Abstract
Purpose
Given the increased incidence of ulcerative colitis worldwide, the current study was designed to investigate the coloprotective potential of CoQ10 against experimentally induced ulcerative colitis (UC) and specify the implicated mechanisms.
Methods
Ulcerative colitis was induced by intracolonic instillation of [2 ml, 3% v/v acetic acid (AA)]. Rats in the different experimental groups received CoQ10 (10 or 30 and 100 mg/kg, orally) for eight consecutive days, either in a protective or curative regimen.
Results
Intracolonic AA instillation significantly increased colon/body weight index, colon weight/colon length ratio, clinical evaluation and macroscopic scoring of UC, serum LDH, C-reactive protein and decreased the serum total antioxidant capacity. Colon MDA, TNF-α and calcium content significantly increased as well, with concomitant reduction in colon GSH, SOD, CAT, Nrf2 and HO-1 contents. Moreover, immunohistochemical staining of colon specimen revealed increased expression of caspase-3 with significant histopathological changes. Coenzyme Q10 suppressed the release of inflammatory biomarkers and restored oxidants/antioxidants hemostasis. In a dose-dependent manner, CoQ10 significantly decreased colon/body weight index, colon weight/colon length ratio, clinical evaluation and macroscopic scoring of UC, serum LDH, C-reactive protein, colon MDA, TNF-α, caspase-3 expression and increased the serum total antioxidant capacity. Colon GSH, SOD, CAT, Nrf2 and HO-1 contents significantly increased. Moreover, coenzyme Q10 significantly preserved tissue histopathological architecture. It appears that the coloprotective effect of CoQ10 was calcium-independent.
Conclusion
Coenzyme Q10 dose-dependently protects against AA-induced UC mainly via modulation of Nrf2/HO-1 and caspase-3 pathways. Antioxidant, anti-inflammatory and anti-apoptotic properties of CoQ10 are implicated in its observed therapeutic benefit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ulcerative colitis (UC) is a major phenotype of inflammatory bowel diseases (IBD), characterized by extensive colon damage involving mucosal and submucosal layers and bloody diarrhea as a primary symptom (Hao et al. 2014; Malago and Sangu 2015). Alternating periods of intense intestinal inflammation and prolonged intervals of remission are considered the major characteristics of the disease (de Melo et al. 2015). Millions of patients all over the world have been reported to be suffering from UC (Liu et al. 2011; Parmar et al. 2014). Inpatient admissions due to UC have been estimated to be around 50.6 per 100,000 populations from 1999 to 2007 (Al-Rejaie et al. 2013).
Hence, the etiology of UC remains enigmatic, with no specific treatment (Vochyanova et al. 2015); current therapies are not completely successful. Dissatisfactory adverse effects and poor adherence usually decrease patient compliance (Biasi et al. 2011; Vochyanova et al. 2015). Moreover, the associated high risk of developing colorectal cancer urges prompt treatment (Kawabata et al. 2012). More importantly, early events of UC usually occur long before symptoms appear making susceptible individuals at higher risk. Such events necessitate studies specifying early events in the pathogenic pathway of the disease and testing new potential therapies (Brito et al. 2014). Sulfasalazine, mesalamine and dietary modification including specific fiber are amongst current treatment options for UC patients.
Even though the exact pathophysiology of UC is still not yet fully elucidated, both reactive oxygen species (ROS) and excessive expression of inflammatory cytokines have been reported to be implicated in the pathogenic pathway of UC (Yao et al. 2010; El Morsy et al. 2015).
Acetic acid (AA)-induced colitis is a well-adopted experimental model for induction of UC (Mustafa et al. 2006). Moreover, it has been reported to closely resemble human colitis (Randhawa et al. 2014) in several aspects including histological changes, inflammatory pathway implicated and response to conventional treatments (Keshavarzian et al. 1992).
Coenzyme (Co) Q10 is a naturally occurring compound. It is a cofactor in the mitochondrial respiratory chain and plays an important role in the production of ATP. Moreover, its reduced hydroquinone form, ubiquinol has been reported to be a potent lipophilic antioxidant capable of recycling and regenerating several antioxidants (Bhagavan and Chopra 2006). Coenzyme Q10 has been appreciated for its antioxidant, anti-inflammatory and ATP-regenerative properties in several experimental models of gastric ulcer, osteoarthritis and diabetes mellitus (Kucharska et al. 2000; El-Abhar 2010; Lee et al. 2013). Interestingly, coenzyme Q10 has an excellent safety record (Bhagavan and Chopra 2006).
The current study protocol was designed and conducted to evaluate the potential therapeutic role of CoQ10 against experimentally induced UC. Several aspects of CoQ10 activity were investigated including its antioxidants, anti-apoptotic and anti-inflammatory potentials.
Materials and methods
Experimental animals
Eighty male Sprague–Dawley rats, weighing 180–220 g, were used. Rats were purchased from “Urology and Nephrology Experimental Research Center”, Mansoura University, Mansoura, Egypt. Rats were maintained under standard environmental and nutritional conditions throughout the experimental period. Of notice, rats were also individually housed and were fasted overnight before induction of UC. The experimental protocol is in accordance with the ethical guidelines for experimental research and was approved by “Research Ethics Committee”, Faculty of Pharmacy, Mansoura University, Egypt.
Drugs and chemicals
Coenzyme Q10 was a generous gift from Arab Company for Pharmaceuticals and Medicinal Plants (Giza, Egypt) and was suspended in (0.5%) carboxymethylcellulose (CMC), which served as the vehicle for oral administration. Acetic acid was supplied by Chemajet chemical company for intracolonic instillation (Alexandria, Egypt).
Induction of colitis
Animals were fasted with free access to water, the night before induction of colitis. Rats were anaesthetized by I.P injection of (20 mg/kg) thiopental sodium. Using a polypropylene tube of 2 mm diameter inserted slowly through the rectum into the colon to a distance of 8 cm, 2 ml of acetic acid in normal saline (3% v/v) was slowly instilled into the colon. Then, the rats were maintained in a supine Trendelenburg position for 30 s. to prevent early leakage of the intracolonic instillate (El Morsy et al. 2015).
