Abstract
An organism’s body size is intrinsically related to its metabolic requirements, life history profile, and ecological niche. Previous work in primates generally, and lemurs specifically, has shown that body size often correlates with ecological parameters related to temperature and energy availability in the environment, although other studies indicate the absence of any such patterns in lemurs. Here we test hypotheses that predict that body mass in Eulemur should covary with 1) overall food availability or resource seasonality and/or 2) temperature, i.e., Bergmann’s rule. We use data from 722 wild true lemurs to identify population-specific body mass for 27 populations representing 11 of the 12 described Eulemur species, and derive climatic data for each population from the WorldClim database. We use phylogenetic generalized least squares models to evaluate these hypotheses and find that body mass significantly negatively correlates with annual mean temperature and positively correlates with standard deviation of temperature, but does not significantly correlate with annual rainfall or number of dry months. These results indicate that body mass distribution across populations in Eulemur is consistent with Bergmann’s rule, but does not track resource seasonality as seen in other lemurs, e.g., Propithecus. We suggest that the fact that body mass in various lemur radiations correlates with different environmental variables may result in these patterns being obscured when such taxa are combined in a single analysis. This may help explain why some previous work has found little evidence of ecogeographical correlates with body mass across all Lemuriformes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A variety of theoretical models predict that some combination of ecological variables may constrain adult body size in mammals. Two broad classes of hypotheses relate body mass to environmental variables. The first group is a collection of resource constraint hypotheses, in which adult body size is limited by resource quality, year round availability, or seasonal availability. The best supported of these hypotheses within primates appears to be a resource seasonality model, in which extended periods of resource scarcity select for smaller body size (Terborgh and van Schaik 1987). This may be mediated through selection for slow growth rates in seasonal environments to minimize the absolute metabolic needs during periods of resource scarcity (Altmann and Alberts 1987, 2005; Leigh and Shea 1996; Leigh and Terranova 1998; Ravosa et al. 1993). Alternatively, it has been suggested that the opposite pattern might occur, in which large body size is selected for in highly seasonal environments because larger individuals have greater reserves to survive periods with inadequate resources (Lindstedt and Boyce 1985).
The second general hypothesis is commonly referred to by the name of an empirical observation known as Bergmann’s rule or the Bergmann effect (Bergmann 1847; Mayr 1956). As originally formulated, this is the observation that within a genus, species living in colder habitats tend to be larger than those in warmer environments, although temperature is often replaced with latitude as a proxy. Bergmann’s proposed mechanism for this relationship is that if body shape stays constant as body size increases, then the ratio of surface area to volume will decrease, resulting in lower heat loss relative to mass (Bergmann 1847). This mechanism is typically referred to as the “heat conservation hypothesis” to distinguish it from other potential mechanisms driving Bergmann’s rule (Blackburn et al. 1999), particularly bccause there have been challenges to this explanation (McNab 2010; Scholander 1955).
Empirical tests of these hypotheses in lemurs have investigated whether body size is dependent on habitat primary productivity, resource seasonality, resource quality, and/or thermoregulation (Albrecht et al. 1990; Gordon et al. 2013; Harcourt and Schreier 2009; Kamilar et al. 2012; Lehman 2007; Lehman et al. 2005; Muldoon and Simons 2007). This research provides a mixed picture as to whether ecological variables might be constraining body mass. Craniodental metrics in both extant and subfossil lemur species typically follow a pattern in which species found in the warmer, drier, and longer dry season ecoregions of Madagascar are smaller than metrics from congeners in regions that are cooler, wetter, and have shorter dry seasons (Albrecht et al. 1990; Godfrey et al. 1990; Muldoon and Simons 2007), consistent with multiple hypotheses relating body size to environmental constraints. In contrast, a recent study which used phylogenetic comparative methods to test several ecological constraint hypotheses across Lemuriformes found no evidence of any relationship between body mass and environmental variables (Kamilar et al. 2012). However, one possible confounding factor in that study is that it included species that varied substantially in size but that came from the same site; in this case, ecogeographic patterns might be obscured by high-magnitude generic- and family-level body size differences associated with identical values for environmental variables (Gordon et al. 2013).
One way to address that concern is to limit the taxonomic breadth of such analyses. For example, multiple studies on day-active indriids have found that species mean body size strongly correlates with proxies for primary productivity and resource seasonality (Lehman 2007; Lehman et al. 2005; Ravosa et al. 1993). These results have recently been borne out by an interspecific study in Propithecus that confirms the relationship between body mass and precipitation-based variables using novel population-level data and phylogenetic comparative methods (Gordon et al. 2013).
Published studies of comparisons at even lower taxonomic levels also exist, although sample sizes are typically too small for significance tests. For example, three populations of Microcebus murinus showed a negative relationship between body mass and temperature, although not between body mass and seasonality (Lahann et al. 2006). Johnson et al. (2005) compared one eastern and one western population of true lemur that were both considered at that time to belong to Eulemur fulvus rufus, although now would be placed in E. rufifrons and E. rufus, respectively. They found that the eastern rain forest population was larger bodied than the western population from a warmer, drier, and more seasonal environment, consistent with both the heat conservation and resource constraint hypotheses.
