Abstract
Exces volumes, \(V^{\mathrm{E}}\), and excess isentropic compressibilities, \(\kappa _{S}^{\mathrm {E}}\), have been reported as a function of composition for binary liquid mixtures of trichloroethylene with ethyl acetate, n-propyl acetate, and n-butyl acetate at 303.15 K. Isentropic compressibilities are calculated using measured sound speeds and density data for pure components and for binary mixtures. Excess volumes and excess isentropic compressibilities are found to be negative for the three systems studied over the entire composition range at 303.15 K, whereas these values become more negative with an increase of carbon chain length. The results are discussed in terms of intermolecular interactions between unlike molecules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Trichloroethylene is a non-flammable liquid and commonly used as an industrial solvent. It is mainly used as a degreasing agent in metal fabricating operations. It had a multitude of uses in many other industries such as dry cleaning, textile, electronics, leather, and rubber. Acute and chronic inhalation exposure to trichloroethylene can affect the human nervous system. Liver, kidney, immunological, and developmental effects have also been reported in humans. In this concern, physical properties of liquid mixtures containing trichloroethylene are required in most calculations where mixing is an important factor in many practical problems concerning mass transport applications.
Studies on molecular interactions in terms of thermodynamic and physical properties of liquid mixtures containing trichloroethylene as a common component have been reported [1–3]. A survey of the literature revealed that the excess volumes, \(V^{\mathrm{E}}\), and excess isentropic compressibilities, \(\kappa _{S}^{\mathrm {E}}\), have not been studied earlier for the present three systems. However, the excess volumes have been reported for 1,1,1-trichloroethane with ketones and ester systems [4]. Hence, in the present paper, the main purpose is to study the solution behavior of trichloroethylene with ethyl acetate, n-propyl acetate, and n-butyl acetate at 303.15 K. Further, the effect of chain length on the molecular interactions is discussed.
2 Materials and Methods
Trichloroethylene, ethyl acetate, n-propyl acetate, and n-butyl acetate (S. D. Fine, India) were purified by the standard methods described in the literature [5]. The water content of solvents used in this work was measured using an Analab (MicroAqua Cal 100) Karl Fischer Titrator and Karl Fisher reagent from Merck. The purity of the samples was ascertained by comparing the measured densities and sound speeds with those reported in the literature [5–9], and the data are given in Table 1 along with CAS number and water content.
Densities of the pure liquids and their mixtures were measured by using a Rudolph Research analytical digital densimeter (DDM-2911 model), and these measurements were carried out by carefully filling the sample in the U tube of the instrument with the help of a medical syringe. We have also ensured that there is no bubble formation during the measurement since cell should be air-free. The density was measured automatically at the specified temperature with an uncertainty of \(0.00005\,\hbox {g}{\cdot }\hbox {cm}^{-3}\). The sound speeds, u, were measured using an ultrasonic interferometer at a fixed frequency of 2 MHz, and the values were accurate to 0.3 %. The sound speed is calculated by using the relation, \(u=\nu \lambda \), where \(\nu \) is the frequency and \(\lambda \) is the wavelength. The wavelength can be computed from the relation, \(d={n\lambda }/2\), where d is the total distance moved by the reflector.
3 Results and Discussion
The excess volume data were calculated from the densities of pure liquids and their mixtures using the following equation:
where \(x_{1}\) and \(x_{2}\) are mole fractions, \(M_{1}\) and \(M_{2}\) are molecular weights, \(\rho _{1}\) and \(\rho _{2}\) are densities of components 1 and 2, respectively, and \(\rho _\mathrm{m}\) is the density of the mixture. The uncertainty in the excess volume measurement is \(0.005\,\hbox {cm}^{3}{\cdot }\hbox {mol}^{-1}\).
The experimental speed of sound (u) and density \((\rho )\) data were used to compute the isentropic compressibility \((\kappa _{s})\) by using the equation,
The isentropic compressibility is dependent on the ultrasonic speed. This quantity is considered as a thermodynamic property if a negligible amount of ultrasonic absorption of the acoustic waves of low frequency and of low amplitude is observed.
The corresponding excess isentropic compressibility \((\kappa _{s}^{\mathrm{E}})\) was calculated employing the following relations [10]:
where \(\kappa _{s}^{\mathrm{id}}\) is the ideal value of the isentropic compressibility and was calculated from the following equation [10]:
Here, \(C_{p,i}\) and \(\alpha _{i}\) are the molar heat capacity and the thermal expansion coefficient of the ith component, respectively. The values of \(C_{p,i}\) and \(\alpha _{i}\) were obtained and evaluated from the literature [11, 12].
Densities, sound speeds, excess volumes, isentropic compressibilities, and excess isentropic compressibilities for the three binary systems are given in Table 2.