Experimental protocols
Evaluation of the protective effect of daily oral coenzyme Q10 (10 or 30 and 100 mg/kg, orally) for 8 consecutive days against acetic acid (2 ml, 3% v/v, intracolonic)-induced ulcerative colitis
Rats were randomly allocated to five groups (8 rats/group) as follows: Normal control: rats received vehicle orally once daily for 8 consecutive days and intracolonic normal saline (0.9% w/v) was instilled on the 8th day of the experiment; acetic acid control: rats received the vehicle orally once daily for 8 consecutive days before induction of colitis; CoQ10 (10 mg/kg), CoQ10 (30 mg/kg) and CoQ10 (100 mg/kg) treated groups: rats received CoQ10 10 or 30 and 100 mg/kg, respectively, for 8 consecutive days with the last dose administrated 1 h prior to induction of colitis.
Evaluation of the curative effect of daily oral coenzyme Q10 (10 or 30 and 100 mg/kg, orally) for 8 consecutive days against acetic acid (2 ml, 3% v/v, intracolonic)-induced ulcerative colitis
Rats were randomly divided into five experimental groups as follows: Normal control: rats received vehicle orally once daily for 8 consecutive days 48 h following normal saline (0.9% w/v) instillation into the colon; acetic acid control: rats received the vehicle once daily orally for 8 consecutive days 48 h following induction of colitis; CoQ10 (10 mg/kg), CoQ10 (30 mg/kg) and CoQ10 (100 mg/kg) treated groups: rats received CoQ10 10 or 30 and 100 mg/kg, respectively, for 8 consecutive days and treatments began 48 h post induction of colitis.
Collection of biological samples
Forty eight hours following intracolonic instillation of AA in the prophylactic protocol or 24 h following the last dose of CoQ10 in the curative protocol, rats were deeply anesthetized by thiopental sodium (40 mg/kg). Blood samples were collected from retro-orbital plexus. Sera were separated by centrifugation for 10 min at 4000 r.p.m. after leaving blood samples to coagulate and were used immediately to estimate the activities of lactate dehydrogenase (LDH), C-reactive protein (CRP) and total antioxidant capacity (TAC).
Body weights, colon weights and colon lengths were measured for calculation of colon/body weight index and colon weight/colon length ratio. Colonic segments were excised, freed of adherent adipose tissue, rinsed with cold normal saline and 6-cm segment of colon was used for macroscopic scoring. Another 2 cm was used for preparation of colon homogenate for assessment of colon GSH concentration, MDA content, SOD activity, catalase activity, calcium content, Nrf2 content, tumor necrosis factor-α (TNF-α) and heme oxygenase-1 (HO-1) activity and 1-cm colonic segments were stored in 10% neutral-buffered formalin for further histopathological examination and immunohistochemical analysis of caspase-3 expression.
Clinical evaluation of UC
To clinically assess AA-induced colitis activity, stool consistency was examined; loose stool and diarrhea, occult and/or bleeding, including hemoccult positivity, gross bleeding and body weight were evaluated. Percentage body weight change for each individual rat was calculated in comparison to the day prior to intracolonic instillation of AA. From these data, a disease activity index was calculated as follows. Acetic acid-colitis activity index was calculated by summation of the scores of weight loss, stool consistency, and bleeding then dividing by 3 (Malago and Nondoli 2008).
.
Score | Weight loss (%) | Stool consistency | Occult/gross bleeding |
|---|---|---|---|
0 | None | Normal | Normal |
1 | 1–5 | ||
2 | 5–10 | Loose stool | Hemoccult-positive |
3 | 10–20 | ||
4 | >20 | Diarrhea | Gross bleeding |
Macroscopic examination scoring
A postmortem laparotomy was conducted, 6 cm of colon extending proximally 2 cm above the anal margin was excised, slit longitudinally, pinned out on a card, and the macroscopic appearances of the colonic mucosa were scored on a scale ranging from 0 to 4 as follows according to Millar et al. (1996).
.
Score | Observation |
|---|---|
(0) | No macroscopic change |
(1) | Mucosal erythema alone |
(2) | Mild mucosal edema, slight bleeding or small erosions |
(3) | Moderate oedema, bleeding ulcers or erosions |
(4) | Severe ulceration/erosions, edema and tissue necrosis |
Preparation of colon homogenate
Homogenization was carried out according to the method described by Buege and Aust (1978) in ice-cold KCl (1.15%, pH 7.4) to yield 10% w/v colon homogenate. The homogenized tissues were centrifuged at 4000 rpm for 30 min at 4 °C. The supernatants were separated and used for further biochemical assessment.
Estimation of serum lactate dehydrogenase (LDH) activity, C-reactive protein (CRP) titre and total antioxidant capacity (TAC)
Serum LDH levels and TAC levels were determined with reference to the methods of Vassault et al. (1999) and Koracevic et al. (2001), respectively, according to the supplied manufacturer’s instructions (Biomed Diagnostics, Egypt) and serum C-reactive protein titer was assessed using CRP-Latex (Chemelex, Spain) as instructed by manufacturer.
Assessment of colonic oxidants/antioxidant stress biomarkers; malondialdehyde (MDA) content, superoxide dismutase (SOD) activity, reduced glutathione (GSH) concentration and catalase activity
Colon MDA content was quantified according to the method described by Ohkawa et al. (1979), SOD activity as described by Nishikimi et al. (1972) and GSH concentration as described by Beutler et al. (1963). Colon catalase activity was assessed as described by Aebi (1984). All tests’ procedures were conducted according to the provided manufacturer’s instructions, using commercially available kit (Biodiagnostic, Giza, Egypt),
Assessment of colon calcium contents
Colon Ca2+ contents were colorimetrically assessed according to the method described by Gindler and King (1972) using colorimetric assay kit, Biodiagnostic Company (Giza, Egypt), according to supplied manufacturer’s instructions.
Assessment of colon tumor necrosis factor-α (TNF-α), nuclear factor, erythroid derived 2 like protein 2 (Nrf2) and heme oxygenase-1 (HO-1) contents
Colon content of inflammatory cytokine, TNF-α was quantified using commercially available ELISA assay kits (eBioscience Inc., San Diego, CA, USA), and colon Nrf2 and HO-1 contents were quantified using ELISA assay kits (Cloud-Clone Corp., Houston, USA). Test procedures were carried out according to the provided manufacturer’s instructions.
Histopathological examination and immunohistochemical analysis of caspase-3 expression
A 3-cm segment of the lower colon was separated and fixed in 10% neutral-buffered formalin solution (pH 7.4). After fixation, 3-µm-thick sections were prepared and stained with haematoxylin and eosin (H&E) for light microscopic evaluation of AA-induced damage especially to colonic mucosal and submucosal layers and response to CoQ10 administration. For immunohistochemical analysis, coated slides were prepared and stained with rat anticaspase-3 antibody (Sigma Aldrich, MO, USA) to assess the extent of caspase-3 expression within colon. The tissues were examined under a microscope in a random order using Olympus CX21 microscope and the histopathologist was blinded to experimental groups.