In the present study we examine the relationship between body mass and environmental variables across multiple populations of Eulemur species to investigate hypotheses relating body size to various environmental constraints. True lemur species (Eulemur spp.) are broadly similar to each other in morphology, behavior, and ecological niche (Overdorff and Johnson 2004), although they range over a variety of forest habitats with different climate characteristics in Madagascar (Kamilar and Muldoon 2010; Mittermeier et al. 2008) and exhibit approximately a twofold difference in mean body mass among the various species of Eulemur (Gordon 2006; Smith and Jungers 1997). Previous work specifically on ecogeographic variation in true lemur body size is limited, although several studies have noted that eastern rain forest true lemurs are larger than their counterparts in drier, warmer western habitats (Albrecht et al. 1990; Gerson 1999, 2000; Godfrey et al. 1990; Johnson et al. 2005).
We evaluate the resource constraint and heat conservation hypotheses in true lemurs by testing the following nonexclusive predictions. First, if body mass is constrained by primary productivity, then linear models should show a significant positive relationship between body mass and annual precipitation, which is typically used in the primate literature as a proxy for primary productivity. Second, if body mass is constrained by the duration of the seasonal periods of low resource availability, then there should be a significant negative relationship between body size and the number of dry months per year. Finally, if body mass is responding to temperature-induced selection on surface area to volume ratios, then body mass should have a negative relationship with annual mean temperature, i.e., Bergmann’s rule.
Methods
Population Identification and Calculation of Population-Specific Variables
Field crews from the Madagascar Biodiversity Partnership (MBP) and Omaha’s Henry Doorly Zoo and Aquarium’s (OHDZA) Conservation Genetics Department collected body mass and GPS coordinate data for 722 individual true lemurs from across Madagascar. These individuals represent all 12 currently recognized species of the genus Eulemur (IUCN 2014; Mittermeier et al. 2008). Data were collected from February 13, 1999 to April 17, 2014 in conjunction with conservation genetics and systematics research (Brenneman et al. 2012; Delmore et al. 2013; Johnson et al. 2008; Tokiniaina et al. 2009). We then assigned individuals to populations or excluded them as isolated individuals using the procedure described in the text that follows.
Geographic Cluster Analysis
We identified populations within each species via a cluster analysis using a geographic distance matrix of capture locations for individual specimens following the procedure described in Gordon et al. (2013). Clusters were identified according to the following conditions: 1) the maximum distance between capture locations within a cluster was 20 km and 2) the distance between the geographic centroids of clusters exceeded 12 km. This procedure excludes isolated specimens from arbitrary inclusion in the nearest cluster, while also ensuring that clusters were geographically distinct. We treated the resulting clusters as distinct populations for identifying population-specific mass and climatic data.
Population-Specific Body Mass
Degree of maturity was not recorded at the time of capture for several individuals in the sample, so mean values of all captured individuals would be biased downward by juveniles in some cases. Following Gordon et al. (2013), we excluded clusters with fewer than four males and four females of known mass to reduce the probability of inclusion of clusters composed exclusively of juveniles, and then we selected the largest female and male to represent the upper end of sex-specific adult mass in each of the remaining populations. We then averaged the male and female values to provide a single value of adult body mass for each population. Some Eulemur species exhibit slight body size dimorphism, although most appear to be effectively monomorphic (Delmore et al. 2011; Johnson et al. 2005; Kappeler 1990, 1991). We compared resulting values against published ranges for adult body mass from wild and free-ranging captive populations (Gordon 2006; Smith and Jungers 1997; Zehr et al. 2014) to ensure that they were plausible body mass values for adults. Application of this procedure resulted in the exclusion of all E. flavifrons, as no geographic cluster for that species met the minimum sample size requirement. Also, as we have noted previously (Gordon et al. 2013), MBP field crews collected data throughout the year and so some individuals would have been weighed during different seasons, but there is no evidence to suggest that there is a systematic bias in sampling times across sampling sites. Thus any seasonal variation in body mass is expected to add noise rather than bias to the relationship between body mass and environmental variables.
Environmental Variables
To facilitate comparison with earlier work, we also followed Gordon et al. (2013) in using four measures of annual tendency and seasonality in rainfall and temperature derived from the WorldClim database (Hijmans et al. 2005): total annual rainfall, number of dry months in the year (≤60 mm of rain per month), annual mean temperature, and standard deviation of monthly mean temperatures. We calculated population-specific values for each variable as the mean of all WorldClim values from 30 arc-second resolution grids that fell within a 10 km radius of each population’s geographic centroid. Environmental variables were not transformed before inclusion in linear models; please refer to Gordon et al. (2013) for a discussion of using correlated environmental variables vs. principal components and nontransformed vs. log-transformed environmental variables.
