Abstract
Primate activity budgets are dictated by food availability and distribution; thus primates living in seasonal environments must adapt their behaviors to accommodate fluctuations in resources. Cat Ba langurs (Trachypithecus poliocephalus), a Critically Endangered Asian colobine and a member of the limestone langur group (francoisi superspecies group within genus Trachypithecus), live only in fragmented and disturbed habitats on Cat Ba Island, northeastern Vietnam. This study aimed to assess the behaviors and diet of Cat Ba langurs by group, age, sex, and season. We predicted they would have high rates of inactivity and foraging, low rates of social behaviors, with seasonal variation that reflects an energy-maximizing strategy. We conducted behavioral observations through scan sampling over an 11-month period and found that Cat Ba langurs spent a significant portion of their day inactive (57 %) followed by foraging (18 %), socializing (13 %), locomoting (10 %), and engaging in “other” behaviors (2 %). Their diet was made up primarily of leaves (83 %) followed by flowers (8 %), fruit (6 %), and stems (3 %). We found groups to differ in diet and activity, which is likely owing to differences in demographics and home range between groups. Seasonally, the animals ate more leaves and spent more time foraging in the dry season than the wet season, suggesting that they are energy maximizers. Cat Ba langurs have activity and dietary budgets similar to those of other limestone langurs, and respond to a presumed seasonal fluctuation in food availability similarly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
All animals have a finite amount of energy with which to fuel daily activities. This means they need to balance the energy exerted to find food with the costs of traveling to find that food. Thus, although physiology is undoubtedly a key factor in determining activity budgets, animals are also largely influenced by diet, making the two quite interdependent (Milton 1980, 1998). Animals that eat primarily leaves, such as colobines, must spend considerable time foraging, to process bulky leaves (Clutton-Brock and Harvey 1977; Decker 1994), and resting, to allow for the digestion of these high-fiber dietary items (Dasilva 1992; Edwards and Ullrey 1999; Kirkpatrick 2007). This also has consequences for social behavior; because leaves are nonmonopolizable and do not require cooperative defense, social behavior is rare for leaf eating primates (Newton and Dunbar 1994). For example, macaques (Macaca spp.), whose diet can include up to 87 % fruit (Thierry 2007), spend 15–32 % of their time inactive and up to a third of their day engaged in social behaviors (Alami et al. 2012; Hambali et al. 2012; Hanya 2004; Kumar et al. 2007), whereas colobines, whose diets can include up to 92 % leaves (Snaith and Chapman 2008), spend as much as 61 % of their time inactive and as little as 0 % of their day in social behaviors (Kirkpatrick 2007; McGraw 1998; Newton 1992; Phiapalath and Suwanwaree 2010; Rawson 2009).
In colobines, activity budgets are known to vary between groups, age classes, and sexes. Given the higher energetic and reproductive costs of adults compared to juveniles, adults often spend more time resting than individuals in younger age categories (Agmen 2014; Li 1992; Li and Rogers 2004; Newton 1992; Phiapalath and Suwanwaree 2010; Rawson 2009; Schneider et al. 2010). Owing to reproductive demands, females also spend significantly more time foraging than males (Clutton-Brock 1977; Dunbar and Dunbar 1988; Workman 2010a). They also spend significantly more time than males engaged in social behaviors (Clutton-Brock 1977; Fashing 2001; Fashing et al. 2007b; Newton 1992; Witte 2011; Workman 2010a), partially owing to the high rates of infant transfers (Jin et al. 2015; Kumar et al. 2005; Yao et al. 2012). Males, in contrast, spend more time being vigilant for predators and outside males (Agmen 2014; Fashing 2001; Workman Workman 2010a), resulting in high rates of inactivity among males (Clutton-Brock 1977; Fashing 2001; Fashing et al. 2007b).
Food availability plays a key role in determining the diet of a species, particularly on a seasonal basis. When food resources are seasonally limited, two typical responses allow animals to maintain energy budgets: conserving energy and being more sedentary, or exerting more energy for increased traveling and foraging than during times of year when food resources are abundant (Hemingway and Bynum 2005; Schoener 1971). The energy conservation, or time minimizing, strategy involves reducing foraging energy expenditure at times of low food availability, while energy maximizing strategies are those that increase time and energy spent trying to find sufficient resources (Schoener 1971). For example, king colobus (Colobus polykomos) spend more time resting and less time feeding when preferred seeds are not available (Dasilva 1992), suggesting that they are adopting an energy conservation strategy. Spectral tarsiers (Tarsius sp.: Gursky 2000) and Yunnan snub-nosed monkeys (Rhinopithecus bieti: (Grueter et al. 2013) forage more in the resource-poor season, with tarsiers also traveling more at this time, suggesting an energy maximizing strategy. Similarly, François’ (Trachypithecus francoisi) and white-headed (T. leucocephalus) langurs feed and travel more in the dry season (Huang et al. 2000, 2003; Yang et al. 2007; Zhou et al. 2007, 2010) when they consume more mature leaves (Hu 2007; Li and Rogers 2006; Zhou et al. 2009).
The limestone langur group (a superspecies group within the Trachypithecus genus) currently includes François’, Delacour’s (T. delacouri), Laotian (T. laotum), Hatinh (T. hatinensis), black (T. ebenus), white-headed, and Cat Ba (T. poliocephalus) langurs (Groves 2007). As with other colobines, limestone langurs have specialized digestive tracts, salivary glands, and dentition to handle the high amount of leaves in their largely folivorous diet (Bauchop 1975; Caton 1999; Kay and Hylander 1975; Oates and Davies 1994).
Cat Ba langurs are endemic to Cat Ba Island, northeastern Vietnam. The Cat Ba langur is listed as Critically Endangered on the IUCN Red List of Threatened Species (Bleisch et al. 2008) and has been consistently listed on the global 25 most endangered primate list since 2000 (Leonard et al. 2016). In the 1960s, the langur population may have been as large as 2400–2700, declining to ca. 135 individuals by 1999 because of intense hunting pressure (Nadler and Ha Thang Long 2000). Estimates after this time placed the population at only ca. 60–70 individuals (Schrudde et al. 2010; Stenke and Chu Xuan Canh 2004), but more recent reanalysis of monitoring data from the period suggests that the population was much lower than this, and may have been not much more than 40 at its low point (Leonard 2014). Based on 2014 figures, the global wild population of the species consists of fewer than 70 individuals and includes two breeding subpopulations (one, containing ca. 31 individuals based in an area known as the sanctuary and the other, consisting of 17–24 individuals, is in the Cua Dong area) and one nonbreeding subpopulation (five adult females) on Cat Ba Island (Lees et al. 2014). There is also an ex situ breeding group of five individuals housed at the Endangered Primate Rescue Centre in Cuc Phuong National Park. The three in situ Cat Ba langur subpopulations are completely isolated from one another. Despite their vulnerability to extinction from extremely low population size (Lees et al. 2014), only one published, peer-reviewed study has been conducted on their behavior, which resulted from only 22 days of observations (Schneider et al. 2010).
