Abstract
Conflicts between individuals are common among animals, with power dynamics often biased toward a particular sex. However, individuals across species exert power differently depending on the primary source of that power. Dominance-based power depends on fighting ability (e.g., greater body size) whereas leverage-based power depends on resources that cannot be taken by force (e.g., mating opportunities). Both can change in relative importance during development. We examined the development and base of female-biased power structures in Verreaux’s sifaka (Propithecus verreauxi) by analyzing the effects of reproductive maturity and body mass on intersexual conflict outcomes. We used data on four social groups at Ankoatsifaka Research Station in Kirindy Mitea National Park collected from 2007 to 2016, which included 483 decided agonistic encounters with both behavioral and morphometric data available. We used generalized linear mixed models to examine the effects of age, body mass, and female reproductive maturity on conflict outcome. Power relationships between mature females and males were unambiguously female biased. Female reproductive maturity, and not body mass, predicted intersexual conflict outcomes. Once reproductively mature, females almost never lost to males, except when females had not yet had an infant survive past weaning. Our results are thus consistent with the hypothesis that female leverage characterizes social structures of adult Verreaux’s sifaka more than female dominance. Future studies are needed to explore the influence of other sources of leverage and dominance, however. Similar studies in other primate species will clarify the role of what is often called “dominance” but may actually be leverage-based power.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Conflicts are common in the social lives of animals, especially for group-living species in which access to food resources and mating opportunities may be limited. These conflicts are often described in terms of dominance relationships, where dominance can be inferred from an asymmetry of aggression and submission in agonistic interactions (Bernstein 1981). However, considerable debate exists as to how dominance should be defined and measured, leaving no standardized operational definition (Bernstein 1981; Drews 1993; Lewis 2002; Rowell 1974). Lewis (2002) attempted to mitigate these issues by proposing the “power framework,” a standardized, operationalizable analytical framework with which asymmetries in dyadic relationships can be characterized while incorporating their dynamic nature.
Within the power framework, “power” refers to the ability of one individual to influence the behavior of another. Power is then based on varying degrees of “dominance” and “leverage” held by individuals within a dyad. Here, dominance arises from the ability to influence others as a result of the use of force, which may be intrinsic or derived. Intrinsic dominance arises from an individual’s own ability to use force (Lewis 2002) and is associated with traits correlated with fighting ability (Clutton-Brock 1985), such as differences in size (e.g., guerzas, Colobus guerza: Harris 2010). Derived dominance is associated with fighting ability outside of an individual’s own physical abilities, such as from coalitionary support (e.g., baboons, Papio spp.: Noë 1990, 1992). In contrast, leverage arises from the ability to influence others based on resources that cannot be taken by force (Lewis 2002), such as kinship (shared genes: de Waal 1986; Hand 1986), services (e.g., grooming, chacma baboons, P. ursinus: Barrett et al.1999), and mating opportunities (Hand 1986; Lewis 2002).
While power dynamics exhibit considerable flexibility in response to shifting degrees of dominance and leverage held by individuals, some species are characterized by power asymmetries that are consistently biased toward either females or males. Female-biased power structures are thought to be less common than male-biased power structures among mammals (Kappeler 1993a; Ralls 1976; cf. Lewis 2018), but appear to be a key characteristic of lemur social structures (Eichmueller et al.2013; Jolly 1984; Kappeler 1993a; Petty and Drea 2015). However, how and why female-biased power manifests in lemurs remains elusive, partly from a lack of consensus on how these patterns should be defined (Jolly 1984; Lewis 2018, unpublished data; Pereira et al.1990; Petty and Drea 2015). Traditionally referred to as “female dominant” social structures, definitions have suffered from the same failings as “dominance” in general (Lewis 2018), likely because the expression of this phenomenon varies considerably across contexts, seasons, and species (Eichmueller et al.2013; Hohenbrink et al.2016). By instead examining this pattern through the power framework, the overarching phenomenon of female-biased power can be acknowledged while systematically characterizing the ways in which its expression varies (Lewis unpublished data). Ascertaining the primary base of female power (i.e., dominance or leverage) within each species represents a key first step in this process.
An ontogenetic approach can aid in determining the relative contributions of dominance and leverage to female power. Relationships between sexes can change drastically during maturation, likely reflecting a concurrent change in the available sources of dominance and leverage. However, studies of the ontogeny of intersexual relationships in primates have been primarily limited to cercopithecines (e.g., Johnson 1987; Kuester and Paul 1988; Lee and Oliver 1979; Markham et al.2015; Nishida 2003; Pereira 2010; Pereira and Fairbanks 1993; Städele et al.2019). In cercopithecine primates, individual fighting ability strongly influences male intrasexual relationships (Pereira 1995), which is a source of intrinsic dominance, whereas female intrasexual relationships depend largely on the rank of their mothers, which aid their daughters via coalitionary support (Cheney 1977; Lee and Oliver 1979), a source of derived dominance. The relative influence of fighting ability and maternal rank on intersexual relationship dynamics, however, varies with developmental stage. During adolescence, for example, males are able only to dominate adult females that rank lower than the male’s mother, but once males approach maximum body size they are able to dominate all females regardless of maternal rank (Lee and Oliver 1979). Taking a similar ontogenetic approach to examining the shifts in power during development in lemurs may thus provide insight regarding how and why female-biased power structures arise as well. Only a handful of studies have examined the development of intersexual relationships in primates characterized by female-biased power structures (Hohenbrink et al.2015; Meredith 2011; O’Mara and Hickey 2014; Pereira 2002), but these studies suggest that reproductive maturity may play a key role. Sex differences in feeding ecology and general behavior develop at or after puberty (Meredith 2011; O’Mara and Hickey 2014), and female power over males similarly seems to develop only after females reach reproductive maturity (Hohenbrink et al.2015; Pereira 2002).