Final Data Set
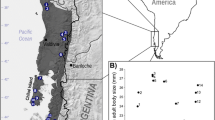
The geographic clustering procedure and minimum population size sampling protocol resulted in 27 populations from 11 species of Eulemur represented by a total of 553 individuals (Fig. 1). Geographic location, body mass, and environmental variables averaged over a 10 km radius circle centered on each population are provided in Table I. Environmental variables correlate among themselves, with annual variables strongly negatively correlated with their own seasonality (r 2 = 0.769 for precipitation variables and 0.754 for temperature variables; Table II, Fig. 2). These correlations must be borne in mind when interpreting the results of linear models, because independent variables that themselves highly correlate with body mass may not have significant regression coefficients when added to models containing other independent variables with which they strongly correlate. However, what is of interest in this study is not the specific regression coefficients themselves, but rather the relative information content of each model (see “Evaluation of Models”).
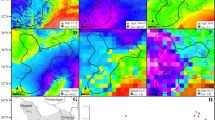
Locations of populations of Eulemur included in this study (N = 27) and environmental variables derived from the WorldClim database (30 arc-second resolution). Numbers in left-most map correspond to population numbers in Table I.
Bivariate plots of seasonality variables against annual variables for temperature (left) and rainfall (right) for all 27 populations of Eulemur included in this study. Pearson and Spearman correlations are highly similar within each pair of variables and are provided in Table II.
Data Analysis
To take into account phylogenetic relationships between populations, we ran phylogenetic generalized least squares (PGLS, Martins and Hansen 1997) models using the ‘caper’ package in R (Orme et al. 2012; R Development Core Team 2014) to investigate the relationship between body mass and environmental variables. We calculated nine models using different combinations of environmental variables as predictors of population-specific body mass (see “Linear Models”). To evaluate the relative strength of different environmental variables in these models, we compared the models using F-ratio significance tests (for nested models) and sample-size corrected Akaike Information Criterion (AICc) weights (for all models). Models with the lowest AICc values are the best performers in terms of maximizing the likelihood of the data given that particular model while exacting a penalty for the number of predictor variables. These AICc values can be transformed into weights between zero and one for a set of models, with higher values indicating better models according to this information criterion perspective; in addition, the relative importance of each predictor variable to the overall relationship between body mass and environmental variation can be estimated by summing the AICc weights for the models in which they appeared (Burnham and Anderson 2002; Symonds and Moussalli 2011).
Linear Models
Following Gordon et al. (2013), we considered nine linear models in which log10 body mass was the dependent variable and one or more environmental variables were the independent variables. In addition to a model that included all four environmental variables and models that included only one environmental variable, we built models that included as predictors either two variables of the same type (i.e., precipitation or temperature variables) or of the same temporal component (i.e., annual trend or seasonality variables). This resulted in the following models: 1) full: all four environmental variables as predictors, (2) annual: annual rainfall and annual mean temperature, 3) seasonality: number of dry months and standard deviation of temperature, 4) rainfall: annual rainfall and number of dry months, 5) temperature: annual mean temperature and standard deviation of temperature, and four models with only one environmental variable each – 6) annual rainfall, 7) number of dry months, 8) annual temperature, and 9) standard deviation of temperature.
Taxonomy and Phylogeny
We follow Mittermeier et al. (2008) for our species-level taxonomy; species-level branching sequence and branch lengths in this study follow Markolf and Kappeler (2013). Because the residuals from linear models are not independent from each other for either species or populations within those species, the error structure of linear models must be adjusted to take into account phylogenetic relationships (Felsenstein 1985). As in Gordon et al. (2013), we modified the species-level phylogeny to include polytomies within each species that contributed multiple populations to the analysis because body mass and environmental variables are specific to populations in this study, not species. We determined consensus branch lengths for the phylogenetic distances between populations across all nine models using a maximum-likelihood method [Electronic Supplementary Material (ESM) Figs. S1, S2]. Once the consensus set of branch lengths was identified, we estimated Pagel’s λ (Pagel 1997, 1999) for all PGLS models using the maximum likelihood estimation in caper (Orme et al. 2012). Pagel’s λ is a parameter that adjusts the degree to which phylogenetic information is incorporated into the error structure of a linear model, and is generally interpreted as a measure of the strength of phylogenetic signal in the residuals of the model (where λ = 1 means that phylogenetic information is fully incorporated into the model, and λ = 0 means that phylogenetic information is ignored and the model is identical to an ordinary least squares model). To directly compare models using nested ANOVA F-ratios, branch lengths and branch scaling parameters such as λ must be identical in all of the models under consideration. We used the mean of λ for the nine models in the recalculation of all models so that they could be directly compared (Gordon et al. 2013).
Evaluation of Models
The goal of this study is to determine whether particular climatic variables are more strongly associated with body mass of Eulemur than others, consistent with the various hypotheses outlined in the Introduction. To address this, we can compare the overall fit of models that include certain climatic variables to models that contain other climatic variables. As mentioned previously, these environmental variables correlate with each other, resulting in some degree of multicollinearity for those models that include more than one independent variable. However, although the inclusion of partially collinear variables will produce biased estimates of regression coefficients within a particular model, it has no effect on the overall information content of a model. For example, consider two linear models: in the first model, body mass is dependent on four correlated predictors (e.g., the four climatic variables in this study), and in the second model, the same set of body mass data is dependent on the four statistically independent principal components that derive from the four correlated predictors. Although the regression coefficients and P-values for those coefficients will differ between models, all statistics pertaining to overall model fit will be exactly the same between the two models: e.g., AICc, r 2, sum of squared error statistics, and overall model significance. Thus multicollinearity has no effect on the determination of whether one model fits the observed data better than another, whether that determination is made using standard ANOVA comparisons of nested models or an information criterion approach.
