Abstract
Long-term population data on endangered species are fundamental to measure conservation implementation objectively, but they are rare, especially in remote forest locations and with total counts. Following the scientific description of the kipunji (Rungwecebus kipunji), we implemented a range of long-term conservation interventions. Thirteen years later, we reassess with a complete count the population size, demography, and distribution of R. kipunji in Tanzania’s Southern Highlands, employing the identical sweep census methods across 1,428 km. We also monitored a habituated group daily over the same period. We report a total of 1,866 individuals in 59 groups (μ = 31.63 ± SE 1.2) in Livingstone Forest (within Kitulo National Park), Mt Rungwe Nature Reserve, and Madehani Village Forest. We estimate a 65% increase in individuals, a 59% increase in group numbers, and a 19% increase in area of occupancy (AoO). Mean group sizes were similar in Mt Rungwe (32.9) and Livingstone (31.9), but lower in the unprotected Madehani (24). The ratio of adult females to adult males was significantly higher in Mt Rungwe than Livingstone. The ratio of subadults/juveniles to adult females, a proxy for survival, was good (1.77), but higher in Livingstone (2.61) than Mt Rungwe (1.11). In the habituated group, we recorded a 121% increase in group size. Signs of human activity fell by 81%, with a 100% and 98% reduction in the number of charcoal pits and timber felling, respectively, in Mt Rungwe. Both temporal and spatial data demonstrate that long-term holistic conservation leads to increased primate numbers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species conservation faces many challenges, not least those associated with financing, political will, and the types of conservation intervention used and why. Objective technical conservation audits are uncommon, and there can be a reluctance for practitioners such as NGOs, government institutions, or academics to submit to close external or empirical examination (Junker et al., 2020). Whilst the value of species-based monitoring systems is understood, they are seldom performed, except perhaps at a global level (IUCN, 2017). The most fundamental means of objectively appraising conservation effort on threatened species is the rigorous collection and analysis of long-term population data, but they too are rare, especially in remote forest locations, with cryptic animals, and those concerning absolute numbers (Kleiman et al., 2001).
Non-human primates are among the most threatened taxa in the world, and they play a critical role in maintaining tropical biodiversity and ecosystem processes and functions (Estrada et al., 2017, 2018). There have, however, been few assessments of the long-term population trends of individual endangered primate species, especially in the context of their response to conservation management (Junker et al., 2020). The best example is the series of censuses of the mountain gorilla (Gorilla beringei beringei) in Bwindi, Uganda (Guschanski et al., 2009; Hickey et al., 2019; McNeilage et al., 2001, 2006; Roy et al., 2014). The global appeal of this subspecies, and the associated tourism revenue have ensured there is a great deal of conservation effort and funds directed their way. However, the relative efficacy of the main methods used (indirect sign through nest counts and genetics) remains contentious (Guschanski et al., 2009; Hickey et al., 2019; Roy et al., 2014), despite nest detection being relatively easy. Long-term demographic data were used on the same subspecies in the Virungas (Rwanda) to demonstrate an increase in G. berengei, but these animals were subject to direct human influences, including habituation, veterinary care, and close monitoring of individuals (Robbins et al., 2011).
Other examples of long-term monitoring of population trends in primates included a 28-year analysis of the density of five primate species in response to logging in Uganda (Chapman et al., 2000); the use of point transects with lures to model subpopulations of golden lion tamarins (Leontopithecus rosalia) (Ruiz-Miranda et al., 2019) and employing indirect signs to determine densities of subpopulations of a subspecies of Mexican howler monkey (Alouatta palliata mexicana), in a small area of forest patches (Alcocer-Rodríguez et al., 2021). None of these examples, however, covered species over time or used absolute numbers. Thus, despite their considerable importance, high-resolution long-term data are uncommon, especially at species level. This is unsurprising given that both the efficacy and consistency of method are essential and expensive.
The endemic primate the kipunji (Rungwecebus kipunji) was discovered to science during conservation survey work in Tanzania’s Southern Highlands in 2003 and Ndundulu in 2004. It was ultimately described as a new genus and named after the forested volcano on which it was first found, Mt Rungwe (Davenport et al., 2006). At that stage, both the animal and its forested habitat were under severe threat, in need of both immediate conservation intervention and ecological and demographic information. Soon after scientific discovery, therefore, we carried out an extensive survey to determine the full extent of R. kipunji’s distribution and abundance, and thus guide conservation measures (Davenport et al., 2008).
R. kipunji are group-living, forest-dependent, and primarily arboreal monkeys (Davenport et al., 2006), although they occasionally leave the forest and move on the ground to raid crops (Davenport, 2005; Davenport et al., 2006). They dwell between 1,300 and 2,450 m asl and are known from two populations separated by ca. 350 km of agricultural land; namely the contiguous montane forests of Mt Rungwe and Livingstone in the Southern Highlands, and the submontane Ndundulu in the southern Udzungwas (Davenport et al., 2006). They form multi-male, multi-female groups, often associating with other monkey species (Davenport & Jones, 2005). Their diet includes over 130 species of fruit, seeds, leaves, bark, pith, lichen, moss, and invertebrates (Bracebridge et al., 2012; Davenport et al., 2006; Davenport & Butynski, 2013) and they tend to be more folivorous during the dry season and more frugivorous during the rains (Bracebridge et al., 2012; Davenport et al., 2010). They have a mean home-range size of 3.06 km2 and are largely not territorial (De Luca et al., 2009).
The systematic surveys of 2007 provided a total estimate of 1,117 individuals, with 93% of the total (1,042 individuals) in Mt Rungwe and Livingstone in the Southern Highlands, and 7% (75 individuals) in Ndundulu in the Udzungwas (Davenport et al., 2008). At that time we categorized R. kipunji as Critically Endangered on the IUCN Red List, but this was later modified to Endangered B1ab(ii, iii, iv, v) + 2ab(ii, iii, iv, v) following changes in the Red List criteria (Davenport, 2019b). That said, R. kipunji is sparsely distributed (Bracebridge et al., 2011, 2013; Davenport, 2019b; Davenport et al., 2006, 2008) and the small areas of habitat where it occurs, and its patchy distribution in Mt Rungwe, Livingstone, and Ndundulu give grounds for considerable conservation concern.
