Abstract
Sexual dimorphism has been widely documented in primates, however craniodental sexual dimorphism among hylobatids has not been well researched. In this study, I investigate interspecific differences in the presence and pattern of craniodental sexual dimorphism among gibbons and siamang using data taken from eight species representing all four hylobatid genera (Hoolock leuconedys, Hylobates agilis, Hy. klossi, Hy. lar, Hy. muelleri, Hy. pileatus, Nomascus gabriellae, and Symphalangus syndactylus). I sampled 17-30 cranial specimens for each species. I quantified craniofacial and upper canine crown height dimorphism using morphometric data taken from 3D surface models and directly from dry specimens to examine the presence and pattern of craniodental sexual dimorphism among hylobatids. Hoolock leuconedys shows statistically significant sex differences across all craniofacial shape and form measures investigated. Although Hy. lar, Ho. leuconedys, and S. syndactylus all show statistically significant cranial form dimorphism, there are interspecific differences in how this dimorphism is expressed. Hoolock leuconedys, Hy. lar, and S. syndactylus are unique in showing upper canine crown height dimorphism, and Ho. leuconedys show a high level of browridge dimorphism in which white fur highlights this region in males, in contrast to their black body and facial pelage. The finding of male-biased sexual dimorphism in only some hylobatid taxa suggests that although male craniofacial morphology of some gibbon and siamang species may be associated with sex-specific agonistic interactions, this effect is not ubiquitous among hylobatids. Further research is required to understand these findings in the context of the socioecology of individual hylobatid taxa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sexual dimorphism (i.e., the morphological differences between males and females) has been widely documented in the primate order, with most primate taxa showing sex differences in body mass, skeletal morphology, canine size, or soft tissue (Balolia et al. 2013, 2017; Clutton-Brock et al. 1977; Cobb and O’Higgins 2007; Dixson et al. 2005; Gordon 2006; Hens 2002, 2003, 2005; Kay et al. 1988; Leutenegger and Cheverud 1982; Leutenegger and Kelly 1977; Leutenegger and Masterson 1989a,b; Leutenegger and Shell 1987; Lockwood 1999; Lynch-Alfaro 2012; Masterson and Leutenegger 1990, 1992; Mitani et al. 1996; O’Higgins et al. 1990a,b, 1993; Oxnard 1983; Plavcan 2001, 2002, 2004, 2011, 2012; Plavcan and van Schaik 1992, 1993, 1997; Schaefer et al. 2004; Wood 1976; Wood et al. 1991). Among sexually dimorphic anthropoids, males tend to be the larger sex and the degree of sexual size differences among taxa varies largely in response to socioecological factors (Clutton-Brock et al. 1977; Leutenegger and Cheverud 1982; Leutenegger and Kelly 1977; Mitani et al. 1996; Plavcan 2004, 2012; Plavcan and van Schaik 1992, 1997). These studies show that variation in sexual body size and canine dimorphism is associated with various surrogate measures of sexual selection including breeding system, male contest competition, socionomic sex ratio, and operational sex ratios. The nature of female relationships can also influence the degree of sexual size dimorphism, where selection for larger body size among females is associated with competition for ecological resources (Gordon 2006; Leigh and Shea 1995; Lindenfors 2002; Martin et al. 1994). Alternatively, smaller female body size may be selected for based on conferred advantages such as faster or earlier breeding and fewer metabolic demands associated with a larger body size (Clutton-Brock and Huchard 2013; Plavcan 2011; Tobias et al. 2012).
Sexual dimorphism in the skull and dentition has been widely documented among the great apes, with low to moderate craniofacial dimorphism observed in Pan paniscus and Pan troglodytes, in contrast to Gorilla spp. and Pongo spp., who show a high degree of craniofacial size dimorphism (Balolia et al. 2013, 2017; Cobb and O’Higgins 2007; Hens 2002, 2003, 2005; Lockwood 1999; Masterson and Leutenegger 1990, 1992; O’Higgins et al. 1990a,b, 1993; Plavcan and van Schaik 1992; Schaefer et al. 2004; Wood 1976; Wood et al. 1991). A similar trend exists for body mass dimorphism among the apes, with Pan showing moderate body mass dimorphism, whereas Gorilla and Pongo show substantial body mass dimorphism (Leutenegger and Cheverud 1982; Leutenegger and Kelley 1977; Plavcan and van Schaik 1997). The pattern of canine dimorphism among these taxa is also similar, with the lowest degree of sexual dimorphism observed in Pan paniscus, and more pronounced canine dimorphism observed in Pan troglodytes, Gorilla gorilla, and Pongo pygmaeus (Leutenegger and Cheverud 1982; Leutenegger and Kelley 1977; Plavcan and van Schaik 1992).
Compared to research documenting craniodental dimorphism among the great apes, sexual dimorphism among hylobatids has been less well studied. This is likely because although the relevance of studying the great apes in the context of human evolution is well established based on their larger bodies and close phylogenetic relationships with hominins, the derived nature of the small apes has made their relevance to understanding human origins less clear (Zichello 2018). Research conducted to date indicates that the hylobatids show slight craniofacial sexual dimorphism relative to the great apes (Plavcan 2001). Among hylobatids, male size exceeds female size for the majority of documented craniofacial measures among Hoolock hoolock, Hylobates agilis, Hy. klossii, Hy. lar, Hy. moloch, Hy. muelleri, Hy. pileatus, Nomascus concolor, and Symphalangus syndactylus (Electronic Supplementary Material [ESM] Table SI; Balolia et al. 2013; Creel and Preuschoft 1976, 1984; Plavcan 2002; Schultz 1940, 1941, 1944, 1962, 1973). For a minority of craniofacial measures, female size exceeds male size among Ho. hoolock, Hy. lar, Hy. moloch, Hy. pileatus, and S. syndactylus, and several measures among Hy. lar, Hy. moloch, Hy. muelleri, Hy. pileatus, N. concolor, and S. syndactylus are monomorphic (Creel and Preuschoft 1976, 1984; Schultz 1973). Male upper canine crown height exceeds female crown height among Ho. hoolock, Hy. agilis, Hy. klossii, Hy. lar, Hy. moloch, Hy. muelleri, Hy. pileatus, N. concolor, and S. syndactylus (Frisch 1963, 1973; Plavcan 2004; Plavcan and van Schaik 1992; Schultz 1973). There are no documented instances in which male upper canine crown height is equal to, or lower than, female upper canine crown height among hylobatids (ESM Table SII). In assessing the strength of evidence for craniodental dimorphism among hylobatids, the craniodental sexual dimorphism estimates that have been published have not been tested for statistical significance (the published data consist of species averages only), and some sexual dimorphism estimates are based on relatively small sample sizes (ESM Tables SI and SII).
