Abstract
The work reported here involved a comparative study regarding the understanding that high school students (16–18 years) have of the concept of chemical elements and their periodic classification. More specifically, the level of knowledge on this topic was compared before and after the completion of baccalaureate studies in a sample of Spanish students. In order to achieve this goal, a questionnaire was developed that included 14 items in an open format, through which various aspects of the students’ understanding of the idea of chemical element and their periodic classification were assessed. In addition, the application of this knowledge to interpret and predict the behaviour and properties of elements and to carry out calculations on the atomic composition of the elements was evaluated. Aspects concerning the acquisition of scientific knowledge, the application of knowledge to different contexts and situations, and the use of scientific evidence to draw conclusions and knowledge about the nature and history of science were evaluated. The questionnaire was previously validated with a large group of students. The results of this study show that improvements occur primarily in addressing higher level cognitive questions (analysis, synthesis and evaluation) in comparison to the lower level tasks (capacity for retention, understanding or direct application of learning). In addition, students who start high school have a very limited understanding of the ideas behind the Periodic Table of the chemical elements and that their lack of understanding, to a large extent, remains upon completion of the baccalaureate. These results suggest that there are real difficulties in understanding this topic and show the limited influence of the studies completed in high school.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The concept of chemical elements, their properties and the law of periodicity are amongst the topics that are now considered as cornerstones both in history (Schmidt, Baumgärtner & Eybe, 2003; Scerri, 2007, 2011; Esteban, 2009) and the teaching of chemistry (Ben-Zvi & Gemut, 1998; Demircioğlua, Demircioğlua & Çalikb, 2009). This situation means that this topic is one of the most frequently discussed in chemical education publications, as evidenced by a review of articles published in journals such as The Journal of Chemical Education (Linares, 2004; Linares & Izquierdo, 2007).
Most publications in this field have focused on historical and epistemological problems or have concerned search-related strategies and resources for teaching at different educational levels. However, less attention has been devoted to identifying the difficulties and shortcomings in student learning or to investigating the effects of proposed strategies and resources to improve the teaching and learning of this subject. As a consequence, with some exceptions (Ben-Zvi & Gemut, 1998; Franco-Mariscal, 2011; Linares, 2004; Taber, 1999; Taber & Tan, 2007; Talanquer, 2006, 2010; Wang & Barrow, 2013), this can be considered to be an area in which very few research studies have been carried out. This situation exists despite the importance of this topic in introductory chemistry courses at the high school and university levels (Scerri, 2007) and despite the fact that students often struggle to learn and understand this topic, indicating that the various obstacles to learning should be analysed and clarified (Franco-Mariscal & Oliva-Martínez, 2012; Schmidt, 1998). As a result, it is of interest to define these shortcomings in teaching/learning and to identify strategies and resources that can contribute to overcoming them.
In the work described here, we carried out a study on the understanding that Spanish students (aged 16–18 years) have the concept of the chemical element and the Periodic Table and compared their knowledge before and after baccalaureate studies. Our aim was to evaluate the performance of the students in this area at the beginning of their university studies and to assess the contribution that baccalaureate studies made to their performance. This type of study is of interest in order to identify gaps in the teaching/learning process and to provide a basis for the development of future innovations aimed at addressing any problems in the learning process.
Theoretical Background
The theoretical foundations of the research described here concern two basic areas. The first area concerns the type of knowledge required and the nature of the questions posed in an assessment of the scientific content. The second area is related to the background that exists in the literature on learning difficulties and problems related to the notion of chemical elements and the Periodic Table. A brief review of the literature on these topics was carried out in order to define and justify the structure and nature of the questionnaire.
The Types of Knowledge and Reasoning Required
In the first area under consideration, both the type of learning to be assessed and the level of cognitive skills required can be analysed by posing questions to gauge the students learning or the learning pursued.
From the point of view of the type of learning, one must return to the classical differentiation made by Ausubel between ‘rote learning’ and ‘meaningful learning’ (Ausubel, Novak & Hanesfan, 1978; Novak & Gowin, 1984). Rote learning is the simple storage of information, which consists of data as facts, statements and definitions that are retained and then recalled in a literal way. In contrast, Ausubel defined meaningful learning as that in which the student assimilates and owns knowledge and relates it to understanding that they already have. Meaningful learning is very desirable since it allows students to understand and gives meaning to knowledge handling in order to provide more stable and enduring knowledge. However, Ausubel stated that rote learning is also necessary. In fact, in terms of the chemical elements and the Periodic Table in particular, rote learning is important to familiarize students with the Periodic Table and it enables them to memorize the names and symbols of the chemical elements and to identify their position in the Periodic Table.
Such rote learning, which is related to the retention and recall of information, encompasses one of the categories listed by Bloom, Engelhart, Fust, Hill & Krathwohl (1956) for the reasoning skills required in assessment tasks. Specifically, this category of ‘knowledge’—as opposed to other types such as comprehension, application, analysis, synthesis and evaluation—increasingly contributes to higher order skills, the development of which would require significant learning to take effect. This hierarchy has been reviewed and reformulated by various authors and has even been combined with other criteria for other forms of categorization (Krathwohl, 2002).