We adopted the species as a conservation flagship for the region’s forests, and along with government partners we carried out applied conservation work across the R. kipunji range. A broad range of landscape-wide conservation interventions was implemented over the last decade and a half. Activities included, but were not restricted to, the gazettement of new protected areas or extensions, law enforcement, snare sweeps, hiring and training of forest rangers, protected area management, the production of park and reserve management plans, community education reaching a quarter of a million people, setting up of village environment committees, wildlife clubs, indigenous tree nurseries and reforestation, livelihood support programs, alternative fuels, honey production, forest leasing, awareness raising, research, surveys, ecological and law enforcement monitoring, by-law enforcement, and fire prevention.
Through providing empirical data we wished to test whether an holistic and long-term approach to site-based forest management alongside species conservation can benefit a species such as the endangered R. kipunji. We predicted that increased long-term protection of relevant primate habitat, in conjunction with the involvement of — and support to — neighboring human communities, would result in more monkeys. Therefore, we repeated the R. kipunji survey using identical methods of 2007 (Davenport et al., 2008), across the Southern Highlands (Livingstone, Mt Rungwe, and Madehani), but excluding Ndundulu, for which we had provided an updated estimate previously (Davenport 2019a). We included all relevant habitat and assessments of human impacts. We asked both spatial and temporal questions. Spatially we ask: a) do forests with more protection support more R. kipunji and contain groups with a higher survival/recruitment rate?, and b) do forests with less human disturbance have more R. kipunji and larger R. kipunji groups? Temporally, we ask: c) has the total population of R. kipunji has grown over time and contemporaneously with conservation action, with an increase in number of individuals, groups and distribution?, d) has a habituated group monitored daily for over a decade showing an increase in numbers and a stable demography?, and e) has there has been a contemporaneous and quantifiable reduction in human impact within the surveyed areas?
Methods
Study Area
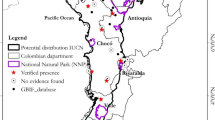
We carried out the study in Tanzania’s Southern Highlands, and the sites were the 191 km2 Livingstone Forest which lies within the 412 km2 Kitulo National Park, the 150 km2 Mt Rungwe Nature Reserve, and Madehani Village Forest Reserve (Fig. 1). The area is located at the northern-most end of the Lake Nyasa Trough. Livingstone, located between 9°00’–9°14’ S and 33°40’–33°57’ E, was gazetted as a catchment forest reserve in 1940 (McKone & Walzern, 1994), and was included as part of Kitulo National Park, gazetted in 2002 (Davenport, 2002; Davenport & Bytebier, 2004). Mt Rungwe is located between 9°03’–9°12’ S and 33°35’–33°45’E, was formerly a catchment forest reserve gazetted in 1949 (McKone & Walzern, 1994), and upgraded to Mt Rungwe Nature Reserve following the discovery of the R. kipunji (Davenport et al., 2008).
Mt Rungwe and Livingstone Forest (in Kitulo) are connected by the Bujingijila Corridor, a 2-km wide degraded forest connection (Davenport, 2005, 2006). R. kipunji inhabits the wetter forest of south Mt Rungwe and isolated groups are scattered in the north and south of Livingstone Forest (Bracebridge et al., 2011; Davenport et al., 2006, 2008). Madehani lies within the Livingstone Mountain range, situated along the northeastern edge of Lake Nyasa between 9°20'-9°21' S and 33°59'–33°60' E. The forest falls under management of three villages Madehani, Iyoka and Lumage, in Bulongwa division. The forest covers 449.77 ha, where the larger part of the forest is in Madehani Village (299 ha), the smaller parts being in Iyoka (83.85 ha) and Lumage (66.82 ha). These forests have steep slopes, which are covered with bamboo vegetation and montane forest. It is surrounded by montane grassland and shrub. R. kipunji habitats comprise submontane, montane, upper montane, and bamboo forests between 1300 and 2600 m (Davenport et al., 2008).
Counting R. kipunji
Forest primates are notoriously challenging to survey and many techniques have been suggested (Brockelman & Ali, 1987; Plumptre & Cox, 2006; Rovero et al., 2006; Whitesides et al., 1988). The cryptic nature of R. kipunji, its scarcity, fear of humans, and predilection for montane forest canopy exacerbated the difficulties. Based on our experience with the species and its habitats, we devised an accurate and comprehensive method to determine the distribution of R. kipunji and to census the population of the species (Davenport et al., 2008). These same methods were subsequently used successfully in other Tanzanian forests with the ashy red colobus (Piliocolobus tephrosceles) in Ufipa (Davenport et al., 2007) and the Zanzibar red colobus (P. kirkii) across Unguja (Davenport et al., 2019). Furthermore, in 2008 we began habituating a group of R. kipunji in Nkuka, a section of western Mt Rungwe that WCS has leased, identifying all individuals and monitoring them daily ever since. This has permitted us a view of long-term demography within one group.
R. kipunji groups are found in isolated subpopulations across the Rungwe–Livingstone forests (Davenport et al., 2008). We divided the study area into four blocks based on R. kipunji group information from previous studies (Bracebridge et al., 2011, 2012; Davenport et al., 2008). The Madehani survey was carried out separately and previously. During the period November 2019 to 2020, various methods were employed to determine distribution and abundance. Distribution data were collected using presence/absence surveys, whereas census data were recorded using total counts made while following groups.
Presence/Absence Surveys
We selected forests for presence/absence surveys based on prior knowledge of the areas, information from previous surveys, village interviews, and the habitat type, quality, and altitudinal range from which R. kipunji were already known. At each site, 3–4 pairs (teams) of observers searched concurrently for R. kipunji along separate pre-planned routes, using 1:50,000 topographic maps (Tanzania Surveys and Mapping Division, Series Y742), global positioning system (GPS) units, and binoculars. Each team comprised a scientist and a field assistant. We considered only direct sightings to be positive indicators of presence. Vocalizations were verified by sightings. New areas were surveyed each day, adjacent to the area covered the previous day. Some areas were revisited if they contained a high density of fruiting trees and other primates, or if inclement weather had hindered earlier work. Survey routes followed wildlife trails, human tracks, and off-track, to survey a large area thoroughly. Each team walked slowly (<2 km per hour) and quietly during 06.30–18.00, no more than 100 m apart, scanning the understorey and canopy for monkeys. Surveys were paused in heavy rain. When an individual or group was detected, the observer remained until he/she was confident that the species was correctly identified, and then the follow would begin.