In this study, we used two complementary lines of evidence to evaluate the various proposed relationships across populations of Eulemur, i.e., whether precipitation variables or temperature variables were more strongly associated with variation in body mass. The first line of evidence was a standard significance testing approach, in which ANOVA comparisons of nested models were used to address whether the fit of a more complete model was significantly better than the fit of a nested model that excluded some variables (e.g., does the addition of temperature variables to a model that includes only precipitation variables significantly improve model fit, and vice versa?). The second line of evidence was based on evidence ratios (ER) derived from an information criterion approach. ER is a measure of how many times more likely the better model (i.e., the model with the lower AICc value) is than the worse model for any pair of models (Burnham and Anderson 2002). The evidence ratio is based on ΔAICc, which is the difference between the AICc value for any particular model and the AICc value for the best model. The ER for any pair of models is calculated as follows (Symonds and Moussalli 2011):
where Δ i is the value for the better model, and Δ j is the value for the worse model.
Ethical Note
All immobilizations, handling, sample collections, and export/import protocols adhered to and were approved by the OHDZA Institutional Animal Care and Use Committee (IACUC), the University of Calgary Animal Care Committee, the Convention on International Trade in Endangered Species, and U.S. Fish & Wildlife Services. Protocols for animal handling and immobilization may be found in previously published research (Delmore et al. 2011, 2013). This research was conducted with permission from the government of Madagascar, following legal requirements.
Results
Phylogenetic Signal in the Error Structure of the Model
Consensus branch lengths as identified by a maximum likelihood procedure resulted in a tree in which populations of the same species diverged from each other at 49.3% of the distance from tree tips to the divergence of each species from other taxa (ESM Fig. S1). Using those consensus branch lengths, we calculated all nine PGLS models using maximum-likelihood to estimate Pagel’s λ for each model. In all cases, λ was estimated as a nonzero value (estimates ranged from 0.590 to 0.720), all 95% confidence intervals excluded λ = 1, and five of nine confidence intervals excluded λ = 0 (Table III). These results indicate the presence of some phylogenetic signal in the error structure of these models.
We recalculated all models using a consensus value of λ set equal to 0.672, the mean of the maximum likelihood value of λ across all models; this value of λ incorporates a majority of phylogenetic information into the error structure of the linear models and falls within the 95% confidence intervals for all estimated values of λ (Table IV). The difference between using model-specific maximum likelihood values of λ and the consensus value had little effect on model fits: the coefficient of determination (r 2) of the nine PGLS models that used the consensus value of λ were all within 0.009 units of the r 2 value for the corresponding model using individual-determined maximum likelihood λ, with five of nine pairs of models having identical r 2 values to three decimal places (compare r 2 values in Tables III and IV).
Ecogeographic Correlates of Body Mass
Variation in population-specific body mass was significantly associated with variation in temperature variables but not rainfall variables (Table IV). Based on AICc weights, the best model in this analysis had body mass dependent solely on standard deviation of temperature, followed closely by body mass dependent solely on annual mean temperature (Table IV). The next two highly weighted models were the seasonality model, in which standard deviation of temperature and number of dry months were both predictors (although only the temperature variable had a significant regression coefficient at α = 0.05), and the temperature model, in which both temperature variables were predictors (Table IV). Although the temperature model was statistically significant (P = 0.001), neither of the two independent variables had a significant regression coefficient (Table IV). This is due to the strong negative correlation between these two variables (r = –0.868, r 2 = 0.754), resulting in neither temperature variable significantly improving model fit when the other temperature variable was already included in the model, despite the fact that both variables individually strongly correlate with body mass.
Both temperature variables had considerably higher summed AICc weights than the precipitation variables (Table IV). This stronger relationship between body mass and temperature rather than precipitation is also shown by the fact that neither model that used only one of the precipitation variables as the sole predictor was significant at α = 0.05, nor was the rainfall model (which was dependent on both precipitation variables). In contrast, all models that included one or both temperature variable(s) were significant.
This overall pattern is also reflected in the pairwise comparison of models. Significance tests of nested models show that the addition of one or more temperature variables to a model that included only precipitation variables always significantly improved model fit, while addition of either precipitation variable to any model never significantly improved model fit (Table V). Evidence ratios derived from AICc values show the same pattern. For any comparison of a model containing only precipitation variables (i.e., the rainfall model, annual rainfall model, and number of dry months model) with a model containing only temperature variables (i.e., the temperature model, annual temperature model, and SD of temperature model), the temperature model is always the better model. Evidence ratios for these comparisons range from 536.2 to 2387.5 (Table V), meaning that the model with only temperature variables is always at least 536 times more likely to estimate the body mass data better than the model with only precipitation variables.
Considered as a whole, these results suggest that population-specific body mass is most strongly associated with standard deviation of temperature, followed closely by annual mean temperature, but with little or no relationship with rainfall variables. In addition, although we do not show the data here, the phylogenetic correlation of population-specific body mass with minimum temperature (which arguably is the most relevant temperature variable for the heat conservation hypothesis for Bergmann’s rule) is even higher than it is with the standard deviation of temperature (r 2 = 0.412 vs. 0.401, respectively).