In the specific case of learning chemistry, there is broad consensus that learning and assessment involve components that are both conceptual and algorithmic in nature, in addition to memory and reasoning skills. As a consequence, various types of questions have been categorized into different classes in terms of their cognitive complexity (Smith, Nakhleh & Bretz, 2010; Stamovlasis, Tsaparlis, Kamilatos, Papaoikonomou & Zarotiadou, 2005; Zoller, Lubesky, Nakhleh, Tessier & Dori, 1995). These different types of question are associated with terminology that does not necessarily mean the same thing in each case and they include a number of categories that are often divergent. Hence, in many cases, categories overlap each other and this makes comparison between rankings difficult, although some interesting approaches to address this issue have been reported (Smith et al., 2010). Selected examples of the taxonomies used by various authors are shown in Table 1.
Although the algorithmic component was not evaluated in this study, it is important to consider this issue here when providing an overview of the areas that can be assessed.
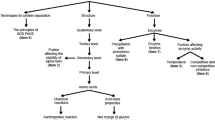
From the point of view of the purpose of the learning to be achieved, one must consider the purposes outlined by Hodson (1992) for learning science. Hodson identified three basic dimensions for the learning of science and these correspond to the tasks of ‘learning science’, ‘learning to do science’ and ‘learning about science’. The first two aspects are included within the taxonomies shown in Table 1. However, the third aspect concerns new areas that must be examined and related to the nature and history of science itself. This aspect is also considered in this study. Finally, according to Millar & Osborne (1998), there are four important areas in learning science: the acquisition of scientific knowledge, the application of knowledge to different contexts and situations, and the use of scientific evidence to draw conclusions and learn about the nature and history of science. In this classification, there are differences in the level of cognitive demand for various types of questions such that only the first category requires knowledge and understanding, the second requires the application of learning, while the third requires a capacity for analysis and synthesis. The fourth category, meanwhile, is likely to involve further capacity for evaluation, namely in matters that require an assessment of the usefulness and limitations of scientific knowledge.
In this study, we opted for the latter classification, which addresses the evaluation of the specific topic in question here. As a consequence, we considered those dimensions that are most appropriate to this area. For example, the algorithmic component, which is very much a part of other topics such as stoichiometry, thermochemistry or balance, is not relevant in the study of issues related to the chemical elements and the Periodic Table. In fact, as discussed below, the focus of research is aimed primarily on the analysis of conceptual learning difficulties or more complex handling of the Periodic Table, either to solve qualitative issues related to the direct application or inferences associated with high-level cognitive skills.
Difficulties Encountered in Learning About the Chemical Elements and Their Periodic Classification
In the limited amount of educational research conducted on issues and gaps in students’ comprehension of the elements and the Periodic Table, we have to distinguish three areas. Firstly, there are studies that focus on the difficulty and complexity of the concept of the chemical element. Secondly, another area relates to the interpretation of properties and the Periodic Table of chemical elements. Finally, there are studies that concern curricular decisions and the methodology adopted by the faculty for the teaching of these topics and the possible difficulties that may be encountered.
The first of these areas concerns the idea of an element as a chemical substance (Furió & Domínguez, 2001, 2007; Raviolo, Garritz & Sosa, 2011) and, in turn, the distinction between elements and compounds (Briggs & Holding, 1986; Franco-Mariscal & Oliva-Martínez, 2012, 2013). Within this profile of learning problems, one must consider the work of Linares (2004), who described how one of the main difficulties with the concept of chemical elements lies in the ambiguous and ambivalent nature of this term. Thus, from the point of view of science, two definitions of ‘chemical element’ are provided by International Union of Pure and Applied Chemistry (IUPAC) (McNaught & Wilkinson, 1997). These two definitions correspond to two different meanings of the concept of chemical element: one of which is conceived in an abstract manner as a kind of atom, and the other understood as a real substance present in daily life and/or in laboratories. Due to this ambiguity in the idea of an element, the Periodic Table is considered both as a table of elements as substances and as a table of atoms of the elements. This double function may confuse students and lead to the use of the term element as a synonym for atom (Schmidt, 1998, 2000).
The second of the profiles outlined above is exemplified by the work of Taber (Taber, 1998, 1999, 2003; Taber & Tan, 2007) and Talanquer (2006, 2007, 2010). These studies concerned the analysis of misconceptions and intuitive explanations used by chemistry students, some of which are particularly relevant in the analysis of the properties of chemical elements and their compounds, as well as on their use and interpretation of the Periodic Table. Thus, in Taber’s studies, students commonly misunderstood the scientific model for explaining and predicting trends in ionisation energy. For example, Taber (2003) described his findings in terms of a number of alternative conceptions. In particular, students commonly used the scientifically invalid ideas that (a) ions with full shells had some special inherent stability (more than octets or noble gas configurations), and (b) a positive nucleus gives rise to a fixed amount of nuclear force that is distributed or shared-out among the electrons present (conservation of force thinking).