Creel and Preuschoft were the first researchers to test the statistical significance of craniofacial dimorphism estimates among hylobatids (Creel and Preuschoft 1976). Based on a dataset of 90 craniofacial landmarks, these authors report that regional samples of 10 hylobatid species (S. syndactylus, Hy. muelleri, Hy. agilis, Hy. lar entelloides, Ho. hoolock, N. concolor, Hy. klossi, Hy. moloch, Hy. lar vestitus, Hy. pileatus) show statistically significant variance–covariance matrix male–female distances, noting that the sample for each species comprises individuals from more than one breeding population, and where some researchers recognize more than one subspecies being included in some taxonomic samples. When conducting their analyses, these authors used an α level of P < 0.1, which exceeds today’s accepted threshold for statistical significance, and used an equation to correct for sample size. Following the application of this equation, male–female distances were recorded as zero in the variance-covariance matrix for Hy. klossii, Hy. lar vestitus, and Hy. pileatus (Creel and Preuschoft 1976). Using the same taxonomic samples, the authors of this study further show that intersexual craniofacial differences are negligible in comparison to interspecific differences, and discriminant function analyses show sexual dimorphism among all hylobatid groups, where S. syndactylus show the most pronounced male-biased sexual dimorphism (males are larger and more robust than females), and Hy. pileatus show the opposite pattern (female mean scores exceed male mean scores) (Creel and Preuschoft 1976). Considering all hylobatids together, these authors show that males, relative to females, show tendencies toward a taller face, a longer and narrower cranial vault, more forward protrusion of the bony bar forming the lateral border of the orbit, a more robust zygomatic arch, more pronounced supramastoid crests, and larger canines (Creel and Preuschoft 1976). More recently, researchers found that although young Hy. lar adults show significant sexual dimorphism in linear measurements of craniofacial size (male size exceeds female size), older adults do not due to female craniofacial growth throughout adult life (Balolia et al. 2013). To date, the presence of canine crown height dimorphism among hylobatids has not been tested for statistically (ESM Table SII).
In addition to hard tissue dimorphism, there is evidence of sexual dimorphism in the soft tissue of hylobatids. Sexual dichromatism has been well documented in some hylobatid taxa, with sex-specific pelage color differences observed in three of the four known hylobatid genera (Bradley and Mundy 2008; Groves 1972; Ma et al. 1988; Mootnick 2006; Mootnick and Fan 2011). All known Nomascus and Hoolock species are sexually dichromatic; by contrast, Hylobates species show no substantial pelage sex differences, except for Hy. pileatus (Mootnick 2006; Mootnick and Fan 2011). All four hylobatid genera show interspecific variation in light facial markings (Geissmann 1993, 2003), but there is currently no consensus as to why facial markings vary among species, or what role sexual dichromatism plays among the hylobatids. Some researchers have suggested that sex differences in pelage coloration in primates are associated with sexual selection or act as indicators of sexual maturity, specifically in the context of dark pelage in males (Bradley and Mundy 2008; Gerald 2003; Neville et al. 1988). Alternatively, research suggests that the lighter colored pelage observed in Hoolock and Nomascus females may be associated with camouflage for the adult females themselves in lightly colored branches, or to camouflage their lightly colored offspring (Mootnick and Fan 2011). Although the selective pressures surrounding the presence and pattern of sexual dichromatism in hylobatids are yet to be elucidated, sex-specific selection exists for pelage and facial coloration among some hylobatid taxa. It is possible that hard tissue morphology in the hylobatid facial skeleton may respond to similar selective pressures.
Although previous studies have shown evidence of slight craniofacial and canine crown height dimorphism among hylobatid taxa (Balolia et al. 2013; Creel and Preuschoft 1976, 1984; Frisch 1963, 1973; Plavcan 2002, 2004; Plavcan and van Schaik 1992; Schultz 1940, 1941, 1944, 1962, 1973), no study has provided a detailed quantitative analysis of variation in the magnitude and pattern of craniodental dimorphism among hylobatids. In the present study, I assess whether there is interspecific variation in the presence and pattern of craniodental sexual dimorphism among hylobatids. I quantify craniofacial and upper canine crown height sexual dimorphism among eight hylobatid species (Ho. leuconedys, Hy. agilis, Hy. klossi, Hy. lar, Hy. muelleri, Hy. pileatus, N. gabriellae, and S. syndactylus). For taxa showing sex differences in cranial and facial form, I investigate whether sexual dimorphism is expressed as size or shape dimorphism, or a combination of both. I further investigate whether there are interspecific differences in the presence of canine dimorphism among hylobatid taxa. Given the well-established association between the degree of sexual size dimorphism and the nature of male intrasexual relationships among primates, findings of interspecific variation in the presence and pattern of craniodental sexual dimorphism among hylobatids would improve our understanding of selection on craniodental morphology in gibbons and siamang in response to socioecological variables.
Methods
Specimens
The sample consists of 187 dentally mature specimens belonging to eight hylobatid species, representing all four hylobatid genera (Table I). I only included specimens with light or moderate canine wear for canine dimorphism analyses. I or J. Michael Plavcan obtained sex information from museum records and confirmed this using pelage coloration where available.
3D Scanning and Processing
I obtained 3D surface models for five of the eight hylobatid species (Ho. leuconedys, Hy. lar, Hy. muelleri, N. gabriellae, S. syndactylus) using a NextEngine 2020i Desktop scanner, a Creaform Go!Scan 20 or from CT data. I processed 3D surface scan data using each scanner’s proprietary software. All specimens were scanned at a resolution of <0.5 mm. Differences in how 3D models were generated are unlikely to have produced substantial measurement error (Balolia and Massey 2021; Fruciano et al. 2017; Marcy et al. 2018).
Data Collection and Craniodental Measures
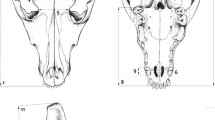
Craniofacial Form and Shape I obtained craniofacial form and craniofacial shape data for Ho. leuconedys, Hy. lar, Hy. muelleri, N. gabriellae, and S. syndactylus from 3D landmarks using Stratovan Checkpoint v. 2017.03.03.0771. I quantified cranial form and shape using 27 cranial landmarks and I quantified facial form and shape using 20 facial landmarks (Table II, Fig. 1).
