Abstract
A comprehensive discussion/analysis of the published viscosity data of ethylene glycol, EG, in the temperature range from 260 (just above the freezing point) to 465 K was recently reported in this journal. It was found that some of the reported data sets significantly deviate from each other, and the largest discrepancies were found at the lower end of the temperature interval examined (Mebelli et al. in Int. J. Thermophys. 42:116, 2021). Hence, in this work, the densities and viscosities of EG were measured in the temperature interval starting in the supercooled region at 248 and extending up to 313 K. Well-established experimental techniques were employed, that is, pycnometry and laminar flow viscometry, the relative precision of which were better than 0.5 and 0.5 %, respectively. The density was found to linearly depend on temperature in the temperature range studied; the cubic expansion coefficient was found to be γ = (5.20 + 3.99 × 10−3 T K− 1) × 10−4 K−1. When our experimental density data were applied to calculate the dynamic viscosity values using the correlation dependence published in the aforementioned review (Mebelli et al. in Int. J. Thermophys. 42:116, 2021), the discrepancy between our experimental data and the calculated values is less than 2 % above the freezing point; however, in the supercooled region, the discrepancy increases up to 4 % at 248 K. When the cooling rate is higher than 10 K min− 1 and the sample mass is less than 5 mg, EG does not freeze; it undergoes glass formation (Tg = − 121 °C) as revealed in our DSC experiments. The Arrhenius plot for viscosity data was found to be nonlinear; from the Angell plot, it was concluded that EG is a moderately fragile liquid with the fragility index = 70.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ethylene glycol (EG) is the primary ingredient in antifreeze, which is used in automobile engines and plumbing to prevent damage due to ice formation. It is also a component in the sprays applied to airplane wings, which prevent the buildup of ice during flight. In addition, ethylene glycol aqueous mixtures are used for the cryopreservation of biological tissues and organs as well as their transportation over long distances. The characterization of the rheological properties of ethylene glycol in a low-temperature range and the clarification regarding the phase diagram of ethylene glycol–water mixtures is of interest for the aforementioned fields, as well as for making contributions to the general theory of liquids. A recent paper has comprehensively reviewed the data in the literature corresponding to the temperature-resolved dynamic viscosities of ethylene glycol [1]. In an effort to resolve the discrepancies between the published data, the authors of Ref. [1] produced a correlation based upon a sum of theoretical contributions to the viscosity, that is, the contribution to the viscosity in the dilute-gas limit, the initial density dependence term for moderately dense gases, a term for the long-range density fluctuations that occur in a fluid near its critical point, and a residual term that accounts for the contribution of all other effects to the viscosity of a fluid at high densities. As illustrated in Figure 5 of Ref. [1], at high (RT and above) temperatures, the deviation of the data reported in Ref. [2] from the correlation is as high as approximately 8 % [2]. At 300 K, the data from two publications deviate as much as 10 % from each other [2, 3]. Below 300 K, the data reported deviate about 5 % from the correlation values. However, the discrepancy between the data reported exceeds the sum of the absolute errors reported by the original authors [28, 29]. In Ref. [1], a comment was made that additional measurements at low temperatures could help resolve these differences. Such data would also be beneficial in the analogous studies of supercooled mixtures that include ethylene glycol as a component. More specifically, since ethylene glycol was first suggested as a primary component of antifreeze [4], there has been a lack of consensus in the scientific literature on its phase diagram with water, particularly regarding the number of eutectic points. Its phase diagram has been reported to contain one, two, or even three eutectic points, while some authors claim that there is a range of compositions in which the mixture will not freeze [4,5,6,7,8,9,10,11,12]. A combined effort by two independent research groups was carried out to incontestably establish the phase diagram of the system; two eutectic points were agreed upon [13]. A later DSC study reported two eutectic points for the mixture, in agreement with the combined effort of the two research groups [14]. Afterward, Fortes and Suard reported that the equimolar EG-water mixture would not freeze unless it was cooled to liquid helium temperature [15].