Census
To ascertain the total R. kipunji population as accurately as possible in 2007, we adapted the complete count method, which is accepted as being the most precise primate census technique (Plumptre & Cox, 2006). Unlike traditional census methods based on complete counts of indirect sign (Harcourt & Fossey, 1981; McNeilage et al. 200, 2006), our methods used direct observations of individuals only. The methods employed in this study, therefore, were those we had devised previously and used successfully for complete census counts of the ashy red colobus in Ufipa (Davenport et al., 2007), R. kipunji in the Southern Highlands (Davenport et al., 2008), and the Zanzibar red colobus (P. kirkii) in Unguja (Davenport et al., 2019). In this way, our calculation of the observed population was neither an estimate nor an extrapolation based on density, but an absolute figure, and one that is directly comparable with the study 13 years before.
To count all individuals directly within each group, we aimed to locate and follow every group for a minimum of 3 consecutive days, tracking all movements and distances with a GPS. When a team located a group, it remained with the group at a distance that was sufficient to maintain contact while minimizing stress on the group (Cipolletta, 2003). Grid reference positions of the group were recorded routinely by GPS every 15 minutes. Teams maintained contact with one another via mobile phone. During the follows, the numbers of individuals, adult males, adult females, infants and subadults/juveniles (Table I) in each group were counted 8 times daily and/or whenever the opportunity arose. The four field assistants had a combined total of 61 years’ experience (μ = 15 ± SE 3.5, N = 4) in R. kipunji research, including in determining sex and age-class (Davenport et al., 2008). Extensive inter-observer reliability training was carried out prior to the study, and the same person did all the counting in each team, reducing potential errors resulting from a change of observers.
Censuses started on the eastern part of Livingstone (Kigulu block), moving towards the western part of Livingstone, with sweeps in each block also starting in the east and moving west to cover the block. The presence of human impacts were recorded and georeferenced throughout the survey. At any particular site, R. kipunji groups were considered to be unique if: (1) they were seen at the same time by different observation teams, spending more than 75% of the observation time at a distance of at least 300 m apart (this was verified a posteriori), (2) one team saw a group other than the one they were following, at least 300 m away, and later verified that no other team had been near the group(s), (3) the groups were recorded > 300 m apart, at the same time, and subsequently moved in different directions. In cases where there was any doubt, at least two teams returned to the location at a later date to verify group identity through location, size and demography.
We used ArcGIS 9.3 (ESRI, Redlands, CA, USA) to analyse observation data from all R. kipunji groups recorded in the census. The species' area of occupancy (AoO) — defined as the area within the species’ extent of occurrence (EoO) that a taxon occupies, excluding cases of vagrancy — was formerly estimated to be 42.4 km2 in Rungwe–Livingstone (Bracebridge et al., 2011) and 25 km2 in Ndundulu (Davenport pers ob. in Marshall et al., 2015) with a combined total of 67.5 km2. Using the new data, we calculated the current AoO, employing the new grid method (IUCN, 2017) whereby a 2 × 2 km grid of cells is overlaid on all observation points and compared it to 2007.
Statistics
Differences in group composition were examined with non-parametrical (Kruskal–Wallis and Mann–Whitney) U tests using R (R Core Team, 2021).
Habituated Group
The Nkuka group, situated in the western sector of Mt Rungwe (#19 on Fig. 2) was habituated between 2009 and 2010 (Bracebridge et al., 2011). Since that time, a rotating team of three people have consistently followed the animals and the group has been monitored daily. All individuals are known and all births, deaths, immigrations, and emigrations are recorded. Group counts were made every week over a 13-year period from May 2008 to February 2021.
Human Impact
In addition, incidences of illegal human impact and activity were observed during the primate surveys, and the location and type of activity recorded. The data were collected in the same manner as 13 years before (Davenport et al., 2008). The activities included charcoal pits, livestock grazing, gardens, logging camps, snares and traps, saw pits and felled trees, and people carrying out such activities. The time since the activity occurred was estimated and those ranked as ‘active’ (active and up to a day old) or ‘recent’ (2 days to 3 months) were used for analysis.
Ethical Note
The field research and village interviews adhered to the legal requirements of the United Republic of Tanzania and met the standards of the IPS Code of Best Practices for Field Primatology. The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request. The authors declare that they have no conflict of interest.
Results
The total effort spent searching for and following R. kipunji was 1,305 team-hours (405 team-days) and covered 1,428 km. The surveys revealed the extent of current R. kipunji distribution across their range in the Southern Highlands (Figs. 2 and 3). Despite extensive surveys, R. kipunji was still not recorded in the northern sectors of Mt Rungwe or the northern and more eastern sections of Livingstone in Kitulo. The species’ absence from these areas was concomitant with earlier findings and was supported by discussions in adjacent villages and (Bracebridge et al., 2011). We recorded R. kipunji between elevations of 1,152 m asl in Kigulu Mlanda to 2,544 m asl in Ibumbigi Lusanje (μ = 1,830 ± SE 2.88, N =2,986), a range of 1,392 m. We estimate the area of occupancy (AoO) of R. kipunji in Mt Rungwe and Livingstone to be 136 km2 based on the 2 × 2 km grid overlay. The total AoO for the Southern Highlands (including Madehani) was 152 km2.