3D landmarks used to quantify craniofacial shape and form, applied to a Hoolock leuconedys 3D surface model. Landmarks are defined in Table II.
Craniofacial size and canine crown height For the five hylobatid taxa for which 3D surface models were available (Ho. leuconedys, Hy. lar, Hy. muelleri, N. gabriellae, S. syndactylus), I collected 20 craniodental linear measurements from 3D landmarks using Stratovan Checkpoint v. 2017.03.03.0771 (Table III). For bilateral measurements (orbital breadth, orbital height, vertical thickness of the supraorbital torus, and canine crown height), I took measurements from both the left and right side. For craniofacial measures, I included the measurements taken from the side with the highest number of well-preserved specimens in statistical analyses. For analyses of canine crown height, I included the maximum upper canine crown height value (i.e., the tooth exhibiting the least amount of dental wear) for each specimen. I collected linear measurements for these five taxa by calculating the interlandmark distances of 3D landmarks obtained from 3D surface models using Microsoft Excel for Office 365 MSO (16.0.12527.20612) (Table III).
To increase the hylobatid taxonomic sample, I obtained additional craniodental data for Hy. klossi, Hy. pileatus, and Hy. agilis specimens from J. Michael Plavcan (Plavcan 1990, 2002, 2003). I used up to nine craniodental linear measurements for each specimen from these datasets (Table III). J. Michael Plavcan collected craniofacial linear measurements using a pair of Fowler digital calipers (Plavcan 2002). The definitions for craniofacial measurements for the dataset compiled using digital calipers refer to the same measurement termination points as those taken by calculating the interlandmark distance of 3D landmarks (Plavcan 2003; Table III). Combining caliper measurements with those taken from 3D surface models is unlikely to introduce error based on the size of specimens being used in this study and the resolution at which these specimens were scanned (Balolia and Massey 2021). J. Michael Plavcan collected canine crown height measurements using a calibrated reticle in the eyepiece of a Wild binocular microscope (Plavcan 1990). The dental measurements derived from this device are comparable to those made using standard calipers, and the differences in data collection technique to the landmark-based approach adopted here are unlikely to introduce error into the combined sample. J. Michael Plavcan measured canine crown height as the distance from the cemento-enamel junction to the apex of the tooth on the mesiobuccal face of the tooth (Plavcan 1990), which is almost identical to the canine crown height measurement using the interlandmark distance (Table III). I quantified cranial size for the seven hylobatid taxa for which craniofacial data were available using the geometric mean of eight linear measurements (Table III). Similarly, I quantified facial size for these same seven hylobatid taxa using the geometric mean of six linear measurements (Table III). I performed geometric mean calculations in Microsoft Excel for Office 365 MSO (16.0.12527.20612).
Cranial Form and Shape Analysis
For the five hylobatid taxa for which 3D surface models were available (Ho. leuconedys, Hy. lar, Hy. muelleri, N. gabriellae, S. syndactylus), I quantified craniofacial shape and form using Generalized Procrustes Analysis (GPA) on 3D landmarks for each taxon separately. For shape analyses, I scaled 3D landmarks to a standard centroid size, and translated and rotated them to minimize the squared distance between landmark sets (Baab et al., 2012; Rohlf and Slice, 1990). For form analyses, I included the natural log of centroid size as part of the GPA. I performed Principal Components Analysis (PCA) to obtain Principal Component (PC) scores. I used these PC scores to conduct Discriminant Function Analysis (DFA) and to visualize the data in form space (PC1 vs. PC2) for sexually dimorphic species. I conducted GPAs and PCAs using the EVAN Toolbox v. 1.71.
Sexual Dimorphism Quantification and Analysis
I quantified sexual size dimorphism using the index of sexual dimorphism (ISD = male mean/female mean) and tested for statistically significant sexual size dimorphism using Student’s t-tests. I tested for statistically significant craniofacial form dimorphism by conducting Students’ t-tests on PC1 scores obtained using the form analysis described in the previous section. I performed Student’s t-tests using SPSS v. 26. I did not calculate sexual form dimorphism estimates because calculating the ISD based on the male and female mean PC scores used for significance testing does not provide meaningful sexual dimorphism estimates. I used Bonferroni corrections to account for the possibility that multiple t-tests of sexual dimorphism yield significant P values (i.e., are a result of type I error).
I calculated sexual shape dimorphism using the male–female Procrustes distance, i.e., the Procrustes distance between the male and female mean shapes, obtained using the GPA procedure described earlier. I used permutation tests (1000 permutations for each analysis) to statistically test for significant sex differences using software developed by the UCL Anatomy Department (Strand Viðarsdóttir et al. 2002).
I performed DFA to assess correct sex classification rates for craniofacial form. For each test, I included PCs representing at least 5% of the sample variation, which resulted in the inclusion of between five and seven PCs in each analysis, representing between 66% and 84% of the variation for each sample. I report “Leave-one-out” (i.e., cross-validated) classification results, which is the most conservative application of this test. I performed DFA analyses in SPSS v. 26.
Ethical Note
No ethical clearance was required for this study.
Data Availability
The data set analyzed during the current study is available from the corresponding author on request.
Results
Craniofacial Form, Shape, and Size Dimorphism: Composite Measures
Of the five species for which craniofacial shape and form data are available, Ho. leuconedys shows statistically significant sex differences across all craniofacial shape and form measures investigated (male > female cranial and facial size by 5%) (Tables IV–VII). Hylobates lar and S. syndactylus also show cranial form dimorphism, which is driven by both size and shape in Hy. lar (male > female cranial size by 4%) and by size alone in S. syndactylus (male > female cranial size by 7%) (Tables IV and V). Hylobates lar shows facial shape dimorphism and S. syndactylus shows facial size dimorphism, similar to what is observed for the cranium (Tables VI and VII). Neither Hy. muelleri nor N. gabriellae show craniofacial size or shape dimorphism. Similarly, neither Hy. klossi nor Hy. pileatus show significant craniofacial size dimorphism (Tables IV–VII).
The three taxa for which cranial form dimorphism is found (Ho. leuconedys, Hy. lar, and S. syndactylus), are visibly separated by sex along the first two PCs (Fig. 2). These taxa show high correct sex classification rates of 88% (Hy. lar), 93% (Ho. leuconedys), and 85% (S. syndactylus). Shape differences include a wider and longer cranium in Ho. leuconedys and Hy. lar males, and a smaller face relative to cranial vault size in Ho. leuconedys females. Hylobates muelleri and N. gabriellae, which do not show statistically significant sex differences in either cranial or facial form, show relatively poor sex classification rates of 42.1% (Hy. muelleri) and 71.4% (N. gabriellae). These results are consistent with the observed lack of craniofacial form dimorphism in Hy. muelleri and N. gabriellae (Tables IV and VI). I did not conduct DFA for Hy. klossi or Hy. pileatus, as form data were not available for these taxa.
