Abstract
To mitigate the temperature distribution gradients on the surface caused by the coolant flowing and heating on both sides, an efficient liquid cooling system is needed to solve the problem of high temperatures in localized regions of the heating surface. A copper foam-based vapor chamber composite cold plate is developed, and its heat thermal performance is experimentally investigated. The evaporation base with the copper foam metal wick is placed on both sides of the cold plate to form vapor chambers. The effects of a range of heating powers and coolant flow rates on the thermal resistance of vapor chambers with different filling ratios on both sides are investigated. The results show that a 0.15 L min−1 coolant flow rate provides excellent heat dissipation, and continuing to increase the flow rate has little impact on the temperature uniformity. The vapor chamber’s 70 % to 80 % filling ratio reflects the best performance in the test heating power range. The structure is easily assembled and suitable for utilization in battery thermal management systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Lithium batteries have gained widespread popularity as energy sources for various electronic devices due to portability, easy installation, and convenience [1]. In an electric vehicle battery pack, numerous battery cells are arranged in series or parallel in each group of batteries [2]. The arrangement of battery cells within a pack can generate heat during charging and discharging, leading to a rapid rise in temperature. In general, \(25\,^{\circ } {\text{C}} \sim 40\,^{\circ } {\text{C}}\) is usually suitable for lithium battery pack operating environment temperature [3]. The optimal temperature difference of less than \(5\,^{\circ } {\text{C}}\) in each cell is required to perform excellently [4]. The higher operating temperature of the battery will accelerate its aging rate and even cause deterioration in local areas. However, the low operating temperature will increase the battery’s internal resistance and reduce the electrochemical reaction rate, leading to a decline in the battery’s working efficiency [5]. Therefore, the performance of the battery thermal management system (BTMS) can be improved according to the actual application scenario, effectively reducing the temperature difference between various areas of the battery so that the battery pack can work efficiently and safely [6].
The BTMS refrigerating medium mainly consists of airg and coolant, and the heat dissipation components are heat pipes and vapor chambers [7, 8]. Air cooling system is cost effective and easy to maintain, but it requires a specific gap between batteries and is ineffective in regulating battery temperatures within a reasonable range [9]. A liquid cooling system typically uses coolants as working mediums. The coolant flows along the flow channel between the liquid cooling plate and the heating surface to achieve heat exchange. When the coolant enters the cold plate, the coolant temperature in the heat source area is higher than in other areas. Under the influence of increasing heat source intensity on the heating surface, the temperature difference continues to increase. Many scholars have addressed this problem by optimizing the design of liquid cooling plate channels and structures [10]. However, such intricate flowing channels necessitate larger sizes and improved machining accuracy of the plate [11]. The serpentine liquid cooling plate is one of the most commonly used liquid flow channels. It has good cooling performance, is simple in design, and is easy to process [12]. However, it results in insufficient temperature uniformity of the coolant across the flow channel and requires enhancements for practical applications.
The vapor chamber is an efficient heat transfer element based on the principle of gas–liquid phase change, which has excellent thermal conductivity [13]. In BTMS, the condensing of the vapor chamber usually uses air or coolant to dissipate heat to improve heat dissipation efficiency. Li et al. [14] attached an aluminum evaporator base to one side of the liquid cooling plate to improve the temperature equalization of the heating surface. By experimenting with vapor chambers of different filling rates and placed at different angles, it was shown that the outer vapor chamber can effectively suppress the temperature difference. Wang et al. [15] placed a serpentine liquid cooling tube in the vapor chamber, and the heat dissipation process of this hybrid structure underwent multiple heat transfer modes. Experimental results show that the bottom temperature rises by only \(2.04\,^{\circ } {\text{C}}\) under optimum heat dissipation conditions, which shows excellent temperature uniformity. Lei et al. [16] designed a cooling method for the condensing section combined with water spray of the heat pipe. Using this cooling method, the overall temperature and temperature difference on the surface of the battery pack at \(I_d=24\) A compared to the battery pack’s temperature without the BTMS system decreased by \(29.2\,^{\circ } {\text{C}}\) and \(8.0\,^{\circ } {\text{C}}\), respectively. Although vapor chambers have been intensively researched for various applications, few studies have focused on their heat dissipation performance with liquid cooling systems under double-sided wall heating conditions.