This study aims to explore the activity and dietary budget of two groups of Cat Ba langurs over an 11-mo period to provide the first detailed description of their behavior that can be compared to closely related species. We first assess overall behavior and dietary differences across groups, ages, and sexes and then explore seasonal changes in activity and dietary budgets. Based on studies of other limestone langurs (Hu 2007; Huang et al. 2000, 2008; Li et al. 2003, 2009; Workman 2010a; Workman and Le Van Dung 2009; Yin et al. 2011; Zhou et al. 2010, 2011, 2013), we predict that leaves would make up most of the Cat Ba langur diet, and, as a result, we would see the majority of the langurs’ day spent inactive and feeding to cope with this low-quality diet. We also predict that, as a result, social behavior will be minimal, as with most folivores. In addition, we assume that resource availability changes throughout the year, as it does in geographically similar habitats (Hu 2007; Li and Rogers 2006; Workman 2010a; Zhou et al. 2009), which leads us to predict that both activity and dietary budgets will change seasonally in accordance with the changing food supply (Huang et al. 2003; Zhou et al. 2007). Based on studies of other limestone langurs (Huang et al. 2000, 2003; Yang et al. 2007; Zhou et al. 2007, 2010), we hypothesize that Cat Ba langurs will be energy maximizers, and predict that they increase energy expenditure through increased foraging and travel times at times of low resource availability to find enough food to meet energy demands.
Methods
Study Site
Cat Ba Island (20°42′–20°55′N; 106°54′–107°10′E) is the largest of the hundreds of limestone karst islands in the Cat Ba archipelago in Ha Long Bay, northeastern Vietnam. In this part of Vietnam, there is a distinct, hot, rainy season from May to October (when 80–90 % of the year’s rain falls: 150–160 cm; Nguyen Quan Van et al. 2010), and a distinct, cool, dry season from November to April; we term these simply wet and dry seasons, respectively, in this study. Established in 1986, roughly half of the 285-km2 island is designated as National Park, a subset of which is a langur sanctuary, a strictly protected core zone within the National Park created for langur protection (Schrudde et al. 2010). Because of the porous nature of limestone karst, there are no permanent freshwater pools on the island (Sterling et al. 2006). Cat Ba Island has tropical moist deciduous and karst forests (Nguyen Quan Van et al. 2010; Nisbett and Ciochon 1993); vegetative growth is stunted, shrubby, and discontinuous (Day and Chenoweth 2004; Liu et al. 2004; Nisbett and Ciochon 1993); and historical logging has left little primary forest remaining (Nadler and Ha Thang Long 2000).
Study Groups
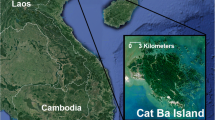
This study focused on the Cua Dong breeding population, located on a peninsula in the southeast corner of Cat Ba and consisting of two social groups. The two groups (Table I) have neighboring home ranges with a slight overlap (Fig. 1). They live in auditory proximity to floating villages, and were habituated to fishing boats and people’s presence at ground level. The larger group (group A, N = 10–13) has a home range that could possibly be expanded into mainland territory, whereas the smaller group’s (group B, N = 7) home range is limited by ocean front and the home range of the larger group (Fig. 1).
Map of home ranges (outlined in white) for Cat Ba langurs (Trachypithecus poliocephalus) living in Cua Dong (southeastern Cat Ba Island, northeastern Vietnam) February 2014–January 2015; group A (10–13 individuals) occupies the northern range and group B (7 individuals) the southern range. Map source: ArcGIS.
Females are identifiable by white patches on their inner thighs and, if parous, pendulous nipples. We identified adults as being full size with species-specific pelage, and immature individuals (ranging from newborns to juveniles) by the state of their natal coat (with newborns being bright orange, and juveniles being mostly adult colored but with orange that extends further down their neck and shoulders than adults).
Data Collection
We collected data from February 2014 to January 2015, with time spent in visual contact ranging from 12.5 to 72.3 h/mo (Table II). As it is very difficult to traverse limestone karst terrain, our observations were made from a boat, following the protocol of previous studies in similar environments (Agmen 2014; Schneider et al. 2010; Workman 2010a). We used stabilizing binoculars to identify the group and age–sex class of individuals from a distance of 30–250 m. We used instantaneous scan sampling to record behaviors at 10-min intervals (Altmann 1974; Martin and Bateson 2009); a total of 245–1655 individual scans were collected each month, with a monthly range of 2.8–4.0 individuals scanned per 10-min interval (Table II). As individual identification was not possible, we were able to note only the age–sex class of each individual. To avoid repeat sampling of the same individual in the same scan, we started at the center of the group and then spiraled out from that point. To ensure the scans were instantaneous, we recorded all information within roughly 30–40 s.
We recorded activities using an ethogram adapted from closely related species (Agmen 2014; Li and Rogers 2004; Workman and Schmitt 2012), and then recoded all behaviors into bigger categories for analysis. Categories of behaviors included inactivity (sunbathing, resting, scanning/vigilance, and autogrooming), foraging (manipulating, searching for, and ingesting food), social behavior (grooming, social play, vocalizing, same- and opposite-sex mounting, embracing, young transferring, presenting, harassing a mounting pair, submission, displacement, and aggression), locomotion (quadrupedal walking or running, climbing, leaping, dropping, and arm swinging), and “other” behaviors (nonsocial play, exploring, struggle against being held, maternal rejection, and masturbation). We classified dietary items as leaves (both young and mature leaves as it was difficult to differentiate between the two), flowers (blossoms), fruits (both immature and ripe fruit; may include unseen seed consumption), or stems (herbaceous stems connecting leaves to branches or nonleafy parts of vines).