Verreaux’s sifaka (Propithecus verreauxi) is a well-studied lemur species living in the spiny deserts and dry forests of western and southwestern Madagascar (Leimberger and Lewis 2017). Like among many other lemurs (Eichmueller et al.2013), intersexual conflicts between adults are consistently decided in favor of females (Richard 1974, 1978; Richard and Nicoll 1987) regardless of context (Jolly 1966; Kubzdela 1997; Palagi et al.2008; Richard and Heimbuch 1975). Verreaux’s sifaka is thus characterized by a female-biased power structure. However, the primary base of this power structure remains unknown. As with many other lemur species (Kappeler 1990), adult females and males exhibit similar average body mass (Ravosa et al.1993; Richard et al.2002) and canine (Richard 1992) measures. Verreaux’s sifaka very rarely form coalitions against other group members (Lewis 2004) and primarily exhibit coalitions during intergroup interactions (Lewis et al.2019). Furthermore, male Verreaux’s sifaka usually disperse from their natal groups before reaching reproductive maturity (Leimberger and Lewis 2017). These characteristics suggest that dominance may play a limited role in adult intersexual relationship dynamics within groups, and leverage arising from sources other than kinship, such as reproductive opportunities, may thus be a more influential base (Lewis unpublished data). However, in some Verreaux’s sifaka populations, females are larger than males (Lewis and Kappeler 2005), and population averages may not always reflect size differences for particular dyads. Given that population averages usually only address adult body sizes, these differences may be especially apparent among conflict partners in different developmental stages (cf. Ravosa et al.1993).
We examined the development and two potential bases of female-biased power in Verreaux’s sifaka. We used body mass as a proxy for dominance and female reproductive maturity as a measure of female leverage. Although body mass and reproductive maturity are not necessarily mutually exclusive bases, one may nonetheless have a greater influence on the expression of female power over males. If female power is based primarily on dominance, we predicted that conflict outcomes are influenced by body mass or body mass differences, but not reproductive maturity. We further predicted that if power is based on dominance, the population is characterized by female-biased size dimorphism and that these dominance-based female-biased power structures develop if and when females become significantly larger than males during development. If power is based primarily on leverage arising from female reproductive maturity, we predicted that conflict outcomes are influenced by female maturity status, but not body mass. Leverage-based female-biased power structures are thus expected to manifest after females reach reproductive maturity, and conflict outcomes for reproductively immature females are the only conflicts expected to be influenced by body mass.
Methods
Study Site and Subjects
The data we used in this study are part of the ongoing and long-term Sifaka Research Project at the Ankoatsifaka Research Station (20°47′17′′S, 44°10′0′′E) in Kirindy Mitea National Park, western Madagascar. Verreaux’s sifaka at the station reside in social groups comprising between 2 and 11 (mean = 6) individuals including 1–3 adult females and 0–3 adult males (Leimberger and Lewis 2017). We observed a total of 31 females and 33 males living in 4 social groups during the study period, with females ranging from 0 to at least 13 yr. of age and males ranging from 0 to at least 10 yr. of age. We identified individuals via individual markings and unique collars and tags, including one radio collar per social group to permit radiotelemetry.
As part of the Sifaka Research Project, we annually anesthetized a portion of the sifaka population inhabiting the study site using tiletamine and zolazepam (15–20 mg/kg), which were remotely injected by disposable dart using a blow pipe or CO2 injection rifle at a distance of 3–6 m or 10–20 m, respectively, depending on the professional darter employed that year. We performed physical and health examinations on sedated animals (Rasambainarivo et al.2014), including measurement of body mass, and assigned individual sex as female or male based on genital morphology. Individuals recovered within a few hours. Because all captured individuals belong to known study groups with known home ranges, we released recovered individuals within 50 m of their social group (located using radiotelemetry) or in the center of their home range (if the individual with the radio collar was sedated).
We considered females as reproductively mature beginning in the mating season of the year in which they were first observed to give birth. Because we rarely observed mating bouts and did not collect data on physiological markers of sexual maturation as part of this long-term study, we designated male reproductive maturity as occurring at 5 yr. of age (Lawler et al.2003; Lewis and van Schaik 2007; Richard et al.2000). Because we did not always know exact birth dates, but >90% of births occur during July and August (Lewis and Kappeler 2005), we assigned individuals to age 1 on September 1 following their birth year. Age category then changed on September 1 of each following year. When we could not estimate birth years, we estimated minimum individual ages via body size, tooth wear, and/or nipple condition recorded during captures.
Behavioral Data
We collected social interactions using continuous focal animal sampling (Altmann 1974) in daily, dawn-to-dusk 1-h samples from June 2007 to October 2016 for a total of 4965 focal hours. Focal animals included all adult and subadult individuals. We did not include behavioral data from 2009 owing to an interruption in data collection caused by Cyclone Fanele (Lewis and Axel 2019; Lewis and Bannar-Martin 2012; Lewis and Rakotondranaivo 2011). We recorded all social behaviors including aggression (e.g., bites, chases, cuffs, lunges) and submission (e.g., chatters, flees, fear grimaces, tail curls) following the Brockman (1994) ethogram. While other behaviors may indicate submission, we were most interested in indicators of the stable layer of the power structure (cf. “formal dominance”: de Waal 1986). Only the chatter vocalization, which is a unidirectional submissive signal in sifaka (Kraus et al.1999), has been demonstrated to be a clear indicator of the formal relationship status (Lewis 2019). We therefore limited this study to only include dyadic interactions in which individuals emitted chatter vocalizations. For all conflicts, we considered the individual that received the chatter vocalization as the conflict “winner” and the individual that exhibited the chatter vocalization as the conflict “loser.”
Information on reproductive maturity was available for all relevant agonistic encounters. Body mass measurements in which both female and male body mass had been documented during the same calendar year of the encounter were available for 483 encounters. Outside of the late dry season, females and males experience similar seasonal fluctuations in body mass (Lewis and Kappeler 2005; Richard et al.2000), and differences in body mass are thus expected to remain approximately similar throughout the year. Because pregnant and nonpregnant females exhibit no significant difference in body mass (Lewis and Kappeler 2005), pregnancy likely did not impact mass measurements.
Relatedness Data
We used both genetic methods and observations of births to assess relatedness between dyads. For genetic analyses, we used ear tissue samples collected during annual captures and fecal samples collected opportunistically during behavioral follows from 2007 to 2012. After collection, we preserved tissue samples in 70–90% ethanol and fecal samples in RNALater solution or a homemade nucleic acid preservation (NAP) buffer for DNA preservation. We stored samples at room temperature until transporting them to the Primate Ecology and Evolution Laboratory at the University of Texas at Austin, where we stored samples at −80 °C until extraction. Detailed information on extraction, genotyping, and parentage protocols is provided in Abondano (2014), with detailed methods regarding the construction of the relatedness matrix outlined in Perofsky et al. (2017). Briefly, we genotyped each individual using 14 microsatellite markers successfully used in other Verreaux’s sifaka populations (Lawler et al.2001; Rakotoarisoa et al.2006). We then constructed a finely resolved relatedness matrix using maximum likelihood methods in CERVUS 3.0 (Kalinowski et al.2007) and KINGROUP2 (Konovalov et al.2004) to estimate the likelihood of pedigree relationships (cousin, half-sibling, full-sibling, and parent–offspring) among dyads. We scored dyads by their pedigree relationships with r-values of 0.5, 0.25, 0.125, or 0, respectively. We assigned known mother–offspring pairs without genetic data an r-value of 0.5. Relatedness data were available for only 58 out of 115 dyads, which prevented us from using them successfully in statistical analyses.