Statistical analysis
The results are expressed as mean ± SE. Statistical analyses were performed using ANOVA followed by Tukey–Kramer multiple comparison tests for statistical comparison between parametric data and Kruskal–Wallis test followed by Dunn’s multiple comparison tests for statistical comparison between non-parametric data. Linear regression analysis for the best fitting line of all the standard points was also performed. p < 0.05 was accepted as being statistically significant.
Results
Effect of either prophylactic or curative CoQ10 (10 or 30 and 100 mg/kg, orally) for 8 consecutive days on acetic acid-induced changes in colon/body weight index and colon weight/colon length ratio
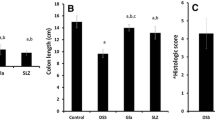
Colon/body weight index and colon weight/colon length ratio of AA control was significantly high compared to normal control group by approximately 2.34- and 4.8-folds, respectively, for prophylactic regimen and approximately 2.31- and 6.3-folds, respectively, for curative regimen (Fig. 1a, b).
Effect of either prophylactic or curative CoQ10 (10 or 30 and 100 mg/kg, orally) for 8 days on acetic acid-induced change in colon/body weight index and colon weight/colon length ratio. Data are expressed as mean ± SEM, n = 8. Statistical analysis was performed using one-way ANOVA followed by Tukey–Kramer multiple comparison test (p < 0.05). *Significantly different from normal control group. #Significantly different from AA-control group
In a dose-dependent way, coenzyme Q10 (10 mg/kg) significantly reduced colon/body weight index and colon weight/colon length ratio by 32.2 and 46.8%, respectively, for prophylactic regimen and by 25.6 and 49.2%, respectively, for curative regimen compared to the AA-control group, (Fig. 1a, b). However, 30 mg/kg CoQ10 significantly reduced colon/body weight index and colon weight/colon length ratio by 41.2 and 61%, respectively, for prophylactic regimen and by 60.5 and 69.2%, respectively, for curative regimen compared to the AA control, (Fig. 1a, b). 100 mg/kg CoQ10 induced further significant decrease in colon/body weight index and colon weight/colon length ratio by 47 and 76%, respectively, in the prophylactic regimen and by 76.3 and 80.4%, respectively, in the curative regimen compared to AA-control group, so that, almost, values observed with normal control were restored (Fig. 1a, b).
Effect of either prophylactic or curative CoQ10 (10 or 30 and 100 mg/kg, orally) for 8 days on acetic acid-induced change in macroscopic scoring and clinical evaluation of ulcerative colitis
In both the prophylactic and curative regimens, macroscopic scoring and clinical evaluation of colitis were strongly correlated. Intracolonic AA instillation forced a significant increase in macroscopic scoring and clinical evaluation of colitis in the prophylactic and curative AA-controls compared to normal control as shown in (Fig. 2a, b).
Effect of either prophylactic or curative CoQ10 (10 or 30 and 100 mg/kg, orally) for 8 days on acetic acid-induced change in macroscopic scoring and clinical evaluation. Data are expressed as mean ± SEM, n = 8. Statistical analysis was performed using Kruskal–Wallis test followed by Dunn’s multiple comparison test (p < 0.05). *Significantly different from normal control group. #Significantly different from AA-control group
Prophylactic and curative daily administration of 10 and 30 mg/kg CoQ10 induced a non-significant reduction in macroscopic scoring and clinical evaluation compared to AA control, (Fig. 2a, b). 100 mg/kg CoQ10 significantly decreased both macroscopic scoring and clinical evaluation by 68 and 90%, respectively, in prophylactic regimen and 95 and 94%, respectively, in curative regimen compared to the AA control as shown in (Fig. 2a, b).
Effect of either prophylactic or curative CoQ10 (10 or 30 and 100 mg/kg, orally) for 8 days on acetic acid-induced change in serum LDH activity and CRP titer
Serum LDH activity significantly increased by approximately 2.4- and 3.2-folds and serum CRP titer also significantly increased by approximately 3.3- and 5.1-folds compared to normal control in prophylactic and curative protocols, respectively, following intracolonic AA instillation (Table 1).
Prophylactic and curative daily oral CoQ10 (10 and 30 mg/kg) for 8 days significantly reduced serum LDH activity by approximately 22 and 38%, respectively, in prophylactic regimen and by approximately 40.8 and 50%, respectively, in curative regimen in comparison to AA control, but LDH activity was still significantly high in comparison to normal control. On the other hand, (100 mg/kg) CoQ10 significantly reduced serum LDH activity by 52.6% in prophylactic regimen and by 66.66% in curative regimen compared to AA control (Table 1).
Likewise, prophylactic and curative daily oral CoQ10 (10 and 30 mg/kg, orally) for 8 days significantly reduced serum CRP titer in comparison to AA control by approximately 36.5 and 41.4%, respectively, in prophylactic regimen and 25 and 57%, respectively, in curative regimen, but titer was still significantly high in comparison to normal control. On the other hand, CoQ10 (100 mg/kg) significantly reduced serum CRP titer by 66% in prophylactic regimen and by 79% in curative regimen compared to the AA control; normal titer was restored (Table 1).
Effect of either prophylactic or curative CoQ10 (10 or 30 and 100 mg/kg, orally) for 8 days on acetic acid-induced change in serum TAC and catalase activity
A-Serum TAC
In either prophylactic or curative regimen, AA (2 ml, 3% v/v, and intracolonic) triggered significant reduction in serum TAC in comparison to normal control. Serum TAC was reduced to about one-third capacity in both prophylactic and curative regimens compared to normal control (Table 2).
Coenzyme Q10 (10 mg/kg) induced a non-significant increase in serum TAC compared to AA control in both prophylactic and curative regimens (Table 2). As demonstrated in Table 2, CoQ10 (30 mg/kg) induced further improvement with a significant increase in serum TAC level by 117 and 130% compared to AA control. Optimum improvement was observed with CoQ10 (100 mg/kg) where a significant increase in serum TAC by 191 and 168% compared to the AA control was observed without any significant difference compared to normal control.
B-Catalase
Intracolonic AA (2 ml, 3% v/v) significantly reduced serum catalase activity in comparison to normal control by about 50.7 and 51.9%, respectively, with both regimens as shown in (Table 2).