In this paper, the structure of a composite copper foam vapor chamber on both sides of a liquid cooling plate is designed. The integrated design avoids the interface contact thermal resistance in the traditional structure. The integrated design involves placing a copper foam metal wick inside the vapor chamber’s shell and directly integrating it with the walls on both sides of the serpentine cold plate. The ceramic heating plate is used as an adjustable heating source. The effects of different factors, such as heating powers, coolant flow rates, and the vapor chamber filling ratios on the heating surface temperature and temperature uniformity are studied. In addition, the influence of the above factors on the transfer process of the gas–liquid coplanar copper foam vapor chamber is also discussed.
2 Design and Thermal Analysis
2.1 Structural Design
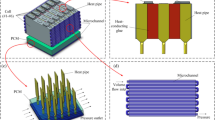
The schematic diagram of the gas–liquid coplanar copper foam vapor chamber composite liquid cooling plate is presented in Fig. 1. The composite structure comprises two evaporator bases, two foam copper wicks, a cold plate cover, and a bottom plate. Coolant enters the plate from the Inlet port, flows through the coolant channel, and exits through the Outlet port. The plate cover and bottom plate are integrated with the evaporator base, respectively.
The evaporator base transfer heat through thermal conduction into the vapor chamber upon heating. The working fluid is vaporized and evaporated after being heated, and the vapor condenses into liquid on the wall of the liquid cooling plate at a lower temperature. The latent heat of vaporization released by condensation transfers heat to the coolant within the channel. The working fluid formed within the chamber under the action of the capillary force of the copper foam wick and vapor pressure returns to the evaporation section. The above process is continuously circulated for heat transfer.
2.2 Theoretical Analysis
A thermal resistance network diagram is constructed for quantitative analysis of the heat transfer process of a cold plate composite vapor chamber (Fig. 2).
\(R_t\) is the thermal resistance from the evaporation base to the coolant in the channel of the cold plate, expressed as follows:
where \(T_h\) and \(T_c\) are the temperatures of the evaporator base and coolant in the channel, respectively.
The evaporator base thermal resistance (\(R_{\text{w}, v}\)) is expressed as follows:
where the thickness, thermal conductivity, and area of the evaporator base are expressed by \(\delta _c\), \(k_s,\) and \(A_w,\) respectively.
The gas–liquid interface thermal resistance (\(R_{ i}\)) in the vapor chamber is calculated from equation [17]:
where \(A_f\) is the area where working fluid immersed in the gas–liquid coplanar wick, and \(h_i\) is the gas–liquid interface heat transfer coefficient.
The thermal resistance (\(R_s\)) of the gas–liquid coplanar copper foam wick is calculated as follows [18]:
where \(h_s\) is the height of the working fluid submerged in the wick, using the \(k_{\text{ eff } }\) equivalent the gas–liquid coplanar wick thermal conductivity.
The thermal resistance (\(R_v\)) of the vapor phase is calculated as follows:
where \(T_v\) and \(\rho _{\text{v}}\) the average temperature and density of the vapor, and \(P_{\text{v}, \text{e}}\) and \(P_{\text{v}, \text{c}}\) are the vapor pressures in the evaporation and condensation sections, respectively.
The coolant flows in the channel with the walls forcing convection heat transfer, described by Newton’s Cooling equation [19]:
where \(A_c\) is the area of forced convective heat transfer, and \(T_w\) and \(T_s\) are the evaporation base temperature and coolant temperature, respectively. In addition, \(\alpha\) is the convective heat transfer coefficient between fluid and solid contact surfaces, which is calculated by [20]
where \(k_f\) and \(d_h\) are the coolant thermal conductivity and the hydraulic diameter of the coolant flow channel, respectively.
