Abstract
Agmatine (AGM), a naturally occurring polyamine derived from L-arginine, has shown significant potential for neuroprotection in Parkinson's Disease (PD) due to its multifaceted biological activities, including antioxidant, anti-inflammatory, and anti-apoptotic effects. This review explores the therapeutic potential of AGM in treating PD, focusing on its neuroprotective mechanisms and evidence from preclinical studies. AGM has been demonstrated to mitigate the neurotoxic effects of rotenone (ROT) by improving motor function, reducing oxidative stress markers, and decreasing levels of pro-inflammatory cytokines in animal models. Additionally, AGM protects against the loss of TH + neurons, crucial for dopamine synthesis. The neuroprotective properties of AGM are attributed to its ability to modulate several key pathways implicated in PD pathogenesis, such as inhibition of NMDA receptors, activation of Nrf2, and suppression of the HMGB1/ RAGE/ TLR4/ MyD88/ NF-κB signaling cascade. Furthermore, the potential of agmatine to promote neurorestoration is highlighted by its role in enhancing neuroplasticity elements such as CREB, BDNF, and ERK1/2. This review highlights agmatine's promising therapeutic potential in PD management, suggesting that it could offer both symptomatic relief and neuroprotective benefits, thereby modifying the disease course and improving the quality of life for patients. Further research is warranted to translate these preclinical findings into clinical applications.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson's Disease (PD) is a progressive neurodegenerative disorder characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and the accumulation of alpha-synuclein protein in the form of Lewy bodies [1].
The exact molecular mechanisms underlying PD pathogenesis are not fully understood, but recent studies have implicated various factors such as neuroinflammation, mitochondrial dysfunction, gut microbiome dysbiosis, oxidative stress, and genetic factors [2,3,4]. Neuroinflammation, in particular, has been identified as a common factor in PD pathogenesis, with evidence suggesting the involvement of both central and peripheral immune systems in a positive feedback loop that exacerbates the disease progression [5]. Oxidative stress is increasingly recognized as a central event in the pathogenesis of PD, contributing significantly to the degeneration of dopaminergic neurons in the SN, which is a hallmark of the disease [6].
Despite significant advances in understanding the pathophysiology of PD, current treatments primarily focus on managing symptoms rather than modifying or reversing the disease [7]. Levodopa (L-dopa), which is considered the gold standard for PD treatment, provides relief from symptoms but does not stop the progression of the disease and is associated with long-term complications such as motor fluctuations and dyskinesias [8, 9]. Additionally, emerging research has explored the potential of novel therapeutic approaches, including immunotherapies, gene editing techniques like CRISPR-Cas9, and nanotechnology-based interventions, to target specific pathways and alleviate PD symptoms [5, 10, 11]. Therefore, there is an urgent need for new therapeutic strategies that not only alleviate symptoms but also have neuroprotective effects to alter the course of the disease.
Agmatine (AGM), a naturally occurring polyamine derived from the decarboxylation of L-arginine, has received significant attention due to its potential neuroprotective properties [12]. Its mechanisms of action are believed to include modulating neurotransmitter systems, inhibiting nitric oxide synthase (NOS), reducing inflammation, and protecting the blood–brain barrier (BBB), all of which contribute to its potential therapeutic benefits [12, 13].
Recent studies have shown that AGM can counteract the neurotoxic effects of rotenone (ROT), a commonly used mitochondrial complex I inhibitor for modeling PD in experiments. These studies have demonstrated that AGM administration enhances motor function, reduces oxidative stress markers, and lowers levels of pro-inflammatory cytokines in animal models of PD. Additionally, AGM has been observed to safeguard against the loss of tyrosine hydroxylase-positive (TH +) neurons, which are crucial for dopamine synthesis [12, 14, 15]. The mechanisms underlying agmatine's neuroprotective effects in PD models include antioxidation, anti-apoptosis, anti-inflammation, and modulation of critical neuroplasticity elements such as CREB, BDNF, and ERK1/2 [16].
AGM exerts its effects by inhibiting NMDA receptor expression, activating the Nrf2 antioxidant pathway, and suppressing the MGB1/RAGE/TLR4/MYD88/NF-κB inflammatory signaling cascade. In a study conducted on rats with ROT-induced lesions, the administration of AGM was found to mitigate dyskinesia while also reducing oxidative stress and inflammation. These significant findings indicate that AGM possesses promising potential as a therapeutic agent in the management of dyskinesia and related symptoms associated with PD and other neurodegenerative disorders [7].
In summary, AGM has received significant attention for its potential therapeutic benefits in the treatment of PD. Compared to other treatment methods, AGM offers unique advantages due to its multifaceted biological activities, which include antioxidant, anti-inflammatory, and neuroprotective effects. This review article aims to explore the potential therapeutic effects of AGM in treating PD, with a focus on its neuroprotective mechanisms and the evidence from preclinical studies. By examining the interaction of AGM with various molecular targets and signaling pathways implicated in PD pathology, we aim to provide insights into how AGM could contribute to neuroprotection and potentially neurorestoration in PD, highlighting its potential as a promising therapeutic agent in managing this debilitating disease.
Overview of Agmatine
AGM (Fig. 1), chemically known as 4 − (aminobutyl)guanidine, is a biogenic amine derived from the amino acid arginine [8,9,10].
The chemical formula of AGM is C5H14N4 [11]. Structurally, AGM contains a guanidine group, similar to arginine, but lacks the carboxylic acid group, distinguishing it from its precursor [12]. The presence of AGM in the mammalian brain was first discovered in the 1990s [13]. It was identified as a decarboxylation product of L-arginine, and since its discovery, a growing body of research has explored its various physiological and pharmacological roles [14].
Agmatine's chemical structure enables it to interact with several receptor systems, including imidazoline receptors, which are implicated in mechanisms like neurotransmission and modulation of insulin secretion. Additionally, AGM acts as an antagonist for the NMDA receptor and as an agonist for the alpha2-adrenergic receptor and the serotonin receptor, which contributes to its potential therapeutic effects in neurological and psychiatric disorders [15].
AGM is not a synthetic molecule; it is a naturally occurring compound. It is produced endogenously in the human body, particularly in the brain, where it functions as a neuromodulator and neurotransmitter [16]. Additionally, AGM is synthesized by gut microbiota within the human gastrointestinal tract, contributing to its systemic levels [17]. Certain foods contain trace amounts of AGM, while fermented foods tend to have higher concentrations. Alcoholic beverages such as beer, wine, and especially sake are rich in AGM due to the fermentation process involving specific bacteria and yeast [17, 18]. In contrast, non-alcoholic fermented beverages like the Turkish drink Şalgam have lower levels of AGM [19].
Given that AGM is derived from arginine, any dietary source rich in arginine could indirectly contribute to the body's AGM levels through metabolic processes [20, 21]. Additionally, the specific mention of AGM conjugated hydroxycinnamic acid as a phenolamide in foods suggests that certain phenolamides, which are compounds found throughout the plant kingdom, might also be related to or contain AGM [22]. However, the direct dietary sources of AGM itself are not detailed in the provided excerpts.
AGM is synthesized from L-arginine through a decarboxylation process catalyzed by the enzyme arginine decarboxylase (ADC). This reaction removes the carboxyl group from L-arginine, resulting in the formation of AGM [23, 24]. This metabolic pathway is crucial for various cellular functions and is conserved across different species, including bacteria, plants, and mammals [17, 25].
In bacteria, ADC can exhibit different characteristics depending on the species. For instance, in the hyperthermophilic methanogen Methanococcus jannaschii, ADC is a pyruvoyl-dependent enzyme that catalyzes the decarboxylation of L-arginine to produce AGM. This enzyme forms a thermostable (αβ)3 complex and is adapted to high temperatures, although the specific pH optimum is not mentioned in the sources [26]. Another study on Selenomonas ruminantium highlights the presence of ADC in the AGM pathway for putrescine synthesis, showing the enzyme's role in polyamine biosynthesis [27].