We were often not able to observe langurs because of dense foliage, which is a common problem for primate researchers, particularly in this habitat type (Agmen 2014; Huang et al. 2003; Li and Rogers 2004, 2006; Li et al. 2009; Rawson 2009; Workman and Schmitt 2012). This may have created a bias in our data against rare or subtle behaviors being recorded, but we obtained the best conservative estimate of behavior, making this study comparable to other limestone langur studies.
Statistical Analysis
We calculated activity and dietary budgets based on 541 contact hours, across 180 days of observation spanning 11 mo in the field (Table II). Activity and dietary budgets are presented as the proportion of scans that included each specific behavior category or the proportion of feeding records that included a specific dietary item. Owing to the irregular distribution of behaviors and consumed food items, we first assessed group, age, sex, and seasonal differences with a Fisher’s Exact test (FET). If we found significant differences with contingency tables (P < 0.05), we carried out post hoc analyses by creating binomials for behavior and food items, then by running each behavior or food item separately within a generalized linear model (GLM). For seasonal analyses, as all categories tested (group, age, and sex) show a similar behavioral and dietary pattern across seasons (Hendershott unpubl. data), they are grouped together. We removed newborns from all analyses because their behaviors are nonindependent. While the foraging category includes water drinking (N = 26), this was not analyzed as part of the dietary budget. We used SPSS 23 for Windows® for all analyses, with significance set to P < 0.05 for two-tailed tests.
Ethical Note
We obtained ethics approval from the Australian National University Animal Experimentation Ethics Committee (Animal Ethics Protocol Number: A2013/18) for July 1, 2013–June 30, 2016. We received research permissions from Cat Ba National Park and the Hai Phong People’s Committee and designed all data collection so as to minimize the impact on the langurs.
Results
Overall Activity and Dietary Budget
Out of a total of 10,879 scans in which behavior could be identified (excluding newborns), inactivity was the most common behavior, occupying 57 % of the overall activity budget. Foraging was the next most common activity, at 18 %, followed by social behaviors (13 %), locomotion (10 %), and “other” behaviors (2 %). Out of a total of 746 scans in which feeding was observed and consumed items identified, the majority were leaves (83 %), followed by flowers (8 %), fruit (6 %), and finally stems (3 %). The langurs were seen to drink ocean water, lick rocks, and lick cavities of rock pools, spending 0.3 % of scans engaged in these drinking/licking behaviors.
Group Differences
The two groups differed in their activity (FET: P < 0.001, N = 10,809; Fig. 2) and dietary (FET: P < 0.001, N = 743; Fig. 3) budgets. The larger group, A, spent significantly more time inactive (GLM: χ2 = 23.771, df = 1, P < 0.001, N = 6111), engaging in social (GLM: χ2 = 5.615, df = 1, P = 0.018, N = 1409) and “other” (GLM: χ2 = 49.650, df = 1, P < 0.001, N = 245) behaviors. Their diet included significantly more leaves (GLM: χ2 = 5.695, df = 1, P = 0.017, N = 615) and almost three times the fruit (GLM: χ2 = 8.213, df = 1, P = 0.004, N = 46) of group B. The smaller group, B, spent significantly more time foraging (GLM: χ2 = 86.440, df = 1, P < 0.001, N = 2000) and locomoting (GLM: χ2 = 6.278, df = 1, P = 0.012, N = 1044), and their diet included significantly more flowers (GLM: χ2 = 21.838, df = 1, P < 0.001, N = 60) than that of group A. Groups did not differ in their consumption of stems (GLM: χ2 = 2.374, df = 1, P = 0.123, N = 22).
Percentage of activity budget (± SE) for each behavior by two groups (group A, N = 10–13; group B, N = 7) of Cat Ba langurs (Trachypithecus poliocephalus) living in Cua Dong (southeastern Cat Ba Island, northeastern Vietnam). An asterisk indicates significant group differences for that activity category as demonstrated through post hoc analyses, which were conducted following an initial analysis for activity budgets that indicated significant group variation.
Percentage of dietary budget (± SE) for each item consumed by two groups (group A, N = 10–13; group B, N = 7) of Cat Ba langurs (Trachypithecus poliocephalus) living in Cua Dong (southeastern Cat Ba Island, northeastern Vietnam). An asterisk indicates significant group differences for that dietary category as demonstrated through post hoc analyses, which were conducted following an initial analysis for dietary budgets that indicated significant group variation.
Age Differences
Behaviors were significantly different between age classes (N = 10186; P < 0.001, FET; Fig. 4), and post hoc analyses indicate that this is true for each individual behavior (inactivity GLM: χ2= 89.276, df = 1, P < 0.001, N = 5722; foraging GLM: χ2 = 38.605, df = 1, P < 0.001, N = 1875; social GLM: χ2 = 28.724, df = 1, P < 0.001, N = 1358; locomotion GLM: χ2 = 64.647, df = 1, P < 0.001, N = 985; “other” GLM: χ2 = 106.283, df = 1, N = 246). There was no significant effect of age on dietary budgets (FET: P = 0.750, N = 716). The general pattern is for social behaviors to increase with age, while locomotion and “other” behaviors decrease with age. Adults spend the most time inactive and subadults forage most often.
Percentage of activity budget (± SE) across ages (adult, subadult, young) for each behavior of Cat Ba langurs (Trachypithecus poliocephalus) living in Cua Dong (southeastern Cat Ba Island, northeastern Vietnam) February 2014–January 2015. An asterisk indicates significant age differences for that activity category as demonstrated through post hoc analyses, which were conducted following an initial analysis for activity budgets that indicated significant age variation.
Sex Differences
Behaviors were significantly different between sex classes (FET: P < 0.001, N = 7289; Fig. 5): post hoc analyses indicate that this pattern holds for inactivity (GLM: χ2 = 10.994, df = 1, P = 0.001, N = 4164), foraging (GLM: χ2 = 6.051, df = 1, P = 0.014, N = 1122), and social behaviors (GLM: χ2 = 3.892, df = 1, P = 0.049, N = 1056). There was no significant effect of sex on locomotion (GLM: χ2 = 1.628, df = 1, P = 0.202, N = 726), “other” behaviors (GLM: χ2 = 3.155, df = 1, P = 0.076, N = 221) or dietary budgets (N = 466, P = 0.483, FET). Males were inactive significantly more than females, while females forage and socialize more than males.