2.3 Fabrication
The working fluid inside near the heating area absorbs heat and vaporizes into steam. The heat released by the steam’s cold condensation inhibits the low-temperature area’s influence on the heating surface’s temperature uniformity. Using a gas–liquid coplanar wick structure, the vapor and liquid channels are arranged on the same plane in the thickness direction and the vapor and liquid flow on the same plane.
Each shell part is made of copper and processed according to the size of Table 1. Fig. 3(a),(b),(c) shows the cold plate base plate and internal flow channel, the vapor chamber shell and internal wick, and the welded structure, respectively.
The cleansing procedure follows: immerse all shells in an ethanol solution, clean by ultrasonic for 5 mins, and rinse shells with deionized water. Then, immerse shells in a diluted hydrochloric acid solution perform ultrasonic cleaning for 5 mins, and once again proceed to rinse with deionized water. Finally, the shells are to be placed in a \(100\,^{\circ } {\text{C}}\) oven for 5 mins.
The inner surface of the cold plate is coated with solder and placed on a \(400\,^{\circ } {\text{C}}\) heating block for encapsulation. In the same step, the evaporation base is encapsulated on both sides of the outer surface of the cold plate. The filling pipe of the evaporation base is punctured using a syringe needle, and the strong adhesive is used to stick and dry. Next, the liquid-cooled vapor chamber composite plate is submerged in water while air is injected through the syringe needle to verify air tightness. The vacuum pump is used to vacuum the interior of the vapor chamber. When the pointer of the vacuum pump is stable and does not rotate, the quantitative working fluid is injected through the syringe needle according to the set filling ratio and then sealed. Finally, a sealing test is conducted by placing the composite plate in warm water to detect bubbles. If no bubbles emerge, it is prepared for use.
3 Experiment
3.1 Experimental Setup
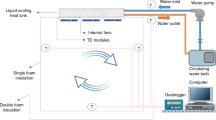
A test system including a heating unit, a cooling unit, and a data unit is set up to test the heat transfer characteristics of the copper foam-based vapor chamber composite liquid cooling system, as shown in Fig. 4.
The heating unit consists of two metal-ceramic heaters with the same resistance, 30 mm long and wide and 2 mm thick, and a DC-adjustable power supply. The heat-conducting silicone grease is coated with a thin layer of thermal grease and placed on the evaporation base, which is convenient for fixing the position of the heating source and reduces the contact thermal resistance between them. The cooling unit comprises a peristaltic pump, flowmeter, and constant temperature water bath. The coolant is extracted from the thermostatic tank by the peristaltic pump, flows through the glass rotor flowmeter, and flows into the cold plate from the bottom inlet. The direction of the inlet and outlet can be switched when the air in the flow channel and tubes has been exhausted. The data acquisition unit comprises a temperature detector, PC, and Pt100 thermocouple. T1–T4 and T5–T6 thermocouples are attached to the evaporator base on both sides, respectively, and the measurement points are distributed, as shown in Fig. 5. The T9 and T10 thermocouples are placed in the water pipe where the coolant enters and leaves the cold plate to detect temperature changes after the coolant enters the structure. The copper foam vapor chamber composite liquid cooling structure is wrapped in the insulation foam and placed in the test box. The temperature of all measuring points was stable within \(\pm 0.1\,^{\circ } {\text{C}}\) for 3 min to record the data at this time.
3.2 Data Processing and Uncertainty Analysis
The maximum temperature differential between the two sides of the heating surface is calculated using equations Eqs. 8 and 9 correspondingly, which serves as a significant indicator for evaluating the temperature uniformity.
where \({T_{i}}\) is the temperature measured by each thermocouple on the wall.
The thermal resistance from the evaporation base to the coolant on one side of the cold plate is calculated by the following formula:
where \(T_{\text{h},\text{ ave }}\) and \(T_{\text{c},\text{ ave }}\) are the average temperature of the evaporation base and coolant inside the cold plate, respectively.
\(T_{\text{h},\text{ ave }}\) and \(T_{\text{c},\text{ ave }}\) are calculated according to Eqs. 11 and 12, respectively.
The filling ratio of one side chamber is calculated as follows:
where \(V_l\) is the volume of anhydrous methanol in the wick at the saturated state, and \(V_p\) is the volume of dry copper foam wick.