In the analysis of the thermodynamic properties of a liquid mixture, it is sometimes useful to determine the ideal behavior of the relevant property (in the context of this paper, the temperature-resolved density/viscosity), which is the weighted linear combination of the data corresponding to each component of the mixture. The comparison of the ideal behavior to the experimental data for the mixture can be used to comment on its corresponding thermodynamic properties. For example, the excess viscosity for a mixture, which is found by taking the difference between the ideal behavior and the experimental viscosity data, is said to be a measure of the strength of the interactions between the different component molecules in a mixture [16]. The Grunberg-Nissan interaction parameter, which is another assessment of the strength of the intermolecular interactions that are present between molecules of each component in a mixture, is also calculated based upon the ideal behavior [17]. Hence, it would be useful to characterize the rheological properties of ethylene glycol at temperatures as low as possible in order to accurately determine the difference between the ideal and measured values in the supercooled region.
Prompted by the findings of Ref. [1] and the aforementioned reasons, this work reports the low-temperature density and viscosity data for ethylene glycol including the values measured in the supercooled region. In addition, the effect of cooling rate on freezing/glass formation of EG was examined using a DSC technique and the data obtained were used to assess the EG fragility.
2 Experimental Methods
Anhydrous ethylene glycol (99.8 %) was purchased from ACROS Organics and used as received. Differential scanning calorimetry experiments were performed using a Q-2000 TA Instruments calorimeter; its calibration was performed by using sapphire and indium standards. The low-temperature calibration was verified using analytical grade purity cyclopentane. Fluke 52 II digital thermometers with K-type thermocouples were calibrated using a secondary standard platinum resistance thermometer.
2.1 Pycnometry
Low-temperature pycnometry was performed using a custom-made setup comprising a pycnometer, a dewar, a thermometer, and a cathetometer. The pycnometer was calibrated using deionized water at 20.0 °C and with toluene at low temperatures. Calibration of the pycnometer was performed five times, and the corresponding calibration diagram was produced to obtain the volume-per-unit-height gradient of the pycnometer stem. The cathetometer (Griffin and George Ltd., London) equipped with a Vernier scale enables a vertical displacement measurement with a 0.01 mm precision (Figs. 1 and 2).
For the measurements to determine the temperature-resolved density of ethylene glycol, the dewar was filled with water (above 278 K) or ethanol (below 278 K) to ensure thermal equilibration of the pycnometer during the measurements. Knowing the mass of the sample, the volume of sample in the reservoir, and the volume of sample in the stem, the density of the sample could be determined at a given temperature by using ρ = m V− 1 [18].
To obtain the densities of the sample in the supercooled region, a modification to the procedure was needed. In a typical experiment, one may find it difficult to avoid the regular crystallization of the sample due to either (a) the slow cooling rate or (b) the rapid contraction of the sample in the stem. To avoid these complications, the bath was first precooled to a desired temperature below the freezing point of ethylene glycol. The pycnometer was kept outside of the dewar until the bath was precooled, at which point the pycnometer was submerged partially up to the bottom of the stem. Since most of the sample is being quenched by the bath, rapid contraction occurs; however, the portion of sample in the stem remains close to room temperature and thus will not crystallize. Once the cooling rate has slowed, the pycnometer can then be lowered into the bath completely and allowed to equilibrate. All our attempts to measure the density of EG liquid below the crystallization temperature resulted in crystallization at approximately − 27 °C.