Percentage of activity budget (± SE) across sexes for each behavior of Cat Ba langurs (Trachypithecus poliocephalus) living in Cua Dong (southeastern Cat Ba Island, northeastern Vietnam) February 2014–January 2015. An asterisk indicates significant sex differences for that activity category as demonstrated through post hoc analyses, which were conducted following an initial analysis for activity budgets that indicated significant sex variation.
Seasonal Differences
Activity (FET: P < 0.0001, N = 10879; Fig. 6) and dietary (FET: P < 0.0001, N = 746; Fig. 7) budgets varied significantly by season. In the wet season (May–October), the langurs were less active (GLM: χ2 = 12.847, df = 1, P < 0.001, N = 6163) and spent more time engaged in social behaviors (GLM: χ2 = 23.358, df = 1, P < 0.001, N = 1413) than in the dry season; at this time they also consumed significantly more fruit (GLM: χ2 = 36.797, df = 1, P < 0.001, N = 46). In the dry season (November–April), Cat Ba langurs spent significantly more time foraging (GLM: χ2 = 55.130, df = 1, P < 0.001, N = 2007), engaging in “other” behaviors (GLM: χ2 = 31.187, df = 1, P < 0.001, N = 246), and consuming leaves (GLM: χ2 = 17.532, df = 1, P < 0.001, N = 618) than in the wet season. Locomotion rates (GLM: χ2 = 1.282, df = 1, P = 0.257, N = 1050) and flower (GLM: χ2 = 0.284, df = 1, P = 0.594, N = 60) and stem (GLM: χ2 = 0.017, df = 1, P = 0.896, N = 22) consumption did not differ significantly between seasons.
Percentage of activity budget (± SE) for each behavior of Cat Ba langurs (Trachypithecus poliocephalus) living in Cua Dong (southeastern Cat Ba Island, northeastern Vietnam) in the wet (May–October) and dry (November–April) seasons February 2014–January 2015. An asterisk indicates significant seasonal differences for that activity category as demonstrated through post hoc analyses, which were conducted following an initial analysis for activity budgets that indicated significant seasonal variation.
Percentage of dietary budget (± SE) for each item consumed by Cat Ba langurs (Trachypithecus poliocephalus) living in Cua Dong (southeastern Cat Ba Island, northeastern Vietnam) in the wet (May–October) and dry (November–April) seasons of February 2014–January 2015. An asterisk indicates significant differences between seasons for that consumed item as demonstrated through post hoc analyses, which were conducted following an initial analysis for dietary budgets that indicated significant seasonal variation.
Discussion
We found the activity budgets for Cat Ba langurs to be similar to those of other, closely related limestone langurs, with rates of inactivity, foraging, and locomotion fitting well within the range for the group (Table III). The high percentage of time inactive supported our prediction, and is likely due to the langurs’ emphasis on leaf-eating (Clutton-Brock and Harvey 1977; Dasilva 1992; Kirkpatrick 2007; Newton 1992; Oates 1977), as high rates of inactivity are expected given the processing time required for breaking down fibrous cell walls into usable energy (Edwards and Ullrey 1999). Feeding is also expected to dominate folivore activity budgets, given that leaf eating requires more processing time compared to other food sources (Clutton-Brock and Harvey 1977; Decker 1994). Our findings support this as leaves were the most common food item ingested and inactivity was the most common behavior, followed by feeding.
As we predicted, time spent in social behaviors is low for Cat Ba langurs compared to fruit-eating species such as macaques. We found that social behavior occupied a similar portion of the activity budget as those reported for white-headed langurs, but much higher than seen in Delacour’s or François’ langurs (Table III). Often social interactions of Asian colobine females are centered on newborns, as they are known to interact because of their attraction to young, and high rates of young transfer (Jin et al. 2015; Kumar et al. 2005; Yao et al. 2012). Accordingly, the high social budget of Cat Ba langurs in our study may be a result of the high number of young present in the study groups. This may have also resulted in higher levels of social play than in other studies, as we also included social play in the social behavior category.
As with activity, the dietary budgets of Cat Ba langurs fell within the range of other limestone langurs (Table IV). Leaves, especially young leaves, are an important source of protein for folivores (Hladik 1978), especially as colobines select leaves with a high protein-to-fibre ratio (Fashing et al. 2007a; Milton 1979; Workman 2010b; Workman and Le Van Dung 2009). The combination of a specialized digestive tract (Caton 1999; Oates and Davies 1994), and the nutritional value, and availability, of leaves explains the extremely high proportion of leaf eating by Cat Ba langurs. However, despite their moniker, leaf-eating colobines consume a wide range of plant parts, e.g., fruit, flowers, seeds, bark, gum and sap, stems and pith, and roots; fungi, e.g., lichen and mushrooms; and animal matter, e.g., insect galls (Kirkpatrick 2007), which we saw in this study, with langurs eating fruits/seeds, flowers, and stems.
Compared to other limestone langurs, we found that Cat Ba Langurs include a high amount of flowers in their dietary budget. Flowers generally have a higher percentage of water (Oftedal 1991) and nitrogen (Waterman and Kool 1994) than mature leaves, and are also relatively high in copper (Behie and Pavelka 2012). The high amount of flower eating may therefore be a method of meeting macronutrient or mineral needs or to serve as a way to increase water consumption.
Fruits are an energy-rich resource, but they contain less protein than leaves (Oftedal 1991; Waterman and Kool 1994). In our study Cat Ba langurs primarily ate green, unripened fruits, which is common among colobines because their gut flora cannot handle the large amount of sugar present in mature fruits (Davies et al. 1999; Waterman and Kool 1994; Workman 2010a; Workman and Le Van Dung 2009). We also found that Cat Ba langurs ate more fruit in the wet season than in the dry season. Similarly, white-headed (Li and Rogers 2006) and Delacour’s (Workman 2010a) langurs eat more fruit at this time of year, when it is more available in karst habitats (Workman 2010a; Zhou et al 2009), compared to times when food is scarce. This suggests that, as with closely related species, Cat Ba langurs are eating fruit in accordance with its availability.
We found group differences in activity and dietary budgets to be likely the result of differences in group size, demographics, and variation between home range size and quality between the two groups. Group A had a higher ratio of adults than group B (46–60 % vs. 43 %), which may explain the higher rate of inactivity and social behaviors. Higher rates of social behavior in group A may also be due to the higher number of females in this group compared to group B (58–70 % vs. 57 %). Whereas other studies of Asian colobines show higher rates of social behaviors in young individuals (Agmen 2014; Li and Rogers 2004; Newton 1992; Phiapalath and Suwanwaree 2010; Schneider et al. 2010), our results found higher social behaviors among older individuals and females. As adult females interact socially in attempts to hold or groom newborns and infants (Jin et al. 2015; Kumar et al. 2005; Yao et al. 2012), it stands to reason that group A’s larger proportion of adult females and newborns may explain the higher degree of social interaction in that group.