It is necessary for the data processing stage to analyze the equipment causing the uncertainty. Uncertainty is usually calculated using Eq. 14 [21]. The uncertainty of power provided by the DC power supply is 0.5 %, and the uncertainty caused by thermocouple (ETA-T-K-30) is 0.7 %.
The vapor chamber composite liquid cooling structure is placed in the test box and filled on each side with insulation cotton with a thermal conductivity of 0.036 \(\text{W} \,(\text{m} \cdot \text{K})^{-1}\) [22]. The maximum heat loss of insulation cotton to DC power supply input power is 4.95 %, and the maximum relative measurement uncertainty of thermal resistance is 6.8 %.
4 Results and Discussion
4.1 Temperature Distribution
As shown in Fig. 6, the temperature distribution on both sides of the evaporator base for heating powers ranges from 5 W to 45 W, where both sides of the vapor chamber filling ratio are 70 % and the coolant flow rate of 0.15 L min−1. In general, the higher the power input to the heating source, the higher the temperature of the heating surfaces on both sides. Moreover, slight differences in temperature between all measurement points at the same power for all tested powers. The temperature difference of the evaporator base testing points is slight when the heating power is low. For example, at 15 W heating power, the temperature difference between the evaporator bases on both sides of the liquid cooling plate is \(0.2\,^{\circ } {\text{C}}\) in the steady state.
Even with increasing power, the temperature distribution on both sides of the evaporator base does not appear extreme phenomenon. At the input power of 40W, the temperature of the outlet is \(2.9\,^{\circ } {\text{C}}\) higher than that of the inlet, but the temperature difference between the evaporator base (T1–T4) side and the (T5–T8) side is only \(1.1\,^{\circ } {\text{C}}\) and \(1.5\,^{\circ } {\text{C}}\).
Figure 7 shows the temperature distribution of the evaporator base on both sides under various flow rates, where the filling ratio is 70% and the heat power is 40 W. The faster the coolant flows, the more the heat is removed and the lower the evaporator base temperature. The overall temperature of the evaporator base is much higher at a coolant flow rate of 0.05 L min−1 than at other flow rates. At flow rates of 0.05 L min−1, 0.1 L min−1, 0.15 L min−1, and 0.2 L min−1, the temperatures at test point T3 are \(33.8\,^{\circ } {\text{C}}\), \(28.8\,^{\circ } {\text{C}}\), \(26.8\,^{\circ } {\text{C}}\), and \(25.5\,^{\circ } {\text{C}}\). Comparing the temperature changes of the evaporator base at different coolant flow rates, the temperature of the evaporator base slowly decreases after the flow rate exceeds 0.15 L min−1. That means that too low a flow rate can result in inadequate cooling, but increasing above a certain threshold reduces the impact on the evaporator base temperature.
Observing Figs. 6 and 7, the temperature distribution on the evaporator base (T1–T4) and (T5–T8) sides exhibit a similar variation trend, with a minor temperature difference between corresponding testing points.
4.2 Temperature Uniformity
Figure 8 shows the temperature difference of the evaporator base on both sides under various flow rates, where the heat power is 30 W. As the coolant flow rate increases, the temperature differential within the test range decreases. When the flow rate is 0.05 L min−1, the temperature difference between the inlet and outlet of the cold plate is nearly \(8.6\,^{\circ } {\text{C}}\), while the maximum temperature difference on the heating surface of the vapor chamber with filling ratios of 60 %, 70 %, 80 %, and 90 % is less than \(3\,^{\circ } {\text{C}}\). That indicates that the vapor chamber on the cold plate’s outer wall can enhance the heating surface’s temperature uniformity.