2.2 Viscometry
Laminar flow viscometry was performed using a set of Cannon–Fenske viscometers (size 100, 200, and 350). Calibration of the viscometers was performed at room temperature using deionized water as well as 91 wt % and 96 wt % glycerol-water solutions; the calibration was also performed at low temperatures using toluene. The viscometers were temperature-controlled using a dewar with a water or ethanol bath, and an iPhone stopwatch with a precision of 0.01 s was used to record the start and stop time. The flow time measurement of the ethylene glycol was repeated five to seven times at each temperature. A modification to the procedure was once again necessary to measure the viscosity of ethylene glycol in the supercooled region. Forcing the sample to flow readily promotes crystallization, but only when the sample is close to the regular crystallization temperature. If the sample is rapidly cooled (in a manner similar to that described in the density measurements), then the regular crystallization can be avoided. In addition to rapid cooling, the sample was drawn up in the capillary leg of the viscometer before quenching and was held in place by applying a stopper on one end of the viscometer. Once the sample was thermally equilibrated below the regular crystallization temperature, the flow time measurements were performed multiple times with no crystallization occurring.
3 Results and Discussion
The main aim of this work was to determine the low-temperature dynamic viscosities of ethylene glycol. Since the experimental method applied (Cannon-Fenske viscometry) allows one to determine the kinematic viscosity, the densities of EG were also determined to obtain dynamic viscosities via the following relation:
where is μ is dynamic viscosity, ρ is density, and ν is kinematic viscosity.
Figure 3 presents the temperature-resolved densities of ethylene glycol from + 40 °C to − 25 °C with a relative precision of 0.5 %. The data follow a clear linear trend in the entire temperature region studied (R2 = 0.99944). The densities of EG were previously reported in the temperature range from + 5 to + 45 °C [19,20,21] and are presented with the data from this work in the insert of Fig. 3; the data are found to agree well in the aforementioned temperature range.
The cubic expansion coefficient for EG was determined based on the density data obtained in this work and is presented in Fig. 4. The following equation was used:
where γ is the cubic expansion coefficient, ρ is the density, and T is temperature. It was found that the cubic expansion coefficient of EG does not change significantly in the temperature range studied, with a value of γ = (5.20 + 3.99 × 10−3 T K− 1) × 10−4 K− 1. To the best of our knowledge, no temperature-resolved cubic expansion coefficient of EG in the low-temperature region has been reported yet, which makes it difficult to assess its validity by a data comparison. Figure 4 shows that the cubic expansion coefficient of EG is significantly smaller than that of ethanol [24,25,26,27] (which is another hydrogen-bond-forming small molecule with a similar molecular mass to ethylene glycol), yet it is larger than that of water [22, 23]. Such differences can be attributed to the intensity of hydrogen bonding (the dominating interaction in these liquids) as illustrated by the corresponding shapes and intensities of the O–H and C–H vibrational bands in their IR spectra (Fig. 5).
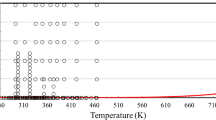
The temperature-resolved dynamic viscosities of ethylene glycol are presented in Fig. 6 in the same temperature range as the density measurements, that is, + 40 °C to − 25 °C; the relative error of the viscosity values presented is 0.5 %. The red line represents the viscosities of ethylene glycol, which are calculated according to the residual viscosity term from the correlation (Eq. 9 in Ref. [1]) and the densities of this work. The insert of Fig. 6 compares the previously published viscosity data of ethylene glycol [28,29,30] and the data produced in this work to the viscosity correlation values, which are presented as the zero dashed line. The following experimental procedure analysis was performed in order to understand the discrepancies found. Reference [28] used a Schott–Geráte AVS 400 viscosity measuring system that utilized a series of Ubbelohde viscometers. The viscometers were cleaned automatically by the instrument and were dried by blowing air through the viscometers. The flow time measurements were also performed automatically by the instrument. The Hagenbach correction for kinetic energy was taken into account in order to determine the absolute viscosities; the corresponding relative error was reported to be 0.1 %. However, no information such as flow times or calibration procedure were reported that will allow for the error evaluation. In Ref. [29], Ubbelohde and Hoeppler viscometers were also used to measure the viscosities with a relative error of 2.0 %, that is, their experimental error is 20 times larger than that of Ref. [28]. The viscometers were calibrated using two standard solutions of known viscosity. The calibration solutions were not identified, and no other information regarding the experimental methods was provided. In Ref. [30], Cannon–Fenske viscometers were used to measure the viscosities with a relative error of 1.5 %; no other information regarding the experimental procedure was provided. In this work, Cannon–Fenske viscometers were also used, and our experimental error is 0.5 %. This was achieved by the proper calibration (as described in the experimental part) and selecting viscometers applied such that the ratio of the accuracy of flow time measurements vs. the corresponding flow time was ≤ 1/200.