In mammals, ADC is involved in the synthesis and storage of AGM in astrocytes. The enzyme ADC is expressed in glial membranes and is crucial for producing AGM, which acts as an endogenous ligand for imidazoline and α2-adrenergic receptors [28]. The activity of ADC in mammals can be regulated by various factors, such as interferon-γ, which increases ADC activity, and lipopolysaccharide, which can decrease ADC activity in certain cell types [28]. AGM is catabolized through two main pathways, which differ depending on tissue localization.
Diamine Oxidase (DAO) Pathway
DAO is an enzyme involved in the metabolism of biogenic amines, including AGM [29]. The DAO pathway plays a crucial role in the degradation of AGM, a biologically active substance derived from the decarboxylation of L-arginine by the enzyme ADC [30]. DAO catalyzes the oxidative deamination of AGM, producing an aminoaldehyde, ammonia, and hydrogen peroxide. This catabolic pathway is crucial for regulating the levels of AGM, which has been implicated in various physiological processes, including renal function and neurotransmission [31]. In mammals, DAO is expressed in various tissues, including the kidneys, intestines, and placenta [32]. Notably, DAO activity has been detected in the brain, suggesting its potential role in regulating AGM levels in the central nervous system[33].
Specifically, DAO catalyzes the oxidative deamination of AGM, resulting in the production of γ-guanidinobutyraldehyde [34, 35]. The reaction proceeds as follows:
Agmatinase Pathway
Agmatinase is essential in the brain because it converts AGM to urea and putrescine. Putrescine, acting as a precursor polyamine, is then transformed into spermine and spermidine, playing vital roles in cellular functions and growth [36, 37]. AGM requires transporter proteins for cellular uptake due to its charged nature, which prevents passive diffusion across membranes. The primary transporters involved are organic cation transporter 2 (OCT2) and extraneuronal monoamine transporter (EMT). These transporters facilitate the bidirectional transport of AGM, which is crucial for maintaining cellular and systemic levels of this amine [38, 39]. AGM has demonstrated significant roles in glucose and lipid metabolism. It enhances insulin secretion from pancreatic β-cells, aiding in managing hyperglycemia, and attenuates insulin resistance in rats [40].
Additionally, AGM modulates lipid metabolism and fatty acid oxidation, which is beneficial in lipid metabolism disorders [41].
In summary, the metabolic pathway of AGM involves its synthesis from L-arginine by ADC, followed by its catabolism through either the DAO pathway or the agmatinase pathway. These pathways regulate AGM levels and contribute to its various physiological roles, including neuroprotection, anti-inflammatory effects, and modulation of neurotransmitter systems.
Figure 2 shows the synthesis and metabolic pathways of AGM and its properties.
AGM is synthesized through the enzymatic action of ADC from L-arginine. AGM can undergo metabolism by agmatinase, resulting in the production of putrescine, a precursor to spermine and spermidine. Alternatively, it can be oxidised by diamine oxidase to γ -guanidinobutyraldehyde. The diagram highlights the neuroprotective effects of AGM, such as its antioxidant, anti-apoptotic, anti-inflammatory properties, as well as its role in BBB protection and brain edema prevention
Neuroprotective Effects of Agmatine
AGM has emerged as a promising neuroprotective agent with potential applications in treating various neurodegenerative diseases, including Alzheimer's disease (AD), Huntington's disease (HD), and PD [28, 30, 42].
Research has demonstrated its ability to protect neurons against a wide range of damaging stimuli, such as neurotoxins, ischemia, and oxidative stress [43].
The neuroprotective effects of AGM are mediated through multiple mechanisms. As an NMDA receptor antagonist, it blocks excessive glutamate signaling, preventing excitotoxicity and neuronal death [44]. AGM also exhibits potent antioxidant properties, reducing oxidative damage in the brain. [45]. Its anti-apoptotic effects involve inhibiting pro-apoptotic proteins like Bax and caspase-3 while promoting anti-apoptotic proteins such as Bcl-2, thereby maintaining neuronal integrity and function [44, 46]. Furthermore, AGM demonstrates anti-inflammatory effects by suppressing pro-inflammatory signaling pathways and reducing levels of inflammatory cytokines, including TNF-α, IL-6, and CCL2 [47]. It modulates NOS signaling, preventing NO-induced neurotoxicity and improving blood flow [36, 48]. AGM also helps maintain calcium homeostasis in neurons, protecting against calcium-induced excitotoxicity [36, 37]. In addition to its neuroprotective properties, AGM enhances neurogenesis, particularly in the hippocampus, by increasing the proliferation and survival of neural stem cells [38]. It also promotes synaptic plasticity, facilitating long-term potentiation and strengthening synaptic connections crucial for learning and memory [39, 40].
Studies in animal models have provided compelling evidence for agmatine's therapeutic potential.
The study conducted by de Souza et al. presents compelling evidence that AGM has significant neuroprotective effects in a mouse model of AD. By alleviating depressive-like behaviors and reducing oxidative stress in the hippocampus, AGM shows promise as a therapeutic agent for improving the quality of life and cognitive function in individuals affected by AD [49]. In another study, the chronic intra-hippocampal administration of AGM prevented memory impairment in mice following the injection of β1-42 amyloid peptide. This treatment also restored neurochemical imbalances in the prefrontal cortex and hippocampus, suggesting a protective role against neuroinflammation. These findings highlight the potential of agmatine to enhance cognitive function through the modulation of BDNF signaling and neuroprotective mechanisms [50].
In a rat model of HD induced by 3-nitropropionic acid, AGM administration significantly improved motor coordination, cognitive function, and mood. It normalized levels of key neurochemicals like GABA, glutamate, and BDNF, while reducing neuronal cell loss and promoting regeneration in the striatum [30].
AGM upregulates hypoxia-inducible factor-1α (HIF-1α), offering neuroprotective effects against ROT-induced toxicity in differentiated SH-SY5Y cells [51].
AGM shows promise in treating ischemic cerebrovascular diseases due to its ability to mitigate the effects of ischemic cascades. The study by Kim, J.M., et al. investigates the anti-inflammatory effects of AGM on transient focal cerebral ischemia in diabetic rats. AGM post-treatment significantly improved neurobehavioral activity and motor function in diabetic rats subjected to middle cerebral artery occlusion. The research demonstrated that AGM reduced the expression of inflammatory cytokines in ischemic brain tissue. Immunohistochemical analysis showed decreased levels of high-mobility group box 1, RAGE, TLR2, and TLR4 following AGM treatment. The findings suggest that AGM has potential therapeutic benefits for reducing cerebral ischemic damage in diabetic conditions [52].
Moreover, AGM has demonstrated efficacy in alleviating stress-induced neuropsychiatric conditions.
The study by Gawali et al. investigates the effects of AGM on anxiety, depression-like behaviors, and cognitive impairment induced by chronic unpredictable mild stress (CUMS) in rats. The researchers found that AGM treatment significantly reduced anxiety and depression-like behaviors in the stressed rats. Additionally, AGM improved cognitive performance, indicating its potential therapeutic benefits. Biochemical analyses revealed that AGM modulated the nitrergic signaling pathway, which is implicated in the pathophysiology of stress-related disorders. These findings suggest that AGM could be a promising treatment for alleviating stress-induced neuropsychiatric conditions by targeting specific biochemical pathways [49].
The neuroprotective properties of AGM extend to peripheral nerve injuries as well. In experimental models of rat peripheral nerve injury, AGM demonstrated antioxidant and antineurotoxic effects, reducing tissue damage in the distal part of the traumatic nerve. The findings suggest that AGM could be a promising therapeutic agent for the treatment of peripheral nerve injuries, potentially aiding in the reduction of nerve damage and enhancing the recovery process [53].
Agmatine's role as an NMDA receptor antagonist and NOS inhibitor contributes to its pain-relieving properties, making it a potential candidate for managing neuropathic pain and chronic pain conditions [54].