Statistical Analysis
To determine when female-biased power asymmetries develop, we first assessed how conflict outcome changed with all possible combinations of female age, male age, and/or female reproductive maturity (Table I, Model 1). This sample included individuals for which we could accurately estimate ages within 2 yr. Because we used the average age at reproductive maturity from males in other populations (Lawler et al.2003; Lewis and van Schaik 2007; Richard et al.2000) and males in this population may reach maturity at a broader range of ages, we excluded male maturity from these models and used male age alone. Next, to determine whether female power is based on leverage or dominance, we examined whether female reproductive maturity, female and male body mass, or the interaction between female and male body mass influenced conflict outcome. Because including only individuals with known ages would limit our data set, we did not include age in these models. We first restricted the analysis to individuals for which body mass data were available for both conflict partners (Table I, Model 2). Second, to further investigate the role of dominance and leverage, we limited the sample to conflicts between mature females with mature males (Table I, Model 3) to determine whether body size had any influence on conflict outcome once female-biased leverage via female reproductive maturity was established. Third, to determine whether body size had any influence on conflict outcome before females gained leverage via reproductive maturity, we limited the sample to immature females and all males (Table I, Model 4). For any conflict in which an immature female is involved, regardless of male maturity status, leverage based on mating opportunities is not expected to be a factor and dominance is then more likely to be the base of any power asymmetry.
We conducted all statistical analyses in R version 3.5.1 (R Development Core Team 2018). We investigated the influence of female and male age, female reproductive maturity, female and male body size, and the interaction between female and male body mass on intersexual conflict outcome using generalized linear mixed models fitted with a binomial distribution with a logit link using the package glmmADMB (Fournier et al.2012; Skaug et al.2016). For analyses, we centered age and mass variables by subtracting the sample mean from all values and z-transformed both variables by dividing the centered values by two standard deviations. We included female and male identities as two random effects for all models. To test the development and primary base of female power we used an information theoretic approach. We created a model set with all combinations of the potentially informative predictors using the dredge function from package MuMin (Bartón 2018). Running all combinations of predictors enables a test of relative variable importance and reduces the risk of missing a potentially informative combination. We compared each model in the set using Akaike’s information criterion (AIC) (Burnham et al.2011; Johnson and Omland 2004; Symonds and Moussalli 2011). We considered factors appearing in the top model as important in addition to factors appearing in subsequent models in which the change in AIC (∆AIC) was <3 compared to the top model. We also used model averaging to assess the coefficient for each factor across all models. Model averaged coefficients that had 95% confidence intervals that did not overlap with zero were considered informative.
Data Availability
The data sets analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Note
The Institutional Animal Care and Use Committee of the University of Texas at Austin, Madagascar National Parks, and Madagascar’s Ad Hoc Committee for Flora and Fauna (CAFF/CORE) approved all capture protocols and field research. All research adhered to the legal requirements of Madagascar. The authors declare that they have no conflicts of interest.
Results
The Development of Female Power
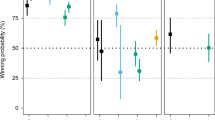
Age was an important predictor of whether a female won contests with a male. By age 3 yr., females won ca. 50% of contests with males, and by age 7 yr., females won nearly 100% of contests with males (Fig. 1). Females with known birth years reached reproductive maturity from ages 3 to 6 yr. (N = 6, median = 4.5) (Electronic Supplementary Material [ESM] Fig. S1), with first observed births from ages 4 to 7 yr. (N = 6; median = 5.5 yr. old) and first offspring to survive past weaning from ages 5 to 9 yr. (N = 5; median = 6 yr. old). When we considered female age, male age, and/or female maturity in various combinations, the best model included female age and male age (model weight = 0.67). Model averaging indicated that female age and male age were both informative predictors: older females exhibited a higher probability of winning contests with males (βFemale Age = 3.30 ± SE 0.68) whereas older males had a lower probability of winning contests with females (βMale Age = −1.61 ± SE 0.39). Other models that contained female age, male age, or reproductive maturity as single variables were not informative (∆AIC > 10, ESM Table SIA).
Individual conflict outcomes (gray) and mean percentage (black) of intersexual conflicts won by female Verreaux’s sifaka (Propithecus verreauxi) in Kirindy Mitea National Park recorded between 2007 and 2016. The horizontal dashed line represents 50% conflicts won by females. The vertical dashed line represents median age at which females reached reproductive maturity. The vertical solid line represents median age at which females gave birth to their first infant to survive past weaning. Conflicts are limited to those for which age data are available for both conflict partners (N = 341).
Dominance Vs. Leverage: Size and Reproductive Maturity
Body mass had a limited impact on conflict outcome between females and males. When we combined female mass, male mass, the interaction between female and male mass, and reproductive maturity into all possible combinations in a candidate model set, the only informative models were those including reproductive maturity (NModels = 5), where in each case reproductively mature females exhibited a higher probability of winning intersexual conflicts than did immature females (ESM Table SIB). Based on model averaging, we found that body mass measures were weak predictors (βFemale Mass = 0.95 ± SE 1.28, βMale Mass = −0.60 ± SE 1.44, βFemale Mass:Male Mass = −0.58 ± SE 1.52) and the only informative predictor of conflict outcome was reproductive maturity (βMature Female = 8.23 ± SE 2.56).