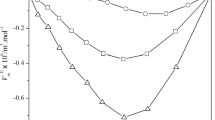
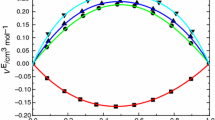
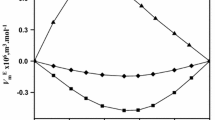
The excess volumes and excess isentropic compressibilities are plotted as a function of mole fraction and volume fraction, respectively, in Figs. 1 and 2.
Excess volumes and excess isentropic compressibilities are negative in all the systems over the whole range of mole fraction. The variation of \(V^{\mathrm{E}}\) with mole fraction is almost symmetrical in the systems, trichloroethylene with n-propyl acetate and n-butyl acetate, whereas in the system of trichloroethylene with ethyl acetate, the values are skewed toward the trichloroethylene-rich region. The excess properties may be explained in terms of (a) the loss of dipolar association in pure components, (b) specific interactions between unlike components, and (c) the difference in size and shape of the components. The negative \(V^{\mathrm{E}}\) and \(\kappa _{S}^{\mathrm {E}}\) indicate that the effect due to specific interactions between unlike components is dominant over the effect due to loss of dipolar association.
The algebraic values of \(V^{\mathrm{E}}\) and \(\kappa _{S}^{\mathrm {E}}\) for the three systems fall in the sequence:
-
n-butyl acetate \(<\) n-propyl acetate \(<\) ethyl acetate
This order is in agreement with the order in molar polarizabilities [5] (ethyl acetate: \(61.65\,\hbox {cm}^{3}{\cdot }\hbox {mol}^{-1}\); n-propyl acetate: \(72.30\,\hbox {cm}^{3}{\cdot }\hbox {mol}^{-1}\); n-butyl acetate: \(78.82\,\hbox {cm}^{3}{\cdot }\hbox {mol}^{-1}\)) of non-common components. This is expected because the non-common component with higher polarizability interacts strongly with trichloroethylene, and hence, \(V^{\mathrm{E}}\) and \(\kappa _{S}^{\mathrm {E}}\) values are more negative in such mixtures.
4 Conclusions
The density and sound speed for three binary mixtures of trichloroethylene with ethyl acetate, n-propyl acetate, and n-butyl acetate at 303.15 K were measured. From the precise experimental density and sound-speed data, excess volumes, \(V^{\mathrm{E}}\), and excess isentropic compressibilities, \(\kappa _{S}^{\mathrm {E}}\), were computed. These derived properties were found to be negative over the entire composition range which can be ascribed to specific interactions between unlike molecules. The computed data and the plots of excess properties were discussed in terms of intermolecular interactions.
References
K. Subramanyam Reddy, D.V.S. Jain, Thermochem. Acta 107, 383 (1986)
K. Purnachandra Rao, K. Subramanyam Reddy, J. Chem. Eng. Data 33, 1980 (1980)
K. Purnachandra Rao, K. Subramanyam Reddy, Fluid Phase Equilib. 34, 265 (1987)
K. Sivakumar, P.R. Naidu, Fluid Phase Equilib. 127, 173 (1997)
J.A. Riddick, W.B. Bunger, T.K. Sakano, Organic Solvents: Physical Properties and Methods of Purification, 4th edn. (Wiley-Inter Science, New York, 1976)
J. Timmermans, Physico-Chemical Constants of Pure Organic Compounds (Elsevier, Amsterdam, 1950)
J.N. Nayak, M.I. Aralaguppi, U.S. Toti, T.M. Aminabhavi, J. Chem. Eng. Data 48, 1483 (2003)
D. Venkatesulu, P. Venkatesu, M.V. Prabhakara Rao, J. Chem. Eng. Data 42, 1145 (1997)
P. Murali Krishna, B. Ranjith Kumar, B. Sathyanarayana, N.K. Satyanarayana, J. Chem. Eng. Data 54, 1069 (2009)
G.C. Benson, O. Kiyohara, J. Chem. Thermodyn. 11, 1061 (1979)
L. Venkatramana, R.L. Gardas, C. Narasimha Rao, K. Sivakumar, K. Dayanand Reddy, J. Solution Chem. 44, 327 (2015)
K. Purnachandra Rao, Ultrasonics 28, 120 (1990)
Acknowledgments
One of the authors (S. Ramanaiah) is grateful to the Council of Scientific and Industrial Research (CSIR), Government of India, New Delhi for the award of Research Associateship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramanaiah, S., Rao, C.N., Nagaraja, P. et al. Excess Volumes and Excess Isentropic Compressibilities of Binary Liquid Mixtures of Trichloroethylene with Esters at 303.15 K. Int J Thermophys 36, 2647–2653 (2015). https://doi.org/10.1007/s10765-015-1927-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10765-015-1927-y