We can use the standard deviation of temperature PGLS model and annual mean temperature PGLS model to estimate expected log10 body mass for “typical” true lemurs (i.e., controlling for phylogenetic variation in the data set) living at the extremes of these two variables in this data set (1.25 and 2.75°C for standard deviation of temperature, 18.16 and 26.80°C for annual mean temperature; Table I). These two models have intercepts of 0.110 and 0.682, respectively; estimated log10 body masses for the extremes of standard deviation of temperature in this data set are 0.224 and 0.361 log10[kg], and estimated mass for the extremes of annual mean temperature are 0.209 and 0.362 log10[kg]. These values correspond to an expected 37% increase in body mass (in kg) associated with an increase in 1.5°C of standard deviation of temperature, and an expected 42% increase in body mass associated with a decrease of 8.64°C of annual mean temperature. These expected differences control for the effects of phylogeny; note that the full range of body mass values in this data set is much greater than these differences in expected mass at environmental extremes, with the highest combined mean mass in Table I being 118% larger than the lowest, but also note that these values are drawn from different species (2.73 kg for Eulemur fulvus and 1.25 kg for E. mongoz).
Discussion
Overall, we found no support for the two resource constraint hypotheses. However, our results suggest that body size variation across Eulemur is generally consistent with Bergmann’s rule, with about 40% of the variation in body size accounted for by variation in temperature across populations in this analysis (see r 2 values in Table IV). The slightly higher correlation of body size with the standard deviation of monthly mean temperatures rather than annual mean temperature is slightly puzzling at first glance. However, there is a strong negative correlation between these two temperature variables, such that those locations with the coolest annual mean temperature also have the greatest variability in monthly mean temperatures on average. Thus a high standard deviation of monthly temperature typically indicates a low baseline temperature with relatively high monthly variability, meaning that those locations will have months with the lowest temperatures overall. This is reflected in the even higher phylogenetic correlation of population-specific body mass with minimum temperature than with standard deviation of temperature. This result is consistent with the expectation of the heat conservation hypothesis that body mass should be highest in those areas that experience the coldest minimum temperatures to minimize the surface area to volume ratio (Bergmann 1847).
A point to consider is whether the lack of support for the resource constraint hypotheses may be due in part to the inclusion of sympatric populations of species with different body size. Owing to our sampling protocol in which we calculate the mean of environmental variables across a circle with a radius of 10 km, no two populations in our study share exactly the same values for all environmental variables (Table I). However, there are five areas where we have partial overlap between populations of different species, resulting in very similar values for those populations. Just as in the Kamilar et al. (2012) study, we might expect that the difference in body mass between populations living in the same place with the same values for environmental variables might obscure underlying patterns, even if the magnitude of body mass differences in sympatric populations is much smaller in this study and we only ever have a maximum of two species living sympatrically in our sample. However, if that is the case, we have no particular reason to believe that this confounding effect would impact precipitation variables more than temperature variables, so it should equally impair our ability to detect relationships between body mass and precipitation on the one hand and temperature on the other. Thus it is probably safe to conclude that even if some small component of body mass variation across Eulemur is constrained by resources, the effect of temperature variation is much greater.
Contextualizing this pattern in true lemurs within the broader pattern of evidence of Bergmann’s rule in nonhuman primates is difficult because there are few such studies. Harcourt and Schreier (2009) investigated Bergmann’s rule in non-Malagasy primates using the common approach of treating latitude as a proxy for temperature. They found that those primate clades whose ranges extended farthest from the equator had patterns consistent with Bergmann’s rule, although this is due in part to a loss of species diversity at higher latitudes that results in the absence of both the smallest and largest bodied species. At lower taxonomic levels, patterns appear to vary. Also using a latitudinal approach, Fernandez-Duque (2011) found that intraspecific and intrapopulation variation in body mass of Aotus follows Bergmann’s rule, while studies in Nycticebus and Macaca show a mixed pattern in which the relationship is observed in some species of these genera but not others (Ito et al. 2014; Ravosa 1998).
There are also few studies of Bergmann’s rule in lemurs, and they tend to show different results at different taxonomic levels and within different clades at the same taxonomic level. An analysis across all lemurs showed no evidence for any ecogeographic effects on body mass (Kamilar et al. 2012), while at the generic level, comparisons of populations of Propithecus representing all extant sifaka species found support for resource constraint hypotheses but little to no support for Bergmann’s rule (Gordon et al. 2013). Intraspecific analyses of some of the smallest bodied lemurs have produced conflicting results: whereas an analysis of three populations of Microcebus murinus showed a negative relationship between body mass and temperature, consistent with Bergmann’s rule (Lahann et al. 2006), an analysis of populations of Cheirogaleus crossleyi showed no such evidence (Blanco and Godfrey 2014). However, the authors of the latter study note that dwarf lemurs are obligate hibernators, unlike mouse lemurs, and thus are not subject to year-round thermal stress. Finally, previous work comparing one population each of Eulemur rufifrons and E. rufus (which have previously been considered to belong to a single subspecies, E. fulvus rufus) showed a pattern consistent with both Bergmann’s rule and resource constraint hypotheses (Johnson et al. 2005). Samples sizes in all of these intraspecific studies are too small to draw meaningful conclusions about statistical significance.