Additionally, in an animal model of attention deficit hyperactivity disorder (ADHD), AGM improved cognitive deficits and did not alter locomotor activity, indicating its potential as a treatment for ADHD without the common side effects of stimulant medications [55].
In summary, the multifaceted neuroprotective mechanisms of agmatine and its demonstrated efficacy in various experimental models highlight its potential as a therapeutic agent for a wide range of neurological and psychiatric disorders. Further research is needed to fully elucidate its clinical applications and optimize its use in treating neurodegenerative diseases.
The Safety of Agmatine
The safety of AGM has been extensively studied in both animal and human models, with numerous studies indicating its overall safety at various dosages and administration routes. According to the search results, there is limited information specifically addressing the safe dose of AGM in animal studies. However, several studies used AGM doses ranging from 10 mg/kg to 75 mg/kg body weight in rats [56,57,58,59,60]. These doses were generally well-tolerated when given intraperitoneally. The route of administration seems to be crucial in determining toxicity. A study comparing subcutaneous and intraperitoneal injection of AGM (80 mg/kg) found that prolonged subcutaneous administration caused delayed dermal reactions in rats, while intraperitoneal administration did not have such effects [61]. It is important to note that while these studies provide valuable insights into the safety profile of agmatine in animal models, they do not establish a definitive "safe dose" for all animal studies. The appropriate dose and route of administration may vary depending on the specific research objectives, treatment duration, animal species, and experimental conditions. AGM sulfate is the sulfate salt form of AGM, which offers improved stability and absorption. This makes it more suitable for use in dietary supplements and clinical studies [62, 63]. In a clinical study, participants were given a daily dose of 2.67 g (2670 mg) of AGM sulfate for a period of two months. This dosage effectively reduced neuropathic pain and was well-tolerated by the participants, as no significant side effects were reported [62].
Keynan et al. reported that AGM sulfate was found to be safe at doses up to 3.560 g per day for a duration of 21 days, with only mild-to-moderate gastrointestinal side effects reported in a few participants. In the randomized, double-blind, placebo-controlled trial, a dose of 2.670 g per day was administered for a period of 14 days, which resulted in significant improvements in pain levels and quality of life when compared to the placebo group. These improvements were most noticeable immediately after the treatment period, but gradually decreased over time. Overall, the findings suggest that AGM sulfate is both safe and effective for short-term use in relieving symptoms of lumbar disc-associated radiculopathy [64].
In the study by GM Gilad and VH Gilad, the researchers investigated the long-term safety of a high daily dosage of dietary AGM sulfate in humans. Participants consumed a daily dose of 2.67 g of AGM sulfate, divided into six capsules taken after meals, for a period of 4–5 years. Throughout the study, participants underwent periodic physical examinations and laboratory blood and urine analyses. The results indicated that all health measurements remained within normal values, demonstrating the extended long-term safety of this high dosage of AGM sulfate [65].
However, it is important to note that while this dose is considered safe and effective in the context of these studies, the safe dosage of AGM may vary depending on an individual's health conditions, other medications taken, and overall health status. Therefore, it is always recommended to consult a medical professional (specialist physician) before starting any new supplement regimen, including AGM sulfate, to determine the appropriate dosage for the specific condition.
Neuroprotective Effects of Agmatine in Experimental Models of Parkinson's Disease: Mechanisms of Action
Antioxidant and Anti-inflammatory Properties
ROT, a pesticide, is commonly used as a model for PD due to its ability to induce neurodegeneration in dopaminergic neurons through mitochondrial dysfunction. It inhibits mitochondrial complex I, resulting in a loss of mitochondrial membrane potential and a decrease in ATP production. This leads to oxidative stress and neuronal cell death [66, 67]. Exposure to ROT causes an overproduction of ROS, which further damages cellular components and worsens neurodegeneration [68]. Furthermore, ROT-induced oxidative stress activates the Nrf2 pathway, which plays a critical role in the cellular antioxidant response [69, 70]. Chronic exposure to ROT also leads to microglial activation, contributing to neuroinflammation and dysfunction of the BBB through the upregulation of matrix metalloproteinases (MMP-2 and MMP-9) [71]. This neuroinflammatory response exacerbates the loss of dopaminergic neurons and further disrupts the BBB, allowing peripheral immune cells to enter the brain and amplify neurodegeneration [71]. Additionally, studies using ROT-induced PD models have shown that ROT triggers the PINK1/Parkin-mediated mitophagy pathway, which is a cellular process that aims to remove damaged mitochondria but can become dysregulated, leading to additional neuronal damage [72].
These molecular and cellular mechanisms collectively contribute to the progressive neurodegeneration of dopaminergic neurons observed in PD. This highlights the complex interplay between mitochondrial dysfunction, oxidative stress, neuroinflammation, and impaired mitophagy in the pathogenesis of the disease.
El-Sayed et al. investigated the neuroprotective properties of AGM in a rat model of PD induced by ROT. The results indicate that AGM treatment effectively improves motor deficits, reduces oxidative stress markers (MDA), and enhances antioxidant defenses (GSH and SOD). Additionally, AGM reduces neuroinflammation by decreasing levels of pro-inflammatory cytokines (TNF-α and IL-1β) and glial activation (GFAP). Importantly, AGM also preserves TH + dopaminergic neurons, suggesting its potential to protect against dopaminergic neurodegeneration. These findings imply that AGM, with its antioxidant and anti-inflammatory properties, shows promise as a therapeutic agent for neurodegenerative diseases such as PD [14].
Increase Neuroplasticity-related Factors and the Signaling Pathway
CREB is a well-recognized pro-survival transcription factor in neurons. Its activity is primarily regulated through phosphorylation at Ser133. In PD, CREB phosphorylation is often impaired, which correlates with decreased expression of crucial target genes like NURR1, essential for the survival of DA neurons. This impairment of CREB function contributes to the degeneration of nigral DA neurons observed in PD [73]. Strategies to restore CREB activity, such as disrupting its interaction with histone deacetylase 1 (HDAC1) and protein phosphatase 1γ (PP1γ), have shown potential in protecting DA neurons in PD models [73].
Strategies to restore CREB activity, such as disrupting its interaction with histone deacetylase 1 (HDAC1) and protein phosphatase 1γ (PP1γ), have shown potential in protecting DA neurons in PD models [74].
BDNF is a neurotrophic factor that supports the survival and function of DA neurons. In PD, levels of BDNF are found to be decreased, particularly in the nigrostriatal pathway, which is critical for motor control. This reduction contributes to the vulnerability of DA neurons to degenerative processes [75]. Enhancing BDNF levels through physical exercise or gene modulation has been shown to have neuroprotective effects in animal models of PD [75].
The ERK1/2 signaling pathway plays a significant role in cell survival and plasticity. In the context of PD, chronic treatment with L-DOPA, a common therapy, often leads to L-DOPA-induced dyskinesia (LID), which is associated with increased activation of the ERK1/2 pathway. Lovastatin, an inhibitor of the enzyme involved in cholesterol biosynthesis, has been shown to reduce the severity of LID in PD models by inhibiting the phosphorylation of ERK1/2 (pERK1/2). This suggests that modulation of the ERK1/2 pathway could be a viable strategy for managing LID in PD patients [50].
Bilge et al. reported the neuroprotective effects of AGM in a rat model of PD induced by ROT. AGM, administered intraperitoneally at a dose of 100 mg/kg, was found to alleviate motor impairments caused by ROT and mitigate oxidative damage. The treatment reduced neuronal loss and TH immunoreactivity while increasing the expression of CREB, BDNF, and ERK1/2 in the striatum, which are crucial for neuroplasticity and neuronal survival. The study utilized various behavioral tests, biochemical analyses, and histological examinations to assess the effects of AGM. Results showed that AGM improved locomotor activity, reduced oxidative stress markers such as MDA and AOPP, and enhanced antioxidant defenses such as SOD and CAT. Additionally, AGM increased the levels of neuroprotective proteins and decreased the loss of DA neurons. The findings suggest that AGM activates cellular pathways that support neuronal health, highlighting its potential as a therapeutic agent for PD by targeting oxidative stress and enhancing neuroplasticity [16].