Temperature-resolved dynamic viscosities of ethylene glycol, including the supercooled region (black open circles). The red solid line is calculated using Eq. 9 from Ref. [1] and the densities measured in this work. Insert shows percentage deviations of the experimental data from the residual viscosity correlation values
In an effort to characterize the thermodynamic properties of EG below its regular crystallization point, a DSC measurement was performed and the thermogram obtained is presented in Fig. 7.
It was found that the regular crystallization of the sample was avoided when the cooling rate was higher than 10 °C per minute, and that the sample instead underwent a glass transition at Tg = − 121 °C (Tg is determined as the arithmetic mean of the two values indicated in Fig. 7). The glass softening was observed on heating at Tg, after which the sample underwent cold crystallization at − 60 °C and subsequent melting which was finished at − 20 °C (because the crystallites produced were small). With our viscosity data and value of Tg obtained in the DSC measurements, the Arrhenius plot and Angell plot of the viscosity data were produced to characterize the rheological properties of ethylene glycol.
The Arrhenius plot of the viscosity of ethylene glycol is presented as an insert in Fig. 8. The red dashed line is included to emphasize that the data exhibit non-Arrhenius behavior, that is, ethylene glycol is not a strong liquid according to Angell’s classification of liquid glass formers [31]. According to Angell’s research results, the viscosity of a glass-forming system is expected to be on the order of 1012 Pa s at the glass transition temperature [31]. Given this, one can produce the corresponding Angell plot in order to determine the fragility index of EG [32], which is defined by Eq. 3:
Its value for ethylene glycol was found to be 70, which is indicative of a moderately fragile liquid. The previous temperature-resolved dielectric spectroscopy study found EG to be a type A glass former, that is, its α-relaxation peak is accompanied on its high frequency side only by the excess wing, and has no secondary relaxation peak, β [33]. In Ref. [33], Tg = 152.0 K was determined by interpolating τα(T) at τg = 100 s and the fragility index m = 50.0 was obtained by evaluating the numerical temperature derivative of the relaxation time data at Tg. The value of Tg coincides with the value determined in this work; however, the fragility index is about 30 % smaller. The error analysis of our DSC experiment indicates that the Tg was determined with the precision of ± 1 °C. The fragility index was determined from the Angell plot shown in Fig. 8. Despite the large interval with no experimental data, the fit is very good (the coefficient of determination, R2 = 0.99998), hence its tangent value error is less than 1 %. Since neither raw dielectric spectroscopy data nor the log(τα) = f(T) relationship corresponding to EG was reported in Ref. [33], it is difficult to comment on the m values discrepancy. In an attempt to assess the validity of our data, the fragility indices of hydrogen bonding glass formers similar to EG are listed in Table 1. When compared to the others, the value for EG does not appear to be an outlier. Linear regression analysis of the fragility index as a function of molar mass, surface tension, number of OH groups, and glass transition temperature was performed. The strongest correlation was found between the fragility index and the surface tension (Fig. 9). This is to be expected because both the viscosity and the surface tension similarly depend on the intermolecular forces.