We found group B to forage more than group A, which may be due to the fact that only group B had subadults, the group found to forage for the highest proportion of scans. This could, however, also represent differences in food tree density between the ranges of the two groups; for example, white-headed langur groups in poor quality habitat fed more and engaged in less social behavior (Li and Rogers 2004). Group B’s higher population density (0.227 ind/ha) compared to group A’s (0.120 ind/ha) may necessitate group B to travel farther to find adequate food resources, as in other taxa (Fashing et al. 2007b; van Schaik et al. 1983; van Schaik and van Noordwijk 1988).
In other limestone langur habitats, fewer preferred foods and fruits are available in the dry than in the wet season (Li and Rogers 2006; Workman 2010a; Zhou et al. 2009). Primates have several approaches to dealing with decreased resource abundance. One approach is to reduce energy expended in search of food, and the other is to put more time into increasing energy intake compared to when food is abundant (Hemingway and Bynum 2005; Schoener 1971). Supporting our prediction, our results suggest that more effort may be needed for foraging in a resource poor environment, as foraging increases, and social behaviors and inactivity decrease, in the dry season compared to during the wet season. This has been documented for other limestone langurs (Huang et al. 2000, 2003; Yang et al. 2007; Zhou et al. 2007, 2010). For example, François’ langurs spend significantly more time feeding in the dry season than in the wet season (26 % vs. 19 %) (Zhou et al. 2007), and white-headed langurs spend significantly more time inactive in the wet season compared to the dry season (84 % vs. 67 %) (Huang et al. 2003). This is most likely due to the poorer quality foods available in the dry season (more nutritious young leaves are more common in the wet season than the dry season: Workman 2010a), when langurs use fallback foods such as mature leaves (Hu 2007; Li and Rogers 2006; Workman 2010a; Zhou et al. 2009). Conversely, in the wet summer season, when more preferred foods are available than in the dry season, François’ and white-headed langurs rest more (53–84 % wet season vs. 40–67 % dry season), with François’ langurs also grooming more at this time (3 % wet season vs. 2 % dry season) (Huang et al. 2003; Yang et al. 2007; Zhou et al. 2007, 2010).
Cat Ba langurs eat more leaves in the dry season than in the wet season, suggesting that they are falling back on the use of leaves when other items are less available. The pattern of increasing activity levels (including foraging and traveling) in the dry season, and reducing resting time (including inactivity and socializing), is considered an energy-maximizing strategy. Cat Ba langurs, and several other limestone langurs (Huang et al. 2000, 2003; Yang et al. 2007; Zhou et al. 2007, 2010), can therefore be considered energy maximizers.
Conclusion
Cat Ba langurs had activity and dietary budgets similar to closely related species living on limestone karst throughout Southeast Asia and southern China. As we predicted, the majority of their day was spent inactive and foraging, with relatively little social time. Cat Ba langurs had a high rate of social behavior compared to other limestone langurs, possibly due to the high percentage of immature individuals in one of the groups (which serve as an attractant for transfers of young and interactions among adult females). Alternatively, this may be due to the variable definitions of “social” behavior across studies. Their diet was predominantly leaves, but other foods played an important role throughout the year, including relatively high rates of flower consumption. Groups showed significant differences in activity patterns due to differences in demographic makeup, and in diet, likely due to differences in home range quality. To accommodate the presumed drop in preferred, valuable resources in the dry season, activity and dietary budgets change for Cat Ba langurs, as we had predicted. The monkeys fall back on a higher rate of leaf eating and foraging in general in the dry season than the wet season, at the expense of inactivity and social time. This qualifies this species as energy maximizers.
Obtaining information on the activity and dietary budgets of species affected by habitat change can allow us to better determine how these threats are impacting behavior and ecology, with implications for long-term survival. This information can, in turn, be used to improve conservation planning. As Cat Ba langurs have activity budgets similar to those of other limestone langurs, this suggests that, although they may be negatively affected by anthropogenically altered habitats, their behaviors are not drastically different from those of animals living in similar circumstances, i.e., they are not putting excessive energy into traveling or feeding compared to other animals in similar habitats. Similarly, Cat Ba langurs respond to seasonal reductions in preferred food availability like other limestone langurs, by exerting more energy, i.e., increasing foraging and activity and decreasing socializing, into finding less preferred foods, i.e., leaves, in the dry season compared to the wet season. Thus, the Cat Ba langur activity and dietary budgets are similar to those of other limestone langurs living in highly fragmented habitats, suggesting they are not more energetically stressed than these other Endangered and Critically Endangered species.
However, there are no limestone langurs living in pristine habitat, so a comparison to their “natural” state is not possible. Nonetheless, monitoring of activity and dietary budgets helps to document the effects of humans on nonhuman primates, and we hope that this study can serve as a baseline for future assessment of how human disturbance affects this Critically Endangered species.
References
Agmen, F. (2014). Conservation strategies for Delacour’s langur (Trachypithecus delacouri) in Vietnam: Behavioural comparisons and reviewing a release. PhD thesis, Australian National University.
Alami, A. E., van Lavieren, E., Rachida, A., & Chait, A. (2012). Differences in activity budgets and diet between semiprovisioned and wild-feeding groups of the endangered Barbary macaque (Macaca sylvanus) in the Central High Atlas Mountains, Morocco. American Journal of Primatology, 74, 210–216.
Altmann, J. (1974). Observational study of behaviour: Sampling methods. Behaviour, 49(3–4), 227–267.
Bauchop, T. (1975). Digestion of leaves in vertebrate arboreal folivores. In G. G. Montgomery (Ed.), The ecology of arboreal folivores: A symposium held at the conservation and research center, National Zoological Park, Smithsonian Institution, May 29–31, 1975 (pp. 193–204). Washington, DC: Smithsonian Institution Press.
Behie, A. M., & Pavelka, M. S. M. (2012). The role of minerals in food selection in a black howler monkey (Alouatta pigra) population in Belize following a major hurricane. American Journal of Primatology, 74, 1054–1063.