When we limited the analyses to reproductively mature females and males, size was not an important predictor of conflict outcome. Model averaging showed that female mass only weakly predicted conflict outcome (βFemale Mass = −2.83 ± SE 1.82), and neither male mass (βMale Mass = −0.30 ± SE 1.46) nor the interaction between female and male mass (βFemale:Male Mass = −0.75 ± SE 2.36) were influential (ESM Table SIC). However, additional examination of the data suggests that the weak influence of female mass indicated by the models is unlikely to be important. Adult females and males did not differ in mean body mass (mean size ratio [female/male body mass] = 1.01 ± SD 0.05; Welch Two Sample t-test: t = 0.540, df = 21.93, P = 0.594), and females and males exhibited no obvious differences in growth over the course of their development (Fig. 2). Furthermore, males submitted to females with a chatter vocalization in 99% of conflicts, with females submitting to males with a chatter vocalization in only three encounters, all with unrelated males. In one case, the female was nearly the same size as the male, and in the other two instances, the female was slightly larger (Fig. 3a). Of the conflicts for which we had kinship data for both conflict partners (N = 262), only 38 conflicts (14.5%) occurred between parents and offspring and 20 conflicts (7.6%) occurred between siblings.
Individual conflict outcomes (N = 483) recorded between 2007 and 2016 in Kirindy Mitea National Park of (a) mature and (b) immature female Verreaux’s sifaka (Propithecus verreauxi) against immature males (light gray) and mature males (black) relative to body mass ratio. Dashed horizontal lines include the 1:1 body mass ratio ± 10% difference in body mass.
When we limited the data to immature females and all males, size had a limited impact on conflict outcome. Overall, immature females won only 28% of conflicts, but tended to lose only when they were > 10% smaller than their male conflict partner (Fig. 3b). In only one conflict did an immature female that was considerably smaller than the male win. Model averaging indicated that the interaction between female and male mass may weakly influence conflict outcome; the larger the female, the less the effect of male size on conflict outcome, and the smaller the male, the less the effect of female size on conflict outcome (βFemale Mass:Male Mass = −11.55 ± SE 9.80) (ESM Table SID). However, neither female nor male mass alone were strong predictors of conflict outcome (βFemale Mass = 7.22 ± SE 7.49, βMale Mass = −2.73 ± SE 3.79). We only had kinship data for 25 out of 68 conflicts, of which only 5 conflicts (20.0%) occurred between parent–offspring pairs and 2 occurred between siblings (8.0%). Owing to the small sample size, the extent to which kinship influences conflicts involving immature females is unknown.
Dominance Vs. Leverage: Case Studies of Parity and Kinship
When we expanded conflict data for mature females and mature males to include those interactions for which body mass data were not available for both conflict partners (NConflicts = 827), females submitted to males on 55 occasions (7%). Forty-four of these instances (80%) occurred between one dyad, in which the female and male were unrelated. Notably, the female in this dyad reached reproductive maturity fairly early for this population at 3 yr. of age, but did not give birth to an offspring that survived past weaning until 6 yr. of age. She began consistently receiving male submissive signals only in the mating season following that birth season. The only other female whose known first offspring did not survive past weaning disappeared soon after giving birth, but the few data available show that after reaching maturity (at age 5 yr), she nevertheless exhibited submissive behavior toward males (NMales = 2) in all intersexual encounters. In addition, mature females were only found to submit to immature males on two occasions. Only one of these instances included body mass data for both individuals, and the female was considerably larger than the male (Fig. 3a). Both instances were between known mother–son pairs.
Discussion
Our findings are consistent with the hypothesis that female-biased power structures in Verreaux’s sifaka are based primarily on leverage and not dominance. Female reproductive maturity strongly influenced conflict outcomes. Regardless of size, mature females won nearly all conflicts with mature males. Only among immature females did body mass appear to have any effect, and even then, both absolute size and the interaction between female and male size were poor predictors of conflict outcome. Age also had a large influence on conflict outcome, with conflicts more often decided toward older females and less often toward older males.
Reproductive maturity represents a key shift in development accompanied by changes in female and male behavior reflecting different reproductive strategies as a result of specific limitations constraining their reproductive success (Emery Thompson 2017; Schulz et al.2009). Competition for mating opportunities and the resources needed for successful reproduction subsequently arise as additional pressures governing interactions between conspecifics (Clutton-Brock 2009; Dahl and Forbes 2010). Because male reproductive success is often more dependent on the number of mating opportunities than is female reproductive success (Clutton-Brock 2007; Trivers 1972), mating opportunities may be considered of higher value to males. Reproductively mature females can exploit this asymmetry when they are able to control male mating access, thus transforming mating opportunities into a source of leverage (Lewis 2002). Even in species where female mate choice may be constrained by male coercion or male-biased dominance derived from larger body size, females may gain short-term advantages over males during fertile periods, including access to services such as increased grooming by males (e.g., chacma baboons, Papio ursinus: Barrett et al.2002; gorillas, Gorilla g. beringei: Sicotte 2002; chimpanzees, Pan troglodytes: Stopka et al.2001; orangutans, Pongo pygmaeus: van Noordwijk and van Schaik 2009). Thus, complete female control over reproduction is unnecessary for females to be able to leverage mating opportunities. Indeed, despite winning nearly every intersexual conflict, mature female Verreaux’s sifaka nevertheless experience harassment and forced copulations by males (Brockman 1999; Brockman and Whitten 1996) as well as male-mediated infanticide (Lewis et al.2003; Littlefield 2010).
Even after reaching reproductive maturity, however, females may differ in the degree to which their sources of leverage yield power over males. These differences may arise from variation in female quality, where indicators of female reproductive potential, such as parity (Setchell and Wickings 2006) or age (Brockman 1999; Muller et al.2006), may affect male behavior toward females (Fitzpatrick and Servedio 2017). In Verreaux’s sifaka, younger, nulliparous females are less likely to give birth to offspring that survive past weaning (Richard et al.2002). In the present study, case studies of females for whom conflict data were available before and after reaching reproductive maturity suggest that female-biased power is not necessarily consistently expressed after reaching reproductive maturity (defined here as the stage of female development reached at the mating season just prior to first observed birth), but instead seems to be more consistently expressed after females give birth to their first infant to survive past weaning. These results may also explain why female age had a stronger influence on conflict outcome than reproductive maturity when these two variables were combined in a single model. Additional longitudinal data on females are required to parse out the relative importance of female reproductive ability, age, and power; however, particularly as females reach senescence. While we were limited in our ability to estimate female ages accurately past 13 yr. of age due to the duration of the study, Verreaux’s sifaka at Beza-Mahafaly Special Reserve no longer give birth to offspring that survive past 12 mo once females reach age 24 yr. (Richard et al.2002) and no longer produce offspring after age 27 yr. (Bronikowski et al.2016). Milne-Edwards’s sifaka (P. edwardsi) show similar patterns, where infants born to females aged 18–27 years are significantly less likely to survive compared to other age groups (Wright et al.2008). If age-related reproductive ability is the primary source of female leverage, we would expect female power over males to decrease during these latter stages of their lifetime.