After the coolant flow rate increases to 0.1 L min−1, the influence on the temperature difference between the evaporator bases on both sides gradually diminishes. Under a filling ratio of 70 %, an increase in flow rate from 0.15 L min−1 to 0.2 L min−1 resulted in a decline in the temperature at testing points T3 and T6 from their previous values of \(27.2\,^{\circ } {\text{C}}\) and \(26.8 \,^{\circ } {\text{C}}\) to \(26\,^{\circ } {\text{C}}\) and \(25.9\,^{\circ } {\text{C}}\), respectively. The utmost discrepancy in temperature witnessed on the T1–T4 side heating surface remains unaltered. At the same time, a slight reduction of only \(0.1\,^{\circ } {\text{C}}\) is noticed in the maximum temperature difference of the T5–T8 side evaporator base. This can be attributed to the fact that the vapor chambers on both sides undergo phase change heat transfer, resulting in a much higher surface heat transfer coefficient than the convective heat transfer of unidirectional fluid. Heat transfer in the vapor chamber is limited when the filling ratio is low, resulting in higher surface temperature and larger temperature difference. However, when the filling ratio is high, some working fluids converge together, occupying the space of the vapor chamber, which lowers the cycle efficiency of the working fluid and causes a deteriorated temperature.
The gas–liquid phase change heat transfer inside the chambers on both sides of the cold plate is one of the most critical factors affecting surface temperature uniformity. Different filling ratios in the chamber lead to varying saturation degrees of the working fluid infiltration wick, which also affects liquid and vapor flow.
Under the low heating power, the temperature of each test point on the side of the vapor chamber with different filling ratio has little change. After the heating power exceeds 20 W, the temperature change of each test point on the heating surface is more obvious. With the heating power increase, the filling ratio’s effect on temperature difference is more significant. As shown in Fig. 9, at the heat power is 5 W, the difference in temperature between the different parts of the heating surface is slight. The temperature difference between the heating surfaces on both sides of the vapor chamber with the filling ratio of 60 % is smaller than that of other filling ratios. However, the temperature difference increases sharply at around 10 W. At the heat power of 40 W and filling ratios of 60 %, 70 %, 80 %, and 90 %, the steady-state temperature differences of the heating surface (T1–T4) are \(1.4\,^{\circ } {\text{C}}\), \(1.1\,^{\circ } {\text{C}}\), \(1.3\,^{\circ } {\text{C}}\), and \(1.8 \,^{\circ } {\text{C}}\), respectively. This phenomenon may be caused by the need to absorb sufficient heat for the vaporization of the working fluid. With a relatively high filling ratio and low heating power, some working fluids are not fully vaporized, resulting in small vapor flow and insufficient heat transfer, leading to a slightly larger surface temperature difference. At 90 % filling ratio, the temperature difference is consistently worse than that of the other filling ratios. That is because too much internal working fluid gathers at the bottom of the chamber and flows inadequately towards the heat source area, resulting in a large temperature difference. The temperature difference between both sides of the heating surface ranges from large to small as 90 % > 60 % > 80 % > 70 % and at 20 W heating power. The ranking remains the same at higher heating powers. That is probably due to the evaporation and reflux rates of the working fluid remaining in relative equilibrium under the capillary force of the gas–liquid co-surface copper foam wick.
4.3 Thermal Resistance
Figure 10 shows the thermal resistance of the 70 % filling ratio vapor chamber on both sides of the cooling plate at different heating power and coolant flow rates. In the tested heating power range, the thermal resistance with flow rates of 0.05 L min−1 and 0.1 L min−1 was significantly larger than that of the other two flow rates. According to its calculation Eq. 10, under the same heating power, the denominator of the calculation formula remains unchanged, and the thermal resistance value is related to the numerator. When the cold plate flow rate is larger, the convective heat transfer capacity is strong, resulting in the average temperature on both sides is lower than the smaller flow rate, while the coolant average temperature change rate is small and the calculated thermal resistance value of using larger flow rate is small.
At 0.05 L min−1 coolant flow rate, the thermal resistance value on the T1–T4 side and T5–T8 side shows a tendency to decline at the beginning and rising up after the heating power of 20 W. This is due to the flow rate being too small and the condensation rate is not sufficient to meet the condensate return in the chamber, resulting in a sharp increase in thermal resistance. When the flow rate increases to 0.15 L min−1, the condensation rate requirements are met, and the gas–liquid two-phase flow cycle in the chamber can be maintained stably, with little change in thermal resistance value with further increases in coolant flow rate.