Talanquer (2006, 2007, 2010) defined a set of heuristic reasoning patterns or different concepts common to the alternatives described in the chemical literature. This is the case for teleological reasoning, through which causal explanations are replaced by tendencies or inclinations of the systems to evolve in such a way that they satisfy some objective or purpose (Talanquer, 2007). For example, for many students, the octet rule becomes an explanation when considering the stability of atoms, so that they ‘tend’ to gain or lose electrons just to ‘get to’ a noble gas electronic structure. This leads to an overestimation of the octet rule as an explanatory model to understand the stability of atoms (Taber, 2001). Closely related to this type of reasoning are other explanations based on anthropomorphic or animistic reasoning, in which phenomena are attributed to the physical and chemical behaviours caused by the feelings and desires of humans. Other heuristic reasoning described by Talanquer (2010) is based on ‘Recognition’ and ‘Representativeness’ schemes. Furthermore, Talanquer (2006, 2010) described a heuristic of the type ‘One-reason decision making’, which is a cognitive shortcut that reduces the number of indicators and alternatives to be considered when making a decision, prompting several choices based on a single indicator. However, this heuristic suffers from a significant drawback when working with periodic properties, the development of which in the Periodic system depends on more than one factor at a time. Furthermore, among the heuristics described by Talanquer, of particular relevance is the one denoted as ‘Periodic trend’, which is based on decisions and comparisons between elements and compounds and only depends on the relative position in the Periodic Table of the different atoms in the substances being compared. For example, the reason that the bromine atom has a larger atomic volume that fluorine is simply ‘because it is lower in the Periodic Table’.
Finally, the third of the profiles outlined above includes, for example, publications by Lehman, Koran & Koran (1984), Agudelo, Marzábal & Izquierdo (2009) and Linares (2004), which can shed light on the learning difficulties that can arise depending on the type of instructional method followed. The research by Lehman et al. (1984) explored the effects that the format of the Periodic Table and complementary written materials had on students when extracting and managing information from these sources. These authors found that the aforementioned factors did influence student performance, with interactions even found between the degree of the student’s previous experience and the degree of understanding shown. For example, the results revealed that subjects with less experience in the use of the Periodic Table showed better performance when they used a version of the table that included more visual information. In contrast, for subjects who were more familiar with the Periodic Table, there were also advantages in the use of more visual tables but only for those with a higher level of verbal comprehension. Meanwhile, those with a lower level of verbal comprehension tended to process more effectively the traditional Periodic Table. The latter group benefited more when the Periodic Table came with additional written material.
Linares (2004) investigated the different ways in which undergraduate textbooks approach teaching the general chemistry of the Periodic Table. Linares identified three different methods, which he called ‘substantialist’, ‘historic’ and ‘quantum mechanical/atomistic’. In the substantialist approach, the observed properties of the substances are used to define the periodicity, whereas the historical path introduces the presentation of the Periodic Table from a historical perspective. Finally, in the atomistic quantum mechanical approach, the atomic structure is employed to explain the configuration of the Periodic Table and the variation in the properties of the elements.
On assessing the studies reviewed in this work, it was found that the most common barriers to learning involve conceptual understanding, problems related to the direct application of concepts and difficulties in the development of complex inferences. Although these studies collectively provide valuable information concerning many areas of learning involved in this topic, major gaps are evident in the aspects investigated to date in this field. These gaps include basic issues such as the presence of chemical elements in our lives, the way in which the elements are arranged in the Periodic Table, the usefulness of the Periodic Table, the criteria by which the elements are organized and the limitations of the current Periodic System. Similarly, there is a dearth of studies that provide an overview to analyse the different causes of learning difficulties for these issues and to evaluate progress made by students as they proceed through the education system, particularly in Spain.
The Periodic Table in the Spanish Educational System
From an educational point of view, the Spanish curriculum (Ministerio de Ciencia y Educación, 2007a, b) addresses content related to the Periodic Table repeatedly and continuously from the 3rd year of Compulsory Secondary Education (CSE) (15–16 years of age)Footnote 1 up to the 2nd year of the baccalaureate in Science (17–18 years)Footnote 2 (see Appendix), albeit with some significant differences between the two cases. For example, in the 3rd year CSE (Year 10), the curriculum begins with a macroscopic description of the chemical element that is addressed by considering the historical predecessor—the ‘simple substance’—and the experimental processes involved in differentiating composite substances. Subsequently, the submicroscopic view is approached by considering Dalton’s atomic theory, the language of symbols and the models proposed by Thomson and Rutherford. In contrast, in the 4th year CSE (Year 11), which is the first course considered in this study, these topics are addressed using a more advanced deductive model in the opposite sense to the previous year, i.e. a more in-depth study of atomic models is presented initially and, from these, the Periodic Table of elements is considered as a basis from which to infer and explain properties.
Although this alternative approach may appear relevant, the change in emphasis may be premature for two reasons. Firstly, the students have not yet acquired sufficient phenomenological experience to understand fully the topics that will be studied in subsequent years. Secondly, considering that, in practice, even in the 3rd year CSE (Year 10), the sequence of instruction is usually based on an operational approach to chemistry that begins by presenting the Periodic Table as a tool to support the rules of chemical nomenclature.
In these circumstances, significant gaps are expected in the knowledge of students who complete high school because the curriculum then moves directly to the analysis of atomic models as a basis for building electronic configurations. The baccalaureate course (Years 12 and 13) generally does not provide sufficient experimental and phenomenological knowledge, albeit in a deductive way, since the properties that are discussed in these courses, in terms of the application of the Periodic Table, usually only concern atomic properties, namely atomic volume, electronegativity, etc., all of which are related to the more abstract idea of the chemical element.
Furthermore, the textbooks do not generally contribute to this area as they tend to be based on conclusions and do not address the ‘heuristic principles’ that facilitate the development of knowledge through the history of science (Niaz, 2005).