Cranial form sexual dimorphism in Hoolock leuconedys, Hylobates lar, and Symphalangus syndactylus. Hoolock leuconedys: males = blue triangles, females = orange circles; Hylobates lar: males = green triangles, females = black circles; Symphalangus syndactylus: males = gray triangles, females = red circles. PC = Principal Component.
Craniofacial Size Dimorphism: Individual Measurements
Three out of the seven taxa for which craniofacial measurements were available show sexual size dimorphism for individual cranial measurements (Table VIII). Hoolock leuconedys shows statistically significant sexual dimorphism for 6 of 19 individual craniofacial size measurements. Five of these measurements represent craniofacial breadth (superior facial breadth, biorbital breadth, interorbital breadth, bizygomatic breadth, and posterior maxillary breadth) and one measurement quantifies supraorbital thickness (Table VIII). Hoolock leuconedys show particularly high ISD values for browridge thickness and the interorbital region (ISD = 1.24 and ISD = 1.12 respectively). I found statistically significant sexual dimorphism for bizygomatic breadth in S. syndactylus, and for anterior maxillary breadth in Hy. pileatus, with male size exceeding female size for both of these measurements (Table VIII). Interpretations of these statistical test results that do not correct for multiple comparisons show that sexual dimorphism may exist for other craniofacial dimensions, particularly those associated with facial breadth in Ho. leuconedys, Hy. klossi, Hy. lar, and S. syndactylus. Similarly, interpretations of these statistical tests results that do not correct for multiple comparisons also show browridge thickness and interorbital region sexual dimorphism in S. syndactylus (ISD = 1.32 and ISD = 1.13 respectively), indicating a similar pattern of dimorphism to what is found in Ho. leuconedys in the upper facial region. This less conservative interpretation of results also suggests the presence of glabellar and alveolar height sexual dimorphism in Ho. leuconedys and Hy. lar and negative sexual dimorphism for orbital height (i.e., female size exceeds male size) in N. gabriellae and S. syndactylus (Table VIII). Consistent with the results of form and shape analyses previously described, these combined results show interspecific differences in the pattern of sexual dimorphism across craniofacial regions among hylobatids.
Canine Crown Height Dimorphism
Hoolock leuconedys, Hy. lar, and S. syndactylus show statistically significant canine crown height dimorphism, with male canine crown height exceeding female canine crown height by 12% for Ho. leuconedys, 19% for Hy. lar, and 20% for S. syndactylus (Fig. 2, Table IX). Statistically significant canine crown height dimorphism is not detected in any other hylobatid species (Table IX).
Discussion
The results of this study show that there is interspecific variation in the presence and pattern of craniodental sexual dimorphism among hylobatids. Hoolock leuconedys ubiquitously shows craniofacial size and shape across all composite measures and canine crown height dimorphism, and shows sexual size dimorphism in six individual craniofacial measurements. Hylobates lar and S. syndactylus also show clear craniofacial dimorphism, where craniofacial form dimorphism among Ho. leuconedys, Hy. lar, and S. syndactylus are differentially driven by size, shape, or a combination of both. Hoolock leuconedys, Hy. lar, and S. syndactylus are the only three taxa to show significant canine crown height dimorphism, and no other hylobatid taxon shows notable sexual dimorphism for any other craniofacial measure. The finding of a relatively high magnitude of sexual dimorphism in S. syndactylus (males are 7% larger than females) is consistent with results of pronounced sexual dimorphism in this taxon relative to other hylobatids (Creel and Preuschoft 1976). Similarly, the finding of no significant sexual size dimorphism in the Hy. lar facial skeleton is consistent with findings of no significant sexual dimorphism in a dentally mature Hy. lar sample (Balolia et al. 2013).
Together, the results of this study show that the presence and pattern of craniodental dimorphism among gibbons and siamang is more diverse than had been previously recognized and suggest that sex-specific selection pressures on craniodental morphology are not uniform across hylobatid groups. This notion can be further considered in the context of ecological and behavioral complexity, flexibility in grouping patterns, and social behavioral plasticity observed among hylobatid taxa (Fuentes 2000; Malone and Fuentes 2009; Morino 2009; Palombit 1996). Under an ecological model of behavioral flexibility, even subtle differences in the Southeast Asian habitats occupied by hylobatids are likely to promote interspecific variation in the nature of their social relationships (Malone and Fuentes 2009; Palombit 1996; Wrangham 1979). The results of this study showing interspecific variation in craniofacial and canine crown height dimorphism is consistent with this proposition. Some authors have argued that the tendency to categorize hylobatids as territorial and pair-living has led to an underestimation of the degree of social variation among taxa, where social organization may be more accurately viewed as a more dynamic process than is currently recognized (Malone and Fuentes 2009; Palombit 1996). Understanding differences in the expression of hylobatid sexual dimorphism using a framework emphasizing that hylobatids live in variable communities, where intergroup behavioral differences are driven by ecological variation (e.g., Fuentes 2000), may provide increased scope to understand morphological differences among hylobatids in the context of the specific circumstances of the population or species under investigation.