Interestingly, we observed two instances of submission by mature females to immature males, both involving mother–son pairs. The adult females in these instances likely lacked leverage derived from the ability to offer mating opportunities. Furthermore, the immature males were smaller than the adult females, but still won, suggesting that dominance does not explain these outcomes. Instead, sons may have held leverage over their mothers via shared genes (de Waal 1986; Hand 1986; Lewis 2002), where mothers may have deferred to their sons in order to gain inclusive fitness benefits (Hand 1986). Kinship-based leverage has been shown to influence agonistic interactions in other primate species as well (Pereira 1995), including other lemur species (Kappeler 1993b). Kinship overall, however, likely does not play a strong role in maintaining female-biased power structures in Verreaux’s sifaka. Among individuals for which kinship data were available, we observed relatively few conflicts between related mature females and mature males, perhaps because males in this population usually disperse from their natal groups prior to reaching reproductive maturity (Leimberger and Lewis 2017). The role of kinship may thus be limited to power dynamics involving immature individuals, but additional data is necessary before the influence of kinship can be accurately distinguished.
Our observations of female losses in this study highlight that even within a population whose power structure is primarily female-biased, variation in power dynamics and their bases still exists across intersexual dyads. Indeed, this variation is present regardless of whether an overarching power structure exists at all (Lewis unpublished data). Adult sex ratios, for example, may affect power dynamics as a source of leverage if changes in sex ratio affects the supply and demand of services provided particular sex (Lewis 2004). Because nearly 100% of intersexual conflicts in our study were decided toward mature females who were interacting with males in variable group compositions, sex ratio is unlikely to play a significant role as a source of leverage in Verreaux’s sifaka. However, brown lemurs (Eulemur fulvus) and red-fronted lemurs (E. rufifrons) lack sex-biased power structures (Kappeler 1993a) and show a clear correlation between sex ratio and the degree of agonism directed toward a particular sex (red-fronted lemurs: Pereira and Kappeler 1997; brown lemurs: Roeder and Fornasieri 1995). Knowledge and general learning abilities may also affect power dynamics as a source of leverage (Lewis unpublished data), but studies examining this correlation across both primates and nonprimates have demonstrated equivocal results (Wascher et al.2018).
Variation in sources of dominance may also give rise to variable power dynamics. Coalitions, for example, are common sources of derived dominance that often influence intersexual power asymmetries (Smuts 1987) and occur across primates (e.g., chimpanzees: Newton-Fisher 2006; red-fronted lemurs: Pereira and Kappeler 1997). Indeed, coalitions are integral to the maintenance of female-biased power structures in spotted hyenas (Crocuta crocuta: Vullioud et al.2019) and potentially in bonobos (Pan paniscus: Parish 1994; White and Wood 2007), although female bonobo coalitions may actually serve to suppress male aggression toward offspring rather than toward the females themselves (Surbeck and Hohmann 2013). Verreaux’s sifaka, however, only rarely exhibit derived dominance via coalitions to win conflicts (Lewis 2004) and we did not observe intragroup coalitions during our study. Consequently, derived dominance is an unlikely source of power in Verreaux’s sifaka. Other sources of intrinsic dominance, however, may play a more substantial role. We examined body mass as a source of intrinsic dominance and found no important influence of body mass on conflict outcome among mature individuals. However, differences in canine size, speed, and agility may also be important for fighting ability (Clutton-Brock 1977, 1985; Kappeler 1990; Plavcan and van Schaik 1997). Additional research is needed to determine whether these other proxies for intrinsic fighting ability have a similarly limited influence on the outcomes of intersexual conflicts.
While no other study has explicitly investigated the base of female-biased power structures in lemurs, the few studies that have examined the development of female power in other lemur species support our findings. In ring-tailed lemurs (Lemur catta), power dynamics among immature individuals are largely determined by differences in body mass regardless of sex, and female-biased power structures do not emerge until after females have reached reproductive maturity (Pereira 2002). Female-biased power structures in gray mouse lemurs (Microcebus murinus) similarly arise only after females reach reproductive maturity (Hohenbrink et al.2015). Furthermore, female breeding experience, but not body mass, has a positive correlation with female-biased conflict outcomes in both gray and Goodman’s mouse lemurs (M. lehilahytsara) (Hohenbrink et al.2016). Combined, these studies suggest that female-biased power structures in lemurs in general may be based on female leverage, and less on female dominance.
Conclusions
Female-biased power structures are common among lemur species (Eichmueller et al.2013; Jolly 1984; Kappeler 1993a; Petty and Drea 2015), but the bases of these power asymmetries (sensu Lewis 2002) have rarely been explicitly investigated (Lewis unpublished data). By examining the development of female power in Verreaux’s sifaka, our study demonstrates that the female-biased power structure in this species more likely arises due to leverage based on reproductive opportunities than due to dominance based on body mass. Dominance seems to contribute to female power only when females are reproductively immature. Female Verreaux’s sifaka likely derive leverage from mating opportunities that become available once females are reproductively mature, as well as additional factors indicating female reproductive potential, such as parity and age. Combined with studies of the development of intersexual relationships in other lemur species, our study suggests that female leverage may be the basis of female-biased power structures in lemurs overall.
References
Abondano, L. A. (2014). Male reproductive skew in multimale social groups of Verreaux’s sifaka (Propithecus verreauxi) at Kirindy Mitea National Park. Madagascar: Master’s Thesis, University of Texas at Austin.
Altmann, J. (1974). Observational study of behavior: Sampling methods. Behaviour, 49(3/4), 227–267.
Barrett, L., Gaynor, D., & Henzi, S. (2002). A dynamic interaction between aggression and grooming reciprocity among female chacma baboons. Animal Behaviour, 63, 1047–1053.
Barrett, L., Henzi, S. P., Weingrill, T., Lycett, J. E., & Hill, R. A. (1999). Market forces predict grooming reciprocity in female baboons. Proceedings of the Royal Society B: Biological Sciences, 266, 665–670.
Bartón, K. (2018). Multi-model inference. R package version 1.15.6.
Bernstein, I. S. (1981). Dominance: The baby and the bathwater. The Behavioral and Brain Sciences, 4, 419–457.