Activation of Nrf2 Pathway and Suppression of NF-κB Signaling
Dyskinesia and abnormal involuntary movements (AIMs) are significant complications that arise from long-term levodopa treatment in PD patients. They manifest as uncontrollable choreic movements and dystonia [76]. These movements are typically repetitive and can appear as twitching, jerking, or twisting motions. Dyskinesia is often associated with certain neurological disorders, such as PD, and can be a side effect of long-term use of specific medications, notably those used to treat PD, like L-dopa. The movements are involuntary and distinct from the normal voluntary movements that the person intends to make [77, 78].
Nrf2 plays a crucial role in the pathogenesis and potential treatment of PD [79, 80]. Activation of the Nrf2 pathway has been shown to protect dopaminergic neurons by increasing the expression of antioxidant and detoxifying enzymes, thereby reducing oxidative damage and neuronal apoptosis [81, 82]. Studies have demonstrated that compounds such as resveratrol and paeoniflorin exert neuroprotective effects in PD models by activating the Nrf2/HO-1 signaling pathway, which enhances cellular antioxidant capacity and inhibits neuroinflammatory responses [83, 84]. Additionally, Nrf2 activation has been associated with the modulation of autophagy, a process crucial for clearing damaged cellular components, further contributing to neuronal survival in PD [85].
The potential of Nrf2 to protect neurons is also demonstrated in its ability to mitigate mitochondrial dysfunction, a characteristic of PD. Nrf2 accomplishes this by preserving the integrity and function of mitochondri [86].
Overall, targeting the Nrf2 pathway represents a promising therapeutic strategy for alleviating the progression of PD by addressing multiple pathogenic mechanisms simultaneously.
Abnormal activation of NF-κB in microglia and astrocytes leads to the production of pro-inflammatory cytokines, contributing to chronic neuroinflammation, which is a hallmark of PD pathology [87, 88]. NF-κB activation is associated with increased oxidative stress, a key factor in the degeneration of DA neurons in PD [89]. The NF-κB pathway is involved in the regulation of apoptotic pathways, contributing to the death of DA neurons in the SN [56, 89]. NF-κB activation is linked to the accumulation and aggregation of α-synuclein, whisch is a hallmark of PD pathology [57].
Azar et al. reported on the neuroprotective effects of AGM in PD and dyskinesia. PD was induced in rats using ROT, followed by treatment with AGM alone or in combination with L-dopa. The study found that ROT caused significant damage in terms of behavior, neurochemistry, and histopathology, while L-dopa induced dyskinesia. AGM improved motor behavior, reduced dyskinetic movements, and inhibited the expression of NMDA receptors in the SN, leading to a decrease in inflammation and oxidative stress. It also increased the number of dopaminergic neurons and levels of dopamine in the striatum, activated antioxidant defenses through Nrf2, and reduced TBARS. Moreover, AGM suppressed the inflammatory signaling pathway HMGB1/RAGE/TLR4/MYD88/NF-κB, decreased pro-inflammatory cytokines, and reduced neuronal apoptosis. The combination of AGM and L-dopa showed enhanced effects, suggesting that agmatine has potential as a therapeutic agent for PD and dyskinesia by targeting multiple pathways involved in neurodegeneration and inflammation [17].
Protecting Mitochondrial Function
Fourier transform infrared (FTIR) spectroscopy is a powerful analytical technique that plays a crucial role in various fields, including biology, medicine, and materials science. It allows for the identification and characterization of molecular structures by measuring the absorption of infrared radiation by the sample [58]. FTIR spectroscopy has been utilized in several studies to investigate PD, particularly in the analysis of biological samples and the evaluation of drug formulations [59].
In a study described in the source, FTIR was used in the development of a quartz tuning fork-based mass sensitive immunosensor for the detection of alpha-synuclein, a biomarker of PD. The study utilized FTIR to evaluate changes in surface morphology and elemental composition, which are crucial for confirming the functionalization of the sensor surfaces and their interaction with the target protein in cerebrospinal fluid samples [60].
Mitochondrial dysfunction is implicated in the degeneration of DA neurons characteristic of PD. This dysfunction can be triggered by genetic mutations, environmental factors, or a combination of both, leading to impaired mitochondrial dynamics, increased oxidative stress, and neuronal death [61,62,63].
Condello et al. showed that AGM, a neuromodulator, has protective effects in a model of PD using ROT-induced damage in human SH-SY5Y neuroblastoma cells. The study utilized FTIR spectroscopy to characterize the changes in cellular damage and found that AGM significantly reduced redox alterations and preserved the cellular redox state. Additionally, AGM administration suppressed ROT effects on cell viability and mitochondrial membrane potential, indicating its potential as a protective agent against oxidative stress and cell damage. The study also demonstrated that AGM may reduce oxidative damage and stabilize mitochondrial function, providing a basis for further research and potential clinical applications [90]. In another study, Condello et al. demonstrated that AGM reduces ROT-induced cellular injury in a dose-dependent manner by reducing oxidative stress and preventing the dissipation of mitochondrial membrane potential. Unlike spermine, AGM also inhibits the nuclear translocation of NF-κB, a key factor in apoptotic signaling. The research shows that AGM significantly decreases apoptotic markers such as caspase 3 activity, Bax expression, and cytochrome c release. These findings suggest that AGM preserves mitochondrial function and suppresses apoptotic signaling mechanisms, highlighting its potential therapeutic value in treating PD by protecting dopaminergic neurons. The study emphasizes the importance of further exploring agmatine's role in neuroprotection and its mechanisms of action in neurodegenerative diseases [66].
Agmatine's Therapeutic Benefits in MPTP models
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is a neurotoxin that has been widely used to create animal models of PD for studying the mechanisms of neurodegeneration and testing potential therapeutic interventions. MPTP specifically affects DA neurons in the SN, resulting in symptoms that resemble those seen in PD, such as motor deficits and loss of DA neurons [91]. This model has greatly contributed to our understanding of the pathophysiology of PD and the development of treatments.
MPTP itself is not toxic but becomes neurotoxic after being metabolized in the brain to 1-methyl-4-phenylpyridinium (MPP +), primarily by the enzyme monoamine oxidase B (MAO-B) in glial cells. MPP + inhibits mitochondrial complex I, leading to impaired mitochondrial function, increased oxidative stress, and ultimately, DA neuronal death [67, 68]. This mechanism closely mimics the mitochondrial dysfunction observed in PD, providing a link between environmental toxins and genetic susceptibility in the disease.
Matheus et al. explore the potential therapeutic benefits of AGM in a mouse model of PD. The study demonstrates that AGM significantly increases the survival rate of aging mice treated with MPTP, a neurotoxin that induces PD-like symptoms. AGM was administered intraperitoneally at a dose of 30 mg/kg for five consecutive days, which improved the general neurological status of the mice. The treatment also attenuated impairments in social memory and locomotor activity, protected against dopaminergic cell loss in the SNpc, and prevented the MPTP-induced decrease in hippocampal glutamate uptake. These findings suggest that AGM may serve as a promising therapeutic tool for managing both cognitive and motor symptoms of PD, highlighting its neuroprotective properties without altering MAO-B activity. The study underscores the importance of further research into agmatine's mechanisms and potential clinical applications for neurodegenerative diseases like PD [69].