There is a paucity in our understanding about whether and how the nature of intermale relationships among hylobatid communities vary. The frequency and intensity of intermale competition are widely acknowledged as being the predominant selective pressures driving body size and canine crown height dimorphism among primates (Plavcan and van Schaik 1992, 1997). The relatively low magnitude of sexual dimorphism that has been previously documented in hylobatids is thought to be a product of males and females undergoing similar selective pressures surrounding body size and canine crown height, with no apparent obvious reproductive advantage for differential selection on male morphology (Plavcan 2001). However, even individuals living in two-adult clusters engage with other adults, participate in intergroup interactions, and individually travel among clusters as part of their daily activities (Brockelman et al. 1998; Fuentes 2000). There is also good evidence to suggest that hylobatid social organization can be explained by the need for males to protect infants from infanticide (Fuentes 2000; Morino 2009; Sommer and Reichard 1997; van Schaik and Dunbar 1990; van Schaik and Kappeler 1997), where even low frequencies of infanticide are sufficient to maintain pair bonds among hylobatids (Fuentes 2000). Alternative arguments suggest that mate guarding may be an important selective pressure to explain hylobatid pair bonds, a behavior that has arisen among hylobatid males because females are widely dispersed and males are able to monopolize mating access to females (Emlen and Oring 1977; Fuentes 2000; Morino 2009; Palombit 1996, 1999). Under both the infanticide prevention hypothesis and the mate guarding hypothesis, there is scope for selection for increased male body size and craniodental features that act as signals of potential aggression. Although the majority of hylobatid taxa live in pairs (Bartlett 2009; Brockelman et al. 2014; Reichard 2003), some hylobatid species live in unifemale/multimale groups, which afford an increased scope for behavioral complexity and the frequency of aggressive encounters, associated with increased body size and canine crown height dimorphism (Plavcan and van Schaik 1992, 1997). Other hylobatid species are known to live in multimale/multifemale groups that allow increased potential for agonistic interactions among males (Malone and Fuentes 2009). Despite a strong theoretical basis to suggest that selection pressures favoring increased sexual dimorphism are present in some hylobatid groups, no research has yet been conducted to systematically investigate whether interspecific differences in the presence and pattern of sexual dimorphism among hylobatids are associated with differences in social organization, mating system, and the nature of intermale or interfemale competition.
A further noteworthy finding is the presence of craniofacial size, shape, and canine crown height dimorphism in Ho. leuconedys, whose males also show increased size in upper orbital regions and aspects of facial breadth relative to females. The underlying causes for the high degree of sexual dimorphism in some craniofacial regions of Ho. leuconedys, and for differences in the pattern of craniodental dimorphism among other gibbon and siamang groups, have not yet been elucidated. However, some evidence suggests that differences in patterns of sexual dimorphism among non-human primates may reflect selection for craniofacial characteristics based on positive allometry for some facial dimensions and increased facial breadth among males of some primate taxa (Balolia et al. 2017; Borgi and Majolo 2016; Klopp 2012; Lefevre et al. 2014; Plavcan 2002, 2003; Weston et al. 2004; Wilson et al. 2020). Recent research also indicates that circumorbital morphology among some colobus monkeys may have evolved in response to intrasexual male competition and that circumorbital dimorphism among anthropoids is not strongly associated with overall sexual size dimorphism (Fannin et al. 2021). These findings further suggest that browridge morphology may play a role in social signaling among primates.
Despite a paucity of behavioral data among Hoolock gibbons, differences have been observed in some specific aspects of Hoolock socioecology compared with the other three hylobatid genera. For example, some Hoolock groups live in a pine/broadleaf forest habitat, where some males form all-bachelor groups of up to five individuals, allowing increased potential for intermale encounters and male–male competition (Geissmann et al. 2013; Malone and Fuentes 2009; Mukjerjee et al. 1991–1992). Furthermore, the finding that browridge thickness in Ho. leuconedys males is 24% larger than that of females can be considered in the context of facial marking dimorphism. Hoolock leuconedys males have white hair accentuating the browridge, in contrast to females, whose white coloring on the entire browridge and crown does not specifically highlight this facial region (Mootnick 2006). The results of the present study additionally show that the males of some hylobatid taxa have a wider bizygomatic region than do females. These findings can be considered in the context of research suggesting that wide faces are associated with increased aggression among primates (Borgi and Majolo 2016; Lefevre et al. 2014; Weston et al. 2004; Wilson et al. 2020). An alternative explanation is that a wide bizygomatic breadth in males could be a dietary adaptation, associated with sex differences in temporalis muscle architecture and size. However, this suggestion seems unlikely as no such dietary sex differences have been documented to date. The available evidence supports the hypothesis that sex differences in hard tissue facial morphology among some hylobatid groups may evolve to facilitate visual communication, similar to the role that large canine crowns play among some primate species (Plavcan 2012; Plavcan and van Schaik 1997).
The findings that among Ho. leuconedys, Hy. lar, and S. syndactylus, male cranial size exceeds that of females, and that all three taxa show male-biased canine crown height dimorphism is consistent with the pattern of dimorphism found in other apes (Balolia et al. 2013; Lockwood 1999; Plavcan and van Schaik 1992; Wood 1976). Among primates, increased male body size and canine crown height is associated with the frequency and intensity of intermale aggression and primate social organization (Clutton-Brock et al. 1977; Kay et al. 1988; Leutenegger and Cheverud 1982; Leutenegger and Kelley 1977; Plavcan 2004, 2012; Plavcan and van Schaik 1992, 1997). Accordingly, the most likely explanation for the findings of the present study is that increased male-biased hard tissue sexual dimorphism in Ho. leuconedys, Hy. lar, and S. syndactylus may be driven by intrasexual male competition. An alternative scenario may be that the observed patterns of sexual dimorphism in these hylobatid species is not exclusively driven by male–male competition, a suggestion that may allow the development of alternative hypotheses surrounding the variation and complexity of hylobatid socioecology and biology and associated morphological variation. However, for the reasons outlined earlier surrounding increased scope for male–male competition and/or agonistic encounters associated with infanticide prevention and mate guarding (Fuentes 2000; Morino 2016; Palombit 1996, 1999; Setchell and Kappeler 2003; Sommer and Reichard 1997; van Schaik and Dunbar 1990; van Schaik and Kappeler 1997), it is likely that the observed presence of sexual dimorphism is driven by intermale competition in some hylobatid groups. The finding that the other hylobatid taxa investigated in this study are sexually monomorphic across the majority of craniodental measures (i.e., do not show sexual dimorphism) suggests that differences in selective pressures among hylobatid taxa exist, even within genera, on male and female craniodental morphology.
Conclusion
This article presents evidence of distinct craniodental sexual dimorphism in three hylobatid species. Although the precise reasons for sex-specific morphological differences in these taxa remain elusive, available information surrounding hylobatid socioecology indicates that aspects of the social behavior of some taxa, including increased scope for competition for access to females, infanticide prevention, and mate guarding, allow increased scope for high rates of intermale aggression and associated selection on male hard tissue morphology. The results presented in this study suggest that sex-specific craniodental morphology among some gibbon and siamang groups may be associated with visual signaling among males and indicate that craniofacial hard tissue morphology may be a useful source of information in reconstructing aspects of social behavior in extant and extinct primates.
References
Baab, K. L., McNulty, K. P., & Rohlf, F. J. (2012). The shape of human evolution: A geometric morphometrics perspective. Evolutionary Anthropology: Issues, News, and Reviews, 21(4), 151–165.
