 × 106 in 400 µl) of HBSS were pre-incubated with increasing concentrations of hydroxytyrosol and oleuropein at 37 °C for 60 min, before stimulation with fMLF (10−6 M) for 2 min. The reaction was stopped by adding 5× Laemmli sample buffer and proteins were denaturated. Samples were subjected to SDS–PAGE followed by immunoblot analysis with anti-phospho-AKT (p-AKT), p-p38, p-ERK1/2, AKT, p38, and ERK1/2 antibodies. Representative of three different experiments
Discussion
Oleuropein and hydroxytyrosol, two polyphenols present in olives and olive oil, are known for their health benefits, particularly in inflammatory diseases. In this work, we confirmed the inhibition of the stimulated production of ROS by oleuropein and hydroxytyrosol, and showed that they are also able to inhibit the neutrophil degranulation and chemotaxis induced by a physiological stimulus, such as the bacterial peptide fMLF, and the fMLF-induced AKT, p38MAPKinase, and ERK1/2 activation.
At inflammatory sites, excessive neutrophil activation can lead to ROS overproduction, excessive release of proteases from granules and recruitment of additional neutrophils to maintain the inflammatory reaction. Others and we have previously reported that the effects of oleuropein and hydroxytyrosol on ROS generation by stimulated neutrophils are mainly due to scavenging of ROS. More specifically, they can scavenge hydrogen peroxide, but not superoxide anion, the product of NOX2 activation and the precursor of hydrogen peroxide (O’Dowd et al. 2004). As expected, oleuropein and hydroxytyrosol were effective at inhibiting ROS production assessed by the luminol-amplified chemiluminescence assay. Other studies have shown that hydroxytyrosol can inhibit other pro-inflammatory processes, such as the production and release of cytokines and other inflammatory agents (Richard et al. 2011; Impellizzeri et al. 2011; Rosignoli et al. 2013; Facchini et al. 2014). As the expression of several pro-inflammatory cytokines is mediated by the activation of NF-kB, a process mediated by hydrogen peroxide, oleuropein and hydroxytyrosol by scavenging hydrogen peroxide can inhibit cytokine production (Zhang et al. 2009).
Interestingly, our results also show that oleuropein and hydroxytyrosol inhibit neutrophil-directed chemotaxis, suggesting that they may limit neutrophil recruitment to the inflammatory site, a major starting step in the inflammatory reaction. Oleuropein and hydroxytyrosol also inhibited the release of specific and azurophilic granules, which may prevent tissue injury from proteases and other toxic peptides effects. These effects were not due to a toxic effect of oleuropein and hydroxytyrosol as no cell death was noted, even at much higher concentrations than the ones required for inhibition of neutrophil function.
Neutrophil stimulation by fMLF induces several signaling cascades, such as the phosphoinositide-3-kinase (PI3K)–AKT pathway, the Rac–p38MAPK pathway and the Ras–ERK1/2 pathway. These pathways are known to be involved in neutrophil ROS production, degranulation, and chemotaxis (Heit et al. 2002; Futosi and Mócsai 2016). Our results show that oleuropein and hydroxytyrosol inhibit AKT, p38MAPK, and ERK1/2 phosphorylation, suggesting that they may inhibit a common upstream pathway such as trimeric G-protein activation or a protein tyrosine kinase activity. The inhibitory effect of oleuropein on AKT activation was reported also in hepatocellular carcinoma (Yan et al. 2015; Liu et al. 2016) and PI3Kinase was suggested to be the target. However, it is unknown whether oleuropein and hydroxytyrosol can directly inhibit the activation of AKT. More work is required to identify the exact target of oleuropein and hydroxytyrosol on the signaling pathways involved in neutrophil activation.
Conclusion
The absorption of olive oil polyphenols has been studied by several groups. Oleuropein and hydroxytyrosol are hydrolyzed and absorbed in the intestinal tract in a dose-dependent manner, and they can reach high concentrations. However, the regular intake of these compounds may provide an effective concentration in blood and improve their effects on cells. In addition, the low cost and low toxicity of olive polyphenols make their use as immune-modulating and anti-inflammatory molecules which is a promising therapy for inflammatory diseases, especially as we demonstrate that their activity is not just limited to their ROS scavenging effect, but may also involve the dampening of neutrophil degranulation and chemotaxis, two major neutrophil pro-inflammatory functions.