Figure 11 shows thermal resistance under different heat powers with various filling ratios, where the flow rate is 0.05 L min−1.The thermal resistance of the 90 % filling ratio vapor chamber on both sides is much larger than that of a 60 % filling ratio. For example, the average thermal resistance of the T1–T4 side at a 90 % filling ratio is 32.2 % larger than that of the 60 % filling ratio. Vapor chambers with a higher filling ratio have thicker liquid layers of the working fluid. The thicker liquid layer within the chamber not only increases the thermal resistance between the shell and the working fluid but also reduces the steam flow channel space, increasing the steam flow rate and the shear resistance between the gas–liquid interface, causing a further increase in thermal resistance.
The vapor chamber on both sides of the cold plate is heated, and the heat is transferred by heat transfer to the working fluid inside. The liquid molecules absorb enough heat, the strong interaction between them is destroyed, and vapor gradually forms. At low heating loads, the heat flux density is insufficient to initiate nucleate boiling of the internal working substance, resulting in a worse impact on thermal performance and increased thermal resistance. As heating power increases, the working substance viscosity decreases, intermolecular motion intensifies, and vaporization speed increases, enhancing heat transfer performance and reducing thermal resistance. At low filling ratios, the thermal resistance value decreases to its lowest value before gradually increasing with a further increase of heating power, the vapor flow rate increases, leading to an increase in gas–liquid interface resistance. Due to vapor taking away part of the reflux condensate, the working fluid in the heating part is reduced. When the filling ratio reaches 90 %, its thermal resistance value is higher than that of a low filling ratio, while the thermal resistance value gradually decreases with the increase in input power without any heat transfer deterioration. Thus, its heat transfer limit is better than that of a low filling ratio.
5 Conclusions
An efficient solution is provided to solve the problem of surface temperature distribution gradient caused by the coolant flowing and heating on both sides. This paper uses a liquid-cooled composite vapor chamber to enhance the wall temperature uniformity and improve heat dissipation efficiency. The effects of a range of heating powers and coolant flow rates on the heat transfer characteristics of vapor chambers with different filling ratios on both sides are investigated. Some conclusions are summarized as follows:
-
1.
An increase in coolant flow rate effectively improves heat dissipation in the condensing section of the vapor chamber, thereby reducing the average temperature of the evaporator base. The flow rate of 0.15 L min−1 shows excellent heat dissipation performance, and continuing to increase the flow rate has little impact.
-
2.
The filling ratio and distribution position of the working fluid in the chamber affects the variable heat transfer in the gas–liquid phase, which can improve the temperature uniformity of the surface. The vapor chamber’s 70 % to 80 % filling ratio reflects the best performance in the test heating power range.
-
3.
When the filling ratio is relatively low, the thermal resistance shows a tendency to decline at the beginning and rising up after 20 W heating power in the test range, while the thermal resistance decreases continuously at a higher filling ratio, and the heat thermal limit is higher than that at low filling ratio.
The conclusions of this paper show that the specially designed vapor chamber composite cold plate structure can improve temperature uniformity and reduce the energy consumption required for cooling. Further work will involve the practical application of this structure to battery packs and experimental investigations.
Data Availability
All the data are available (if requested).