CoQ10 (10 mg/kg) triggered a significant increase in catalase activity by 40.8 and 41.6% compared to respective AA control. Prophylactic and curative CoQ10 (30 and 100 mg/kg) significantly restored serum catalase activity; serum catalase increased by 88 and 93% in the prophylactic regimen and 76 and 92.3% with curative regimen, respectively, compared to respective AA control without any significant difference in comparison to respective normal control as seen in (Table 2).
Effect of either prophylactic or curative CoQ10 (10 or 30 and 100 mg/kg, orally) for 8 days on acetic acid-induced change in colonic oxidants/antioxidant stress markers; malondialdehyde (MDA) content, superoxide dismutase (SOD) activity and reduced glutathione (GSH) concentration
A-Prophylactic regimen
Intracolonic AA instillation (2 ml, 3% v/v) induced a threefold increase in colon MDA content with significant reduction in colon SOD activity and GSH concentration to about one-third (0.32) and (0.17) values observed with either respective normal controls as shown in (Table 3).
Coenzyme Q10 (10 or 30 and 100 mg/kg, orally) significantly ameliorated AA-induced damage to different extents. Coenzyme Q10 (10 mg/kg) reduced colonic MDA content and restored SOD activity by 17 and 57%, respectively, but without significant increase in GSH concentration compared to AA control, while CoQ10 (30 mg/kg) significantly reduced colonic MDA content and increased SOD activity and GSH concentration by 20, 156.6 and 175%, respectively, compared to AA control. Optimum improvement was observed with CoQ10 (100 mg/kg) with significant reduction in colonic MDA content and increase of SOD activity and GSH concentration by 56, 196 and 455%, respectively, compared to AA control (Table 3).
B-Curative regimen
Intracolonic AA instillation (2 ml, 3% v/v,) induced a 3.4-fold increase in colon MDA content with significant reduction in colon SOD activity and GSH concentration to about one-third (0.3) and by (0.06) values observed in either respective normal controls (Table 3).
Coenzyme Q10 (10 mg/kg) reduced colonic MDA content and restored SOD activity with significant increase in GSH concentration by (0.7), (1.8) and (5) values observed in either respective AA control (Table 3), while CoQ10 (30 mg/kg) significantly reduced colonic MDA content and increased SOD activity and GSH concentration by (0.65), (2.7) and (10) values observed in either respective AA control (Table 3). Further improvement was observed with CoQ10 (100 mg/kg) with a significant reduction in colonic MDA content and increase in SOD activity and GSH concentration by (0.36), (3.4) and (15) values observed in either respective AA control, without any significant difference from normal control (Table 3).
Effect of either prophylactic or curative CoQ10 (10 or 30 and 100 mg/kg, orally) for 8 days on acetic acid-induced change in Colon TNF-α content and Ca2+ levels
In either prophylactic or curative regimens, intracolonic AA instillation (2 ml, 3% v/v) induced a significant increase in colon TNF-α content by about 3.1- and 4-folds, respectively, compared to normal control (Table 4). However, CoQ10 (10 or 30 and 100 mg/kg, orally) for 8 days in both prophylactic and curative regimen resulted in a dose-dependent reduction in colon TNF-α content compared to AA control; colon TNF-α content significantly declined by approximately 43, 59, 67% in prophylactic regimen and 20, 63, 74% in curative regimen, respectively, compared to AA control, (Table 4).
In either prophylactic or curative regimens, intracolonic AA instillation (2 ml, 3% v/v,) induced a significant increase in Ca2+ content by about 1.9- and 1.8-folds, respectively, compared to normal control (Table 4). Coenzyme Q10 (10 or 30 and 100 mg/kg, orally) in both prophylactic and curative regimens failed to induce any significant reduction in Ca2+ content compared to AA control.
Effect of either prophylactic or curative CoQ10 (10 or 30 and 100 mg/kg, orally) for 8 days on acetic acid-induced changes in colon Nrf2 and HO-1 contents
As demonstrated in Table 5, colon Nrf2 and HO-1 contents significantly declined following intracolonic instillation of AA (2 ml, 3% v/v) compared to normal control. Colon Nrf2 content significantly declined to about one-third (0.35) and one-fifth (0.21) of normal contents in prophylactic and curative regimens, respectively, compared to normal control (Table 5). Likewise, HO-1 content significantly declined to about (0.44) and one-fifth (0.2) of normal contents in prophylactic and curative regimens, respectively, compared to normal control (Table 5).
Coenzyme Q10 (10 or 30 and 100 mg/kg, orally) for 8 days either in prophylactic or curative regimen induced a dose-dependent restoration of colon Nrf2 and HO-1 contents compared to AA control; colon Nrf2 contents significantly increased by 87, 120, 254% in prophylactic regimen and 182, 274, 428% in curative regimen. Also, HO-1 significantly increased by 8.5, 31, 113% in prophylactic regimen and 24, 75, 345% in curative regimen (Table 5).
Effect of either prophylactic or curative CoQ10 (10 or 30 and 100 mg/kg, orally) for 8 days on acetic acid-induced histopathological changes in hematoxylin and eosin (H&E)-stained colon specimen
A-Prophylactic regimen
As shown in (Fig. 3), colon sections from the normal control stained with H&E revealed normal colon architecture with normal mucosa and submucosa without any evidence of any necro-inflammatory damage or tissue injury (Fig. 3a). However, colon specimen from AA control revealed widely ulcerated mucosa with multiple epithelial erosions and severe inflammatory cells infiltration (Fig. 3b) (Table 6).
Haematoxylin-and-eosin-stained colonic sections showing the effect of prophylactic CoQ10 (10 or 30 and 100 mg/kg, orally) for 8 days on AA-induced ulcerative colitis (×200). a Normal control group showing normal mucosa and underlying submucosa. b AA control showing widely ulcerating mucosa and severe inflammatory cells infiltrate. c AA/CoQ10 (10 mg/kg orally) showing mucosal ulceration with epithelial loss and severe inflammatory cells infiltrate. d AA/CoQ30 (30 mg/kg orally) showing repairing mucosal epithelial injury with mild inflammatory cells infiltrate. e AA/CoQ100 (100 mg/kg orally) showing healing mucosa with minimal inflammatory infiltrate. mu mucosal epithelium, u ulceration, I infiltration
Coenzyme Q10 (10 mg/kg) elicited mild improvement; mucosal ulcers with epithelial loss and severe inflammatory cells infiltrate were detected (Fig. 3c). CoQ10 (30 mg/kg) induced further recovery; mucosal epithelial injury was downregulated with fewer inflammatory cells (Fig. 3d). On the other hand, (100 mg/kg) CoQ10 for 8 days induced optimal improvement where reparative epithelial changes were observed, healed ulcer (Fig. 3e) (Table 6).