MPP + affects synaptic transmission in the hippocampus by modulating the GABAergic system, which is involved in regulating mood. The acute application of MPP + initially enhances and then reduces synaptic transmission at Schaffer collaterals-CA1 synapses in the hippocampus. This effect is mediated by increased activity of the GABAergic system [92]. In addition, MPP + lesions in mice result in emotional, memory/learning, and striatal neurochemical dysfunctions, which are indicative of depressive-like states [93]. Moretti et al. discovered the potential antidepressant effects of AGM in a mouse model of PD. The study focused on male C57BL6 mice treated with AGM, followed by an intracerebroventricular injection of MPP + , a neurotoxin that mimics PD symptoms. Behavioral assessments, such as the tail suspension test (TST) and splash test, were conducted to evaluate depressive-like behaviors. The results showed that MPP + increased the amount of time the mice spent immobile and induced anhedonic behavior, both of which were prevented by AGM pre-treatment. Additionally, MPP + increased the levels of TH in the striatum, an effect that was also mitigated by AGM. However, neither MPP + nor AGM had an impact on locomotor activity or BDNF levels in the striatum and frontal cortex. The findings suggest that AGM has antidepressant-like effects and may provide protection against neurotoxin-induced depressive behaviors, highlighting its potential as a therapeutic agent for PD-associated depression [94].
Effect of Agmatine on HIF-1α
HIF-1α plays a crucial role in the cellular response to hypoxia and has been increasingly studied for its implications in neurodegenerative diseases, including PD [70, 71].
A study investigated the effectiveness of hydralazine, a medication used to treat high blood pressure, in a cellular model of PD using SH-SY5Y cells exposed to 6-hydroxydopamine (6-OHDA). The study found that hydralazine reduced cell death caused by apoptosis and markers of oxidative stress, while also increasing the expression of HIF-1α and its downstream genes. These findings suggest that activating the hypoxia-signaling pathway could be beneficial in PD [95]. Ferlazzo et al. studied the protective effects of AGM against ROT-induced toxicity in differentiated SH-SY5Y cells, focusing on the role of HIF-1α. The researchers discovered that AGM treatment increased the expression of HIF-1α, a transcription factor involved in cellular responses to hypoxia and neuroprotection. This increase in HIF-1α by AGM was associated with improved cell viability and reduced apoptosis in SH-SY5Y cells exposed to ROT. The study demonstrated that agmatine's neuroprotective effects involve the activation of HIF-1α-mediated pathways, which enhance the expression of target genes that contribute to cell survival and resistance to stress [79].
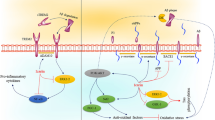
Figure 3 and Table 1 briefly show the neuroprotective effects of AGM in in vivo and in vitro experimental models of PD.
Neuroprotective effects of AGM in in vivo and in vitro experimental models of PD. AGM enhances antioxidant defense by increasing the activity of enzymes like SOD and catalase, and reducing MDA levels. It also interacts with glutamate receptors and modulates NOS activity. AGM exerts anti-inflammatory actions by affecting various inflammatory mediators, thereby reducing inflammation. AGM protects against apoptosis, contributing to neuronal survival. AGM increases the expression of proteins related to neuroplasticity and neuroprotection, such as CREB, BDNF, and eERK1/2. The combined effects of these mechanisms lead to improvements in motor function and reduced neurodegeneration in PD. AGM helps in maintaining the integrity of the BBB and prevents brain edema. AGM activates Nrf2, enhancing antioxidant defense. AGM upregulates HIF-1α, which plays a role in the cellular response to low oxygen levels. Overall, the figure highlights the multifaceted neuroprotective effects of AGM, making it a promising therapeutic agent for PD
How Can the Combination of Agmatine with L-dopa be Effective in the Treatment of Parkinson's Disease?
AGM, an endogenous polyamine, has shown promising neuroprotective effects in various preclinical studies related to PD. When combined with the standard treatment of L-dopa, AGM may provide additional benefits in managing PD symptoms and potentially slowing disease progression. Here are some key points on how AGM can complement L-dopa therapy:
-
1.
Anti-oxidant and anti-inflammatory effects: AGM exhibits potent antioxidant and anti-inflammatory properties, which can help protect dopaminergic neurons from oxidative stress and neuroinflammation, two major pathological processes in PD [47].
-
2.
NMDA receptor modulation: AGM acts as an NMDA receptor antagonist, which can prevent excitotoxicity and neuronal death caused by excessive glutamate levels in PD [51, 72].
-
3.
Mitochondrial protection: AGM has been shown to improve mitochondrial function and prevent mitochondrial dysfunction, a key factor in the degeneration of dopaminergic neurons in PD [47].
Comparison of Agmatine with other Neuroprotective Agents and Clinical Potential
Nucleic Acid-Based Therapies
Nucleic acid-based therapies, including antisense oligonucleotides, microRNA, short interfering RNA, and gene therapy, have shown promise in targeting genetic dysregulations in PD. These therapies primarily focus on downregulating the α-synuclein gene, which is implicated in the formation of Lewy bodies and neurites, key pathological features of PD [73]. While these therapies offer targeted approaches to modify disease progression, they often face challenges related to delivery, stability, and potential off-target effects. Unlike nucleic acid-based therapies, AGM does not require complex delivery systems and has a broader range of protective effects.
Alpha-synuclein Targeting Therapies
Therapies targeting α-synuclein, a protein that aggregates in PD, aim to prevent its pathological accumulation and spread. These include immunotherapies and small molecules designed to inhibit α-synuclein aggregation [98]. Agmatine's broad-spectrum protective effects can complement α-synuclein targeting therapies, potentially enhancing overall neuroprotection.
Polyphenols
-
Antioxidant Activity: Polyphenols such as resveratrol, quercetin, and naringenin neutralize reactive oxygen species (ROS) and reduce oxidative stress, a major factor in PD pathogenesis [76, 99].
-
Anti-Inflammatory Effects: They modulate inflammatory pathways, which can help protect neurons from chronic inflammation seen in PD [77].
-
Mitochondrial Function: Polyphenols like resveratrol enhance mitochondrial function and promote mitophagy, reducing mitochondrial dysfunction [78, 100].
-
Neurotrophic Factors: Polyphenols increase the concentration of neurotrophic factors, aiding in neuronal survival and plasticity [81, 101].
-
Gut-Brain Axis: Polyphenols interact with the gut microbiota, potentially influencing neuroinflammation and neuroprotection indirectly [82, 102].
Both AGM and polyphenols are promising as neuroprotective agents in PD, but they act through different or even similar mechanisms such as anti-inflammatory or antioxidant properties. Further research, especially clinical trials, is needed to fully understand their potential and optimize their use in the treatment of PD.
Potential and Challenges in Clinical Applications
Potential
-
Broad-Spectrum Neuroprotection: Agmatine's ability to reduce oxidative stress, inflammation, and apoptosis makes it a versatile neuroprotective agent.
-
Safety Profile: Preclinical studies suggest that AGM has a favorable safety profile, which could translate to fewer side effects in clinical use.
-
Combination Therapy: Agmatine's mechanisms of action make it a suitable candidate for combination with other neuroprotective agents, potentially enhancing therapeutic outcomes.
Challenges
-
Clinical Efficacy: Demonstrating the clinical efficacy of AGM in PD patients is crucial. This requires well-designed clinical trials to establish its benefits and optimal dosing.
-
Long-Term Safety: Long-term safety studies are necessary to ensure that chronic administration of AGM does not lead to adverse effects.
-
Regulatory Approval: Navigating the regulatory pathways for approval as a treatment for PD involves rigorous testing and validation, which can be time-consuming and costly.
Conclusion
AGM, an endogenous polyamine, has been found to possess neuroprotective properties in various neurological diseases, including PD. Its mechanisms of action include antioxidant, anti-apoptotic, and anti-inflammatory activities, as well as protection of the BBB. In ROT-induced models of PD, AGM has demonstrated the ability to mitigate oxidative damage, alleviate motor impairments, reduce neuronal loss, and increase the expression of neuroplasticity-related proteins in the brain. Additionally, AGM exhibits strong antioxidant properties, enhances the activity of antioxidant enzymes, and reduces levels of oxidative stress markers. It also has anti-inflammatory effects, reducing levels of pro-inflammatory cytokines elevated in PD. AGM inhibits the expression of NMDA receptors, increases the number of nigral TH immunoreactive cells, and enhances the antioxidant defense mechanism, potentially slowing the disease progression and improving patients' quality of life. Furthermore, AGM has shown promise in protecting against mitochondrial dysfunction and stabilizing mitochondrial function, indicating its potential as a protective agent against oxidative stress and cell damage. These findings suggest that AGM may represent a new therapeutic tool for managing cognitive and motor symptoms of PD, in addition to its neuroprotective potential.