Research Design
The main aim of this work was to conduct a comparative study into the understanding of a sample of Spanish school students (16–18 years) of the concept of the chemical element and the Periodic Table, both before and after completing their studies. To achieve this aim, a questionnaire was developed and validated, a process that is also described in this article. Thus, the objectives of the study were twofold:
-
1.
To validate an exploratory questionnaire to assess students’ knowledge on the concept of the chemical element and the Periodic Table.
-
2.
To use the questionnaire to assess the students’ knowledge before and after completing their baccalaureate and to evaluate the changes due to the teaching received prior to university entrance.
The questionnaire consisted of a total of 12 questions in open format (Table 2). The questions were designed to assess the understanding of the idea of the chemical element and its periodic classification and the application of this knowledge to interpret and predict phenomena and properties and to perform calculations on the atomic composition of the elements. Two of these questions were broken down into two sections, meaning that the total number of items evaluated was 14. These questions addressed four areas around the Periodic Table that are considered important in learning science (Millar & Osborne, 1998): the acquisition of scientific knowledge (K), the application of knowledge to different contexts and situations (A), the use of scientific evidence to draw conclusions (U) and knowledge about the nature and history of science (N). The composition of the questions and the writing style was developed, by consensus, by the first two authors of this paper and by a teacher of Chemistry at the University of Cádiz (Spain) who has extensive teaching and research experience.
Analysis of the responses obtained for each item was conducted to develop a typology of responses through a system of categories for each individual item (Franco-Mariscal, 2011). The categorization process was developed jointly by two judges who had previously analysed the criteria and had agreed on a process for their implementation. After this initial categorization process, we performed a subsequent analysis in which the responses were classified into three levels: appropriate responses, partially appropriate responses and inappropriate responses (or blanks). A summary of the different situations is presented in Table 3, which shows the nature of the questions and the evaluation criteria used to assess student responses.
The questionnaire was used to carry out two studies, one for each of the proposals made. The first study was conducted to validate the instrument used and for this purpose the questionnaire was completed, without a time limit, by a sample of 176 students aged between 15 and 18 years. The students came from six different secondary schools in southern Spain with students of a middle socioeconomic level. All of the students had received traditional teaching from the methodological point of view.
In the second study, the questionnaire was given at the end of the course to a sample of 136 students. Of these, 67 came from three different high schools and they were enrolled in the optional subject Chemistry in the 4th year CSE (Year 11). The remaining 69 students were in five classes in the 2nd year of the Baccalaureate in Science (Year 13), which included the subject of Chemistry in the curriculum. Three of these groups were from the same secondary schools as mentioned previously, whereas the other two institutions had very similar characteristics. As in the previous case, all of the students had received traditional education that was not innovative.
The results obtained by the students in the 4th year CSE (Year 11) were adopted to assess the level of students in these subjects at the start of their baccalaureate. The results obtained by the students in the 2nd year of the baccalaureate (Year 13) were adopted to define the level of knowledge at the end of this educational stage and, therefore, the level for those who would enter university. The difference between these levels can therefore be regarded as an index to assess the evolution between the two points, i.e. the improvement during baccalaureate studies.
The data were processed with the statistical software package SPSS 21.0 using descriptive analysis, principal components analysis, scale reliability analysis and tests to compare independent parametric (ANOVA) and non-parametric (Mann–Whitney U) groups.
Results and Discussion
Study 1: Validation of the Questionnaire
For validation of the questionnaire, we considered two types of validity. Firstly, the internal validity was assessed through factor analysis and a subsequent study of the internal consistency and, secondly, the external validity was evaluated by correlation with a small sample of students through their academic performance.
The first step in the analysis involved a descriptive study of the results for each item taken individually. The percentages obtained in each of the three categories of response (inappropriate or blank, partially appropriate and appropriate) in the analysis of each item are shown in Table 4.
It can be seen that student performance varies markedly from one item to another. The best results were obtained for items 5A, 6 and 7, for which around two thirds of the students provided acceptable answers. These items concern, respectively, knowledge about characteristic properties of the chemical elements, the students’ ability to classify a group given elements from the Periodic Table and their appreciation of the historical nature of, and changes to, the Periodic Table. In contrast, inappropriate responses were given by over two thirds of the students for item 11. This item concerned the idea of isotopes of elements to explain atomic masses that are not integers. Items 1, 3 and 9 fall in the middle, with the majority of students (around two thirds) falling in the central category of the distribution. These items concerned their understanding of the difference between element and chemical compound, the universality of the chemical elements and their relationship to atomic number as an identifier of the nature of each element, and the ranking of elements by atomic volumes. Many inappropriate or partially appropriate responses corresponded with memory failure or a lack of understanding of the content studied. However, an important part of the answers also reflected several of the ideas and misconceptions about this topic described by other authors (Furió & Domínguez, 2007; Raviolo et al., 2011; Taber, 1999, 2003; Talanquer, 2006, 2007).
Overall, it appears that the questions concerned the use of evidence to draw conclusions (U) and these proved to be more complex for the students as they involved high level cognitive tasks.