Bleisch, B., Le Xuan Canh, Covert, B., & Yongcheng, L. (2008). Trachypithecus poliocephalus. In Version 2015.2 IUCN Red List of Threatened Species. Retrieved from: www.icunredlist.org. Accessed 3 June 2015.
Caton, J. M. (1999). Digestive strategy of the Asian colobine genus Trachypithecus. Primates, 40(2), 311–325.
Clutton-Brock, T. H. (1977). Some aspects of intraspecific variation in feeding and ranging behaviour in primates. In T. H. Clutton-Brock (Ed.), Primate ecology: Studies of feeding and ranging behaviour in lemurs, monkeys and apes (pp. 539–556). London: Academic Press.
Clutton-Brock, T. H., & Harvey, P. H. (1977). Species differences in feeding and ranging behaviour in primates. In T. H. Clutton-Brock (Ed.), Primate ecology: Studies of feeding and ranging behaviour in lemurs, monkeys and apes (pp. 557–584). London: Academic Press.
Dasilva, G. L. (1992). The western black-and-white colobus as a low-energy strategist: Activity budgets, energy expenditure and energy intake. Journal of Animal Ecology, 61(1), 79–91.
Davies, A. G., Oates, J. F., & Dasilva, G. L. (1999). Patterns of frugivory in three West African colobine monkeys. International Journal of Primatology, 20(3), 327–357.
Day, M. J., & Chenoweth, M. S. (2004). The karstlands of Trinidad and Tobago, their land use and conservation. The Geographical Journal, 170(3), 256–266.
Decker, B. S. (1994). Effects of habitat disturbance on the behavioral ecology and demographics of the Tana River red colobus (Colobus badium rufomitratus). International Journal of Primatology, 15(5), 703–737.
Dunbar, R. I. M., & Dunbar, P. (1988). Maternal time budgets of gelada baboons. Animal Behaviour, 36, 970–980.
Edwards, M. S., & Ullrey, D. E. (1999). Effect of dietary fiber concentration on apparent digestibility and digesta passage in non-human primates. II. Hindgut- and foregut-fermenting folivores. Zoo Biology, 18, 537–549.
Fashing, P. J. (2001). Activity and ranging patterns of guerezas in the Kakamega Forest: Intergroup variation and implications for intragroup feeding competition. International Journal of Primatology, 22(4), 549–577.
Fashing, P. J., Dierenfeld, E. S., & Mowry, C. B. (2007a). Influence of plant and soil chemistry on food selection, ranging patterns, and biomass of Colobus guereza in Kakamenga Forest, Kenya. International Journal of Primatology, 28(3), 673–703.
Fashing, P. J., Mulindahabi, F., Gakima, J. B., Masozera, M., Plumptre, A. J., & Nguyen, N. (2007b). Activity and ranging patterns of Colobus angolensis ruwenzorii in Nyungwe Forest, Rwanda: Possible costs of large group size. International Journal of Primatology, 28(3), 529–550.
Groves, C. P. (2007). Speciation and biogeography of Vietnam’s primates. Vietnamese Journal of Primatology, 1, 27–40.
Grueter, C. C., Li, D., Ren, B., & Li, M. (2013). Overwintering strategy of Yunnan snub-nosed monkeys: Adjustments in activity scheduling and foraging patterns. Primates, 54(2), 125–135.
Gursky, S. (2000). Effects of seasonality on the behavior of an insectivorous primates, Tarsius spectrum. International Journal of Primatology, 21(3), 477–495.
Hambali, K., Ismail, A., & Md-Zain, B. M. (2012). Daily activity budget of long-tailed macaques (Macaca fascicularis) in Kuala Selangor Nature Park. International Journal of Primatology, 12(4), 47–52.
Hanya, G. (2004). Seasonal variations in the activity budget of Japanese macaques in the coniferous forest of Yakushima: Effects of food and temperature. American Journal of Primatology, 63, 165–177.
Hemingway, C. A., & Bynum, N. (2005). The influence of seasonality on primate diet and ranging. In D. K. Brockman & C. P. van Schaik (Eds.), Seasonality in primates: Studies of living and extinct human and non-human primates (pp. 57–104). Cambridge: Cambridge University Press.
Hladik, C. M. (1978). Adaptive strategies of primates in relation to leaf-eating. In G. G. Montgomery (Ed.), The ecology of arboreal folivores: A symposium held at the conservation and research center, National Zoological Park, Smithsonian Institution, May 29–31, 1975 (pp. 373–395). Washington, DC: Smithsonian Institution Press.
Hu, G. (2007). Socioecology and behavioural flexibility of François’ langur (Trachypithecus françoisi) in Mayanghe Nature Reserve, Southwest China. PhD thesis, Australian National University.
Huang, C., Sun, R., Xue, Y., Wei, S., & Li, Y. (2000). The research on dietary and feeding time budget of white-headed leaf monkey. Acta Anthropologica Sinica, 19(1), 65–72.
Huang, C., Wei, F., Li, M., Li, Y., & Sun, R. (2003). Sleeping cave selection, activity pattern and time budget of white-headed langurs. International Journal of Primatology, 24(4), 813–824.
Huang, C., Wu, H., Zhou, Q., Li, Y., & Cai, X. (2008). Feeding strategy of François’ langur and white-headed langur at Fusui, China. American Journal of Primatology, 70, 320–326.
Jin, T., Wang, D., Pan, W., & Yao, M. (2015). Nonmaternal infant handling in wild white-headed langurs (Trachypithecus leucocephalus). International Journal of Primatology, 36(2), 269–287.
Kay, R. F., & Hylander, W. L. (1975). The dental structure of mammalian folivores with special reference to primates and phalangeroidea (Marsupialia). In G. G. Montgomery (Ed.), The Ecology of arboreal folivores: A symposium held at the conservation and research center, National Zoological Park, Smithsonian Institution, May 29–31, 1975 (pp. 173–191). Washington, DC: Smithsonian Institution Press.
Kirkpatrick, R. C. (2007). The Asian colobines: diversity among leaf-eating monkeys. In C. Campbell, A. Fuentes, K. MacKinnon, S. Bearder, & R. Stumpf (Eds.), Primates in perspective (2nd ed., pp. 186–200). Oxford: Oxford University Press.
Kumar, A., Solanki, G. S., & Sharma, B. K. (2005). Observations on parturition and allomothering in wild capped langur (Trachypithecus pileatus). Primates, 46, 215–217.