A total of 59 R. kipunji groups were identified (31 in Livingstone, 23 in Mt Rungwe, and five in Madehani, Table II) and groups were counted 891 times (μ = 15.1 ± SE 1.87, N = 59). We estimate the total R. kipunji population in the Southern Highlands to be 1,866 individuals, with 12 to 51 individuals per group (μ = 31.63 ± SE 1.2, N = 59): 990 individuals in Livingstone (Kitulo National Park), 756 in Mt Rungwe (Nature Reserve), and 120 in Madehani (Village Forest). Mean group size was considerably higher in Mt Rungwe (μ = 32.87 ± SE1.93, N = 23) and Livingstone (μ = 31.94 ± SE 1.66, N = 31) than Madehani (μ = 24.00 ± SE 3.05, N = 5), although this difference was not statistically significant (Kruskal–Wallis: χ2 = 4.092, df = 2, P = 0.129).
The total number of adult females was 521 (29.8% of the total), with a mean of 9.65 ± SE 0.7 adult females per group (N = 54, Table III). The total number of adult males was 209 (11.2%), with a mean of 3.87 ± SE 0.2 per group (N = 54). These data show a combined ratio of 2.49 adult females to adult males in both sites (Table IV). However, the total ratios of 1.97 in Livingstone versus 5.04 in Mt Rungwe differed significantly (Mann–Whitney U test: W = 165, P = 0.0008). Whilst there was no significant difference (Mann–Whitney U test: W = 301, N = 54, P = 0.324) in the number of adult males per group in Livingstone (μ = 3.77; N = 31; ± SE 0.32) compared to Mt Rungwe (μ = 0.40; N = 23; ± SE 0.22), there were significantly more adult females per group in Mt Rungwe (μ = 12.65; N = 23; ± SE 1.15) than Livingstone (μ = 7.42; N = 31; ± SE 0.64), (Mann–Whitney U test: W = 160, N = 54, P = 0.0006).
The total number of subadults and juveniles was 924 (52.9% of the total), with a mean of 17.11 ± SE 1.04 per group (N = 54), and the total number of infants was 92 (4.9%), with a mean of 1.7 ± SE 0.23 per group (N = 54), yielding ratios of 0.18 infants to adult females, and 1.77 subadults/juveniles to adult females across both sites. The mean ratio of infants/neonates to adult females per group between Livingstone (μ = 0.24; N = 31; ± SE 0.05) and Mt Rungwe (μ = 0.19; N = 23; ± SE 0.03) showed no significant difference (Mann–Whitney U test: W = 375.5, P = 0.7491). However, the mean ratio per group of subadults/juveniles to adult females in Livingstone (μ = 3.26; N = 31; ± SE 0.05) was significantly higher than in Mt Rungwe (μ = 1.47; N = 23; ± SE 0.26; Mann–Whitney U test: W = 577, P = 0.0001). There were significantly more subadults/juveniles per group in Livingstone (μ = 19.39; N = 31; ± SE 1.37) than in Mt Rungwe (μ = 14.04; N = 23; ± SE 1.37; Mann–Whitney U test: W = 499, P = 0.013).
In the habituated Nkuka group (#19 on Fig. 2) between 2008 and 2021, there were 31 births, seven deaths, two immigrations and 28 emigrations. The group size ranged from a minimum of 19 and a maximum of 44 individuals with a mean of 28.4 (± SE 0.57, N = 156, Fig. 4, Table V). After a group split in 2011, between May 2011 and August 2020 there was a 121% increase in the habituated group size over 10 years.
The data are compared with those collected 13 years earlier (Table VI) with a total increase of 67.5% individuals and 58.8% of number of groups. In Livingstone, the total number of individuals rose from 541 to 990 (83%) and the number of groups from 18 to 31 (72.2%). New groups had formed in Ibumbihi, Kalasa, and Ikubo. In Mt Rungwe, the total number of monkeys had risen from 501 to 756 (50.9%) between 2007 and 2020 with the number of groups rising from 16 to 23 (43.8%), with a new group having formed in Mwaikole. In both forests, the mean group size remained constant, both over time and between sites.
Signs of human activity in the study area appear to have decreased both in frequency and type (Table VII). A total reduction in active and recent human impacts by 81% (N = 657) was recorded, with a 91% (N = 495) drop in Mt Rungwe, and a 37% (N = 162) drop in Livingstone. Most significantly, whilst snaring continues to be a challenge, there was a 100% drop (N = 84) in charcoal pits in Mt Rungwe from 4.9/100 km to 0, and a 56% and 98% reduction in saw pits and cut trees in Livingstone (N = 101) and Mt Rungwe (N = 338) respectively. The total reduction in timber activity observations was 90% (N = 439).
Discussion
After many years of practical and holistic conservation, we report an 81% decrease in illegal activities in the forests of Mt Rungwe and Livingstone and a concomitant 1.65-fold increase (65%) in the population of the Tanzanian endemic Rungwecebus kipunji between 2007 and 2020. The conservation status of R. kipunji, the immediacy of threats the primate faces, and our need to enable direct comparisons with the survey of 13 years before (Davenport et al., 2008) guided the decision to perform again a complete count by sweep census. This allowed us to test our predictions that more than a decade of active and all-inclusive conservation work in and around the forests would result in an increased R. kipunji population. Our results show this to be the case. Whilst it is not possible to measure the effectiveness of any one conservation intervention alone (Junker et al., 2020), our data show the validity, not only of the conservation strategy used in Mt Rungwe and Livingstone, but also of a means by which to show and quantify protection efficacy.
The methods have been employed successfully on two other primate species (Davenport et al., 2007, 2019), and our aim was to ensure as precise a population assessment as possible and one consistent with the previous survey. The complete count relies on locating and following every group (Davenport et al., 2008). Despite the considerable effort undertaken, there is a small possibility that under-counting or double-counting may have occurred as a result of challenges associated with dense steep habitat, fission–fusion, sampling bias and group overlap. Similarly, the greater experience of field technicians may have improved detection of R. kipunji.
We recorded R. kipunji groups across southern Mt Rungwe and the more western and central parts of Livingstone in Kitulo (Fig. 2, Table II). Whilst there was range extension, this was consistent with the earlier surveys where groups mostly inhabited the areas of forest with higher rainfall and a closed canopy (Davenport, 2019b). However, much of Mt Rungwe and Livingstone contains heterogeneous forest, a fragmented mix of primary, secondary, and regenerating forest reflecting a history of disturbance (Davenport et al., 2006; De Luca et al., 2009). This suggests that while not optimal habitat, R. kipunji tolerates disturbed habitat relatively well (Bracebridge et al., 2011).