However, only one of the two questions that assess aspects of the nature of science (N) yielded poor results, as one would expect. This question required students to evaluate the limitations of the Periodic Table (item 8). The other item (item 7) yielded significantly better results, probably because the answer to the question can be limited to a descriptive analysis that simply requires various examples of periodic classifications used throughout history—an area that was studied in class. The second step consisted of a correlation analysis between items to check whether global assessments should be made in the questionnaire to provide more reliable results than the individual items. To achieve this goal, the responses were quantified on a scale that assigned 2 points to completely appropriate responses, 1 point to partially appropriate responses and 0 points to inappropriate responses or blanks. All Pearson coefficients between items were positive and were in the range 0.74–0.10, although these were only statistically significant in two out of three correlations. These results led us to perform a principal components analysis in order to study the underlying structure of these correlations. The Kaiser–Meyer–Olkin sampling adequacy ratio had a value of 0.76, which can be considered medium-high, with χ 2 = 528.1 (d.f. = 91) for the Bartlett’s sphericity test, which is statistically significant (p < 0.001). These results support the applicability of this analysis.
The exploratory analysis yielded a solution with four factors, which together explained 53 % of the total variance. This means that more than half of the information provided by the questionnaire items can be summarized by the combination of only four mutually independent factors. A Varimax rotation analysis revealed some difficulties to define the meaning of each factor. First of all, the factor loadings values were only moderate, with 0.72 as highest value. Second of all, a significant part of the items loaded on two or more factors simultaneously. And finally, all the factors saturated on items of a very different nature, making difficult to obtain patterns in order to define each factor. Furthermore, tests performed showed that the scales constructed from these factors were not sufficiently reliable (Cronbach’s alpha coefficients between 0.62 and 0.45). In fact, the Scree plot (Fig. 1) shows a pronounced gap between the first and second factors and this indicates the conservation of a single factor. This factor accounted for 28 % of the variance. For all these reasons, it was hard to establish a connection between the content of the questionnaire and the statistical information. Consequently, we did not define partial scales based on these four factors and instead define a single global scale. It is important to note that these dimensions did not correspond to any of the four areas in which the questionnaire was structured.
The factor matrix for a single-factor analysis is shown in Table 5. All items show a positive and acceptable factor loading on the first factor, indicating that this is a common factor to all test items. This finding demonstrates the overall applicability of the assessment questionnaire.
The reliability of the test was measured by Cronbach’s alpha coefficient, which had a value of 0.80. This value can be considered as moderately high and shows the internal consistency of the scale (DeVellis, 1991).
Therefore, the data support the construction of a single scale to summarize the information from the various questionnaire items through the same overall score obtained from the sum of the 14 items. This scale, whose possible values range from 0 to 28 points, yielded a mean of 15.2 ± 0.4, with a minimum of 3.0 and a maximum of 25.0.
In addition to the analysis of internal consistency for the scale obtained from the questionnaire, we also assessed the external validity. To do this, based on Cohen & Swerdlik (2001), we constructed a subsample of 38 students from 4th year CSE (Year 11) and correlated the scale values built with the marks obtained in the subject of Chemistry in the corresponding quarter. These students corresponded to two groups taught by the first author. The students were monitored in order to assess the learning outcomes bearing in mind different sources of information: exams, portfolios, participation in class, etc. The results showed a Pearson correlation coefficient of r = 0.60, which is a relatively high value and is statistically significant (p < 0.001). This demonstrates the predictive value of the test, thus providing another indicator of validity.
Study 2: Change of the Knowledge of Students During Their Baccalaureate
In order to analyse the change of the students’ knowledge during their baccalaureate, a cross-sectional study was carried out in which we compared the responses of students who had finished their 4th year CSE and 2nd year baccalaureate, respectively, using variables that were dependent on the results of the previously validated questionnaire. The percentages obtained for each of the items are shown in Table 6 and, as described above, the responses were differentiated into appropriate responses, partially appropriate responses and inappropriate responses (or blanks). The results of the Mann–Whitney U test are also shown as this is the most suitable basis for the comparison of ordinal data from two independent groups. It is indicated in each case whether or not significant differences, and to what extent, were found between the two groups of students.
It can be seen from the results in Table 6 that half of the items have statistically significant differences and, in some cases, significant changes were found in the percentage distributions between the three levels considered. The results show, at least in these cases, positive developments in the knowledge of students during their studies. In the other half of items, however, the improvements observed are not large, although small variations that are detected finally reach statistical significance thresholds.
In relative terms, fewer cases of significant progress were found for students answering questions related to the acquisition and application of knowledge (K and A) than for the development of inferences from evidence (U) or questions that address the nature of science (N). This finding indicates that improvements occur primarily through questions that address aspects with a higher level of cognitive demand (analysis, synthesis and evaluation), while those that concern the capacity for retention, understanding or direct application of learning are associated with lower levels of progress. This improvement should be considered as a substantial progress, despite the results are still quite poor in the 2nd year of baccalaureate studies (Year 13).
It should also be noted that the smallest differences are obtained for items 1 to 4 and these are related to more elemental content and structuring for later learning and they do have an impact on the concept of the chemical element. This area is covered in the Spanish curriculum from 3rd year CSE (Year 10), suggesting that the knowledge of the baccalaureate students did not improve on that acquired at the more basic level.
An alternative analysis for comparisons between groups would be to consider the scale constructed from the overall scores from the questionnaire, which would be a scale from 0 to 28 points. For this subsample, the reliability of the scale was measured by Cronbach’s alpha coefficient, which had a value of 0.79. This value indicates that the scale has a reasonably high reliability.