References
Andreadou I, Benaki D, Efentakis P, Bibli SI, Milioni AI, Papachristodoulou A, Zoga A, Skaltsounis AL, Mikros E, Iliodromitis EK (2015) The natural olive constituent oleuropein induces nutritional cardioprotection in normal and cholesterol-fed rabbits: comparison with preconditioning. Planta Med 81:655–663
Babior BM (2000) Phagocytes and oxidative stress. Am J Med 109:33–44
Barbaro B, Toietta G, Maggio R, Arciello M, Tarocchi M, Galli A, Balsano C (2014) Effects of the olive-derived polyphenol oleuropein on human health. Int J Mol Sci 15:18508–18524
Bedouhene S, Hurtado-Nedelec M, Sennani N, Marie JC, El-Benna J (2014) Polyphenols extracted from olive mill wastewater exert a strong antioxidant effect in human neutrophils. Int J Waste Resour 4:161
Belambri SA, Dang PM, El-Benna J (2014) Evaluation of p47phox phosphorylation inhuman neutrophils using phospho-specific antibodies. Methods Mol Biol 1124:427–433
Bradley PP, Priebat DA, Christensen RD, Rothstein G (1982) Measurement of cutaneous inflammation; estimation of neutrophil content with an enzyme marker. J Invest Dermatol 78:206–209
Brazil JC, Parkos CA (2016) Pathobiology of neutrophil-epithelial interactions. Immunol Rev 273:94–111
El-Benna J, Dang PM (2007) Analysis of protein phosphorylation in human neutrophils. Methods Mol Biol 412:85–96
El-Benna J, Hurtado-Nedelec M, Marzaioli V, Marie JC, Gougerot-Pocidalo MA, Dang PM (2016) Priming of the neutrophil respiratory burst: role in host defense and inflammation. Immunol Rev 273:180–193
Facchini A, Cetrullo S, D’Adamo S, Guidotti S, Minguzzi M, Facchini A, Borzì RM, Flamigni F (2014) Hydroxytyrosol prevents increase of osteoarthritis markers in human chondrocytes treated with hydrogen peroxide or growth-related oncogene α. PLoS One 9:e109724
Faurschou M, Borregaard N (2003) Neutrophils granules and secretory vesicles in inflammation. Microbes Infect 14:1317–1327
Futosi K, Mócsai A (2016) Tyrosine kinase signaling pathways in neutrophils. Immunol Rev 273:121–139
Giner E, Recio MC, Rios JL, Cerdá-Nicolás JM, Giner RM (2016) Chemopreventive effect of oleuropein in colitis-associated colorectal cancer in c57bl/6 mice. Mol Nutr Food Res 60:242–255
Heit B, Tavener S, Raharjo E, Kubes P (2002) An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J Cell Biol 159:91–102
Impellizzeri D, Esposito E, Mazzon E, Paterniti I, Di Paola R, Bramanti P, Morittu VM, Procopio A, Britti D, Cuzzocrea S (2011) The effects of oleuropein aglycone, an olive oil compound, in a mouse model of carrageenan-induced pleurisy. Clin Nutr 30:533–540
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Liu M, Wang J, Huang B, Chen A, Li X (2016) Oleuropein inhibits the proliferation and invasion of glioma cells via suppression of the AKT signaling pathway. Oncol Rep 36:2009–2016
Nauseef WM (2007) How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev 219:88–102
Nauseef WM, Borregaard N (2014) Neutrophils at work. Nat Immunol 15:602–611
Nelson RD, Quie PG, Simmons RL (1975) Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol 115:1650–1656
Nordenfelt P, Tapper H (2011) Phagosome dynamics during phagocytosis by neutrophils. J Leukoc Biol 90:271–284
O’Dowd Y, Driss F, Dang PM, Elbim C, Gougerot-Pocidalo MA, Pasquier C, El-Benna J (2004) Antioxidant effect of hydroxytyrosol, a polyphenol from olive oil: scavenging of hydrogen peroxide but not superoxide anion produced by human neutrophils. Biochem Pharmacol 68:2003–2008
Owen RW, Giacosa A, Hull WE, Haubner R, Würtele G, Spiegelhalder B, Bartsch H (2000) Olive-oil consumption and health: the possible role of antioxidants. Lancet Oncol 1:107–112
Paige L (2006) Mechanisms of degranulation in neutrophils. Allergy Asthma Clin Immunol 2:98–108
Richard N, Arnold S, Hoeller U, Kilpert C, Wertz K, Schwager J (2011) Hydroxytyrosol is the major anti-inflammatory compound in aqueous olive extracts and impairs cytokine and chemokine production in macrophages. Planta medica 77(17):1890–189734
Rosignoli P, Fuccelli R, Fabiani R, Servili M, Morozzi G (2013) Effect of olive oil phenols on the production of inflammatory mediators in freshly isolated human monocytes. J Nutr Biochem 24:1513–1519
Tan SY, Weninger W (2016) Neutrophil migration in inflammation: intercellular signalrelay and crosstalk. Curr Opin Immunol 44:34–42
Tuck KL, Hayball PJ (2002) Major phenolic compounds in olive oil: metabolism and health effects. J Nutr Biochem 13:636–644
Visioli F, Bernardini E (2011) Extra virgin olive oil’s polyphenols: biological activities. Curr Pharm Des 17:786–804
Visioli F, Bellomo G, Galli C (1998) Free radical-scavenging proprieties of olive oil polyphenols. Biochem Biophy Res Commun 247:60–64
Visioli F, Poli A, Galli C (2002) Antioxidant and other biological activities of phenols from olives and olive oil. Med Res Rev 22:65–75
Waterman E, Lockwood B (2007) Active components and clinical applications of olive oil. Altern Med Rev 12:331–342
Yan CM, Chai EQ, Cai HY, Miao GY, Ma W (2015) Oleuropein induces apoptosis via activation of caspases and suppression of phosphatidylinositol 3 kinase/protein kinase B pathway in HepG2 human hepatoma cell line. Mol Med Rep. 11:4617–4624
Zhang X, Cao J, Jiang L, Zhong L (2009) Suppressive effects of hydroxytyrosol on oxidative stress and nuclear Factor-kappaB activation in THP-1 cells. Biol Pharm Bull 32:578–582
Acknowledgements
This research was supported by Institut National de la Santé et de la Recherche Médicale (INSERM), Centre National de la Recherche Scientifique (CNRS), Labex-INFLAMEX, University Denis-Diderot Paris7 and Vaincre La Mucoviscidose (VLM). The authors thank Martine Torres, Ph.D. for her critical leasing of the manuscript and editorial help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing conflict of interest.
Rights and permissions
About this article
Cite this article
Bedouhene, S., Moulti-Mati, F., Dang, P.MC. et al. Oleuropein and hydroxytyrosol inhibit the N-formyl-methionyl-leucyl-phenylalanine-induced neutrophil degranulation and chemotaxis via AKT, p38, and ERK1/2 MAP-Kinase inhibition. Inflammopharmacol 25, 673–680 (2017). https://doi.org/10.1007/s10787-017-0367-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-017-0367-7