Predictions based on a comparison between an interpolation analysis for habitat suitability and the AoO suggested that the majority of the most suitable areas in Mt Rungwe and Livingstone were already occupied by R. kipunji (Bracebridge et al., 2011). Ten years later however, the AoO has increased from 42.5 km2 to 136 km2. This is partly a result of the very different calculation methods employed. However, if the same 2 × 2 km grid method is performed, the AoO of 42.5 becomes 100 km2, and thus there has (still) been an increase in the last decade of 36 km2 (36%). Interestingly, the data show that this increase in AoO is exclusively in Livingstone and is a reflection of range expansion due to primate population growth and forest improvement. The total AoO for the Southern Highlands (including Madehani) is 152 km2 and for the species as a whole (including Ndundulu) is 177 km2. R. kipunji were also recorded between altitudes of 1,152 and 2,544 m asl, representing the highest and the lowest elevations the species has been found, and a range of 1,392 m. The species has expanded in number, distribution, and elevation.
We recorded 990 individuals in Livingstone (Kitulo National Park), 756 in Mt Rungwe (Nature Reserve), and 120 in Madehani (Village Forest), now representing 95% of the total population of R. kipunji (Table III). With the additional 100 animals in Ndundulu in Kilombero Nature Reserve we reported previously (Davenport, 2019a), we estimate a global population of R. kipunji of 1,966 animals. Whilst this remains very small for a total primate population, it represents a 65% increase over the last 13 years (excluding the ‘new’ Madehani population). Whilst it is possible that we have become more adept at finding the animals, this increase is not a reflection of new areas having been investigated, but a genuine growth in numbers (with new fission groups formed adjacent to old known ones). All areas of both forests were surveyed in both 2007 and 2020. The data from the habituated Nkuka group demonstrate that such a marked population growth of the species under optimal conditions is entirely possible (Fig. 4), with a 121% increase in that group’s numbers over the same time period.
These figures represent a significant conservation success and are the result of a long-term, concerted effort to protect the R. kipunji and its habitat. Over the last 20 years and especially since first describing the genus (Davenport et al., 2006), a broad range of conservation interventions have been implemented focusing on protected area management, adjacent community support and science. In terms of protected areas, these have included raising of the status of Livingstone as part of Kitulo NP, Mt Rungwe as a nature reserve, and Nkuka as a privately leased forest, and the provision of financial and technical support in the management of all three. Law enforcement, management planning, snare removal, boundary demarcation, ranger training and hiring, the enforcement of by-laws, and community participatory ranger patrols have all helped to reduce destructive activities and reduce encroachment.
Contemporaneously, the initiation and implementation of a range-wide environmental education program with school wildlife clubs and village environment committees, public relations, awareness on issues such as rainfall, water, soil conservation and pollination, tourism support, bee keeping and honey marketing projects, alternative fuel options, tree planting, nursery development, and woodlots for domestic fuel wood use (reducing dependence on the reserve for fuel) have all contributed to community support. With regard to science, R. kipunji and biodiversity research and monitoring, ecological and socio-ecological monitoring, the encouragement of applied research by national and international students, carbon quantification through REDD+, and international publications have all provided an interest and human presence and raised the area’s profile.
The result of these activities during a decade and a half of conservation investment is clear in terms of both a considerable increase in R. kipunji and improvement in habitat, caused by a marked reduction in illegal human use. The dramatic fall in human activities over the last 13 years is reflected in the total reduction of 81% in active and recent human impacts across both forests. Interestingly, Mt Rungwe demonstrated the most stark reduction (91%), for example with a 100% drop in charcoal pits and a 98% reduction in saw pits and cut trees. This is in part a function of the very poor condition of Mt Rungwe in the early 2000s. There were increases in incidences of cattle grazing within Livingstone, from 0.06/100 km in 2007 to 0.63/100 km in 2020. Similarly, there was an uptick in observations of gardens in Livingstone, from 0/100 km in 2007 to 0.49/100 km in 2020. However, these are both relatively modest, especially considering the matrix immediately surrounding the park is home to ~350 people per km2 (Davenport et al., 2008), and the gardens were small (<25 m2). Incidences in Mt Rungwe were negligible. That said, animal snares and traps are still being found across the landscape and this needs addressing.
Group size in primates is determined by predation pressure (including hunting), habitat quality, and social dynamics (Schülke & Ostner, 2012; Struhsaker, 2010). Crowned eagles (Stephanoaetus coronatus), leopards (Panthera pardus), and African rock pythons (Python sebae) are the only non-human predators of R. kipunji, and before any conservation work was initiated, hunting by people was not uncommon. Predation has been shown to result in smaller group sizes in P. tephrosceles, although the effect of predation tends to be less strong than that of habitat (Stanford, 1998). Larger primate groups usually occur in large forest blocks of good habitat, whereas smaller groups typically occur in small forest patches or degraded forest (Struhsaker, 2010). Our finding smaller mean group sizes in Madehani (μ = 24.00 ± SE 3.05; N = 5), compared to Mt Rungwe (μ = 32.87 ± SE1.93; N = 23) and Livingstone (μ = 31.94 ± SE 1.66; N = 31), is consistent with this, and is doubtless as a result of fragmentation, reduced resources, and higher levels of hunting by people, as found in other primates (Chapman et al., 2002; Struhsaker et al., 2004). The mean group sizes in both Livingstone and Mt Rungwe (Table V) are largely unchanged compared with 2007, and this is likely due to the large areas of previously unoccupied forest that has become higher quality over the last 20 years.