Balolia, K. L., & Massey, J. S. (2021). How does scanner choice and 3D model resolution affect data accuracy? Journal of Anatomy, 238(3), 679–692.
Balolia, K. L., Soligo, C., & Lockwood, C. A. (2013). Sexual dimorphism and facial growth beyond dental maturity in great apes and gibbons. International Journal of Primatology, 34(2), 361–387.
Balolia, K. L., Soligo, C., & Wood, B. (2017). Sagittal crest formation in great apes and gibbons. Journal of Anatomy, 230(6), 820–832.
Bartlett, T. Q. (2009). Gibbon socioecology. In W. S. a. N. Vasey (Ed.), The gibbons of Khao Yai: Seasonal variation in behaviour and ecology (pp. 120–147). Routledge.
Borgi, M., & Majolo, B. (2016). Facial width-to-height ratio relates to dominance style in the genus Macaca. PeerJ, 4, e1775.
Bradley, B. J., & Mundy, N. I. (2008). The primate palette: The evolution of primate coloration. Evolutionary Anthropology: Issues, News, and Reviews, 17(2), 97–111.
Brockelman, W. Y., Nathalang, A., Greenberg, D. B., & Suwanvecho, U. (2014). Evolution of small-group territoriality in gibbons. In J. Y. a. L. Karczmarski (Ed.), Primates and cetaceans: Field research and conservation of complex mammalian societies (pp. 213–230). Springer Japan.
Brockelman, W. Y., Reichard, U., Treesucon, U., & Raemaekers, J. J. (1998). Dispersal, pair formation and social structure in gibbons (Hylobates lar). Behavioral Ecology and Sociobiology, 42(5), 329–339.
Clutton-Brock, T. H., Harvey, P. H., & Rudder, B. (1977). Sexual dimorphism, socionomic sex ratio and body weight in primates. Nature, 269(5631), 797–800.
Clutton-Brock, T. H., & Huchard, E. (2013). Social competition and its consequences in female mammals. Journal of Zoology, 289(3), 151–171.
Cobb, S. N., & O'Higgins, P. (2007). The ontogeny of sexual dimorphism in the facial skeleton of the African apes. Journal of Human Evolution, 53(2), 176–190.
Creel, N., & Preuschoft, H. (1976). Cranial morphology of the lesser apes: A multivariate statistical study. Gibbon and Siamang, 4, 219–303.
Creel, N., & Preuschoft, H. (1984). Systematics of the lesser apes: A quantitative taxonomic analysis of craniometric and other variables. In H. Preuschoft, D. J. Chivers, W. Y. Brockelman, & N. Creel (Eds.), The lesser apes: Evolutionary and behavioural biology (pp. 562–613). Edinburgh University Press.
Dixson, A., Dixson, B., & Anderson, M. (2005). Sexual selection and the evolution of visually conspicuous sexually dimorphic traits in male monkeys, apes, and human beings. Annual Review of Sex Research, 16(1), 1–19.
Emlen, S. T., & Oring, L. W. (1977). Ecology, sexual selection, and the evolution of mating systems. Science, 197(4300), 215–223.
Fannin, LD, Plavcan, JM, Daegling, DJ, McGraw, WS. (2021). Oral processing, sexual selection, and size variation in the circumorbital region of Colobus and Piliocolobus. American Journal of Physical Anthropology, 175(3), 559– 576.
Frisch, J. E. (1963). Sex-differences in the canines of the gibbon (Hylobates lar). Primates, 4(2), 1–10.
Frisch, J. E. (1973). The hylobatid dentition. Gibbon and Siamang, 2, 55–95.
Fruciano, C., Celik, M. A., Butler, K., Dooley, T., Weisbecker, V., & Phillips, M. J. (2017). Sharing is caring? Measurement error and the issues arising from combining 3D morphometric datasets. Ecology and Evolution, 7(17), 7034–7046.
Fuentes, A. (2000). Hylobatid communities: Changing views on pair bonding and social organization in hominoids. American Journal of Physical Anthropology, 113(S31), 33–60.
Geissmann, T. (1993). Evolution of communication in gibbons (Hylobatidae). PhD thesis, Anthropological Institute, Philosoph. Faculty II, University of Zurich.
Geissmann, T. (2003). Circumfacial markings in siamang and evolution of the face ring in the Hylobatidae. International Journal of Primatology, 24(1), 143–158.
Geissmann, T., Grindley, M. E., Lwin, N., Aung, S. S., Aung, T. N., Htoo, S. B., & Momberg, F. (2013). The conservation status of hoolock gibbons in Myanmar. Gibbon Conservation Alliance, Zürich, Switzerland.
Gerald, M. (2003). How color may guide the primate world: Possible relationships between sexual selection and sexual dichromatism. In Jones, C. B. (Ed.), Sexual selection and reproductive competition in primates: New perspectives and directions (Vol. 3, Special Topics in Primatology, pp. 141–171). American Society of Primatologists.
Gordon, A. D. (2006). Scaling of size and dimorphism in primates II: Macroevolution. International Journal of Primatology, 27(1), 63–105.
Groves, C. P. (1972). Systematics and phylogeny of gibbons. Gibbons and Siamang, 1, 1–89.
Hens, S. M. (2002). A geometric approach to cranial sexual dimorphism in the orang-utan. Folia Primatologica, 73(4), 165–174.
Hens, S. M. (2003). Growth and sexual dimorphism in orangutan crania: A three-dimensional approach. American Journal of Physical Anthropology, 121(1), 19–29.
Hens, S. M. (2005). Ontogeny of craniofacial sexual dimorphism in the orangutan (Pongo pygmaeus). I: Face and palate. American Journal of Primatology, 65(2), 149–166.
Kay, R. F., Plavcan, J. M., Glander, K. E., & Wright, P. C. (1988). Sexual selection and canine dimorphism in new world monkeys. American Journal of Physical Anthropology, 77(3), 385–397.
Klopp, E. B. (2012). Craniodental features in male Mandrillus may signal size and fitness: An allometric approach. American Journal of Physical Anthropology, 147(4), 593–603.
Lefevre, C. E., Wilson, V. A. D., Morton, F. B., Brosnan, S. F., Paukner, A., & Bates, T. C. (2014). Facial width-to-height ratio relates to alpha status and assertive personality in capuchin monkeys. PLOS ONE, 9(4), e93369.
Leigh, S. R., & Shea, B. T. (1995). Ontogeny and the evolution of adult body size dimorphism in apes. American Journal of Primatology, 36(1), 37–60.