The mean values obtained for this scale were 12.8 ± 0.5 for 4th year CSE and 15.7 ± 0.7 for 2nd year baccalaureate (see Fig. 2). A parametric analysis of variance revealed the presence of statistically significant differences between the two groups of students (ANOVA: F 1,134 = 10.281, p < 0.001), although the homogeneity of variances test allowed us to reject the null hypothesis (Levene = 4.055, d.f.1 = 1, d.f.2 = 134, p < 0.05), which prompted us to carry out a comparison by the Mann–Whitney test. This test also showed significant differences between groups, thus indicating a statistically significant evolution in knowledge (Mann–Whitney U: U = 1504.5, n 1 = 67, n 2 = 69, p < 0.001). However, the averages obtained for the overall scale of the questionnaire show small differences in relative terms, since a value of only 10 % is reached at the total of the scale and 23 % of the initial value. The variations observed clearly appear to be insufficient bearing in mind the emphasis that the Spanish curriculum places on these topics, which are covered in the 2 years of the baccalaureate (Years 12 and 13).
It can be inferred from the results discussed above that the education received by students only partially succeeds in cementing the studied content and leads to only a slight improvement in the capabilities that have greater cognitive demand. This improvement occurs without an improvement in the basic skills of the students and the skills of lower level students are not developed significantly, with serious deficiencies remaining. This finding indicates a clear and significant failing in the education offered. This situation is probably due to the premature transition in the Spanish education system, as discussed above, from the first contact with this topic through an inductive and phenomenological approach to the more deductive approach that is focused on linking the properties of elements and their position in the Periodic Table with electronic configuration. Moreover, despite the fact that from Year 11 to Year 13 the Spanish curriculum (Ministerio de Ciencia y Educación, 2007a, b) focuses on the sub-microscopic aspects, specifically the internal structure of atoms, it appears that insufficient emphasis is placed on the expected learning outcomes.
Conclusions
The first contribution of this work was the validation, with a sample of 176 high school students aged between 15 and 18, of a 14-item questionnaire that enables an assessment of the level of understanding of students around the concept of the chemical element and the Periodic Table. This is one of the most important topics in high school chemistry. Satisfactory results were obtained from the point of view of internal and external validity. The first of these techniques was assessed by principal components analysis and evaluation of the reliability or internal consistency, and the second technique was assessed through its correlation with academic performance in a small sample of students.
The second contribution of this research concerns the existence of significant differences between the sample of students in the 4th year CSE (Year 11) and the 2nd year of baccalaureate studies (Year 13) in half of the questionnaire items. Specifically, the results suggest that improvements occur primarily in addressing higher level cognitive questions (analysis, synthesis and evaluation) compared to the lower level tasks (capacity for retention, understanding or direct application of learning). This finding clearly indicates that a breakthrough occurred for students completing their baccalaureate in comparison to those in the 4th year CSE. However, significant progress was not identified in the other half of the items, indicating a lack of progress in learning for the students in a significant proportion of the topics studied. It can be seen that it is in the first half of the questionnaire where the differences between groups are less marked, coinciding precisely with the items whose content it is assumed that students should have learned at the end of compulsory secondary education. It is possible that the teachers have paid less attention in subsequent courses and the learning difficulties that they encounter may have been underestimated.
Overall, the results indicate a limited degree of progression in students’ knowledge around the notion of the chemical elements and their classification during Baccalaureate schooling. This limited progress can be interpreted by considering the existence of significant difficulties and obstacles in understanding the concept of the chemical element in its different aspects, a problem that would require a particular didactic treatment to overcome it. In contrast, the Spanish school curriculum does not devote sufficient time to these areas in the early grades through a descriptive phenomenological study of the subject matter. Furthermore, subsequent courses the curriculum do not provide mechanisms to review learning or offset shortcomings and gaps in knowledge.
The study has some limitations that should be considered when drawing conclusions and educational implications. The wide variety of proposed topics and the limited number of items set for each topic are factors that complicate the task of inferring what students actually learnt after completing their studies, beyond providing general quantitative data on the overall level of performance achieved. However, despite everything, what does seem clear is that the traditional teaching method is not able, in its own right, to overcome these difficulties (Taber, 2001; Çalık, 2005; Fernández-González, 2013). This highlights the need to identify strategies and procedures to provide more stimulating and creative teaching methods that are particularly sensitive to these difficulties (Levine, 1990; Schmidt, 2000; Farrer, Monk, Heron, Lough & Sadler, 2010). In particular, we are interested in the development and use of strategies and resources committed to the following areas:
-
Promote a more active learning environment that is more participative and challenging than the methods commonly used in class
-
Consistent with the above, to take into account the students’ explanatory models and the need to contribute to their evolution through strategies that refer to scientific modelling processes
-
Contribute to an improvement in the motivation of students in the study of these topics through approaches and recreational teaching resources and by linking the content with daily life. The use of educational games, informative videos, analogies and practical work, such as small research projects, are amongst the areas that support our proposals
-
Encourage collaborative work in the classroom and carry out work in small groups
-
Develop a context of continuous interaction between students and the teacher in a climate that facilitates ongoing dialogue and feedback between the two
From this standpoint, we are currently developing teaching methods that articulate all of the above aspects. We intend to present the results obtained with these methods in future publications through the assessment of the progress made by students.