That said, there are at least two proposed general relationships that may account for the different patterns observed in lemurs. The first is that variation in pelage thickness and density should have a greater impact on thermoregulation in larger mammals than in smaller mammals, and thus Bergmann’s rule should be more evident in small mammals than in large mammals (Steudel et al. 1994). If correct, this might help explain why support for Bergmann’s rule is found in mouse lemurs and true lemurs but not in sifakas. However, broad studies across mammals that find evidence for Bergmann’s rule do not find evidence for a difference in the pattern between small and large mammals (Ashton et al. 2000; Blackburn and Hawkins 2004). A second possibility is that there is a more complex relationship among body mass, temperature, and resource seasonality. In their study of ecogeographic body mass variation in Microcebus murinus, Lahann et al. (2006) suggest that temperature negatively correlates with body mass, whereas resource seasonality positively correlates with longevity and negatively correlates with reproductive rate. It is likely that the relationship of all of these variables to each other vary depending on the specific constraints on life history parameters for any given species, considered in conjunction with the spatial and temporal distribution of food resources. For example, the difference in dietary niche between Eulemur and Propithecus species (the former incorporating mostly fruit into their diet, and the latter being more folivorous) may have an interaction effect with climatic variables that produces the different patterns of ecogeographic body mass variation observed in these two genera; dietary differences also appear to affect ecogeographic relationships with body mass in carnivores (Meiri et al. 2007). Whether a particular model can be developed that successfully predicts adult body mass based on environmental parameters, diet, and life history parameters (other than growth rate and duration) remains to be seen.
Regardless of why some lemur taxa appear to follow Bergmann’s rule or to support resource constraint hypotheses while others do not, the results of this study considered in conjunction with previous work on Propithecus suggest that comparative analyses of ecogeographic body mass variation in high-level taxa should be interpreted with caution. Kamilar et al. (2012) found no evidence any relationship between body mass and environmental variables, and we suggested that this might be due to multiple species sharing the same values for environmental variables but highly different body mass values (Gordon et al. 2013). An additional reason why such studies may not find a relationship between body mass and environmental factors is that different relationships may be present in various lower level taxa (e.g., temperature relationships in true lemurs and resource seasonality relationships in sifakas), and these different relationships may be due to unmeasured factors (e.g., interactions with dietary differences). The inclusion of these multiple taxa with their differing patterns into a single analysis will likely obscure those relationships. As a consequence, we recommend that future comparative studies attempt to determine whether patterns (or absence of patterns) observed at high taxonomic levels are consistent with those observed at lower taxonomic levels within the same data sets (Gordon 2006; Smith and Cheverud 2002), and if not, attempt to determine whether additional factors might be involved in these relationships, e.g., dietary niche, activity pattern, life history variables.
References
Albrecht, G. H., Jenkins, P. D., & Godfrey, L. R. (1990). Ecogeographic size variation among the living and subfossil prosimians of Madagascar. American Journal of Primatology, 22, 1–50.
Altmann, J., & Alberts, S. (1987). Body mass and growth rate in a wild primate population. Oecologia, 72, 15–20.
Altmann, J., & Alberts, S. C. (2005). Growth rates in a wild primate population: ecological influences and maternal effects. Behavioral Ecology and Sociobiology, 57, 490–501.
Ashton, K. G., Tracy, M. C., & de Queiroz, A. (2000). Is Bergmann’s rule valid for mammals? The American Naturalist, 156, 390–415.
Bergmann, C. (1847). Uber die verhaltnisse der warmeokonomie der thiere zu ihrer grosse. Göttinger Studien, 595–708.
Blackburn, T. M., & Hawkins, B. A. (2004). Bergmann's rule and the mammal fauna of northern North America. Ecography, 27, 715–724.
Blackburn, T. M., Gaston, K. J., & Loder, N. (1999). Geographic gradients in body size: a clarification of Bergmann’s rule. Diversity and Distributions, 5, 165–174.
Blanco, M., & Godfrey, L. (2014). Hibernation patterns of dwarf lemurs in the high altitude forest of eastern Madagascar. In N. B. Grow, S. Gursky-Doyen, & A. Krzton (Eds.), High altitude primates (pp. 23–42). New York: Springer Science+Business Media.
Brenneman, R. A., Johnson, S. E., Bailey, C. A., Ingraldi, C., Delmore, K. E., Wyman, T. M., Andriamaharoa, H. E., Ralainasolo, F. B., Ratsimbazafy, J. H., & Louis, E. E. (2012). Population genetics and abundance of the endangered grey-headed lemur Eulemur cinereiceps in south-east Madagascar: assessing risks for fragmented and continuous populations. Oryx, 46, 298–307.
Burnham, K. P., & Anderson, D. R. (2002). Model selection and multimodel inference (2nd ed.). New York: Springer Science+Business Media.