Kumar, R. S., Mishra, C., & Sinha, A. (2007). Foraging ecology and time-activity budget of the Arunachal macaque Macaca munzala—a preliminary study. Current Science, 93(4), 532–539.
Lees, C., Rawson, B. M., Behie, A. M., Hendershott, R. L., & Leonard, N. (2014). Preliminary population viability analysis for the critically endangered Cat Ba langur (Trachypithecus poliocephalus). Hanoi: IUCN SSC Conservation Breeding Specialist Group, Fauna & Flora International.
Leonard, N. (2014). A summary of the Hang Cai langur population & the voting options for the second TWG meeting. Cat Ba Langur Conservation Project, unpublished report.
Leonard, N., Passaro, R. J., Schrudde, D., Stenke, R., Phan, T. D., Raffel, M., et al. (2016). Golden-headed or Cat Ba langurs Trachypithecus poliocephalus poliocephalus (Trouessart 1911) Vietnam. In C. Schwitzer, R. A. Mittermeier, A. B. Ryaldns, F. Chiozza, & E. A. Williamson (Eds.), Primates in peril: The world’s 25 most endangered primates 2014–2016 (pp. 56–57). Arlington, VA: IUCN SSC Primate Specialist Group, International Primatological Society, Conservation International, and Bristole Zoological Society.
Li, Z. (1992). Time budgets of Presbytis leucocephalus. Acta Theriologica Sinica, 12(1), 7–13.
Li, Z., & Rogers, E. (2004). Habitat quality and activity budgets of white-headed langurs in Fusui, China. International Journal of Primatology, 25(1), 41–54.
Li, Z., & Rogers, M. E. (2006). Food items consumed by white-headed langurs in Fusui, China. International Journal of Primatology, 27(6), 1551–1567.
Li, Z., Wei, Y., & Rogers, E. (2003). Food choice of white-headed langurs in Fusui, China. International Journal of Primatology, 24(6), 1189–1205.
Li, Y., Ding, P., Huang, C., Jiang, P., & Wood, C. (2009). Dietary response of a group of François’ langur Trachypithecus francoisi in a fragmented habitat in the county of Fusui, China: Implications for conservation. Wildlife Biology, 15, 137–146.
Liu, Z., Groves, C., Yuan, D., & Meiman, J. (2004). South China karst aquifer storm-scale hydrochemistry. Ground Water, 42(4), 491–499.
Martin, P., & Bateson, P. (2009). Measuring behaviour: An introductory guide (3rd ed.). Cambridge: Cambridge University Press.
McGraw, W. S. (1998). Posture and support use of old world monkeys (Cercopithecidae): The influence of foraging strategies, activity patterns, and the spatial distribution of preferred food items. American Journal of Primatology, 46(3), 229–250.
Milton, K. (1979). Factors influencing leaf choice by howler monkeys: A test of some hypotheses of food selection by generalist herbivores. The American Naturalist, 114(3), 362–378.
Milton, K. (1980). The foraging strategy of howler monkeys: A study in primate economics. New York: Columbia University Press.
Milton, K. (1998). Physiological ecology of howlers (Alouatta): Energetic and digestive considerations and comparison with the Colobinae. International Journal of Primatology, 19, 513–548.
Nadler, T., & Long, H. T. (2000). The Cat Ba langur: Past, present, and future: The definitive report on Trachypithecus poliocephalus, the world’s rarest primate. Hanoi: Forestry Inventory and Planning Institute.
Newton, P. N. (1992). Feeding and ranging patterns of forest Hanuman langurs (Presbytis entellus). International Journal of Primatology, 13(3), 245–285.
Newton, P. N., & Dunbar, R. I. M. (1994). Colobine monkey society. In A. G. Davies & J. F. Oates (Eds.), Colobine monkeys: Their ecology, behaviour and evolution (pp. 311–346). Cambridge: Cambridge University Press.
Nisbett, R. A., & Ciochon, R. L. (1993). Primates in northern Viet-Nam: A review of the ecology and conservation status of extant species, with notes on pleistocene localities. International Journal of Primatology, 14(5), 765–795.
Oates, J. F. (1977). The guereza and its food. In T. H. Clutton-Brock (Ed.), Primate ecology: Studies of feeding and ranging behaviour in lemurs, monkeys and apes (pp. 276–321). New York: Academic Press.
Oates, J. F., & Davies, A. G. (1994). What are the colobines? In A. G. Davies & J. F. Oates (Eds.), Colobine monkeys: Their ecology, behaviour and evolution (pp. 1–9). Cambridge: Cambridge University Press.
Oftedal, O. T. (1991). The nutritional consequences of foraging in primates: The relationship of nutrient intakes to nutrient requirements. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 334, 161–170.
Phiapalath, P., & Suwanwaree, P. (2010). Time budget and activity of red-shanked douc langurs (Pygathrix nemaeus) in Hin Namno National Protected Area, Lao PDR. In T. Nadler, B. M. Rawson, & N. Van Thinh (Eds.), Conservation of primates in Indochina (pp. 171–178). Hanoi: Frankfurt Zoological Society, Endangered Primate Rescue Center, Cuc Phuong National Park, and Conservation International, Indo-Burma Program.
Rawson, B. M. (2009). The socio-ecology of the black-shanked douc (Pygathrix nigripes) in Mondulkiri Province, Cambodia. PhD thesis, Australian National University.
Schneider, I., Tielen, I. H. M., Rode, J., Levelink, P., & Schrudde, D. (2010). Behavioural observations and notes on the vertical ranging pattern of the critically endangered Cat Ba langur (Trachypithecus poliocephalus poliocephalus) in Vietnam. Primate Conservation, 25, 111–117.
Schoener, T. W. (1971). Theory of feeding strategies. Annual Review of Ecology and Systematics, 2, 369–404.
Schrudde, D., Levelink, P., & Raffel, M. (2010). Protection of the Cat Ba langur (Trachypithecus [poliocephalus] poliocephalus) through the ‘Cat Ba langur Conservation Project’. In T. Nadler, B. M. Rawson, & N. Van Thinh (Eds.), Conservation of primates in Indochina (pp. 237–243). Hanoi: Frankfurt Zoological Society, Endangered Primate Rescue Center, Cuc Phuong National Park, and Conservation International, Indo-Burma Program.
