Abstract
New experimental data for the viscosity of molten lithium nitrate from its melting point up to about 700 K are reported. The measurements were taken, for the first time, with an oscillating-cup viscometer with an estimated uncertainty of 3 %. The obtained data were compared with previous works. It was concluded that there are still large discrepancies between different sets of data. Our data follow the Arrhenius behavior with an activation energy for viscous flow of \(E_{\eta } \hbox {= 19.3}\) \(\hbox {kJ}{\cdot }\hbox {mol}^{-1}\), slightly higher than previous recommendations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ionic liquids, a class of fluids containing only ions, include the low-temperature ionic liquids (RTILs), with melting temperature lower than \(\hbox {100 }^\circ \)C, and the high-temperature ionic liquids (HTIL’s or molten salts) with higher melting points. The former have captured, in recent years, the attention of the scientific community due to their large range of applications in the chemical industry. However, this distinction is artificial as both are Coulombic fluids with academic and industrial interest, as recently reported for pure molten alkali nitrates and mixtures including lithium nitrate, used in a large range of high-temperature applications, like heat storage for solar power plants [1,2,3,4]. The optimal technological design of the chemical processes involving ionic liquids requires the characterization of their thermodynamic and transport properties, namely viscosity. In a previous work [5], we proposed a set of reference data for the viscosity of molten sodium nitrate. Preliminary studies [6] on the viscosity of lithium nitrate showed differences between different laboratories (10–15 %) indicating systematic errors in the available measurements. This kind of observation, common to RTILs properties [7], leads us to revise the viscosity of this melt with two fundamental purposes, to add new data that can be used to establish reference data, and the elucidation of the mechanisms of momentum transfer in the liquid phase. In this work, we present new data on the viscosity of molten lithium nitrate between the melting point and about 700 K, using for the first time, an oscillating-cup viscometer.
Some of the applications of the molten lithium nitrate also include metal coatings at high temperature, for gas separation, phase change material for heat transfer and others. The melting point of \(\hbox {LiNO}_{3}\) is 537 K (or circa \(\hbox {264 }^\circ \)C) which fills the gap between the low-temperature ionic liquids and salts that melt at higher temperatures, for example the chlorides or carbonates. Therefore, the accurate characterization of the fluid properties is a must, for both scientific and technological reasons.
We have shown in the past [5] that the previous recommendations about the viscosity of molten \(\hbox {NaNO}_{3}\) were, at least, doubtful, and a preliminary reference correlation for the viscosity of molten sodium nitrate, and a set of reference data were proposed. In the case of lithium nitrate, the first recommendation of the Molten Salts Data Centre [8], based on an early work by Dantuma [9], was found to be seriously flawed [10]. The actual recommendation [11] is based on the later work of Murgulescu and Zuca [12, 13] and Protsenko and Razumovskaya [14]. However, as can be seen in these references, the last work published about the viscosity of molten \(\hbox {LiNO}_{3 }\)is due to Janz et al. [10], in 1978. The recommended equation by the MSDC, limited to the temperature range from 540 K to 650 K, with an estimated uncertainty of 3 % [11] is:
where \(\eta \) is the absolute viscosity in \(\hbox {mPa}{\cdot }\hbox {s}\), R is the gas constant, in \(\hbox {J}{\cdot }\hbox {K}^{-1}{\cdot }\hbox {mol}^{-1}\) and T is the absolute temperature in K. However, an analysis of experimental data shows that the deviations between different sets of data are greater than the claimed mutual uncertainty. This fact, apart from the reanalysis of the existing data, justifies the accurate measurement of the viscosity.
2 Experimental
The experimental apparatus (oscillating-cup viscometer) and working equations utilized in the present work were described in detail elsewhere [15]. Briefly, the method is based on the damped oscillations of a torsion wire (Pt92W8) connected to a rod (Mo) and a stainless steel cup (SS) containing the fluid. The viscosity is computed from the measured period and logarithmic decrement (in units of \(\Delta =\delta \hbox {/2}\pi \)) of oscillations, with the empty and filled cup, \(T_{0}\), \(\Delta _{0}\), and T and \(\Delta \), respectively. The only measurements needed are mass, temperature and time that can be obtained with great accuracy.
The sample (\(\hbox {LiNO}_{3}\) from Fluka), with a minimum stated purity of 98.5 %, was dried overnight and the cup was successively filled in a separate furnace, to permit the compactness of solid sample and the elimination of air in the sample. The cup was then finally closed in a dry box under nitrogen atmosphere. The measurements were restricted to approximately 700 K because, due to high polarizing power of the cation, slight decomposition is noted 40 K above the melting point, becoming appreciable above 720 K [16]. During the measurements, the viscometer was purged with dry nitrogen to prevent oxidation phenomena.