B-Curative regimen
As shown in (Fig. 4), colon sections from the normal control groups stained with H&E revealed normal colon architecture and normal mucosa, submucosa without any evidence of any necro-inflammatory damaged or tissue injury (Fig. 4a). But colon specimen following intracolonic instillation of AA (2 ml, 3% v/v) revealed massive ulcerative hemorrhagic colitis, loss of entire necrotic mucosa with significant inflammatory infiltrate (Fig. 4b) (Table 6).
Hematoxylin–and-eosin-stained colonic sections showing the effect of curative CoQ10 (10 or 30 and 100 mg/kg, orally) for 8 days on AA-induced UC (×200). a Normal control group showing normal mucosa and underlying submucosa. b AA control showing mucosal ulceration and severe inflammatory cells infiltrate. c AA/CoQ10 (10 mg/kg orally) showing ulcerated mucosa and severe inflammatory cells infiltrate. d AA/CoQ30 (30 mg/kg orally) showing repairing mucosal epithelial injury with mild inflammatory cells infiltrate. e AA/CoQ100 (100 mg/kg orally) showing healed mucosal epithelium with minimal inflammatory infiltrate. mu mucosal epithelium, u ulceration, I infiltrate
Coenzyme Q10 (10 mg/kg) resulted in minimal improvement; mucosal ulcers, epithelial loss and severe inflammation were detected (Fig. 4c). Coenzyme Q10 (30 mg/kg) ameliorated mucosal epithelial injury; only mild inflammatory cells infiltration was detected (Fig. 4d). Finally, (100 mg/kg) CoQ10 for 8 days induced complete mucosal ulcers healing with minimal inflammatory infiltration (Fig. 4e) (Table 6).
Effect of either prophylactic or curative CoQ10 (10 or 30 and 100 mg/kg, orally) for 8 days on acetic acid-induced activation of colon apoptosis; immunohistochemical analysis of caspase-3 expression
Normal control in either prophylactic or treatment regimen revealed negative immunostaining for caspase-3 (Figs. 5a, 6a), respectively. On the contrary, intracolonic instillation of AA (2 ml, 3% v/v) either in prophylactic or curative regimen induced strong caspase-3 expression confirming significant apoptotic activity in colon tissue (Figs. 5b, 6b).
Effect of prophylactic CoQ10 (10 or 30 and 100 mg/kg, orally) for 8 days on AA-induced activation of colon apoptosis; immunohistochemical analysis of caspase-3 expression (×200). a Normal control showing negative immunostaining. b AA control showing strong caspase-3 expression. c AA/CoQ10 (10 mg/kg orally) showing severe to moderate caspase-3 expression. d AA/CoQ30 (30 mg/kg orally) showing moderate caspase-3 expression. e AA/CoQ100 (100 mg/kg orally) showing mild caspase-3 expression
Effect of curative CoQ10 (10 or 30 and 100 mg/kg, orally) for 8 days on acetic acid-induced activation of colon apoptosis; immunohistochemical analysis of caspase-3 expression (×200). a Normal control group showing negative immunostaining. b AA control showing strong caspase-3 expression. c AA/CoQ10 (10 mg/kg orally) showing severe to moderate caspase-3 expression. d AA/CoQ30 (30 mg/kg orally) showing moderate caspase-3 expression. e AA/CoQ100 (100 mg/kg orally) showing negative caspase-3 expression
Prophylactic and curative daily oral CoQ10 (10 or 30 and 100 mg/kg) dose-dependently downregulated colon caspase-3 expression. Severe to moderate caspase-3 expression was detected in colonic specimen from rats treated with (10 mg/kg) CoQ10 either in prophylactic or curative regimens (Figs. 5c, 6c). Coenzyme Q10 (30 mg/kg) in prophylactic and curative regimens downregulated caspase-3 expression; only moderate expression (Figs. 5d, 6d) was detected in the examined specimen. Only mild caspase-3 expression was detected in specimen isolated from rats treated with CoQ10 (100 mg/kg) in both prophylactic and curative regimens (Figs. 5e, 6e).
Effect of either prophylactic or curative CoQ10 (10 or 30 and 100 mg/kg, orally) for 8 days on correlation between TAC and clinical evaluation, macroscopic scoring or MDA of ulcerative colitis
Prophylactic and curative CoQ10 (10 or 30 and 100 mg/kg, orally) administration revealed strong significant correlation between TAC and clinical evaluation, macroscopic scoring or MDA. In prophylactic regimen, the correlation between TAC and clinical evaluation, macroscopic scoring or MDA was −0.98, −0.95 or −0.96, respectively, (Fig. 7). Also, in curative regimen, the correlation between TAC and clinical evaluation, macroscopic scoring or MDA was −0.97, −0.96 or −0.93, respectively (Fig. 8).
Effect of either prophylactic or curative CoQ10 (10 or 30 and 100 mg/kg, orally) for 8 days on correlation between MDA and Nrf2, HO-1 or TNF-α
Prophylactic and curative CoQ10 (10 or 30 and 100 mg/kg, orally) showed a strong and significant correlation between MDA and Nrf2, HO-1 or TNF-α. In prophylactic regimen, the correlation coefficient between MDA and Nrf2, HO-1 or TNF-α was −0.89, −0.98 or 0.82, respectively (Fig. 9). While in the curative regimen, the correlation between MDA and Nrf2, HO-1 or TNF-α was −0.95, −0.92 or 0.91, respectively (Fig. 10).
Effect of either prophylactic or curative CoQ10 (10 or 30 and 100 mg/kg, orally) for 8 days on correlation between TAC and Nrf2, HO-1 or TNF-α
Prophylactic and curative CoQ10 (10 or 30 and 100 mg/kg, orally) showed a strong significant correlation between TAC and Nrf2, HO-1 or TNF-α. In prophylactic regimen, correlation between TAC and Nrf2, HO-1 or TNF-α was 0.94, 0.89 or −0.98, respectively (Fig. 11). In the curative regimen, correlation between TAC and either Nrf2, HO-1 or TNF-α was 0.906, 0.96 or −0.879, respectively (Fig. 12).
Discussion
The current study sheds light on the coloprotective effect of CoQ10 against experimentally induced UC. Moreover, the results of the current study highlight dose-dependent protective effect of CoQ10 and define potential mechanisms implicated in the observed coloprotective effect. The observed coloprotective effect was proven to be attributed to anti-inflammatory, antioxidant and anti-apoptic effects mainly through modulatory effect on Nrf2/HO-1 pathway.