Mean recruitment/survivorship (proportion of adult females to subadults/juveniles) in the Southern Highlands is relatively high at 1.77, compared, for example, to 0.25 for the P. kirkii (Davenport et al., 2019) where habitat loss is significant. These R. kipunji data indicate a population still on the increase, and the figure correlates with the 1.65-fold increase in total number of individuals. The 1.11 recruitment rate in Mt Rungwe is lower than Livingstone at 2.61, possibly a reflection of the less remote and originally more disturbed nature of the forest in the former. Indeed, there were significantly more subadults/juveniles in Livingstone. The current recruitment in Nkuka is not high, as reflected by a subadult/juvenile to adult female ratio of 0.91 (Table IV), compared to 1.77 for the whole population, although this is probably a reflection of the fact that 11 individuals including subadults and juveniles recently emigrated from the group. Given all these data, there is considerable room for optimism, especially in terms of expansion. If current habitat protection continues, one could estimate a further doubling of the population in the next 25 years. It has been argued that where predators are common there are more males in primate groups and thus lower sex ratios (Van Schaik & Hörstermann, 1994). Our surveys showed that the adult female: male ratios were considerably and significantly higher in Mt Rungwe (5.04) than Livingstone (1.97), with an overall mean of 2.49. This may be a reflection of greater hunting by villagers in Mt Rungwe as retribution for raiding crops, and Livingstone being more inaccessible.
Outside Mt Rungwe and Livingstone, we identified five R. kipunji groups and estimated a total of 120 individuals in Madehani, with 17–32 individuals per group (μ = 24, +SE 3.04). Despite being just 449.77 ha this forest is of importance, as it comprises approximately 6.1% of the global R. kipunji population and is an isolated genetic pool. Greater protection for this forest is therefore warranted. Our close study of the habituated Nkuka group over 13 years (in an area where good conservation intervention resulted in very few illegal activities), has shown what R. kipunji are demographically capable of. Between 2008 and 2021, there were 31 births, seven deaths, two immigrations and 28 emigrations. The group size ranged from a minimum of 19 to a maximum of 44 individuals, with a mean of 28.4. After the split and between May 2011 and August 2020, there was a 121% increase over 10 years. Significantly, there were two major splits in this time, when the group number reached 33 in 2009 and 44 in 2020.
Temporally, this new survey reveals a 65% increase in individual R. kipunji over 13 years. This may be compared, for example, with a 3% growth recorded for (P. tephrosceles) in eight sites in Kibale over 45 years (Chapman et al., 2018). Moreover, we show an increase in group numbers by 59% and a 19% increase in AoO. The AoO only increased in Livingstone (by 38.5%). In Livingstone, individual numbers rose by 83% and group numbers by 72%. In Mt Rungwe, individuals rose by 51% and groups by 44%. Human impacts fell significantly by 81%. Over the same time period, the habituated group in Nkuka increased by 121%.
Spatially, mean group sizes are the same in Mt Rungwe and Livingstone (although much lower in Madehani). The ratio of adults females to adult males is significantly higher in Mt Rungwe, and there are significantly more adult females in Mt Rungwe too. The ratio of subadults/juveniles (SAs/Js) to adult females is significantly lower in Mt Rungwe and there are significantly fewer SAs/Js in Mt Rungwe. Survival is currently, therefore, higher in Livingstone.
The time period is precisely consistent with the implementation of broad-scale conservation, and thus the temporal data strongly suggests the positive impact on human impact and monkey numbers. The spatial data augments that conclusion, but also demonstrates that increases in numbers and range is concomitant with the degree of protection. Nkuka is a small (800 ha) forest patch leased and managed solely by WCS, albeit surrounded on three sides by — and contiguous with — Mt Rungwe. Livingstone is within a national park, the highest level of government protection, followed by Mt Rungwe. Madehani is a village forest with very little investment.
Conservation has an obligation to substantiate its methods and justify its financing, and to do so in a transparent and empirical manner. This is essential for donors, but also for the wider global community so that positive and negative lessons may be learned (Redford & Taber, 2000). These results are very positive and demonstrate clear and quantitative conservation success. They also justify the change in the IUCN Red List status for this species from ‘Critically Endangered’ to ‘Endangered’ (Davenport, 2019b). The species still faces serious challenges, and with 1,966 individuals in total remains very rare by most primate standards, even in Tanzania (Davenport et al., 2014). However, numbers have significantly increased since 2007 on the back of a persistent and holistic conservation approach. Both conservation and monitoring need to continue.
References
Alcocer-Rodríguez, M., Arroyo-Rodríguez, V., Galán-Acedo, C., Cristóbal-Azkarate, J., Asensio, N., Rito, K. F., Hawes, J. E., Veà, J., & Dunn, J. C. (2021). Evaluating extinction debt in fragmented forests: the rapid recovery of a critically endangered primate. Animal Conservation, 24, 432–444. https://doi.org/10.1111/acv.12648.
Bracebridge, C. E., Davenport, T. R. B., & Marsden, S. J. (2011). Can we extend the area of occupancy of the kipunji, a critically endangered African primate? Animal Conservation, 14, 687–696.
Bracebridge, C. E., Davenport, T. R. B., & Marsden, S. J. (2012). The impact of forest disturbance on the seasonal foraging ecology of a critically endangered African primate. Biotropica, 44, 560–565.
Bracebridge, C. E., Davenport, T. R. B., Mbofu, V. F., & Marsden, S. J. (2013). Is there a role for human-dominated landscapes in the long-term conservation management of the critically endangered kipunji (Rungwecebus kipunji). International Journal of Primatology, 34, 1122–1136.
Brockelman, W. Y., & Ali, R. (1987). Methods of surveying and sampling forest primate populations. In C. W. March & R. A. Mittermeier (Eds.), Primate Conservation in the Tropical Rain Forest (pp. 23–62). Alan R. Liss.
Chapman, C. A., Balcomb, S. R., Gillespie, T. R., Skorupa, J. P., & Struhsaker, T. (2000). Long-term effects of logging on African primate communities: a 28-year comparison from Kibale National Park. Uganda. Conservation Biology, 14(1), 207–217.
Chapman, C. A., Chapman, L. J., Bjorndal, K. A., & Onderdonk, D. A. (2002). Application of protein-to-fiber ratios to predict colobine abundance on different spatial scales. International Journal of Primatology, 23, 283–310.
Chapman, C. A., Bortolamiol, S., Matsuda, I., Omeja, P. A., Paim, F. P., Reyna-Hurtado, R., Sengupta, R., & Valenta, K. (2018). Primate population dynamics: variation in abundance over space and time. Biodiversity and Conservation, 27(5), 1221–1238.