Snaith, T. V., & Chapman, C. A. (2008). Red colobus monkeys display alternative behavioral responses to the costs of scramble competition. Behavioral Ecology, 19, 1289–1296.
Stenke, R., & Canh, C. X. (2004). The golden-headed langur (Trachypithecus poliocephalus poliocephalus) on Cat Ba Island: Status, threat factors and recovery options. In T. Nadler, U. Streicher, & H. T. Long (Eds.), Conservation of primates in Vietnam (pp. 72–77). Hanoi: Frankfurt Zoological Society, Help for Threatened Wildlife, Vietnam Primate Conservation Programme.
Sterling, E. J., Hurley, M. M., & Minh, L. D. (2006). Vietnam: A natural history. New Haven, CT: Yale University Press.
Thierry, B. (2007). The macaques: A double-layered social organization. In C. J. Campbell, A. Fuentes, K. C. MacKinnon, M. Panger, & S. K. Bearder (Eds.), Primates in perspective (pp. 224–239). New York and Oxford: Oxford University Press.
Van Nguyen, Q., Tran, T. D., & Dinh, H. V. (2010). Landscapes and ecosystems of tropical limestone: Case study of the Cat Ba islands, Vietnam. Journal of Ecology and Field Biology, 33(1), 23–36.
van Schaik, C. P., & van Noordwijk, M. A. (1988). Scramble and contest in feeding competition among female long-tailed macaques (Macaca fascicularis). Behaviour, 105(1–2), 77–98.
van Schaik, C. P., van Noordwijk, M. A., de Boer, R. J., & den Tonkelaar, I. (1983). The effect of group size on time budgets and social behaviour in wild long-tailed macaques (Macaca fascicularis). Behavioral Ecology and Sociobiology, 13, 173–181.
Waterman, P. G., & Kool, K. M. (1994). Colobine food selection and plant chemistry. In A. G. Davies & J. F. Oates (Eds.), Colobine monkeys: Their ecology, behaviour and evolution (pp. 251–284). Cambridge: Cambridge University Press.
Witte, A. (2011). Grooming dynamics among captive African (Colobus angolensis) and Asian (Trachypithecus cristatus) colobines. Honors thesis, Ohio State University.
Workman, C. (2010a). Diet of the Dealcour’s langur (Trachypithecus delacouri) in Van Long Nature Reserve, Vietnam. American Journal of Primatology, 72, 317–324.
Workman, C. (2010a). The foraging ecology of the Delacour’s langur (Trachypithecus delacouri) in Van Long Nature Reserve, Vietnam. PhD thesis, Duke University.
Workman, C., & Schmitt, D. (2012). Positional behavior of Delacour’s langurs (Trachypithecus delacouri) in northern Vietnam. International Journal of Primatology, 33, 19–37.
Workman, C., & Van Dung, L. (2009). The chemistry of eaten and uneaten leaves by Delacour’s langurs (Trachypithecus delacouri) in Van Long Nature Reserve, Vietnam. Vietnamese Journal of Primatology, 3, 29–36.
Yang, L., Minghai, Z., Jianzhang, M., Ankang, W., Shuangxi, W., & Shusen, Z. (2007). Time budget of daily activity of François’ langur (Trachypithecus francoisi francoisi) in disturbance habitat. Acta Ecological Sinica, 27(5), 1715–1722.
Yao, M., Yin, L., Zhang, L., Liu, L., Qin, D., & Pan, W. (2012). Parturitions in wild white-headed langurs (Trachypithecus leucocephalus) in the Nongguan Hills, China. International Journal of Primatology, 33, 888–904.
Yin, L., Lie, W., Zhao, Q., Qin, D., Li, X., et al. (2011). A video-aided study of the diet of wild white-headed langurs (Trachypithecus leucocephalus). Folia Primatologica, 82, 33–44.
Zhou, Q., Wei, F., Huang, C., Li, M., Ren, B., & Luo, B. (2007). Seasonal variation in the activity patterns and time budgets of Trachypithecus francoisi in the Nonggang Nature Reserve, China. International Journal of Primatology, 28, 657–671.
Zhou, Q., Huang, Z., Wei, X., Wei, F., & Huang, C. (2009). Factors influencing interannual and intersite variability in the diet of Trachypithecus francoisi. International Journal of Primatology, 30, 583–599.
Zhou, Q., Huang, H., Tang, X., & Huang, C. (2010). Seasonal variation in the activity budgets of the white-headed langur. Acta Theriologica Sinica, 30(4), 449–455.
Zhou, Q., Tang, X., Huang, H., & Huang, C. (2011). Factors affecting the ranging behaviour of white-headed langurs (Trachypithecus leucocephalus). International Journal of Primatology, 32, 511–523.
Zhou, Q., Tang, Z., Li, Y., & Huang, C. (2013). Food diversity and choice of white-headed langur in fragmented limestone hill habitat in Guangxi, China. Acta Ecological Sinica, 33, 109–113.
Acknowledgments
This study would not have been possible without the support and cooperation of the Cat Ba Langur Conservation Project and Cat Ba National Park. Thank you to Mr. Nguyen Cam (Cat Ba National Park ranger) for daily boat driving and langur spotting. Mr. Neahga Leonard (Cat Ba Langur Conservation Project Manager) provided on-the-ground support, for which we are very grateful. Mr. Nguyen The Cuong (Hai Phong Province Coordinator for Fauna & Flora International) assisted in translations between the authors and the National Park. Dr. Teresa Newman in the Statistical Consulting Unit of ANU provided help in analyses. We would like to thank Professor Colin Groves, Dr. Joanna Setchell, and two anonymous reviewers for valuable feedback on earlier drafts of this manuscript. Australian National University Research Training Scheme funding, Primate Action Fund (PAF 14-15; CI Contract 1000575), WildInvest, and Critical Ecosystem Partnership Fund (Grant 64587) provided grants. Hai Phong People’s Committee and Cat Ba National Park provided research permits.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare they have no conflicts of interest.
Additional information
Handling Editor: Joanna M. Setchell
An erratum to this article is available at http://dx.doi.org/10.1007/s10764-017-9954-0.
Rights and permissions
About this article
Cite this article
Hendershott, R., Behie, A. & Rawson, B. Seasonal Variation in the Activity and Dietary Budgets of Cat Ba Langurs (Trachypithecus poliocephalus). Int J Primatol 37, 586–604 (2016). https://doi.org/10.1007/s10764-016-9923-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-016-9923-z