Brockman, D. K. (1994). Reproduction and mating system of Verreaux’s sifaka, Propithecus verreauxi, at Beza Mahafaly. Madagascar: PhD Thesis, Yale University.
Brockman, D. K. (1999). Reproductive behavior of female Propithecus verreauxi at Beza Mahafaly, Madagascar. International Journal of Primatology, 20(3), 375–398.
Brockman, D. K., & Whitten, P. L. (1996). Reproduction in free-ranging Propithecus verreauxi: Estrus and the relationship between multiple partner matings and fertilization. American Journal of Physical Anthropology, 100, 57–69.
Bronikowski, A. M., Cords, M., Alberts, S. C., Altmann, J.(1974) Brockman, D. K., et al (2016). Female and male life tables for seven wild primate species. Scientific Data, 3 3:160006.
Burnham, K. P., Anderson, D. R., & Huyvaert, K. P. (2011). AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behavioral Ecology and Sociobiology. 65, 23–35.
Cheney, D. L. (1977). The acquisition of rank and the development of reciprocal alliances among free-ranging immature baboons. Behavioral Ecology and Sociobiology, 2, 303–318.
Clutton-Brock, T. H. (1977). Sexual dimorphism, socionomic sex ration and body weight in primates. Nature, 269, 797–800.
Clutton-Brock, T. H. (1985). Size, sexual dimorphism, and polygyny in primates. In W. L. Jungers (Ed.), Size and scaling in primate biology (pp. 51–60). New York: Springer Science+Business Media.
Clutton-Brock, T. H. (2007). Sexual selection in males and females. Science, 318(5858), 1882–1885.
Clutton-Brock, T. H. (2009). Sexual selection in females. Animal Behaviour, 77(1), 3–11.
Dahl, R. E., & Forbes, E. E. (2010). Pubertal development and behavior: Hormonal activation of social and motivational tendencies. Brain and Cognition, 72(1), 66–72.
de Waal, F. B. M. (1986). The integration of dominance and social bonding in primates. The Quarterly Review if Biology, 61(4), 459–479.
Drews, C. (1993). The concept and definition of dominance in animal behaviour. Behaviour, 125(3–4), 283–313.
Eichmueller, P., Thorén, S., & Radespiel, U. (2013). The lack of female dominance in golden-brown mouse lemurs suggests alternative routes in lemur social evolution. American Journal of Physical Anthropology, 150(1), 158–164.
Emery Thompson, M. (2017). Energetics of feeding, social behavior, and life history in non-human primates. Hormones and Behavior, 91, 84–96.
Fitzpatrick, C. L., & Servedio, M. R. (2017). Male mate choice, male quality, and the potential for sexual selection on female traits under polygyny. Evolution, 71(1), 174–183.
Fournier, D. A., Skaug, H. J., Ancheta, J., Ianelli, J., Maunder, M. N., et al (2012). AD Model Builder: Using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optimization Methods and Software, 27(2), 233–249.
Hand, J. L. (1986). Resolution of social conflicts: Dominance, egalitarianism, spheres of dominance, and game theory. The Quarterly Review of Biology, 61(2), 201–220.
Harris, T. R. (2010). Multiple resource values and fighting ability measures influence intergroup conflict in guerezas (Colobus guereza). Animal Behaviour, 79, 89–98.
Hohenbrink, S., Koberstein-Schwarz, M., Zimmermann, E., & Radespiel, U. (2015). Shades of gray mouse lemurs: Ontogeny of female dominance and dominance-related behaviors in a nocturnal primate. American Journal of Primatology, 77(11), 1158–1169.
Hohenbrink, S., Schaarschmidt, F., Bünemann, K., Gerberding, S., Zimmermann, E., & Radespiel, U. (2016). Female dominance in two basal primates, Microcebus murinus and Microcebus lehilahytsara: Variation and determinants. Animal Behaviour, 122, 145–156.
Johnson, J. A. (1987). Dominance rank in juvenile olive baboons, Papio anubis: the influence of gender, size, maternal rank and orphaning. Animal Behaviour, 35(6), 1694–1708.
Johnson, J. B., & Omland, K. S. (2004). Model selection in ecology and evolution. Trends in Ecology and Evolution Trends in Ecology and Evolution, 19, 101–108.
Jolly, A. (1966). Lemur behavior. Chicago: University of Chicago Press.
Jolly, A. (1984). The puzzle of female feeding priority. Female Primates: Studies by Women Primatologists, 1, 197–205.
Kalinowski, S. T., Taper, M. L., & Marshall, T. C. (2007). Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Molecular Ecology, 16(5), 1099–1106.
Kappeler, P. M. (1990). The evolution of sexual size dimorphism in prosimian primates. American Journal of Primatology, 21, 201–214.
Kappeler, P. M. (1993a). Female dominance in primates and other mammals. In P. Bateson, P. Klopfer, & N. Thompson (Eds.), Perspectives in ethology (pp. 143–158). New York: Plenum Press.
Kappeler, P. M. (1993b). Variation in social structure: The effects of sex and kinship on social interactions in three lemur species. Ethology, 93, 125–145.
Konovalov, D. A., Manning, C., & Henshaw, M. T. (2004). KINGROUP: A program for pedigree relationship reconstruction and kin group assignments using genetic markers. Molecular Ecology Notes, 4(4), 779–782.
Kraus, C., Heistermann, M., & Kappeler, P. M. (1999). Physiological suppression of sexual function of subordinate males: A subtle form of intrasexual competition among male sifakas (Propithecus verreauxi)? Physiology and Behavior, 66(5), 855–861.
Kubzdela, K. S. (1997). Female reproductive success, and female dispersal patterns in white sifaka, Propithecus verreauxi verreauxi. PhD Thesis, University of Chicago.
Kuester, J., & Paul, A. (1988). Rank relations of juvenile and subadult natal males of barbary macaques (Macaca sylvanus) at affenberg salem. Folia Primatologica, 51(1), 33–44.
Lawler, R. R., Richard, A. F., & Riley, M. A. (2001). Characterization and screening of microsatellite loci in a wild lemur population (Propithecus verreauxi verreauxi). American Journal of Primatology, 55(4), 253–259.
Lawler, R. R., Richard, A. F., & Riley, M. A. (2003). Genetic population structure of the white sifaka (Propithecus verreauxi verreauxi) at Beza Mahafaly Special Reserve, southwest Madagascar (1992–2001). Molecular Ecology, 12(9), 2307–2317.