Delmore, K. E., Louis, E. E., & Johnson, S. E. (2011). Morphological characterization of a brown lemur hybrid zone (Eulemur rufifrons × E. cinereiceps). American Journal of Physical Anthropology, 145, 55–66.
Delmore, K. E., Brenneman, R. A., Lei, R., Bailey, C. A., Brelsford, A., Louis, E. E., & Johnson, S. E. (2013). Clinal variation in a brown lemur (Eulemur spp.) hybrid zone: combining morphological, genetic and climatic data to examine stability. Journal of Evolutionary Biology, 26, 1677–1690.
Felsenstein, J. (1985). Phylogenies and the comparative method. The American Naturalist, 125, 1–15.
Fernandez-Duque, E. (2011). Rensch's rule, Bergmann's effect and adult sexual dimorphism in wild monogamous owl monkeys (Aotus azarai) of Argentina. American Journal of Physical Anthropology, 146, 38–48.
Gerson, J. S. (1999). Size in Eulemur fulvus rufus from western Madagascar: sexual dimorphism and ecogeographic variation. American Journal of Physical Anthropology (Supplement), 28, 134.
Gerson, J. S. (2000). Social relationships in wild red-fronted brown lemurs (Eulemur fulvus rufus). Ph.D. dissertation, Duke University.
Godfrey, L. R., Sutherland, M. R., Petto, A. J., & Boy, D. S. (1990). Size, space, and adaptation in some subfossil lemurs from Madagascar. American Journal of Physical Anthropology, 81, 45–66.
Gordon, A. D. (2006). Scaling of size and dimorphism in primates II: macroevolution. International Journal of Primatology, 27, 63–105.
Gordon, A. D., Johnson, S. E., & Louis, E. E., Jr. (2013). Females are the ecological sex: sex-specific body mass ecogeography in wild sifaka populations (Propithecus spp.). American Journal of Physical Anthropology, 151, 77–87.
Harcourt, A. H., & Schreier, B. M. (2009). Diversity, body mass, and latitudinal gradients in primates. International Journal of Primatology, 30, 283–300.
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G., & Jarvis, A. (2005). Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology, 25, 1965–1978.
Ito, T., Nishimura, T., & Takai, M. (2014). Ecogeographical and phylogenetic effects on craniofacial variation in macaques. American Journal of Physical Anthropology, 154, 27–41.
IUCN (2014). IUCN Red List of Threatened Species. Version 2014.3.
Johnson, S. E., Gordon, A. D., Stumpf, R. M., Overdorff, D. J., & Wright, P. (2005). Morphological variation in populations of Eulemur albocollaris and E. fulvus rufus. International Journal of Primatology, 26, 1399–1416.
Johnson, S. E., Lei, R., Martin, S. K., Irwin, M. T., & Louis, E. E. (2008). Does Eulemur cinereiceps exist? Preliminary evidence from genetics and ground surveys in southeastern Madagascar. American Journal of Primatology, 70, 372–385.
Kamilar, J. M., & Muldoon, K. M. (2010). The climatic niche diversity of Malagasy primates: a phylogenetic approach. PLoS ONE, 5, e11073.
Kamilar, J. M., Muldoon, K. M., Lehman, S. M., & Herrera, J. P. (2012). Testing Bergmann's rule and the resource seasonality hypothesis in Malagasy primates using GIS-based climate data. American Journal of Physical Anthropology, 147, 401–408.
Kappeler, P. M. (1990). The evolution of sexual size dimorphism in prosimian primates. American Journal of Primatology, 21, 201–214.
Kappeler, P. M. (1991). Patterns of sexual dimorphism in body weight among prosimian primates. Folia Primatologica, 57, 132–146.
Lahann, P., Schmid, J., & Ganzhorn, J. U. (2006). Geographic variation in populations of Microcebus murinus in Madagascar: resource seasonality or Bergmann's rule? International Journal of Primatology, 27, 983–999.
Lehman, S. M. (2007). Ecological and phylogenetic correlates to body size in the Indriidae. International Journal of Primatology, 28, 183–210.
Lehman, S. M., Mayor, M., & Wright, P. C. (2005). Ecogeographic size variations in sifakas: a test of the resource seasonality and resource quality hypotheses. American Journal of Physical Anthropology, 126, 318–328.
Leigh, S. R., & Shea, B. T. (1996). Ontogeny of body size variation in African apes. American Journal of Physical Anthropology, 99, 43–65.
Leigh, S. R., & Terranova, C. J. (1998). Comparative perspectives on bimaturism, ontogeny, and dimorphism in lemurid primates. International Journal of Primatology, 19, 723–749.
Lindstedt, S. L., & Boyce, M. S. (1985). Seasonality, fasting endurance, and body size in mammals. The American Naturalist, 125, 873–878.
Markolf, M., & Kappeler, P. M. (2013). Phylogeographic analysis of the true lemurs (genus Eulemur) underlines the role of river catchments for the evolution of micro-endemism in Madagascar. Frontiers in Zoology, 10, 70.
Martins, E. P., & Hansen, T. F. (1997). Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. The American Naturalist, 149, 646–667. Erratum 153:448.
Mayr, E. (1956). Geographical character gradients and climatic adaptation. Evolution, 10, 105–108.