Visual inspection of the sample after the measurements showed a white crystalline solid, with some slight contamination of a yellow solid, probably lithium nitrite or other products from the SS cup. The mass loss was smaller than 0.1 g (of a total mass of about 27 g), or 0.4 %, which supports the assumption that the sample decomposition was minimal. Further analysis of the pH of \(\hbox {LiNO}_{3}\) sample aqueous solutions proved that nitrite anion presence was less than 0.5 %. X-ray powder diffraction tests demonstrated that the several fusion and recrystallization processes increased its crystallinity and even the purity, because the initial 10 % amount of hydrate lithium nitrate was reduced to only 1 %. Phase identification was carried out by X-ray powder diffraction using a Philips PW 1730 diffractometer, operating with a monochromatized Cu-K radiation in Bragg–Brentano geometry and scanning (\(\hbox {0.02}^{o}\) step in 2\(\theta \)) over the range \(\hbox {10}^{o} \quad \le \hbox { 2}\theta \quad \le \hbox { 70}^{o}\).
3 Results and Discussion
Some of the physical parameters of the oscillating system were redetermined and are listed in Table 1.
The viscosity was measured from melting point up to about 700 K, utilizing density data taken from the literature [11]. Table 2 summarizes the obtained data. The total uncertainty, calculated from the root mean square deviations of the different contributions, already including an uncertainty of 0.5 % in density, was estimated to be 3 %, as shown in Table 3. The accuracy relative to previous publication [5] degraded to the mentioned 3 % mainly due to the uncertainty in the value of \(\delta \). Additionally, a higher value of damping for the empty cup was found in the present measurements which also may difficult the convergence of the working equations. Data points were obtained on both increasing and decreasing temperature, and the values agree within ± 3 %.
Figure 1 shows the comparison with other previous authors. The available data are scarce and data of Brovkina et al. [17], Smotrakov et al. [18] are smoothed data, not experimental data points.
Comparison of present data with previous authors. \(\bigcirc \) - Present work; \(*-\) Protsenko et al. [14]; \(\diamondsuit \) - Murgulescu et al. [12]; \(\lozenge \) - Zuca [13]; \(\bigtriangleup \) - Janz et al.[10]; \(\cdot \cdot \cdot \cdot \cdot \cdot \) Smotrakov et al. [18];- - - - - Brovkina et al. [17]
As referred before, the fist recommendations of the MSDC [8] was made taking into account the work of Dantuma [9] by the oscillating ball technique. The choice was made because the paper of Murgulescu and Zuca [12] presented lack of details on sample preparation and purity and Protsenko and Razumovskaya [14] reported a higher melting point for their sample what could imply an error on thermometry. The uncertainty of the viscosity values was estimated to be about 3.5 %.
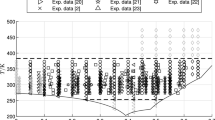
Percent deviation between experimental sets of data and MSDC recommendation. \(\bigcirc \) - Present work; \(*\) - Protsenko et al. [14]; \(\diamondsuit \) - Murgulescu et al. [12]; \(\lozenge \) - Zuca [13]; \(\bigtriangleup \) - Janz et al. [10]; \(\cdot \cdot \cdot \cdot \cdot \) Smotrakov et al. [18];- - - - - - Brovkina et al. [17]
Later, Janz et al. [10] measured the viscosity of molten lithium nitrate with a modified Ostwald capillary viscometer, with an accuracy of ± 1.5 %, and found that the results of Dantuma [9] were in serious error. Additionally, the differences between capillary and oscillational methods show large divergences, amounting to a maximum of 22 %. The results of Zuca [13] by the oscillating sphere method also were in disagreement with the previous recommendations. The actual recommendation of the MSDC [11] is based on the work of Janz et al. [10] together with Murgulescu and Zuca [12, 13] and Protsenko and Razumovskaya data [14]. Figure 2 compares the data shown in Fig. 1. Included are also the data of Brovkina et al. [17] and Smotrakov et al. [18], not mentioned in the papers of Janz.
As it can be seen, there are still large discrepancies between different sets of data, and deviations are positive or negative to current recommended data. Data points of Zuca above 670 K show a different deviation relative to points at lower temperature. Our data are systematically higher than the MSDC correlation. More, the previous recommendations are completely out of scale as they show a difference to the data of Janz, ranging from –15 % to –30 %.