A well-standardized experimental model was used for induction of UC. Acetic acid is an important colitis-inducing agent (El Morsy et al. 2015). Progression of AA-induced UC was associated with significant colonic macroscopical and biochemical alterations; increased serum LDH activity, CRP titer and colon content of MDA, TNF-α and Ca2+ with concomitant reduction in serum TAC, colon SOD activity, CAT activity, GSH concentration, Nrf2 and HO-1 contents. Ultimately, this was accompanied by significant histopathological colonic damage and increased colonic expression of caspase-3. These results are consistent with previous observations of Al-Rejaie et al. (2013) and Malago and Sangu (2015).
Giris et al. (2008) reported increased oxidative stress and apoptotic activity to be implicated in the pathogenesis of UC with reduced antioxidant capacity and increased free radical production such as ROS (Lih-Brody et al. 1996). Free radicals have been detected in colorectal specimens of animal models of UC (Bitiren et al. 2010). Moreover, Seidelin (2015) confirmed apoptosis to be implicated in the pathogenic pathway of UC.
A therapeutic agent, that can significantly interfere with critical checkpoints within the pathogenic pathway of UC, of note, increased oxidative stress, inflammatory cytokines expression and apoptosis, can be expected to offer significant protection against AA-induced cellular, biochemical and functional damage of colon.
In the current study, CoQ10 dose-dependently decreased the colon MDA content, suggesting inhibitory effect on lipid peroxidation and generation of reactive oxygen species (ROS), paralleled with increased serum TAC, colon content of GSH, SOD and catalase activities suggesting significant boost of antioxidant defenses. Such improvements in oxidative/antioxidant balance were paralleled with significant reduction in serum LDH activity and CRP titer, suggesting concomitant cytoprotective impact.
Significant reduction in colon TNFα content confirms anti-inflammatory and immune-modulatory effect of CoQ10 especially at higher doses. The particular anti-inflammatory effect of CoQ10 is confirmed by mitigation of AA-induced histological alterations; of particular importance is the significant downregulation of apoptic and inflammatory reaction. Mild caspase-3 expression was detected with high dose of CoQ10 indicating retraction of apoptosis. These finding is in line with El-Sheikh et al. (2012). In context, Papucci et al. (2003) reported CoQ10-mediated anti-apoptotic activity as an essential mechanism in its therapeutic merits.
The inflamed colon is related to oxidative stress (Al-Rejaie et al. 2013). With this in mind, it could be presumed that downregulation of ROS production and enhancement of antioxidant defense can contribute to preservation of cellular integrity and subsequent structural, biochemical and physiological improvements (Said et al. 2016).
Antioxidant properties of CoQ10 were accompanied by significant reduction in AA-induced pathological changes with restoration of normal biochemical balance and cellular hemostasis. Coenzyme Q10 has been reported to possess a gastro-protective effect mediated primarily via its antioxidant properties (El-Abhar 2010).
Coenzyme Q10 is a cofactor in the mitochondrial respiratory chain and plays an important role in ATP production (Bhagavan and Chopra 2006). Most ROS are generated in the mitochondria, which generate ATP and supply cellular energy requirements. Of notice, mitochondria are a very important target for oxidative stress (Greco et al. 2011). Increased mitochondrial oxidative stress increases oxidative injury and suppresses electron transport of the respiratory chain, ultimately reducing ATP production (Chi et al. 2015), driving metabolic failure, oncosis and apoptosis (Lee et al. 2001). Hence, by replenishing ATP stores and preserving ATP production, CoQ10 can be presumed to protect mitochondria against AA-induced increased ROS production, metabolic failure and apoptosis.
A physiological function of catalase is to counteract oxidative stress which plays an important role in colitis induction. Colon catalase activity declined following intracolonic AA instillation. Several antioxidants, amongst other substances used as experimental treatments for UC, are proposed to protect against free radical-induced injury in various bowel diseases. Enhanced catalase activity mitigated the harmful effects of free radicals and reduced the number of reactive metabolites driven by AA (Al-Rejaie et al. 2013).
Enzymes such as SOD and CAT, and also non-enzymatic antioxidants like GSH, constitute the antioxidant system, protecting the cell against oxidative damage. GSH is an essential contributor in cellular antioxidant defenses, and acts by scavenging free radicals and other ROS. Acetic acid-induced oxidative stress depleted GSH stores (Cetinkaya et al. 2005), and the reduction of GSH may, in turn, aggravate AA-induced UC, probably via weakening of the antioxidant defense.
Maintenance of reduced GSH concentration is strongly believed to block lipid peroxidation and to restore cellular defense mechanisms and thereby protect against oxidative damage to the tissues. In accordance with these findings, the current results verify that CoQ10 maintained and boosted colonic GSH concentrations. The coloprotective action of the CoQ10 can be proposed to be mediated, in part, by replenishing the depleted GSH.
SOD is another key enzyme in cellular antioxidant defenses. It inactivates superoxide ion by converting it into its more stable metabolite, hydrogen peroxide (Cetinkaya et al. 2006). It restrains lipid peroxidation in colon by eliminating free radicals and ROS. Accumulated researches indicated that decreased SOD activity in colon tissues aggravated mucosal injury. Coenzyme Q10 significantly suppressed lipid peroxidation, prevented AA-induced reduction of SOD activity and restored MDA levels. The coloprotective effect of CoQ10 can be attributed to its ability to inhibit the generation of ROS. This result is in agreement with previous result reporting similar observations (El-Sheikh et al. 2012; Kandhare et al. 2013).
Nrf2 is the key transcription factor regulating the antioxidant response crucial for cytoprotection against extracellular stresses (Khor et al. 2006). Nrf2 is a cellular sensor of oxidative stresses. It controls expression and programmed induction of various defensive genes encoding antioxidant proteins and detoxifying enzymes. Thus, disruption or loss of Nrf2 signaling is believed to enhance susceptibility not only to oxidative and electrophilic stresses but also to tissue injuries (Khor et al. 2006). Moreover, in vivo study of Rangasamy et al. (2005) proved Nrf2 implication in the regulation of inflammatory process.
Coordinated expression of cytoprotective and anti-oxidative genes by activation of Nrf2 signaling is essential for body’s protection against various inflammatory tissue injuries. Recent studies have reported Nrf2/ARE signaling to be involved in attenuation of inflammation-associated pathogenesis of several disorders including colitis (Kim et al. 2010). In the current study, colonic Nrf2 expression was remarkably restored with CoQ10 administration. Coenzyme Q10 not only prevented AA-induced reduction in colon Nrf2 content, but also increased its levels in a dose-dependent manner.