Data Availability
No datasets were generated or analysed during the current study.
Abbreviations
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- CREB:
-
CAMP response element-binding protein
- BDNF:
-
Brain-derived neurotrophic factor
- ERK:
-
Extracellular signal-regulated kinase
- HMGB1:
-
High mobility group box 1
- RAGE:
-
Receptor for advanced glycation end products
- TLR2:
-
Toll-like receptor 2
- TLR4:
-
Toll-like receptor 4
- MYD88:
-
Myeloid differentiation primary response 88
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NMDA:
-
N-methyl-D-aspartate
- CRISPR/Cas9:
-
Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated protein 9
- MDA:
-
Malondialdehyde
- NO:
-
Nitric oxide
- TNF-α:
-
Tumor necrosis factor alpha
- GSH:
-
Glutathione
- SOD:
-
Superoxide dismutase
- MMP-2:
-
Matrix metalloproteinase-2
- MMP-9:
-
Matrix metalloproteinase-9
- GABA:
-
Gamma-aminobutyric acid
- Bcl-2:
-
B-cell lymphoma 2
- Bax:
-
Bcl-2-associated X protein
- IL-6:
-
Interleukin 6
- CCL2:
-
C–C motif chemokine ligand 2
- NURR1:
-
Nuclear receptor related 1 protein
References
Kumar, S., L. Goyal, and S. Singh. 2022. Tremor and rigidity in patients with Parkinson’s disease: Emphasis on epidemiology, pathophysiology and contributing factors. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders) 21 (7): 596–609.
Huang, M., et al. 2022. Impact of environmental risk factors on mitochondrial dysfunction, neuroinflammation, protein misfolding, and oxidative stress in the etiopathogenesis of parkinson’s disease. International journal of molecular sciences 23 (18): 10808.
Mani, S., et al. 2021. A systematic review of molecular approaches that link mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurological Sciences 42 (11): 4459–4469.
Johnson, M.E., et al. 2019. Triggers, facilitators, and aggravators: Redefining Parkinson’s disease pathogenesis. Trends in Neurosciences 42 (1): 4–13.
Wang, T., et al. 2022. Neuroinflammation in Parkinson’s disease: Triggers, mechanisms, and immunotherapies. The Neuroscientist 28 (4): 364–381.
Guo, J.D., et al. 2018. Damage to dopaminergic neurons by oxidative stress in Parkinson’s disease. International Journal of Molecular Medicine 41 (4): 1817–1825.
Azar, Y.O., et al. 2022. Agmatine-mediated inhibition of NMDA receptor expression and amelioration of dyskinesia via activation of Nrf2 and suppression of HMGB1/RAGE/TLR4/MYD88/NF-κB signaling cascade in rotenone lesioned rats. Life Sciences 311: 121049.
Kosonen, R., et al. 2021. Role of agmatine in the application of neural progenitor cell in central nervous system diseases: Therapeutic potentials and effects. Anatomy & Cell Biology 54 (2): 143.
Williams, B.J., et al. 2010. Discovery of an operon that participates in agmatine metabolism and regulates biofilm formation in Pseudomonas aeruginosa. Molecular Microbiology 76 (1): 104–119.
Piletz, J.E., et al. 2013. Agmatine: Clinical applications after 100 years in translation. Drug Discovery Today 18 (17–18): 880–893.
Ikeuchi, Y., et al. 2010. Agmatine-conjugated cytidine in a tRNA anticodon is essential for AUA decoding in archaea. Nature Chemical Biology 6 (4): 277–282.
Galea, E., et al. 1996. Inhibition of mammalian nitric oxide synthases by agmatine, an endogenous polyamine formed by decarboxylation of arginine. Biochemical Journal 316 (1): 247–249.
Uzbay, T.I. 2012. The pharmacological importance of agmatine in the brain. Neuroscience & Biobehavioral Reviews 36 (1): 502–519.
Laube, G., and H.-G. Bernstein. 2017. Agmatine: Multifunctional arginine metabolite and magic bullet in clinical neuroscience? Biochemical Journal 474 (15): 2619–2640.
Halaris, A., and J.E. Piletz. 2003. Relevance of imidazoline receptors and agmatine to psychiatry: A decade of progress. Annals of the new York Academy of Sciences 1009 (1): 1–20.
Saha, P., et al. 2023. Neuroprotection by agmatine: Possible involvement of the gut microbiome? Ageing Research Reviews 91:102056.
Rafi, Hira, Hamna Rafiq, and Muhammad Farhan. 2024. Pharmacological profile of agmatine: An in-depth overview. Neuropeptides 105:102429.
Akasaka, N., et al. 2018. Agmatine production by Aspergillus oryzae is elevated by low pH during solid-state cultivation. Applied and Environmental Microbiology 84 (15): e00722-e818.
Özdestan, O.Z.l. and A. Uren. 2010. Biogenic amine content of shalgam (salgam): a traditional lactic acid fermented Turkish beverage. Journal of agricultural and food chemistry 58(4): 2602–2608.
Flynn, N., et al. 2002. The metabolic basis of arginine nutrition and pharmacotherapy. Biomedicine & Pharmacotherapy 56 (9): 427–438.
Molderings, G.J., and B. Haenisch. 2012. Agmatine (decarboxylated L-arginine): Physiological role and therapeutic potential. Pharmacology & Therapeutics 133 (3): 351–365.
Wang, W., H.D. Snooks, and S. Sang. 2020. The chemistry and health benefits of dietary phenolamides. Journal of Agricultural and Food Chemistry 68 (23): 6248–6267.
Arena, M., et al. 2011. Expression of Lactobacillus brevis IOEB 9809 tyrosine decarboxylase and agmatine deiminase genes in wine correlates with substrate availability. Letters in Applied Microbiology 53 (4): 395–402.
Murakami, Y., et al. 2024. Identification and enzymatic properties of arginine decarboxylase from Aspergillus oryzae. Applied and Environmental Microbiology 90 (5): e00294-e324.
Wang, J., et al. 2020. Effects of NaCl on gene expression of agmatine deiminase pathway genes of putrescine in Lactobacillus delbrueckii and Escherichia coli. Journal of Food Processing and Preservation 44 (11): e14875.
Giles, T.N., and D.E. Graham. 2007. Characterization of an acid-dependent arginine decarboxylase enzyme from Chlamydophila pneumoniae. Journal of bacteriology 189 (20): 7376–7383.
Liao, S., et al. 2008. Occurrence of agmatine pathway for putrescine synthesis in Selenomonas ruminatium. Bioscience, biotechnology, and biochemistry 72 (2): 445–455.
Bilge, S.S., et al. 2020. Neuroprotective action of agmatine in rotenone-induced model of Parkinson’s disease: Role of BDNF/cREB and ERK pathway. Behavioural Brain Research 392: 112692.
Šebela, M., M. Tylichová, and P. Peč. 2007. Inhibition of diamine oxidases and polyamine oxidases by diamine-based compounds. Journal of Neural Transmission 114: 793–798.
Katariya, R., et al. 2024. Agmatine mitigates behavioral abnormalities and neurochemical dysregulation associated with 3-Nitropropionic acid-induced Huntington’s disease in rats. Neurotoxicology 102: 12–28.
Li, B., and S. Lu. 2020. The importance of amine-degrading enzymes on the biogenic amine degradation in fermented foods: A review. Process Biochemistry 99: 331–339.
Tan, Z., et al. 2022. Advances in the clinical application of histamine and diamine oxidase (DAO) activity: A review. Catalysts 13 (1): 48.