Figure 3 shows the obtained viscosity as a function of absolute temperature. The present data (one data point was removed at T = 554.5 K) can be fitted to the Arrhenius equation which is also represented in Fig. 4. The least-squares fit to the Arrhenius law gives then the following equation:
With the quantities expressed in the same units than in Eq. (1) and a regression coefficient of 0.99, the activation energy for viscosity, \(E_{\eta }\) = 19.3 \(\hbox {kJ}\) \(\cdot \) \(\hbox {mol}^{-1}\), was found to be about 4 % higher that the value given by the MSDC recommendation [11].
4 Conclusions
New data for the viscosity of molten lithium nitrate between 540 K and 690 K are reported. These data were obtained, for the first time, with an oscillating-cup viscometer, with an estimated uncertainty of 3 %. These data were compared with previous values obtained by different techniques (capillary and oscillating sphere). New data can help to establish new recommended data as the previous values were obtained by different techniques (capillary and oscillating sphere). We stated in the past [19] that the transport of charge and momentum is done by individual ions or by locally ordered complexes. In the case of lithium salt, due to the polarization of anion by the strong electric field of the \(\hbox {Li}^{+}\) ion, the complexes also participate in the transport of momentum. These fluids are Coulombic in nature, and some of the considerations outlined here can be naturally extended to the low-temperature ionic liquids (RTILs).
References
J.G. Cordaro, N.C. Rubin, R.W. Bradshaw, J. Sol. Energy Eng. 133, 011014 (2011)
R.W. Bradshaw, Sandia Report, SAND2010-1129 (2010)
T. Wang, D. Mantha, R.G. Reddy, Appl. Energy 102, 1422 (2013)
A.G. Fernandez, S. Ushak, H. Galleguillos, F.J. Pérez, Appl. Energy 119, 131 (2014)
V.M.B. Nunes, M.J.V. Lourenço, F.J.V. Santos, C.A. Nieto de Castro, Int. J. Thermophys. 27, 1638 (2006)
V.M.B. Nunes, M.J.V. Lourenço, F.J.V. Santos, C.A.Nieto de Castro, Viscosity of molten lithium nitrate in Euchem Conference on Molten Salts and ionic Liquids, Hammamet, Tunísia (2006)
C.A. Nieto de Castro, F.J.V. Santos, Chim. Oggi Chem. Today 25, 20 (2007)
G.J. Janz, F.W. Dampier, G.R. Lakshiminarayanan, P.K. Lorenz, R.P.T. Tomkins, Natl. Stand. Ref. Data Ser., Natl. Bur. Stand. 15, 25 (1968)
R.S. Dantuma, Z. Anorg. Chem. 175, 1 (1928)
G.J. Janz, S.W. Lurie, G.L. Gardner, J. Chem. Eng. Data 23, 14 (1978)
G.J. Janz, NIST Standard Reference Database, vol 27, (Gaithersburg, MD, 1991)
I.G. Murgulescu, S. Zuca, Electrochim. Acta 11, 1383 (1966)
S. Zuca, Rev. Roum. Chim. 15, 1277 (1970)
P.I. Protsenko, O.N. Razumovskaya, Zhur. Prikl. Khim. 38, 2355 (1965)
V.M.B. Nunes, F.J.V. Santos, Nieto de Castro, C. A. Int. J. Thermophys. 19, 427 (1998)
K.H. Stern, J. Phys. Chem. Ref. Data 1, 747 (1972)
I. Brovkina, A. Farmakovskaya, V. Khokhlov, Electrochem. Molten Solid Electrolytes 21, 4 (1974)
V.G. Smotrakov, N.P. Popovskaya, V.A. Tereshenko, Zhur. Prikl. Khim 45, 2627 (1972)
V.M.B. Nunes, M.J.V. Lourenço, P. Panandiker, F.J.V. Santos, C.A. Nieto de Castro, in Proceedings of the 7th International Symposium on Molten Salts Chemistry and Technology, ed. by P. Taxil et al., vol. 2 (Toulouse, France, 2005), pp. 783–786
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nunes, V.M.B., Lourenço, M.J.V., Santos, F.J.V. et al. Measurements of the Viscosity of Molten Lithium Nitrate by the Oscillating-cup Method. Int J Thermophys 38, 13 (2017). https://doi.org/10.1007/s10765-016-2150-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-016-2150-1