Lee, P. C., & Oliver, J. I. (1979). Competition, dominance and the acquisition of rank in juvenile yellow baboons (Papio cynocephalus). Animal Behaviour, 27, 576–585.
Leimberger, K. G., & Lewis, R. J. (2017). Patterns of male dispersal in Verreaux’s Sifaka (Propithecus verreauxi) at Kirindy Mitea National Park. American Journal of Primatology, 79, e22455.
Lewis, R. J. (2002). Beyond dominance: The importance of leverage. The Quarterly Review of Biology, 77(2), 149–164.
Lewis, R. J. (2004). Male–male relationships in sifaka (Propithecus verreauxi verreauxi): Power, conflict, and cooperation. PhD Thesis, Duke University.
Lewis, R. J. (2018). Female power in primates and the phenomenon of female dominance. Annual Review of Anthropology, 47, 31.
Lewis R. J. (2019). Subordination signals improve the quality of social relationships in Verreaux’s sifaka: Implications for the evolution of power structures and social complexity. American Journal of Physical Anthropology, 169, 599–607.
Lewis, R. J., & Axel, A. C. (2019). Using vegetation phenology and long-term demographic data to assess the impact of Cyclone Fanele on a lemur population in Madagascar. In A. M. Behie & N. Malone (Eds.), Primate research and conservation in the Anthropocene (pp. 216–236). Cambridge: Cambridge University Press.
Lewis, R. J., & Bannar-Martin, K. H. (2012). The impact of Cyclone Fanele on a tropical dry forest in Madagascar. Biotropica, 44(2), 135–140.
Lewis, R. J., & Kappeler, P. M. (2005). Seasonality, body condition, and timing of reproduction in Propithecus verreauxi verreauxi in the Kirindy Forest. American Journal of Primatology, 67, 347–364.
Lewis, R. J., & Rakotondranaivo, F. (2011). The impact of Cyclone Fanele on sifaka body condition and reproduction in the tropical dry forest of western Madagascar. Journal of Tropical Ecology, 27, 429–432.
Lewis, R. J., & van Schaik, C. P. (2007). Bimorphism in male verreaux’s sifaka in the Kirindy Forest of Madagascar. International Journal of Primatology, 28, 159–182.
Lewis, R. J., Razafindrasamba, S. M., & Tolojanahary, J. P. (2003). Observed infanticide in a seasonal breeding prosimian (Propithecus verreauxi verreauxi) in Kirindy Forest, Madagascar. Folia Primatologica, 74(2), 101–103.
Littlefield, B. L. (2010). Infanticide following male takeover event in Verreaux’s sifaka (Propithecus verreauxi verreauxi). Primates, 51(1), 83–86.
Markham, A. C., Lonsdorf, E. V., Pusey, A. E., & Murray, C. M. (2015). Maternal rank influences the outcome of aggressive interactions between immature chimpanzees. Animal Behaviour, 100, 192–198.
Meredith, S. L. (2011). The development of adult sex-typed social behavior in Lemur catta. PhD Thesis, Arizona State University.
Muller, M. N., Thompson, M. E., & Wrangham, R. W. (2006). Male chimpanzees prefer mating with old females. Current Biology, 16, 2234–2238.
Newton-Fisher, N. E. (2006). Female coalitions against male aggression in wild chimpanzees of the Budongo Forest. International Journal of Primatology, 27(6), 1589–1599.
Nishida, T. (2003). Harassment of mature female chimpanzees by young males in the Mahale Mountains. International Journal of Primatology, 24(3), 503–514.
Noë, R. (1990). A veto game played by baboons: A challenge to the use of the prisoner’s dilemma as a paradigm for reciprocity and cooperation. Animal Behaviour, 39, 78–90.
Noë, R. (1992). Alliance formation among male baboons: Shopping for profitable partners. In A. H. Harcourt & F. B. M. de Waal (Eds.), Coalitions and alliances in humans and other animals (pp. 285–321). New York: Oxford University Press.
O’Mara, M. T., & Hickey, C. M. (2014). The development of sex differences in ring-tailed lemur feeding ecology. Behavioral Ecology and Sociobiology, 68, 1273–1286.
Palagi, E., Antonacci, D., & Norscia, I. (2008). Peacemaking on treetops: First evidence of reconciliation from a wild prosimian (Propithecus verreauxi). Animal Behaviour, 76(3), 737–747.
Parish, A. (1994). Sex and food control in the “uncommon chimpanzee”: How bonobo females overcome a phylogenetic legacy of male dominance. Ethology and Sociobiology, 15(3), 157–179.
Pereira, M. E. (1995). Development and social dominance among group-living primates. American Journal of Primatology, 37, 143–175.
Pereira, M. E. (2002). Agonistic interaction, dominance relation, and ontogenetic trajectories in ringtailed lemurs. In M. E. Pereira & L. A. Fairbanks (Eds.), Juvenile primates: Life history, development, and behavior (pp. 285–305). Chicago: University of Chicago Press.
Pereira, M. E. (2010). Agonistic interactions of juvenile savanna baboons. Ethology, 79(3), 195–217.
Pereira, M. E., & Fairbanks, L. A. (Eds.) (1993). Juvenile primates: Life history, development, and behavior. New York: Oxford University Press.
Pereira, M. E., & Kappeler, P. M. (1997). Divergent systems of agonistic behaviour in lemurid primates. Behaviour, 134(3/4), 225–274.
Pereira, M. E., Kaufman, R., Kappeler, P. M., & Overdorff, D. J. (1990). Female dominance does not characterize all of the Lemuridae. Folia Primatologica, 55, 96–103.
Perofsky, A. C., Lewis, R. J., Abondano, L. A., Difiore, A., & Meyers, L. A. (2017). Hierarchical social networks shape gut microbial composition in wild verreaux’s sifaka. In Proceedings of the Royal Society B: Biological Sciences, 284(1868). Supplemental: Material.
Petty, J. M. A., & Drea, C. M. (2015). Female rule in lemurs is ancestral and hormonally mediated. Scientific Reports, 5, 1–5.
Plavcan, J. M., & van Schaik, C. P. (1997). Intrasexual competition and body weight dimorphism in anthropiod primates. American Journal of Physical Anthropology, 68, 37–68.
R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org.
Rakotoarisoa, G., Shore, G. E., Mcguire, S. M., Engberg, S. E., Louis, E. E., & Brenneman, R. A. (2006). Characterization of 13 microsatellite marker loci in Verreaux’s sifaka (Propithecus verreauxi). Molecular Ecology Notes, 6(4), 1122–1125.