Shaff, R.E., and M.A. Beaven. 1976. Turnover and synthesis of diamine oxidase (DAO) in rat tissues. Studies with heparin and cycloheximide. Biochemical Pharmacology. 25 (9): 1057–1062.
Mayo-Yáñez, M., et al. 2023. Diamine Oxidase Activity Deficit and Idiopathic Rhinitis: A New Subgroup of Non-Allergic Rhinitis? Life 13 (1): 240.
Benítez, J., et al. 2018. Metabolic strategies for the degradation of the neuromodulator agmatine in mammals. Metabolism 81: 35–44.
Raghavan, S.A., and M. Dikshit. 2004. Vascular regulation by the L-arginine metabolites, nitric oxide and agmatine. Pharmacological Research 49 (5): 397–414.
Barua, S., et al. 2019. Therapeutic effect of agmatine on neurological disease: Focus on ion channels and receptors. Neurochemical Research 44: 735–750.
Song, H.W., et al. 2011. Agmatine enhances neurogenesis by increasing ERK1/2 expression, and suppresses astrogenesis by decreasing BMP 2, 4 and SMAD 1, 5, 8 expression in subventricular zone neural stem cells. Life Sciences 89 (13–14): 439–449.
Fairbanks, C.A., et al. 2000. Agmatine reverses pain induced by inflammation, neuropathy, and spinal cord injury. Proceedings of the National Academy of Sciences 97 (19): 10584–10589.
Guerra, G.P., M.A. Rubin, and C.F. Mello. 2016. Modulation of learning and memory by natural polyamines. Pharmacological Research 112: 99–118.
Zhang, Y., et al. 2021. Agmatine and glycolipid metabolism. Zhong nan da xue xue bao. Yi xue ban= Journal of Central South University. Medical Sciences. 46 (8): 889–893.
Kotagale, N., et al. 2020. Agmatine ameliorates manifestation of depression-like behavior and hippocampal neuroinflammation in mouse model of Alzheimer’s disease. Brain Research Bulletin 160: 56–64.
Kotagale, N.R., B.G. Taksande, and N.N. Inamdar. 2019. Neuroprotective offerings by agmatine. Neurotoxicology 73: 228–245.
Katariya, R.A., et al. 2024. Agmatine as a Novel Intervention for Alzheimer’s Disease: Pathological Insights and Cognitive Benefits. Ageing Research Reviews 96: 102269.
de Souza, A.C.G., et al. 2018. Agmatine attenuates depressive-like behavior and hippocampal oxidative stress following amyloid β (Aβ1-40) administration in mice. Behavioural Brain Research 353: 51–56.
Arndt, M.A., et al. 2009. The arginine metabolite agmatine protects mitochondrial function and confers resistance to cellular apoptosis. American Journal of Physiology-Cell Physiology 296 (6): C1411–C1419.
Xu, W., et al. 2018. Neuroprotective role of agmatine in neurological diseases. Current Neuropharmacology 16 (9): 1296–1305.
Mun, C.H., et al. 2010. Regulation of endothelial nitric oxide synthase by agmatine after transient global cerebral ischemia in rat brain. Anatomy & Cell Biology 43 (3): 230.
Gawali, N.B., et al. 2017. Agmatine attenuates chronic unpredictable mild stress-induced anxiety, depression-like behaviours and cognitive impairment by modulating nitrergic signalling pathway. Brain Research 1663: 66–77.
Schuster, S., et al. 2008. The 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor lovastatin reduces severity of L-DOPA-induced abnormal involuntary movements in experimental Parkinson’s disease. Journal of Neuroscience 28 (17): 4311–4316.
Ferlazzo, N., et al. 2020. Up-regulation of HIF-1α is associated with neuroprotective effects of agmatine against rotenone-induced toxicity in differentiated SH-SY5Y cells. Amino Acids 52: 171–179.
Kim, J.M., et al. 2016. The anti-inflammatory effects of agmatine on transient focal cerebral ischemia in diabetic rats. Journal of Neurosurgical Anesthesiology 28 (3): 203–213.
Sezer, A., et al. 2014. Neuroprotective effects of agmatine ın experimental peripheral nerve ınjury ın rats: a prospective randomized and placebo-controlled trial. https://doi.org/10.5137/1019-5149.JTN.7324-12.1
Dhokne, M.D., et al. 2023. Agmatine as a Novel Treatment Option for Neuropathies: Experimental Evidences. INNOSC Theranostics and Pharmacological Sciences 5 (2): 1–10.
França, A.P., et al. 2022. Agmatine improves olfactory and cognitive deficits in Spontaneously Hypertensive Rats (SHR): An animal model of Attention Deficit Hyperactivity Disorder (ADHD). Behavioral Neuroscience 136 (2): 139.
Chou, T.-W., et al. 2021. Fibrillar α-synuclein induces neurotoxic astrocyte activation via RIP kinase signaling and NF-κB. Cell Death & Disease 12 (8): 756.
Alrouji, M., et al. 2023. NF-κB/NLRP3 inflammasome axis and risk of Parkinson’s disease in Type 2 diabetes mellitus: A narrative review and new perspective. Journal of Cellular and Molecular Medicine 27 (13): 1775–1789.
Chen, Y., et al. 2015. Applications of micro-fourier transform infrared spectroscopy (FTIR) in the geological sciences—a review. International Journal of Molecular Sciences 16 (12): 30223–30250.
Araki, K., et al. 2015. Synchrotron FTIR micro-spectroscopy for structural analysis of Lewy bodies in the brain of Parkinson’s disease patients. Scientific Reports 5 (1): 17625.
Karaboğa, M.N.S., et al. 2024. An innovative method for the detection of alpha synuclein, a potential biomarker of Parkinson’s disease: Quartz tuning fork-based mass sensitive immunosensor design. Physical Chemistry Chemical Physics 26 (6): 5106–5114.
Moradi Vastegani, S., et al. 2023. Mitochondrial dysfunction and Parkinson’s disease: Pathogenesis and therapeutic strategies. Neurochemical Research 48 (8): 2285–2308.
Ahn, E.H., et al. 2021. Mitochondrial dysfunction triggers the pathogenesis of Parkinson’s disease in neuronal C/EBPβ transgenic mice. Molecular Psychiatry 26 (12): 7838–7850.
Blagov, A., et al. 2024. Significance of Mitochondrial Dysfunction in the Pathogenesis of Parkinson’s Disease. Frontiers in Bioscience-Landmark 29 (1): 36.
Keynan, O., et al. 2010. Safety and efficacy of dietary agmatine sulfate in lumbar disc-associated radiculopathy An open-label, dose-escalating study followed by a randomized, double-blind, placebo-controlled trial. Pain Medicine 11 (3): 356–368.
Gilad, G.M., and V.H. Gilad. 2014. Long-term (5 years), high daily dosage of dietary agmatine—evidence of safety: A case report. Journal of medicinal food 17 (11): 1256–1259.
Condello, S., et al. 2011. Agmatine effects on mitochondrial membrane potential andNF-κB activation protect against rotenone-induced cell damage in human neuronal-like SH-SY5Y cells. Journal of Neurochemistry 116 (1): 67–75.
Schapira, A. 1994. Evidence for mitochondrial dysfunction in Parkinson’s disease—a critical appraisal. Movement Disorders 9 (2): 125–138.
Sayre, L., et al. 1986. Mechanism of induction of Parkinson’s disease by 1 methyl-4-phenyl-1,2, 3, 6-tetrahydropyridine (MPTP). Chemical and electrochemical characterization of a geminal-dimethyl-blocked analog of a postulated toxic metabolite. Journal of the American Chemical Society 108 (9): 2464–2466.
Matheus, F.C., et al. 2012. Neuroprotective effects of agmatine in mice infused with a single intranasal administration of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP). Behavioural brain research 235 (2): 263–272.