Notes
Equivalent to 4th Form or Year 10 (General Certificate of Secondary Education) in the UK.
Equivalent to Upper 6th Form or Year 13 in the UK.
References
Agudelo, C., Marzábal, A. & Izquierdo, M. (2009). Distintas narrativas para un mismo contenido: la Tabla Periódica en los libros de texto [Different narrative for the same content: Periodic Table in textbooks]. Enseñanza de las Ciencias, Número Extra VIII Congreso Internacional sobre Investigación en Didáctica de las Ciencias, Barcelona, pp. 2892–2895.
Ausubel, D., Novak, J. & Hanesfan, H. (1978). Educational psychology. New York, NY: Holt, Rinehart, and Winston.
Ben-Zvi, N. & Gemut, S. (1998). Uses and limitations of scientific models: The Periodic Table as an inductive tool. International Journal of Science Education, 20(3), 351–360.
Briggs, H. & Holding, B. (1986). Aspects of Secondary students’ understanding of elementary ideas in chemistry: Summary report. Childen’s Learning in Science Project. Centre for Studies in Science and Mathematics Education: University of Leeds.
Çalık, M. (2005). A cross-age study of different perspectives in solution chemistry from junior to senior high school. International Journal of Science and Mathematics Education, 3(4), 791–796.
Cohen, R. & Swerdlik (2001). Pruebas y Evaluación Psicológicas. Introducción a las Pruebas y a la Medición [Psychological Testing and Evaluation. Introduction to Test and Measurement]. Mexico: McGraw Hill.
Demircioğlua, H., Demircioğlua, G. & Çalikb, M. (2009). Investigating the effectiveness of storylines embedded within a context-based approach: The case for the Periodic Table. Chemistry Education Research and Practice, 10, 241–249.
DeVellis, R. F. (1991). Scale development theory and applications. Newberry Park, CA: Sage.
Esteban, S. (2009). La historia del Sistema Periódico [The history of the Periodic System]. Madrid, Spain: Cuadernos de la UNED.
Farrer, N. J., Monk, N., Heron, J., Lough, J. A. & Sadler, P. J. (2010). (RSC)2: Chemistry, performance, and pedagogy—an interactive approach to periodic trends. Chemistry Education Research and Practice, 11, 308–313.
Fernández-González, M. (2013). La formulación química en la formación inicial del profesorado: Concepciones y propuestas [The chemical formulation in initial teacher education: Concepts and proposals]. Revista Eureka sobre Enseñanza y Divulgación de las Ciencias, 10, 678–693. Retrieved from http://hdl.handle.net/10498/15621
Franco-Mariscal, A.J. (2011). El juego educativo como recurso didáctico en la enseñanza de la clasificación periódica de los elementos químicos en Educación Secundaria [The educational game as a didactic resource for teaching the periodic table of the chemical elements in secondary education] (PhD Thesis). University of Cádiz, Cádiz, Spain.
Franco-Mariscal, A.J. & Oliva-Martínez, J.M. (2012). Dificultades de comprensión de nociones relativas a la clasificación periódica de los elementos químicos: La opinión de profesores e investigadores en educación química [Difficulties in understanding concepts concerning the Periodic Table of the Elements: The opinion of teachers and researchers in chemistry education]. Revista Científica, 16(2), 53–71.
Franco-Mariscal, A.J. & Oliva-Martínez, J.M. (2013). Evolución en el alumnado de la idea de elemento químico a lo largo del bachillerato [Changes in students the idea of chemical element along the baccalaureate] . Revista Eureka sobre Enseñanza y Divulgación de las Ciencias, 10(3), 353–376. Retrieved from http://hdl.handle.net/10498/15443
Furió, C. & Domínguez, M.C. (2001). Conocer la historia de la ciencia para comprender las dificultades de los estudiantes sobre el concepto de sustancia química. Enseñanza de las Ciencias, Número Extra VI Congreso Internacional sobre Investigación en la Didáctica de las Ciencias, 55–56.
Furió, C. & Domínguez, M. C. (2007). Usual teaching deficiencies when explaining the macroscopic concepts of substance and chemical change. Journal of Science Education, 8(2), 84–92.
Hodson, D. (1992). In search of a meaningful relationship: An exploration of some issues relating to integration in science and science education. International Journal of Science Education, 14(5), 541–566.
Krathwohl (2002). A revision of Bloom’s taxonomy: An overview. Theory Into Practice, 41(4), 212–218.
Lehman, J. R., Koran, J. J. & Koran, M. L. (1984). Interaction of learner characteristics with learning from three models of the Periodic Table. Journal of Research in Science Teaching, 21(9), 885–893.
Levine, E. H. (1990). Create your own Periodic Table. Journal of Chemical Education, 67, 1045–1046.
Linares, R. (2004). Elemento, átomo y sustancia simple. Una reflexión a partir de la enseñanza de la Tabla Periódica en los cursos generales de Química [Element, atom and simple substance. A reflection from the teaching of the Periodic Table in the general courses Chemistry] (Unpublished Ph.D. Thesis). Universidad Autónoma de, Barcelona, Spain.
Linares, R. & Izquierdo, M. (2007). La Tabla Periódica en el [In the Periodic Table]. Journal of Chemical Education a través del siglo XX. Tecné, Episteme y Didaxis, 21, 7–23.