Interestingly, HO-1 is believed to be amongst enzymes upregulated post Nrf2 stimulation. HO-1 is believed to exhibit anti-inflammatory activities by inhibiting the production of pro-inflammatory mediators in a variety of cells (Kim et al. 2014). It has pronounced anti-inflammatory as well as anti-oxidative properties. Also, it is a critical regulator and modulator of innate immunity and inflammation. HO-1 has been reported to be upregulated in acute inflammatory disorders (Yalniz et al. 2012), specifically intestinal inflammation (Naito et al. 2004). In the current study, CoQ10 administration restored HO-1 levels in a dose-dependent manner. This result confirms the previous findings reporting HO-1 to mediate important homeostatic pathway with anti-inflammatory effects in different experimental models of colitis (Sheikh et al. 2011; Yalniz et al. 2012).
In association with the observed improvement in colonic Nrf2 and HO-1 contents, colon TNF-α content significantly declined in a dose-dependent manner with CoQ10 administration, proving the association between antioxidant and anti-inflammatory potential of CoQ10 in the observed therapeutic effect.
In conclusion, coenzyme Q10 significantly ameliorated AA-induced UC. The observed amelioration of colon function and physiology with CoQ10 treatment could be proposed to be mediated via multiple mechanisms including: (1) reduction of colitis-induced augmentation of oxidative stress; (2) blockade of colitis-induced inhibition of antioxidant activity; (3) blockade of colitis-induced elevation of pro-inflammatory cytokines TNF-α; (4) inhibiting colitis-induced depletion of antioxidant defense cytokines, Nrf2 and HO-1; (5) reduction of colitis-induced apoptosis and caspase-3 expression. It is worth mentioning that anti-inflammatory, antioxidant and anti-apoptic potentials of CoQ10 are dose-dependent. It appears that modulatory effect of CoQ10 on Nrf2/HO-1 is the major mechanism implicated in the observed therapeutic potential (Table 6).
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Al-Rejaie SS, Abuohashish HM, Al-Enazi MM, Al-Assaf AH, Parmar MY, Ahmed MM (2013) Protective effect of naringenin on acetic acid-induced ulcerative colitis in rats. World J Gastroenterol 19(34):5633–5644
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Bhagavan HN, Chopra RK (2006) Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res 40(5):445–453
Biasi F, Astegiano M, Maina M, Leonarduzzi G, Poli G (2011) Polyphenol supplementation as a complementary medicinal approach to treating inflammatory bowel disease. Curr Med Chem 18(31):4851–4865
Bitiren M, Karakilcik AZ, Zerin M, Ozardali I, Selek S, Nazligul Y, Ozgonul A, Musa D, Uzunkoy A (2010) Protective effects of selenium and vitamin E combination on experimental colitis in blood plasma and colon of rats. Biol Trace Elem Res 136(1):87–95
Brito TV, Neto JP, Prudencio RS, Batista JA, Junior JS, Silva RO, Franco AX, Aragao KS, Soares PM, Souza MH, Chaves LS, Freitas AL, Medeiros JV, Barbosa AL (2014) Sulfated-polysaccharide fraction extracted from red algae Gracilaria birdiae ameliorates trinitrobenzenesulfonic acid-induced colitis in rats. J Pharm Pharmacol 66(8):1161–1170
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Cetinkaya A, Bulbuloglu E, Kurutas EB, Ciralik H, Kantarceken B, Buyukbese MA (2005) Beneficial effects of N-acetylcysteine on acetic acid-induced colitis in rats. Tohoku J Exp Med 206(2):131–139
Cetinkaya A, Bulbuloglu E, Kantarceken B, Ciralik H, Kurutas EB, Buyukbese MA, Gumusalan Y (2006) Effects of l-carnitine on oxidant/antioxidant status in acetic acid-induced colitis. Dig Dis Sci 51(3):488–494
Chi X, Zhang R, Shen N, Jin Y, Alina A, Yang S, Lin S (2015) Sulforaphane reduces apoptosis and oncosis along with protecting liver injury-induced ischemic reperfusion by activating the Nrf2/ARE pathway. Hepatol Int 9(2):321–329
de Melo MN, Soares LA, Porto CR, de Araujo AA, Almeida M, de Souza TP, Petrovick PR, de Araujo RF Jr, Guerra GC (2015) Spray-dried extract of Phyllanthus niruri L. reduces mucosal damage in rats with intestinal inflammation. J Pharm Pharmacol 67(8):1107–1118
El Morsy EM, Kamel R, Ahmed MA (2015) Attenuating effects of coenzyme Q10 and amlodipine in ulcerative colitis model in rats. Immunopharmacol Immunotoxicol 37(3):244–251
El-Abhar HS (2010) Coenzyme Q10: a novel gastroprotective effect via modulation of vascular permeability, prostaglandin E(2), nitric oxide and redox status in indomethacin-induced gastric ulcer model. Eur J Pharmacol 649(1–3):314–319
El-Sheikh AA, Morsy MA, Mahmoud MM, Rifaai RA, Abdelrahman AM (2012) Effect of coenzyme-q10 on doxorubicin-induced nephrotoxicity in rats. Adv Pharmacol Sci 2012:981461
Gindler EM, King JD (1972) Rapid colorimetric determination of calcium in biologic fluids with methylthymol blue. Am J Clin Pathol 58(4):376–382
Giris M, Depboylu B, Dogru-Abbasoglu S, Erbil Y, Olgac V, Alis H, Aykac-Toker G, Uysal M (2008) Effect of taurine on oxidative stress and apoptosis-related protein expression in trinitrobenzene sulphonic acid-induced colitis. Clin Exp Immunol 152(1):102–110
Greco T, Shafer J, Fiskum G (2011) Sulforaphane inhibits mitochondrial permeability transition and oxidative stress. Free Radic Biol Med 51(12):2164–2171
Hao Y, Nagase K, Hori K, Wang S, Kogure Y, Fukunaga K, Kashiwamura S, Yamamoto S, Nakamura S, Li J, Miwa H, Noguchi K, Dai Y (2014) Xilei san ameliorates experimental colitis in rats by selectively degrading proinflammatory mediators and promoting mucosal repair. Evid Based Complement Altern Med eCAM 2014:569587
Kandhare AD, Ghosh P, Ghule AE, Bodhankar SL (2013) Elucidation of molecular mechanism involved in neuroprotective effect of coenzyme Q10 in alcohol-induced neuropathic pain. Fundam Clin Pharmacol 27(6):603–622