References
L.D. Couto, M. Charkhgard, B. Karaman, N. Job, M. Kinnaert, Energy 263, 125966 (2023). https://doi.org/10.1016/j.energy.2022.125966
B. Wang, C. Ji, S. Wang, J. Sun, S. Pan, D. Wang, C. Liang, Appl. Therm. Eng. 168, 114831 (2020). https://doi.org/10.1016/j.applthermaleng.2019.114831
Y. Liu, S. Xu, Y. Wang, H. Dong, Int. J. Thermophys. 43, 131 (2022). https://doi.org/10.1007/s10765-022-03058-1
M. Al-Zareer, I. Dincer, M.A. Rosen, J. Power Sources. 363, 291–303 (2017). https://doi.org/10.1016/j.jpowsour.2017.07.067
D. Burow, K. Sergeeva, S. Calles, K. Schorb, A. Boerger, C. Roth, P. Heitjans, J. Power Sources. 307, 806–814 (2016). https://doi.org/10.1016/j.jpowsour.2016.01.033
W. Wu, S. Wang, W. Wu, K. Chen, S. Hong, Y. Lai, Energy Convers. Manag. 182, 262–281 (2019). https://doi.org/10.1016/j.enconman.2018.12.051
H.M. Daraghmeh, C.-C. Wang, Appl. Therm. Eng. 114, 1224–1239 (2017). https://doi.org/10.1016/j.applthermaleng.2016.10.093
B.H.J.W. Han, Int. J. Thermophys. 43, 148 (2022). https://doi.org/10.1007/s10765-022-03088-9
J. Chen, S. Kang, E. Jiaqiang, Z. Huang, K. Wei, B. Zhang, H. Zhu, Y. Deng, F. Zhang, G. Liao, J. Power Sources. 442, 227228 (2019). https://doi.org/10.1016/j.jpowsour.2019.227228
J. Zhao, Z. Rao, Y. Li, Energy Convers. Manag. 103, 157–165 (2015). https://doi.org/10.1016/j.enconman.2015.06.056
Z. An, K. Shah, L. Jia, Y. Ma, Appl. Therm. Eng. 154, 593–601 (2019). https://doi.org/10.1016/j.applthermaleng.2019.02.088
M. Li, F. Liu, B. Han, J. Guo, Y. Xu, Ionics 27, 2685–2695 (2021). https://doi.org/10.1007/s11581-021-04033-w
M. Bulut, S.G. Kandlikar, N. Sozbir, Heat Transf. Eng. 40, 1551–1573 (2019). https://doi.org/10.1080/01457632.2018.1480868
W. Li, L. Li, W. Cui, M. Guo, Int. J. Heat Mass Transf. 170, 121026 (2021). https://doi.org/10.1016/j.ijheatmasstransfer.2021.121026
H. Wang, P. Bai, R. Cai, Y. Luo, X. Chen, S. Li, G. Wu, Y. Tang, G. Zhou, Energy Convers. Manag. 244, 114499 (2021). https://doi.org/10.1016/j.enconman.2021.114499
S. Lei, Y. Shi, G. Chen, Int. J. Heat Mass Transf. 163, 120494 (2020). https://doi.org/10.1016/j.ijheatmasstransfer.2020.120494
K.L. Lawrence, Comput. Syst. Sci. Eng. 3, 691 (1992). https://doi.org/10.1016/0956-0521(92)90020-J
S.W. Chi, Heat Pipe Theory and Practice (Hemisphere, New York, 1976)
J. Xie, M. Zang, S. Wang, Z. Ge, Proc. Inst. Mech. Eng. Part D (2017). https://doi.org/10.1177/0954407016685457
V. Gnielinski, vol. 16, pp. 359–367 (1976)
G. Huang, W. Liu, Y. Luo, Y. Li, H. Chen, Int. J. Therm. Sci. 170, 107145 (2021). https://doi.org/10.1016/j.ijthermalsci.2021.107145
Z. Zhao, L. Li, Y. Wang, Y. Wang, Y. Hui, AIP Adv. (2023). https://doi.org/10.1063/5.0134402
Acknowledgement
This work is partly supported by the National Science Foundation of China (NSFC, Grant No.62162035) and Yunnan Fundamental Research Projects (Grant No. CB23031C047A).
Author information
Authors and Affiliations
Contributions
Z. Securing funding and conducting a thorough review and revision of the initial draft. X. Writing the first draft, which includes substantive translation, and designing the experiments. B. Providing study materials, reagents, and necessary laboratory instrumentation. Y. Collecting and curating data/evidence. C. Managing and coordinating the research activity planning and execution, as well as polishing the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Z., Chen, X., Li, B. et al. Heat Transfer Performance of Copper Foam-Based Vapor Chamber Composite Liquid Cooling System Under Double-Sided Heating. Int J Thermophys 45, 19 (2024). https://doi.org/10.1007/s10765-023-03308-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-023-03308-w