Leutenegger, W., & Cheverud, J. (1982). Correlates of sexual dimorphism in primates: Ecological and size variables. International Journal of Primatology, 3(4), 387–402.
Leutenegger, W., & Kelly, J. T. (1977). Relationship of sexual dimorphism in canine size and body size to social, behavioral, and ecological correlates in anthropoid primates. Primates, 18(1), 117–136.
Leutenegger, W., & Masterson, T. J. (1989a). The ontogeny of sexual dimorphism in the cranium of Bornean orang-utans (Pongo pygmaeus pygmaeus): I. Univariate analyses. Zeitschrift für Morphologie und Anthropologie, 78(1), 1–14.
Leutenegger, W., & Masterson, T. J. (1989b). The ontogeny of sexual dimorphism in the cranium of Bornean orang-utans (Pongo pygmaeus pygmaeus): II. Allometry and heterochrony. Zeitschrift für Morphologie und Anthropologie, 78(1), 15–24.
Leutenegger, W., & Shell, B. (1987). Variability and sexual dimorphism in canine size of Australopithecus and extant hominoids. Journal of Human Evolution, 16(4), 359–367.
Lindenfors, P. (2002). Sexually antagonistic selection on primate size. Journal of Evolutionary Biology, 15(4), 595–607.
Lockwood, C. A. (1999). Sexual dimorphism in the face of Australopithecus africanus. American Journal of Physical Anthropology, 108(1), 97–127.
Lynch-Alfaro, J. W., Silva Jr., J. D. S. E., & Rylands, A. B. (2012). How different are robust and gracile capuchin monkeys? An argument for the use of Sapajus and Cebus. American Journal of Primatology, 74(4), 273–286.
Ma, S., Wang, Y., & Poirier, F. E. (1988). Taxonomy, distribution, and status of gibbons (Hylobates) in Southern China and adjacent areas. Primates, 29(2), 277–286.
Malone, N., & Fuentes, A. (2009). The ecology and evolution of hylobatid communities: Causal and contextual factors underlying inter- and intraspecific variation. In D. Whittaker & S. Lappan (Eds.), The gibbons: New perspectives on small ape socioecology and population biology (pp. 241–264). Springer New York.
Marcy, A. E., Fruciano, C., Phillips, M. J., Mardon, K., & Weisbecker, V. (2018). Low resolution scans can provide a sufficiently accurate, cost- and time-effective alternative to high resolution scans for 3D shape analyses. PeerJ, 6, e5032.
Martin, R. D., Willner, L. A., & Dettling, A. (1994). The evolution of sexual size dimorphism in primates. In R. V. Short & E. Balaban (Eds.), The differences between the sexes (pp. 159–200). Cambridge University Press.
Masterson, T. J., & Leutenegger, W. (1990). The ontogeny of sexual dimorphism in the cranium of Bornean orang-utans (Pongo pygmaeus pygmaeus) as detected by principal-components analysis. International Journal of Primatology, 11(6), 517–539.
Masterson, T. J., & Leutenegger, W. (1992). Ontogenetic patterns of sexual dimorphism in the cranium of Bornean orang-utans (Pongo pygmaeus pygmaeus). Journal of Human Evolution, 23(1), 3–26.
Mitani, J. C., Gros-Louis, J., & Richards, A. F. (1996). Sexual dimorphism, the operational sex ratio, and the intensity of male competition in polygynous primates. The American Naturalist, 147(6), 966–980.
Mootnick, A. R. (2006). Gibbon (Hylobatidae) species identification recommended for rescue or breeding centers. Primate Conservation, 2006(21), 103–138 136.
Mootnick, A. R., & Fan, P.-F. (2011). A comparative study of crested gibbons (Nomascus). American Journal of Primatology, 73(2), 135–154.
Morino, L. (2009). Monogamy in mammals: Expanding the perspective on hylobatid mating systems. In D. Whittaker & S. Lappan (Eds.), The gibbons: New perspectives on small ape socioecology and population biology (pp. 279–311). Springer New York.
Morino, L. (2016). Dominance relationships among siamang males living in multimale groups. American Journal of Primatology, 78(3), 288–297.
Mukherjee, R. P., Chaudjiru, S., & Murmu, A. (1991–1992). Hoolock gibbons (Hylobates hoolock) in Arunachel Pradesh, northeast India: The Lohit district. Primate Conservation, 12–13, 31–33.
Neville, M. K. (1988). The howling monkeys, genus Alouatta. Ecology and Behavior of Neotropical Primates, 2, 349–453.
O’Higgins, P., Johnson, D. R., Moore, W. J., & Flinn, R. M. (1990a). The variability of patterns of sexual dimorphism in the hominoid skull. Experientia, 46(7), 670–672.
O’Higgins, P., Moore, W. J., Johnson, D. R., McAndrew, T. J., & Flinn, R. M. (1990b). Patterns of cranial sexual dimorphism in certain groups of extant hominoids. Journal of Zoology, 222(3), 399–420.
Oxnard, C. E. (1983). Sexual dimorphisms in the overall proportions of primates. American Journal of Primatology, 4(1), 1–22.
Palombit, R. A. (1996). Pair bonds in monogamous apes: A comparison of the siamang Hylobates syndactylus and the white-handed gibbon Hylobates Lar. Behaviour, 133(5–6), 321–356.
Palombit, R. A. (1999). Infanticide and the evolution of pair bonds in nonhuman primates. Evolutionary Anthropology: Issues, News, and Reviews, 7(4), 117–129.
Plavcan, J. M. (1990). Sexual dimorphism in the dentition of extant anthropoid primates. PhD dissertation, Duke University, University of Michigan Microfilms.
Plavcan, J. M. (2001). Sexual dimorphism in primate evolution. American Journal of Physical Anthropology, 116(S33), 25–53.
Plavcan, J. M. (2002). Taxonomic variation in the patterns of craniofacial dimorphism in primates. Journal of Human Evolution, 42(5), 579–608.
Plavcan, J. M. (2003). Scaling relationships between craniofacial sexual dimorphism and body mass dimorphism in primates: implications for the fossil record. American Journal of Physical Anthropology, 120(1), 38–60.
Plavcan, J. M. (2004). Sexual selection, measures of sexual selection, and sexual dimorphism in primates. In P. M. Kappeler & C. P. van Schaik (Eds.), Sexual selection in primates (pp. 230–252). Cambridge University Press.
Plavcan, J. M. (2011). Understanding dimorphism as a function of changes in male and female traits. Evolutionary Anthropology: Issues, News, and Reviews, 20(4), 143–155.