Fragility index as a function of surface tension for the compounds listed in Table 1. The parameters related to the linear fit, Y = A + B * X, are: A = 33.25 ± 10.5, B = 581.6 ± 270, R = 0.63115
4 Conclusions
The densities and viscosities of EG were measured in the temperature interval starting in the supercooled region at 248 (12 K below the regular crystallization point) and extending up to 313 K. The density was found to linearly depend on temperature in the entire range studied; no deviation from linearity was observed around the crystallization point and the cubic expansion coefficient was found to be γ = (5.20 + 3.99 × 10−3 T K−1) × 10−4 K−1. When our experimental density data were applied to calculate the viscosity values using the correlation dependence published in Ref. [1], the discrepancy between our experimental data and the calculated values was less than 2 % above the freezing point; however, in the supercooled region, the discrepancy increases up to 4 % at 248 K. When the cooling rate is higher than 10 K min−1 and the sample mass is less than 5 mg, EG does not freeze; it undergoes glass formation (Tg = − 121 °C) as revealed in our DSC experiments. The Arrhenius plot for viscosity data was found to be nonlinear; from the Angell plot, it was concluded that EG is a moderately fragile liquid with the fragility index = 70.
Data Availability
No datasets were generated or analyzed during the current study.
References
M. Mebelli, D. Velliadou, M.J. Assael, M.L. Huber, Reference correlation for the viscosity of ethane-1,2-diol (ethylene glycol) from the triple point to 465 K and up to 100 MPa. Int. J. Thermophys. 42, 116 (2021). https://doi.org/10.1007/s10765-021-02867-0
T. Lech, G. Czechowski, J. Jazdun, Viscosity of the series of 1, n-alkanediols. J. Chem. Eng. Data 46, 725 (2001). https://doi.org/10.1021/je0003750
T. Zhao, J. Zhang, B. Guo, F. Zhang, F. Sha, X. Xie, X. Wei, Density, viscosity and spectroscopic studies of the binary system of ethylene glycol plus dimethyl sulfoxide at T = (298.15 to 323.15) K. J. Mol. Liq. 207, 315 (2015). https://doi.org/10.1016/j.molliq.2015.04.001
G.O. Curme Jr., C.O. Young, Ethylene glycol—a contribution of chemistry to the automobile antifreeze problem. Ind. Eng. Chem. 17(11), 1117–1120 (1925). https://doi.org/10.1021/ie50191a006
J.C. Olsen, A.S. Brunjes, J.W. Olsen, Freezing and flow points for glycerol, prestone, denatured alcohol, and methanol. Ind. Eng. Chem. 22, 1315–1317 (1930). https://doi.org/10.1021/ie50252a019
J.A. Spangler, E.C.H. Davies, Freezing points, densities, and refractive indexes of system glycerol-ethylene glycol-water. Ind. Eng. Chem. Anal. Ed. 15, 96–99 (1943). https://doi.org/10.1021/i560114a004
F.H. Conrad, E.F. Hill, E.A. Ballman, Freezing points of the system ethylene glycol–methanol–water. Ind. Eng. Chem. 32, 542–543 (1940). https://doi.org/10.1021/ie50364a023
M.B. Ewert, Theory of concentrated solutions: freezing of aqueous solutions of organic compounds. Bull. Soc. Chim. Belg. 46, 90 (1937)
R.M. Tombaugh, H.S. Choguill, Some physical properties of the ternary system: ethylene glycol: diethylene glycol: water. Trans. Kansas Acad. Sci. 54, 411–419 (1951). https://doi.org/10.2307/3625724
G.O. Curme, Jr., Ed., F. Johnston, Assoc. Ed., Glycols (Reinhold Publishing Corporation, New York, 1952)