Ralls, K. (1976). Mammals in which females are larger than males. The Quarterly Review of Biology, 51(2), 245–276.
Rasambainarivo, F. T., Junge, R. E., & Lewis, R. J. (2014). Biomedical evaluation of Verreaux’s sifaka (Propithecus verreauxi) from Kirindy Mitea National Park in Madagascar. Journal of Zoo and Wildlife Medicine, 45(2), 247–255.
Ravosa, M. J., Meyers, D. M., & Glander, K. E. (1993). Relative growth of the limbs and trunk in sifakas: Heterochronic, ecological, and functional considerations. American Journal of Physical Anthropology, 92, 499–520.
Richard, A. F. (1974). Intra-specific variation in the social organization and ecology of Propithecus verreauxi. Folia Primatologica, 22, 178–207.
Richard, A. F. (1978). Behavioral variation: Case study of a Malagasy lemur. Lewisburg: Bucknell University Press.
Richard, A. F. (1992). Aggressive competition between males, female-controlled polygyny and sexual monomorphism in a Malagasy primate, Propithecus verreauxi. Journal of Human Evolution, 22, 395–406.
Richard, A. F., & Heimbuch, R. (1975). An analysis of the social behavior of three groups of Propithecus verreauxi. In I. Tattersall & R. W. Sussman (Eds.), Lemur biology (pp. 313–333). New York: Plenum Press.
Richard, A. F., & Nicoll, M. E. (1987). Female social dominance and basal metabolism in a Malagasy primate, Propithecus verreauxi. American Journal of Primatology, 12, 309–314.
Richard, A. F., Dewar, R. E., Schwartz, M., & Ratsirarson, J. (2000). Mass change, environmental variability and female fertility in wild Propithecus verreauxi. Journal of Human Evolution, 39(4), 381–391.
Richard, A. F., Dewar, R. E., Schwartz, M., & Ratsirarson, J. (2002). Life in the slow lane? Demography and life histories of male and female sifaka (Propithecus verreauxi verreauxi). Journal of Zoology, 256, 421–436.
Roeder, J.-J., & Fornasieri, I. (1995). Does agonistic dominance imply feeding priority in lemurs? A study in Eulemur fulvus mayottensis. International Journal of Primatology, 16(4), 629–642.
Rowell, T. E. (1974). The concept of social dominance. Behavioral Biology, 11(2), 131–154.
Schulz, K. M., Molenda-Figueira, H. A., & Sisk, C. L. (2009). Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Hormones and Behavior, 55(5), 597–604.
Sicotte, P. (2002). The function of male aggressive displays towards females in mountain gorillas. Primates 43, 277–289.
Setchell, J. M., & Wickings, E. J. (2006). Mate choice in male mandrills. Ethology, 112, 91–99.
Skaug, H., Fournier, D., Bollker, B., Magnusson, A., & A., N. (2016). Generalized linear mixed models using “AD Model Builder.” R package version 0.8.3.3.
Smuts, B. (1987). Gender, aggression, and influence. In B. B. Smuts, D. L. Cheney, R. M. Seyfarth, R. W. Wrangham, & T. T. Struhsaker (Eds.), Primate societies (pp. 400–412). Chicago: University of Chicago Press.
Städele, V., Roberts, E. R., Barrett, B. J., Strum, S. C., Vigilant, L., & Silk, J. B. (2019). Male–female relationships in olive baboons (Papio anubis): Parenting or mating effort? Journal of Human Evolution, 127, 81–92.
Stopka, P. Johnson. D. P. & Barrett, L. (2001). ‘Friendship’ for fitness or ‘friendship’ for friendship’s sake? Animal Behaviour, 61, 19–21.
Surbeck, M., & Hohmann, G. (2013). Intersexual dominance relationships and the influence of leverage on the outcome of conflicts in wild bonobos (Pan paniscus). Behavioral Ecology and Sociobiology, 67(11), 1767–1780.
Symonds, M. R. E., & Moussalli, A. (2011). A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behavioral Ecology and Sociobiology, 65, 13–21.
Trivers, R. L. L. (1972). Parental investment and sexual selection. In B. Campbell (Ed.), Sexual selection and the descent of man, 1871–1971 (pp. 136–179). Chicago: Aldine Publishing Company.
van Noordwijk, M. & van Schaik, C. P. (2009). Intersexual food transfer among orangutans: Do females test makes for coercive tendency? Behavioral Ecology and Sociobiology, 63, 883–890.
Vullioud, C., Davidian, E., Wachter, B., Rousset, F., Courtiol, A., & Höner, O. P. (2019). Social support drives female dominance in the spotted hyaena. Nature Ecology and Evolution, 3(1), 71–76.
Wascher, C. A. F., Kulahci, I. G., Langley, E. J. G., & Shaw, R. C. (2018). How does cognition shape social relationships? Philosophical Transactions of the Royal Society B: Biological Sciences, 373(1756), 15–18.
White, F. J., & Wood, K. D. (2007). Female feeding priority in bonobos, Pan paniscus, and the question of female dominance. American Journal of Primatology, 69, 837–850.
Wright, P. C., King, S., Baden, A., & Jernvall, J. (2008). Aging in wild female lemurs: Sustained fertility with increased infant mortality. Interdisciplinary Topics in Gerontology, 36, 17–28.
Acknowledgments
We thank the Ankoatsifaka Research Station staff, whose work provided the majority of the data analyzed here. We would also like to thank the Madagascar government and Madagascar National Parks for permission to conduct this research and the University of Antananarivo and MICET for assistance in research facilitation. Further thanks go to three anonymous reviewers for their helpful comments on a previous version of this manuscript and to Professor Jo Setchell for her editorial assistance. Our research was financed by the University of Texas at Austin, The Leakey Foundation, Primate Conservation, Inc., and multiple private donors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Joanna M. Setchell
KMO is no longer affiliated with the University of Texas at Austin.
Electronic supplementary material
ESM 1
(DOCX 184 kb)
Rights and permissions
About this article
Cite this article
Voyt, R.A., Sandel, A.A., Ortiz, K.M. et al. Female Power in Verreaux’s Sifaka (Propithecus verreauxi) Is Based on Maturity, Not Body Size. Int J Primatol 40, 417–434 (2019). https://doi.org/10.1007/s10764-019-00096-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-019-00096-9