Feng, Y., et al. 2014. Neuroprotection by Orexin-A via HIF-1α induction in a cellular model of Parkinson’s disease. Neuroscience letters 579: 35–40.
Mehrabani, M., et al. 2020. Protective effect of hydralazine on a cellular model of Parkinson’s disease: A possible role of hypoxia-inducible factor (HIF)-1α. Biochemistry and Cell Biology 98 (3): 405–414.
Gilad, G.M., et al. 2005. Neurochemical evidence for agmatine modulation of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) neurotoxicity. Neurochemical Research 30: 713–719.
Pandey, S.K., and R.K. Singh. 2022. Recent developments in nucleic acid-based therapies for Parkinson’s disease: Current status, clinical potential, and future strategies. Frontiers in Pharmacology 13: 986668.
Xu, X., et al. 2022. CREB inactivation by HDAC1/PP1γ contributes to dopaminergic neurodegeneration in Parkinson’s disease. Journal of Neuroscience 42 (22): 4594–4604.
Palasz, E., et al. 2020. BDNF as a promising therapeutic agent in Parkinson’s disease. International journal of molecular sciences 21 (3): 1170.
Singh, A., et al. 2020. Promising polyphenols in Parkinson’s disease therapeutics. Neurochemical Research 45: 1731–1745.
Aquilano, K., et al. 2008. Role of nitric oxide synthases in Parkinson’s disease: A review on the antioxidant and anti-inflammatory activity of polyphenols. Neurochemical Research 33: 2416–2426.
Ferretta, A., et al. 2014. Effect of resveratrol on mitochondrial function: implications in parkin-associated familiar Parkinson’s disease. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 1842 (7): 902–915.
Fan, W., and J. Zhou. 2023. Icariside II suppresses ferroptosis to protect against MPP+-Induced Parkinson’s disease through Keap1/Nrf2/GPX4 signaling. Journal of Physiological Investigation 66 (6): 437–445.
Rehman, I.U., et al. 2022. Neuroprotective effects of nicotinamide against mptp-induced parkinson’s disease in mice: Impact on oxidative stress, neuroinflammation, nrf2/ho-1 and tlr4 signaling pathways. Biomedicines 10 (11): 2929.
Uddin, M.S., et al. 2020. Neuroprotective role of polyphenols against oxidative stress-mediated neurodegeneration. European Journal of Pharmacology 886: 173412.
Riegelman, E., et al. 2024. Gut-Brain Axis in Focus: Polyphenols, Microbiota, and Their Influence on α-Synuclein in Parkinson’s Disease. Nutrients 16 (13): 2041.
Zamanian, M.Y., et al. 2023. Targeting Nrf2 signaling pathway and oxidative stress by resveratrol for Parkinson’s disease: an overview and update on new developments. Molecular Biology Reports 50 (6): 5455–5464.
Zhang, J., et al. 2023. Paeoniflorin protects 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced Parkinson’s disease mice by inhibiting oxidative stress and neuronal apoptosis through activating the Nrf2/HO-1 signaling pathway. Neuroreport 34 (5): 255–266.
Shirgadwar, S.M., et al. 2023. Neuroprotective effect of Phloretin in rotenone-induced mice model of Parkinson’s disease: modulating mTOR-NRF2-P62 mediated autophagy-oxidative stress crosstalk. Journal of Alzheimer's Disease 94 (s1): S109–S124.
Bento-Pereira, C., and A.T. Dinkova-Kostova. 2021. Activation of transcription factor Nrf2 to counteract mitochondrial dysfunction in Parkinson’s disease. Medicinal Research Reviews 41 (2): 785–802.
Singh, S.S., et al. 2020. NF-κB-mediated neuroinflammation in Parkinson’s disease and potential therapeutic effect of polyphenols. Neurotoxicity Research 37: 491–507.
Li, T., et al. 2023. Neferine exerts anti-inflammatory activity in BV-2 microglial cells and protects mice with MPTP-induced Parkinson’s disease by inhibiting NF-κB activation. Molecular Medicine Reports 28 (6): 1–9.
Guo, L., et al. 2022. Shikonin ameliorates oxidative stress and neuroinflammation via the Akt/ERK/JNK/NF-κB signalling pathways in a model of Parkinson’s disease. Clinical and Experimental Pharmacology and Physiology 49 (11): 1221–1231.
Condello, S., et al. 2012. Protective effects of agmatine in rotenone-induced damage of human SH-SY5Y neuroblastoma cells: Fourier transform infrared spectroscopy analysis in a model of Parkinson’s disease. Amino Acids 42: 775–781.
Qureshi, Hamid Y., and Hemant K. Paudel. 2011. Parkinsonian neurotoxin 1-methyl-4-phenyl-1, 2, 3, 6- tetrahydropyridine (MPTP) and α-synuclein mutations promote tau protein phosphorylation at Ser262 and destabilize microtubule cytoskeleton in vitro. Journal of Biological Chemistry 286 (7): 5055–5068.
Suzuki, E., and T. Okada. 2007. Regional differences in GABAergic modulation for TEA-induced synaptic plasticity in rat hippocampal CA1, CA3 and dentate gyrus. Neuroscience Research 59 (2): 183–190.
Cunha, M.P., et al. 2017. MPP+-lesioned mice: an experimental model of motor, emotional, memory/learning, and striatal neurochemical dysfunctions. Molecular Neurobiology 54:6356–6377.
Moretti, M., et al. 2015. Effects of agmatine on depressive-like behavior induced by intracerebroventricular administration of 1-Methyl-4-phenylpyridinium (MPP+). Neurotoxicity research 28: 222–231.
Mehrabani, M., et al. 2020. Protective effect of hydralazine on a cellular model of Parkinson’s disease: a possible role of hypoxia-inducible factor (HIF)-1α. Biochemistry and Cell Biology 98 (3): 405–414.
Condello, S., et al. 2012. Protective effects of agmatine in rotenone-induced damage of human SH-SY5Y neuroblastoma cells: Fourier transform infrared spectroscopy analysis in a model of Parkinson’s disease. Amino Acids 42: 775–781.
Condello, S., et al. 2011. Agmatine effects on mitochondrial membrane potential andNF-κB activation protect against rotenone-induced cell damage in human neuronal-like SH-SY5Y cells. Journal of Neurochemistry 116 (1): 67–75.
Xu, L. and J. Pu. 2016. Alpha-synuclein in Parkinson’s disease: from pathogenetic dysfunction to potential clinical application. Parkinsons Dis. 2016: 1720621. https://pubmed.ncbi.nlm.nih.gov/27610264
Kujawska, M., and J. Jodynis-Liebert. 2018. Polyphenols in Parkinson’s disease: A systematic review of in vivo studies. Nutrients 10 (5): 642.
Kung, H.-C., et al. 2021. Oxidative stress, mitochondrial dysfunction, and neuroprotection of polyphenols with respect to resveratrol in Parkinson’s disease. Biomedicines 9 (8): 918.
Arias-Sánchez, R.A., L. Torner, and B. Fenton Navarro. 2023. Polyphenols and neurodegenerative diseases: potential effects and mechanisms of neuroprotection. Molecules 28 (14): 5415.
Zhang, Y., et al. 2022. The interaction of polyphenols and the gut microbiota in neurodegenerative diseases. Nutrients 14 (24): 5373.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
M.Y.Z, N.N and M.N: conception, design, writing, and revising the manuscript. L.G.K, N.T and M.S.I: revising and editing the manuscript and graphic drawing. S.V.M and B.H: data gathering and editing the manuscript. K.P and I.PV were involved in gathering data, preparing the initial draft of the manuscript, and creating the tables. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethical Approval and Informed Consent from Patients
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zamanian, M.Y., Nazifi, M., Khachatryan, L.G. et al. The Neuroprotective Effects of Agmatine on Parkinson’s Disease: Focus on Oxidative Stress, Inflammation and Molecular Mechanisms. Inflammation (2024). https://doi.org/10.1007/s10753-024-02139-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10753-024-02139-7