McNaught, A. D. & Wilkinson, A. (1997). IUPAC. Compendium of chemical terminology. Oxford: Blackwell Scientific Publications.
Millar, R. & Osborne, J. (Eds.). (1998). Beyond 2000: Science education for the future. London, England: King’s College, School of Education.
Ministerio de Educación y Ciencia (2007a). Orden ECI/2220/2007, de 12 de julio, por la que se establece el currículo y se regula la ordenación de la Educación Secundaria Obligatoria. (BOE num. 174, 21 de julio de 2007).
Ministerio de Educación y Ciencia (2007b). Real Decreto 1467/2007, de 2 de noviembre, por el que se establece la estructura del bachillerato y se fijan sus enseñanzas mínimas. (BOE num. 266, 6 de noviembre de 2007).
Niaz, M. (2005). Por que los libros de química general no cambian y siguen una ‘retórica de conclusiones’ [For the general chemistry textbooks do not change and follow a ‘rhetoric of conclusions’]. Educacion Quimica, 16(3), 410–415.
Novak, J. D. & Gowin, D. B. (1984). Learning how to learn. New York: Cambridge University Press.
Raviolo, A., Garritz, A. & Sosa, P. (2011). Sustancia y reacción química como conceptos centrales en química. Una discusión conceptual, histórica y didáctica [Substance and chemical reaction as central concepts in chemistry. a discussion conceptual, historical and didactic]. Revista Eureka sobre Enseñanza y Divulgación de las Ciencias, 8(3), 240–254. Retrieved from http://hdl.handle.net/10498/14388
Scerri, E. R. (2007). The Periodic Table. Its story and its significance. New York, NY: Oxford University Press.
Scerri, E. R. (2011). Who is a theorist? Revista Eureka sobre Enseñanza y Divulgación de las Ciencias, 8(3), 231–239. Online at: http://hdl.handle.net/10498/14387.
Schmidt, H. J. (1998). Does the Periodic Table refer to chemical elements? School Science Review, 80(290), 71–74.
Schmidt, H. J. (2000). Should chemistry lessons be more intellectually challenging? Chemistry Education Research and Practice, 1(1), 17–26.
Schmidt, H. J., Baumgärtner, T. & Eybe, H. (2003). Changing ideas about the Periodic Table of elements and students’ alternative concepts of isotopes and allotropes. Journal of Research in Science Teaching, 40(3), 257–277.
Smith, K. C., Nakhleh, M. B. & Bretz, S. L. (2010). An expanded framework for analyzing general chemistry exams. Chemistry Education Research & Practice, 11, 147–153.
Stamovlasis, D., Tsaparlis, G., Kamilatos, C., Papaoikonomou, D. & Zarotiadou, E. (2005). Conceptual understanding versus algorithmic problem solving: Further evidence from national chemistry examination. Chemistry Education Research & Practice, 6, 104–118.
Taber, K. S. (1998). The sharing-out of nuclear attraction: Or I can’t think about physics in chemistry. International Journal of Science Education, 20(8), 1001–1014.
Taber, K. S. (1999). Ideas about ionisation energy: A diagnostic instrument. School Science Review, 81(295), 97–104.
Taber, K. S. (2001). Building the structural concepts of chemistry: Some considerations from educational research. Chemistry Education Research and Practice, 2(2), 123–158.
Taber, K. S. (2003). Understanding ionisation energy: Physical, chemical and alternative conceptions. Chemistry Education Research and Practice, 4(2), 149–169.
Taber, K. S. & Tan, K. C. D. (2007). Exploring learners’ conceptual resources: Singapore a level students’ explanations in the topic of ionisation energy. International Journal of Science and Mathematics Education, 5(3), 375–392.
Talanquer, V. (2006). Commonsense chemistry: A model for understanding student’s alternative conceptions. Journal of Chemical Education, 83, 811–816.
Talanquer, V. (2007). Explanations and teleology in chemistry education. International Journal of Science Education, 29, 853–870.
Talanquer, V. (2010). Pensamiento Intuitivo en Química: Suposiciones Implícitas y Reglas Heurísticas [Intuitive Thinking in Chemistry: Assumptions Heuristics implicit and Rules]. Enseñanza de las Ciencias, 28(2), 165–174.
Wang, C. Y. & Barrow, L. H. (2013). Exploring conceptual frameworks of models of atomic structures and periodic variations, chemical bonding, and molecular shape and polarity: A comparison of undergraduate general chemistry students with high and low levels of content knowledge. Chemistry Education Research and Practice, 14, 130–146.
Zoller, U., Lubesky, A., Nakhleh, M. B., Tessier, B. & Dori, J. (1995). Success on algorithmic and LOCS vs. conceptual chemistry exam questions. Journal of Chemical Education, 72, 987–989.
Acknowledgments
This research was partially supported with funds from the Educational Innovation Team “KIMIKA” (EIEU26), of the University of Cádiz (Spain).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Franco-Mariscal, AJ., Oliva-Martínez, J.M. & Almoraima Gil, M.L. Understanding the Idea of Chemical Elements and Their Periodic Classification in Spanish Students Aged 16–18 Years. Int J of Sci and Math Educ 14, 885–906 (2016). https://doi.org/10.1007/s10763-014-9614-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10763-014-9614-1