Kawabata K, Tung NH, Shoyama Y, Sugie S, Mori T, Tanaka T (2012) Dietary crocin inhibits colitis and colitis-associated colorectal carcinogenesis in male ICR mice. Evid Based Complement Altern Med eCAM 2012:820415
Keshavarzian A, Haydek J, Zabihi R, Doria M, D’Astice M, Sorenson JR (1992) Agents capable of eliminating reactive oxygen species. Catalase, WR-2721, or Cu(II)2(3,5-DIPS)4 decrease experimental colitis. Dig Dis Sci 37(12):1866–1873
Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, Kong AN (2006) Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res 66(24):11580–11584
Kim J, Cha YN, Surh YJ (2010) A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res 690(1–2):12–23
Kim JH, Park GY, Bang SY, Park SY, Bae SK, Kim Y (2014) Crocin suppresses LPS-stimulated expression of inducible nitric oxide synthase by upregulation of heme oxygenase-1 via calcium/calmodulin-dependent protein kinase 4. Mediators Inflamm 2014:728709
Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V (2001) Method for the measurement of antioxidant activity in human fluids. J Clin Pathol 54(5):356–361
Kucharska J, Braunova Z, Ulicna O, Zlatos L, Gvozdjakova A (2000) Deficit of coenzyme Q in heart and liver mitochondria of rats with streptozotocin-induced diabetes. Physiol Res 49(4):411–418
Lee JM, Moehlenkamp JD, Hanson JM, Johnson JA (2001) Nrf2-dependent activation of the antioxidant responsive element by tert-butylhydroquinone is independent of oxidative stress in IMR-32 human neuroblastoma cells. Biochem Biophys Res Commun 280(1):286–292
Lee J, Hong YS, Jeong JH, Yang EJ, Jhun JY, Park MK, Jung YO, Min JK, Kim HY, Park SH, Cho ML (2013) Coenzyme Q10 ameliorates pain and cartilage degradation in a rat model of osteoarthritis by regulating nitric oxide and inflammatory cytokines. PLoS ONE 8(7):e69362
Lih-Brody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, Weissman GS, Katz S, Floyd RA, McKinley MJ, Fisher SE, Mullin GE (1996) Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci 41(10):2078–2086
Liu Y, Xiang J, Liu M, Wang S, Lee RJ, Ding H (2011) Protective effects of glycyrrhizic acid by rectal treatment on a TNBS-induced rat colitis model. J Pharm Pharmacol 63(3):439–446
Malago JJ, Nondoli H (2008) Sodium arsenite reduces severity of dextran sulfate sodium-induced ulcerative colitis in rats. J Zhejiang Univ Sci B 9(4):341–350
Malago JJ, Sangu CL (2015) Intraperitoneal administration of butyrate prevents the severity of acetic acid colitis in rats. J Zhejiang Univ Sci B 16(3):224–234
Millar AD, Rampton DS, Chander CL, Claxson AW, Blades S, Coumbe A, Panetta J, Morris CJ, Blake DR (1996) Evaluating the antioxidant potential of new treatments for inflammatory bowel disease using a rat model of colitis. Gut 39(3):407–415
Mustafa A, El-Medany A, Hagar HH, El-Medany G (2006) Ginkgo biloba attenuates mucosal damage in a rat model of ulcerative colitis. Pharmacol Res 53(4):324–330
Naito Y, Takagi T, Yoshikawa T (2004) Heme oxygenase-1: a new therapeutic target for inflammatory bowel disease. Aliment Pharmacol Ther 20(Suppl 1):177–184
Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46(2):849–854
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Papucci L, Schiavone N, Witort E, Donnini M, Lapucci A, Tempestini A, Formigli L, Zecchi-Orlandini S, Orlandini G, Carella G, Brancato R, Capaccioli S (2003) Coenzyme q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J Biol Chem 278(30):28220–28228
Parmar AR, Trivedi PP, Jena GB (2014) Dextran sulfate sodium-induced ulcerative colitis leads to testicular toxicity in mice: role of inflammation, oxidative stress and DNA damage. Reprod Toxicol 49C:171–184
Randhawa PK, Singh K, Singh N, Jaggi AS (2014) A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol 18(4):279–288
Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S (2005) Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med 202(1):47–59
Said E, Elkashef WF, Abdelaziz RR (2016) Tranilast ameliorates cyclophosphamide-induced lung injury and nephrotoxicity. Can J Physiol Pharmacol 94(4):347–358
Seidelin JB (2015) Regulation of antiapoptotic and cytoprotective pathways in colonic epithelial cells in ulcerative colitis. Scand J Gastroenterol 50(Suppl 1):1–29
Sheikh SZ, Hegazi RA, Kobayashi T, Onyiah JC, Russo SM, Matsuoka K, Sepulveda AR, Li F, Otterbein LE, Plevy SE (2011) An anti-inflammatory role for carbon monoxide and heme oxygenase-1 in chronic Th2-mediated murine colitis. J Immunol 186(9):5506–5513
Vassault A, Grafmeyer D, de Graeve J, Cohen R, Beaudonnet A, Bienvenu J (1999) Quality specifications and allowable standards for validation of methods used in clinical biochemistry. Ann Biol Clin 57(6):685–695
Vochyanova Z, Bartosova L, Bujdakova V, Fictum P, Husnik R, Suchy P, Smejkal K, Hosek J (2015) Diplacone and mimulone ameliorate dextran sulfate sodium-induced colitis in rats. Fitoterapia 101:201–207
Yalniz M, Demirel U, Orhan C, Bahcecioglu IH, Ozercan IH, Aygun C, Tuzcu M, Sahin K (2012) Nadroparin sodium activates Nrf2/HO-1 pathway in acetic acid-induced colitis in rats. Inflammation 35(3):1213–1221
Yao J, Wang JY, Liu L, Li YX, Xun AY, Zeng WS, Jia CH, Wei XX, Feng JL, Zhao L, Wang LS (2010) Anti-oxidant effects of resveratrol on mice with DSS-induced ulcerative colitis. Arch Med Res 41(4):288–294
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Khodir, A.E., Atef, H., Said, E. et al. Implication of Nrf2/HO-1 pathway in the coloprotective effect of coenzyme Q10 against experimentally induced ulcerative colitis. Inflammopharmacol 25, 119–135 (2017). https://doi.org/10.1007/s10787-016-0305-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-016-0305-0