H.K. Ross, Cryoscopic studies—concentrated solutions of hydroxy compounds. Ind. Eng. Chem. 46, 601–610 (1954). https://doi.org/10.1021/ie50531a054
J.B. Ott, J.R. Goates, J.D. Lamb, Solid-liquid phase equilibria in water + ethylene glycol. J. Chem. Thermodyn. 4, 123–126 (1972). https://doi.org/10.1016/S0021-9614(72)80015-6
D.R. Cordray, L.R. Kaplan, P.M. Woyciesjes, T.F. Kozak, Solid–liquid phase diagram for ethylene glycol + water. Fluid Phase Equilib. 117, 146–152 (1996). https://doi.org/10.1016/0378-3812(95)02947-8
S.S.N. Murthy, Some insight into the physical basis of the cryoprotective action of dimethyl sulfoxide and ethylene glycol. Cryobiology 36, 84–96 (1998). https://doi.org/10.1006/cryo.1997.2064
A.D. Fortes, E. Suard, Crystal structures of ethylene glycol and ethylene glycol monohydrate. J. Chem. Phys. 135, 234501 (2011). https://doi.org/10.1063/1.3668311
R.J. Fort, W.R. Moore, Viscosities of binary liquid mixtures. Trans. Faraday Soc. 62, 1112–1119 (1966). https://doi.org/10.1039/TF9666201112
L. Grunberg, A.H. Nissan, Mixture law for viscosity. Nature 164, 799–800 (1949). https://doi.org/10.1038/164799b0
The symbols and abbreviations used in this work are in accordance with the IUPAP recommendations: E.R. Cohen, P. Giacomo, Symbols, units, nomenclature and fundamental constants in physics. Physica. 146A, 1–68 (1987)
G. Douheret, A. Pal, H. Høiland, O. Anowi, M.I. Davis, Thermodynamic properties of (ethan-1,2-diol + water) at the temperature 298.15 K I: Molar volumes, isobaric molar heat capacities, ultrasonic speeds, and isentropic functions. J. Chem. Thermodyn. 23, 569–580 (1991). https://doi.org/10.1016/S0021-9614(05)80100-4
J. Huot, E. Battistel, R. Lumry, G. Villeneuve, J. Lavallee, A. Anusiem, C. Jolicoeur, A comprehensive thermodynamic investigation of water-ethylene glycol mixtures at 5, 25, and 45°C. J. Sol. Chem. 17, 601–636 (1988). https://doi.org/10.1007/BF00645974
E.A. Crespo, J.M.L. Costa, Z.B.M.A. Hanafiah, K.A. Kurnia, M.B. Oliveira, F. Llovell, L.F. Vega, P.J. Carvalho, J.A.P. Coutinho, New measurements and modeling of high-pressure thermodynamic properties of glycols. Fluid Phase Equilib. 436, 113–123 (2017). https://doi.org/10.1016/j.fluid.2017.01.003
D.E. Hare, M.C. Sorensen, The density of supercooled water: II—bulk samples cooled to the homogeneous nucleation limit. J. Chem. Phys. 87, 4840 (1987). https://doi.org/10.1063/1.453710
M. Tanaka, G. Girard, R. Davis, A. Peuto, N. Bignell, Recommended table for the density of water between 0 °C and 40 °C based on recent experimental reports. Metrologia 38, 301 (2001). https://doi.org/10.1088/0026-1394/38/4/3
J.J.P. dos Santos Junior, R.G. Pereira, M. Rosendahl, A.J.S.M. de Mendonça, D.M. do Espirito Santo Filho, J.M. Gouveia, T.S. Mazioli, Measurements and correlations of density, isothermal compressibility factor, and thermal expansion coefficient of anhydrous ethanol fuel as a function of temperature and pressure. Int. J. Thermophys. 42, 78 (2021). https://doi.org/10.1007/s10765-021-02825-w
F.A.M.M. Gonçalves, A.R. Trindade, C.S.M.F. Costa, J.C.S. Bernardo, I. Johnson, I.M.A. Fonseca, A.G.M. Ferreira, PVT, viscosity, and surface tension of ethanol: New measurements and literature data evaluation. J. Chem. Thermodyn. 42, 1039–1049 (2010). https://doi.org/10.1016/j.jct.2010.03.022