Cipolletta, C. (2003). Ranging patterns of a western gorilla group during habituation to humans in the Dzanga-Ndoki National Park, Central African Republic. International Journal of Primatology, 24, 1207–1226.
Davenport, T. R. B. (2002). Tanzania’s new national park to protect orchids. Oryx, 36(3), 224.
Davenport, T. R. B. (2005). Finding kipunji. Africa Geographic, 13(7), 56–61.
Davenport, T. R. B. (2006). Plants, primates, and people. Conservation in the Southern Highlands of Tanzania. Miombo, 28, 7–8.
Davenport, T.R.B. (2019a). Kipunji (Rungwecebus kipunji). 32–36. In: Primates in Peril: The World’s 25 Most Endangered Primates 2018–2020 (pp. 129). Washington, DC: IUCN SSC Primate Specialist Group, International Primatological Society, Global Wildlife Conservation, and Bristol Zoological Society,.
Davenport, T. (2019b). Rungwecebus kipunji. The IUCN Red List of Threatened Species 2019: e.T136791A17961368. https://doi.org/10.2305/IUCN.UK.2019-3.RLTS.T136791A17961368.en. Downloaded on 02 September 2021.
Davenport, T. R. B., & Butynski, T. M. (2013). The Kipunji Rungwecebus kipunji. In T. M. Butynski, J. Kingdon, & J. Kalina (Eds.), The Mammals of Africa, Primates (Vol. II, pp. 213–217). Bloomsbury Publishing.
Davenport, T. R. B., & Bytebier, B. (2004). Kitulo Plateau, a first park for orchids. Orchid Review, 112, 160–165.
Davenport, T. R. B., & Jones, T. (2005). The highland mangabey — Africa's first new monkey for 20 years further illustrates the exceptional value of Tanzania's forests. Arc Journal, 18, 1–6.
Davenport, T. R. B., Stanley, W. T., Sargis, E. J., De Luca, D. W., Mpunga, N. E., Machaga, S. J., & Olson, L. E. (2006). A new genus of African monkey, Rungwecebus: morphology, ecology and molecular phylogenetics. Science, 312, 1378–1384.
Davenport, T. R. B., Mpunga, N. E., & Machaga, S. J. (2007). Census and conservation assessment of the red colobus (Procolobus rufomitratus tephrosceles) on the Ufipa Plateau, southwest Tanzania: newly discovered, threatened and extinct populations. Primate Conservation, 22, 97–105.
Davenport, T. R. B., De Luca, D. W., Jones, T., Mpunga, N. E., Machaga, S. J., Kitegile, A., & Picton Phillipps, G. (2008). The critically endangered kipunji Rungwecebus kipunji of southern Tanzania: first census and conservation status assessment. Oryx, 42, 352–359.
Davenport, T. R. B., De Luca, D. W., Bracebridge, C. E., & C.E., Machaga, S.J., Mpunga, N.E., Kibure, O., & Abeid, Y.S. (2010). Diet and feeding patterns in the kipunji (Rungwecebus kipunji) in Tanzania’s Southern Highlands: a first analysis. Primates, 51(3), 213–220.
Davenport, T. R. B., Nowak, K., & Perkin, A. (2014). Priority primate areas in Tanzania. Oryx, 48, 39–51.
Davenport, T. R. B., Fakih, S. A., Kimiti, S. P., Kleine, L. U., Foley, L. S., & De Luca, D. W. (2019). Zanzibar's endemic red colobus Piliocolobus kirkii: first systematic and total assessment of population, demography an distribution. Oryx, 53(1), 36–44. https://doi.org/10.1017/S003060531700148X.
De Luca, D. W., Picton Phillipps, G., Machaga, S. J., & Davenport, T. R. B. (2009). Home range, core areas and territoriality in the ‘critically endangered’ kipunji (Rungwecebus kipunji) in southwest Tanzania. African Journal of Ecology, 48, 895–904.
Estrada, A., Garver, P. A., Rylands, A. B., Roos, C., Fernandez-Duque, E., Di Fiore, A., Nekaris, K. A.-I., et al (2017). Impending extinction crisis of the world’s primates: Why primates matter. Science Advances, 3, e1600946. https://doi.org/10.1126/sciadv.1600946.
Estrada, A., Garber, P. A., Mittermeier, R. A., Wich, S., Gouveia, S., Dobrovolski, R., Nekaris, K. A. I., Nijman, V., Rylands, A. B., Maisels, F., Williamson, E. A., et al (2018). Primates in peril: the significance of Brazil, Madagascar, Indonesia and the Democratic Republic of the Congo for global primate conservation. Biodiversity and Conservation PeerJ, 6, e4869. https://doi.org/10.7717/peerj.4869.
Guschanski, K., Vigilant, L., McNeilage, A., Gray, M., Kagoda, E., & Robbins, M. M. (2009). Counting elusive animals: Comparing field and genetic census of the entire mountain gorilla population of Bwindi Impenetrable National Park, Uganda. Biological Conservation, 142(2), 290–300.
Harcourt, A. H., & Fossey, D. (1981). The Virunga gorillas: decline of an ‘island’ population. African Journal of Ecology, 19, 83–97.
Hickey, J. R., Uzabaho, E., Akantorana, M., Arinaitwe, J., Bakebwa, I., Bitariho, R., Eckardt, W., Gilardi, K. V., Katutu, J., Kayijamahe, C., Kierepka, E. M., Mugabukomeye, B., Musema, A., Mutabaazi, H., Robbins, M. M., Sacks, B. N., & Zikusoka, G. K. (2019). Bwindi Sarambwe 2018 Surveys: monitoring mountain gorillas, other select mammals, and human activities (p. 40). GVTC, IGCP & partners.
IUCN (2017) The IUCN Red List of Threatened Species. http://www.iucnredlist.org. Accessed 6 May 2017.