Plavcan, J. M. (2012). Sexual size dimorphism, canine dimorphism, and male-male competition in primates. Human Nature, 23(1), 45–67.
Plavcan, J. M., & van Schaik, C. P. (1992). Intrasexual competition and canine dimorphism in anthropoid primates. American Journal of Physical Anthropology, 87(4), 461–477.
Plavcan, J. M., & van Schaik, C. P. (1993). Canine dimorphism. Evolutionary Anthropology: Issues, News, and Reviews, 2(6), 208–214.
Plavcan, J. M., & van Schaik, C. P. (1997). Intrasexual competition and body weight dimorphism in anthropoid primates. American Journal of Physical Anthropology, 103(1), 37–68.
Reichard, U. H. (2003). Social monogamy in gibbons: The male perspective. In U. H. Reichard & C. Boesch (Eds.), Monogamy: Mating strategies and partnerships in birds, humans and other mammals (pp. 190–213). Cambridge University Press.
Rohlf, F. J., & Slice, D. (1990). Extensions of the procrustes method for the optimal superimposition of landmarks. Systematic Biology, 39(1), 40–59.
Schaefer, K., Mitteroecker, P., Gunz, P., Bernhard, M., & Bookstein, F. L. (2004). Craniofacial sexual dimorphism patterns and allometry among extant hominids. Annals of Anatomy - Anatomischer Anzeiger, 186(5), 471–478.
Schultz, A. H. (1940). The size of the orbit and of the eye in primates. American Journal of Physical Anthropology, 26(1), 389–408.
Schultz, A. H. (1941). The relative size of the cranial capacity in primates. American Journal of Physical Anthropology, 28(3), 273–287.
Schultz, A. H. (1944). Age changes and variability in gibbons: A morphological study on a population sample of a man-like ape. American Journal of Physical Anthropology, 2(1), 1–129.
Schultz, A. H. (1962). Metric age changes and sex differences in primate skulls. Zeitschrift für Morphologie und Anthropologie, 52(3), 239–255.
Schultz, A. H. (1973). The skeleton of the Hylobatidae and other observations on their morphology. Gibbon and Siamang, 2, 1–54.
Setchell, J. M., & Kappeler, P. M. (2003). Selection in relation to sex in primates. Advances in the Study of Behavior, 33(877173), 33003–33007.
Sommer, V., & Reichard, U. (1997). Group encounters in wild gibbons (Hylobates lar): Agonism, affiliation, and the concept of infanticide. Behaviour, 134(15–16), 1135–1174.
Strand Viðarsdóttir, U., O'Higgins, P., & Stringer, C. (2002). A geometric morphometric study of regional differences in the ontogeny of the modern human facial skeleton. Journal of Anatomy, 201, 211–229.
Tobias, J. A., Montgomerie, R., & Lyon, B. E. (2012). The evolution of female ornaments and weaponry: social selection, sexual selection and ecological competition. Philosophical Transactions of the Royal Society B: Biological Sciences, 367(1600), 2274–2293.
van Schaik, C., & Dunbar, R. I. M. (1990). The evolution of monogamy in large primates: a new hypothesis and some crucial tests. Behaviour, 115(1–2), 30–61.
van Schaik, C. P., & Kappeler, P. M. (1997). Infanticide risk and the evolution of male–female association in primates. Proceedings of the Royal Society of London B: Biological Sciences, 264(1388), 1687–1694.
Weston, E. M., Friday, A. E., Johnstone, R. A., & Schrenk, F. (2004). Wide faces or large canines? The attractive versus the aggressive primate. Proceedings of the Royal Society of London B: Biological Sciences, 271, S416–S419.
Wilson, V., Weiss, A., Lefevre, C. E., Ochiai, T., Matsuzawa, T., Inoue-Murayama, M., Freeman, H., Herrelko, E. S., & Altschul, D. (2020). Facial width-to-height ratio in chimpanzees: Links to age, sex and personality. Evolution and Human Behavior, 41(3), 226–234.
Wood, B. A. (1976). The nature and basis of sexual dimorphism in the primate skeleton. Journal of Zoology, 180(1), 15–34.
Wood, B. A., Li, Y., & Willoughby, C. (1991). Intraspecific variation and sexual dimorphism in cranial and dental variables among higher primates and their bearing on the hominid fossil record. Journal of Anatomy, 174, 185–205.
Wrangham, R. (1979). On the evolution of ape social systems. Information (International Social Science Council), 18(3), 336.
Zichello, J. M. (2018). Look in the trees: Hylobatids as evolutionary models for extinct hominins. Evolutionary Anthropology: Issues, News, and Reviews, 27(4), 142–146.
Acknowledgments
This article is dedicated to Charles A. Lockwood and to Colin Groves, whom I thank for their mentorship and for valuable discussions surrounding sexual dimorphism and gibbon biology respectively. I thank the museum curators whose institutions house the hylobatid crania used in this study and J. Michael Plavcan for sharing his cranial and dental hylobatid data (NSF SBR 9616671) used in this study. I also thank the Smithsonian's Division of Mammals (Dr. Kristofer Helgen) and Human Origins Program (Dr. Matt Tocheri) for the scans of USNM specimens used in this research (http:// humanorigins.si.edu/evidence/3d-collection/primate). These scans were acquired through the generous support of the Smithsonian 2.0 Fund and the Smithsonian’s Collections Care and Preservation Fund. I thank Bernard Wood, Alison Behie, and Eric Lewitus for comments on an earlier draft of this article, and Jo Setchell, J. Michael Plavcan, and an anonymous reviewer for comments and feedback that improved the manuscript.
Author information
Authors and Affiliations
Contributions
KLB conceived and designed the study, analyzed the data, interpreted the results, and wrote the article.
Corresponding author
Additional information
Handling Editor: Joanna M. Setchell
Supplementary Information
ESM 1
Supporting Information is available online: Table SI: Summary of primary research to date documenting hylobatid craniofacial sexual dimorphism. Table SII: Summary of primary research to date documenting hylobatid canine sexual dimorphism. Table SIII: Descriptive statistics, index of sexual dimorphism (ISD) values, and Student’s t-test results for individual cranial measurements for seven hylobatid taxa (XLSX 46.2 kb)
Rights and permissions
About this article
Cite this article
Balolia, K.L. Craniodental Sexual Dimorphism Among Hylobatids. Int J Primatol 42, 737–758 (2021). https://doi.org/10.1007/s10764-021-00233-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-021-00233-3