Y. Takiguchi, M. Uematsu, Densities for liquid ethanol in the temperature range from 310 K to 480 K at pressures up to 200 MPa. J. Chem. Thermodyn. 28, 7–16 (1996). https://doi.org/10.1006/jcht.1996.0003
T.F. Sun, J.A. Schouten, S.N. Biswas, Determination of the thermodynamic properties of liquid ethanol from 193 to 263 K and up to 280 MPa from speed-of-sound measurements. Int. J. Thermophys. 12, 381–395 (1991). https://doi.org/10.1007/BF00500759
A. Marchetti, C. Preti, M. Tagliazucchi, L. Tassi, G. Tosi, The n, n-dimethylformamide ethane-1,2-diol solvent system—density, viscosity, and excess molar volume at various temperatures. J. Chem. Eng. Data 36, 360 (1991). https://doi.org/10.1021/je00004a005
D. Bohne, S. Fischer, E. Obermeier, Thermal, conductivity, density, viscosity, and Prandtl-numbers of ethylene glycol-water mixtures. Ber. Bunsenges. Phys. Chem. 88, 739–742 (1984). https://doi.org/10.1002/bbpc.19840880813
T.A. Litovitz, R. Higgs, R. Meister, J. Chem. Phys. 22, 1281–1283 (1954). https://doi.org/10.1063/1.1740382
C.A. Angell, Structural instability and relaxation in liquid and glassy phases near the fragile liquid limit. J. Non Cryst. Solids 102, 205–221 (1988). https://doi.org/10.1016/0022-3093(88)90133-0
R. Böhmer, K.L. Ngai, C.A. Angell, D.J. Plazek, Nonexponential relaxations in strong and fragile glass formers. J. Chem. Phys. 99, 4201–4209 (1993). https://doi.org/10.1063/1.466117
T. Blochowicz, C. Gainaru, P. Medick, C. Tschirwitz, E.A. Rössler, The dynamic susceptibility in glass forming molecular liquids: the search for universal relaxation patterns II. J. Chem. Phys. 124, 134503 (2006). https://doi.org/10.1063/1.2178316
1,2,6-Hexanetriol (1,2,6-trihydroxyhexane) (Technical Bulletin for Aldrich Chemical Company Inc., Milwaukee 1996)
A.W. Adamson, A.P. Gast, Physical Chemistry of Surfaces, 6th edn. (Wiley, 1997)
J. Wu, X. Liu, R. Zhang, L. Kong, F. Li, Z. Wu, J. Zhang, Thermodynamic data, excess properties, and intermolecular interactions of the 1,3-butanediol + 1,2-propane diamine binary system. J. Solution Chem. 51, 890–916 (2022). https://doi.org/10.1007/s10953-022-01177-9
Physical Properties of Glycerine and Its Solutions (Glycerine Producers’ Association, New York, 1963)
https://matmake.com/materials-data/propylene-glycol-properties.html (accessed 7 Aug 2024)
R. Tahery, H. Modarress, J. Satherley, Density and surface tension of binary mixtures of acetonitrile + 1-alkanol at 293.15 K. J. Chem. Eng. Data 51, 1039–1042 (2006). https://doi.org/10.1021/je050519g
Isobutanol, Technical data sheet for The DOW Chemical Company: Midland (2012)
Acknowledgments
We thank the Pennsylvania State University Department of Chemistry for support.
Funding
This study was partially supported by the Pennsylvania State University Department of Chemistry.
Author information
Authors and Affiliations
Contributions
B.H.M. developed analytical tools. M.L. performed experiments, processed data, and wrote the manuscript. B.H.M. supervised the project and edited the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Leonard, M., Milosavljevic, B.H. Low-Temperature Study of Thermodynamic and Rheological Properties of Ethylene Glycol Including the Supercooled Region. Int J Thermophys 45, 134 (2024). https://doi.org/10.1007/s10765-024-03426-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-024-03426-z