McNab, B. K. (2010). Geographic and temporal correlations of mammalian size reconsidered: a resource rule. Oecologia, 164, 13–23.
Meiri, S., Yom-Yov, Y., & Geffen, E. (2007). What determines conformity to Bergmann’s rule? Global Ecology and Biogeography, 16, 788–794.
Mittermeier, R. A., Ganzhorn, J. U., Konstant, W. R., Glander, K., Tattersall, I., Groves, C. P., Rylands, A. B., Hapke, A., Ratsimbazafy, J., Mayor, M. I., Louis, E. E., Jr., Rumpler, Y., Schwitzer, C., & Rasoloarison, R. M. (2008). Lemur diversity in Madagascar. International Journal of Primatology, 29, 1607–1656.
Muldoon, K. M., & Simons, E. L. (2007). Ecogeographic size variation in small-bodied subfossil primates from Ankilitelo, southwestern Madagascar. American Journal of Physical Anthropology, 134, 152–161.
Orme, D., Freckleton, R., Thomas, G., Petzoldt, T., Fritz, S., Isaac, N., & Pearse, W. (2012). Caper: Comparative analyses of phylogenetics and evolution in R. R package version 0.5.
Overdorff, D. J., & Johnson, S. E. (2004). Eulemur, true lemur. In S. M. Goodman & J. Benstead (Eds.), The natural history of Madagascar (pp. 1320–1324). Chicago: University of Chicago Press.
Pagel, M. (1997). Inferring evolutionary processes from phylogenies. Zoologica Scripta, 26, 331–348.
Pagel, M. (1999). Inferring the historical patterns of biological evolution. Nature, 401, 877–884.
R Development Core Team. (2014). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Ravosa, M. J. (1998). Cranial allometry and geographic variation in slow lorises (Nycticebus). American Journal of Primatology, 45, 225–243.
Ravosa, M. J., Meyers, D. M., & Glander, K. E. (1993). Relative growth of the limbs and trunk in sifakas: heterochronic, ecological, and functional considerations. American Journal of Physical Anthropology, 92, 499–520.
Scholander, P. F. (1955). Evolution of climatic adaptation in homeotherms. Evolution, 9, 15–26.
Smith, R. J., & Cheverud, J. M. (2002). Scaling of sexual dimorphism in body mass: a phylogenetic analysis of Rensch's rule in primates. International Journal of Primatology, 23, 1095–1135.
Smith, R. J., & Jungers, W. L. (1997). Body mass in comparative primatology. Journal of Human Evolution, 32, 523–559.
Steudel, K., Porter, W. P., & Sher, D. (1994). The biophysics of Bergmann's rule: a comparison of the effects of pelage and body size variation on metabolic rate. Canadian Journal of Zoology, 72, 70–77.
Symonds, M. E., & Moussalli, A. (2011). A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behavioral Ecology and Sociobiology, 65, 13–21.
Terborgh, J., & van Schaik, C. P. (1987). Convergence vs. non-convergence in primate communities. In J. H. R. Gee & P. S. Giller (Eds.), Organization of communities, past and present (pp. 205–226). Oxford: Blackwell.
Tokiniaina, H., Bailey, C., Shore, G., Delmore, K., Johnson, S., Louis, E., & Brenneman, R. (2009). Characterization of 18 microsatellite marker loci in the white-collared lemur (Eulemur cinereiceps). Conservation Genetics, 10, 1459–1462.
Zehr, S. M., Roach, R. G., Haring, D., Taylor, J., Cameron, F. H., & Yoder, A. D. (2014). Life history profiles for 27 strepsirrhine primate taxa generated using captive data from the Duke Lemur Center. Scientific Data, 1, 140019.
Acknowledgments
We are grateful to Giuseppe Donati for inviting us to contribute to this issue, and we thank him, Joanna Setchell, and three anonymous reviewers for their valuable comments on earlier versions of this manuscript. We also thank the government of Madagascar for permission to conduct the original research that was used for this analysis. We thank Omaha’s Henry Doorly Zoo and Aquarium Center for Conservation and Research, the Madagascar Biodiversity Partnership, and Madagascar Institut pour la Conservation des Écosystèmes Tropicaux (MICET) for assistance in original data collection, along with many individuals involved in original research (including Kira Delmore, Sheila Holmes, Christina Ingraldi, Annemarie Rued, and Hobinjatovo Tokiniaina). Grant sponsors for original research included the National Geographic Society (6613.99); Margot Marsh Biodiversity Fund; Conservation International; Primate Conservation, Inc.; the Natural Science and Engineering Research Council of Canada; the University of Calgary; the American Society of Primatologists; and the Ahmanson Family Foundation. A. D. Gordon also thanks the participants of the 2014 AnthroTree Workshop (supported by the National Science Foundation and the National Evolutionary Synthesis Center, NSF grants BCS-0923791 and EF-0905606) for discussions on phylogenetic comparative methods.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Gordon, A.D., Johnson, S.E. & Louis, E.E. Environmental Correlates of Body Mass in True Lemurs (Eulemur spp.). Int J Primatol 37, 89–108 (2016). https://doi.org/10.1007/s10764-015-9874-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-015-9874-9