Junker, J., Petrovan, S. O., Arroyo-Rodríguez, V., Boonratana, R., Byler, D., Chapman, C. A., Chetry, D., Cheyne, S. M., Cornejo, F. M., Cortés-Ortiz, L., et al (2020). A severe lack of evidence limits effective conservation of the world's primates. Bioscience, 70(9), 794–803. https://doi.org/10.1093/biosci/biaa082.
Kleiman, D. G., Reading, R. P., Miller, B. J., Clark, T. W., Scott, J. M., Robinson, J., Wallace, R. L., Cabin, R. J., & Fellman, F. (2001). Improving the evaluation of conservation programs. Conservation Biology, 14(2), 356–365.
Marshall, A. R., Lemos de Figueiredo, R. J. R., Gereau, R. E., Abeid, Y., Ahrends, A., Jones, T., Lovett, J. C., Marshall, C. J., Davenport, T. R. B., & Bracebridge, C. E. (2015). Evaluating the habitat of the critically endangered kipunji. Journal of East African Natural History, 104(1&2), 169–193.
McKone, D.J. & Walzern, V.P. (1994). A brief survey of catchment forest reserves Mbeya region, Tanzania. Report for Government of Tanzania/EEC Agroforestry, Soil and Water Conservation Project, Mbeya and the Regional Natural Resources Office Mbeya, Tanzania.
McNeilage, A., Plumptre, A. J., Brock-Doyle, A., & Vedder, A. (2001). Bwindi Impenetrable National Park, Uganda: gorilla census 1997. Oryx, 35, 39–47.
McNeilage, A., Robbins, M. M., Gray, M., Olupot, W., Babaasa, D., Bitariho, R., et al (2006). Census of the mountain gorilla Gorilla beringei beringei population in Bwindi Impenetrable National Park, Uganda. Oryx, 40, 419–427.
Plumptre, A. J., & Cox, D. (2006). Counting primates for conservation: primate surveys in Uganda. Primates, 47, 65–73.
R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing https://www.R-project.org. Accessed 21 Sept 2021.
Redford, K. H., & Taber, A. (2000). Writing the wrongs: developing a safe-fail culture in conservation. Conservation Biology, 14, 1567–1568.
Robbins, M. M., Gray, M., Fawcett, K. A., Nutter, F. B., Uwingeli, P., et al (2011). Extreme conservation leads to recovery of the Virunga mountain gorillas. PLoS ONE, 6(6), e19788. https://doi.org/10.1371/journal.pone.0019788.
Rovero, R., Struhsaker, T. T., Marshall, A. R., Rinne, T. A., Pedersen, U. B., & Butynski, T. M. (2006). Abundance of diurnal primates in Mwanahina Forest, Udzungwa Mountains, Tanzania: a multi-observer comparison of line-transect data. International Journal of Primatology, 27, 675–697.
Roy, J., Vigilant, L., Gray, M., Wright, E., Kato, R., Kabano, P., Basabose, A., Tibenda, E., Kühl, H. S., & Robbins, M. M. (2014). Challenges in the use of genetic mark-recapture to estimate the population size of Bwindi mountain gorillas (Gorilla beringei beringei). Biological Conservation, 180, 249–261.
Ruiz-Miranda, C. R., de Morais Jr., M. M., Dietz, L. A., Rocha Alexandre, B., Martins, A. F., Ferraz, L. P., et al (2019). Estimating population sizes to evaluate progress in conservation of endangered golden lion tamarins (Leontopithecus rosalia). PLoS ONE, 14(6), e0216664. https://doi.org/10.1371/journal.pone.0216664.
Schülke, O., & Ostner, J., (2012). Ecological and social influences on sociality. In Mitani et al. (eds.) The Evolution of Primate Societies (pp. 195–219). University of Chicago Press.
Stanford, C. B. (1998). Chimpanzees and Red Colobus: The Ecology of Predator and Prey. Harvard University Press.
Struhsaker, T. T. (2010). The Red Colobus Monkeys: Variation in Demography, Behavior, and Ecology of Endangered Species. Oxford University Press.
Struhsaker, T. T., Marshall, A. R., Detwiler, K. M., Siex, K., Ehardt, C. L., Lisbjerg, D. D., & Butynski, T. M. (2004). Demographic variation among Udzungwa red colobus in relation to gross ecological and sociological parameters. International Journal of Primatology, 25, 615–658.
Van Schaik, C., & Hörstermann, M. (1994). Predation risk and the number of adult males in a primate group: a comparative test. Behavioral Ecology & Sociobiology, 35, 261–272.
Whitesides, G. H., Oates, J. F., Green, S. M., & Kluberdanz, R. P. (1988). Estimating primate densities from transects in a West African rainforest: a comparison of techniques. Journal of Animal Ecology, 57, 345–367.
Acknowledgements
This work was funded by the Margot Marsh Biodiversity Foundation, the Mohamed bin Zaid Species Conservation Fund (MBZ GEM 192520770) and the Wildlife Conservation Society. Research permission was granted by the Tanzania Wildlife Research Institute, Tanzania Commission for Science and Technology, Tanzania National Parks, Tanzania Forestry Services, and the Rungwe District Council. We thank Alexander Georgiev for assistance with data analysis and comments on an earlier version of the manuscript. We thank Liz Williamson and Daniela De Luca for technical advice and Atupakisye Mwaibanje, Christopher Mwampetele, Asifiwe Omary, and Samweli Mwalima for invaluable assistance in the field. Many thanks to the Editor and two anonymous referees for very useful comments.
Author information
Authors and Affiliations
Contributions
TD conceived the research, raised the funds, designed the methods, directed the fieldwork, analyzed the data, and wrote the article. SM analyzed data and carried out GIS. SM, NM, SK, WM, OM, PM coordinated with village authorities, led teams in the field, managed data collection, and edited text.
Corresponding author
Additional information
Handling Editor: Joanna Setchell
Rights and permissions
About this article
Cite this article
Davenport, T.R.B., Machaga, S.J., Mpunga, N.E. et al. A Reassessment of the Population Size, Demography, and Status of Tanzania’s Endemic Kipunji Rungwecebus kipunji 13 Years on: Demonstrating Conservation Success. Int J Primatol 43, 317–338 (2022). https://doi.org/10.1007/s10764-022-00281-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-022-00281-3