Abstract
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by systemic polyarticular pain, and its main pathological features include inflammatory cell infiltration, synovial fibroblast proliferation, and cartilage erosion. Immune cells, synovial cells and neuroendocrine factors play pivotal roles in the pathophysiological mechanism underlying rheumatoid arthritis. Biological clock genes regulate immune cell functions, which is linked to rhythmic changes in arthritis pathology. Additionally, the interaction between biological clock genes and neuroendocrine factors is also involved in rhythmic changes in rheumatoid arthritis. This review provides an overview of the contributions of circadian rhythm genes to RA pathology, including their interaction with the immune system and their involvement in regulating the secretion and function of neuroendocrine factors. A molecular understanding of the role of the circadian rhythm in RA may offer insights for effective disease management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
BACKGROUND
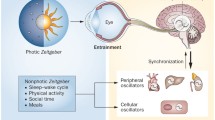
Rheumatoid arthritis (RA) is a chronic inflammatory disease with an unknown etiology and a high disability rate characterized by inflammatory cell infiltration, synovial fibroblast proliferation, and cartilage erosion [1,2,3,4]. Although both genetic and environmental factors have been proven to contribute to the development of RA [5,6,7,8,9], the underlying mechanisms remain unclear. The progression of RA involves two interactional events: genetic susceptibility leads to an exacerbated immune response, and the presence of infection or injury factors leads to abnormal humoral and cellular immune responses that destroy autologous tissues [3, 6, 7]. Recent research has focused on the role of the biological clock in autoimmune diseases [8,9,10]. Loss of clock genes induces a proinflammatory response that increases the incidence of autoimmune disease [8, 11], abnormal circadian clock has an impact on the function of immune cells [12, 13]. RA patients exhibits diurnal variation in symptoms such as morning stiffness that improves after exercise [14, 15]. Joint pain and stiffness patterns in RA patients correlate with the secretion levels of inflammatory factors [16, 17]. In the serum of patients with rheumatoid disease, IL-6 lever shows circadian oscillations and peaks in the early morning [16, 17]. Moreover, the peak serum level of circulating melatonin in RA patients appears earlier at night, and the morning peak in cortisol levels occurs later than that of inflammatory factors [17, 18] (Fig. 1A). Interestingly, night shift workers who experience inverted day‒night cycles have a greater incidence of rheumatoid arthritis [19,20,21]. The body's internal clock is controlled by the central clock, which relays information to peripheral clocks throughout the body to control physiological functions, including sleep–wake cycle regulation, heart rate, blood pressure, hormoneproduction, and immunity [22,23,24,25]. This review provides a comprehensive overview of the impact of the biological clock on the progression of rheumatoid arthritis and investigates the molecular mechanisms underlying the circadian rhythm to identify potential targets for the prevention and treatment of this disease.
Rheumatoid arthritis exhibits daily fluctuations in symptoms and inflammatory markers, while time therapy can effectively ameliorate the condition (by Figdraw). A: In the serum of patients with rheumatoid disease, IL-6 level shows circadian oscillations and peaks in the early morning, which was similar to the daily change of morning stiffness symptoms in RA patients. The symptoms of morning stiffness in patients with RA are alleviated following early exercise, which is associated with the diurnal peak of cortisol levels. B: Modified release (delayed-release) prednisone given at 22 o'clock in the evening is more effective in relieving arthritis than immediate-release prednisone given at 6–8 o'clock in the morning. Modified release (delayed-release) prednisone Compared with immediate-release prednisone in the morning, modified release prednisone in the evening can significantly improve joint inflammation for a duration 12 months). IR-treatment: immediate-release prednisone treatment; MR-treatment: modified-release prednisone treatment.
THE CIRCADIAN CLOCK
Light and nutrients are crucial external factors for the entrainment of organism biorhythms. The oscillatory nature of the circadian clock serves as the physiological foundation for the activity of an organism aligns with the environmental changes that occur over a 24-h period. The hypothalamic suprachiasmatic nucleus (SCN) has been identified as the primary circadian pacemaker driving behavior. Light stimulates the retina, which transmits the visual information back to the SCN [26]. The peripheral clocks in each organ receive instructions from the brain to regulate cyclic metabolic activities. While these biological rhythms in peripheral organs are synchronized by the central clock, they can also be entrained by external environmental cues such as temperature, light sources, and nutrition [27, 28]. The molecular mechanism underlying the biological clock involves a self-regulating negative-feedback transcription network (Fig. 2) [27, 29]. At its core are two genes, circadian locomotor output cycle kaput (CLOCK) and brain and muscle ARNT-like 1 (BMAL1), which encode proteins that dimerize and act as transcriptional activators that bind to E-box elements on the promoters of clock genes period 1/2/3 (PER1/2/3) and cryptochrome 1/2 (CRY1/2) [30]. Transcribed PER and CRY produce protein dimers that enter the nucleus, providing negative feedback to regulate CLOCK/BMAL1 transcription. The degradation of PER and CRY relieve the inhibition of CLOCK/BMAL1 transcription, and CLOCK/BMAL1 initiate a new circadian transcription cycle. This core circadian clock transcription circuit is typically reinforced by a secondary circuit involving nuclear receptor subfamily 1 group D member (NR1D1/2, encoding reverse-erythroblastosis virus α/β (REV-ERBα/β)) and retinoic acid-related orphan receptors α/β/γ (RORα/β/γ) activated by the CLOCK/BMAL1 complex. RORs compete with REV-ERBs for activating or repressing BMAL1 transcription through their action on specific DNA sequences, terms ROR response elements (RORES), located on the Bmal1 promoter region. The biorhythm feedback loop is also modulated by additional transcription factors, including the Albumin gene D-site binding protein (DBP), which is differentially expressed in chondrocytes protein (DEC), E4 protein-binding protein (E4BP4), Hepatic leukemia factor (HLF), Thyrotroph embryonic factor (TEF), and nuclear factor interleukin-3 regulated (NFIL3) [31,32,33]. These integrated feedback loops collectively orchestrate the precise regulation of biorhythms.
A molecular clock is the basis for regulating the circadian rhythm of the body (by Figdraw). The central clock in the SCN and peripheral clocks in many other tissues have a clock based on autoregulatory feedback loops. External factors, such as temperature, light source, and food, exert an influence on the central clock regulator of the suprachiasmatic nucleus (SCN), thereby modulating the peripheral clock in peripheral tissues. The peripheral tissue's biological clock loop is capable of perceiving and adapting to changes in the external environment. This clock is centered on BMAL1 and CLOCK. CLOCK/BMAL1 heterodimers act as transcriptional activators binding to E-box elements on promoters of PER and CRY. The transcribed PER and CRY proteins enter the nucleus, form a multimeric complex and inhibit CLOCK/BMAL-mediated transcription. This core circadian clock transcription circuit is typically reinforced by a secondary circuit involving REV-ERBs and ROR activated by CLOCK/BMAL1 complex. REV-ERB and ROR proteins compete for a response element (RORE) on the promoter of BMAL1, thereby repressing and activating transcription accordingly. BMAL1: Brain and muscle ARNT-like 1; CLOCK: Circadian locomotor output cycles kaput; PER: Period; CRY: Cryptochrome; NR1D: nuclear receptor subfamily 1 group D member; REV-ERB: Reverse-erythroblastosis virus; ROR: Retinoic acid-related orphan receptors; RORE: ROR response element
THE CIRCADIAN CLOCK IN IMMUNITY
The immune system, which is composed of an intricate network of cells and effector molecules, aims to protect the body and eliminate of pathogens. Autoimmunity triggered by overactivity in the adaptive immune system, specifically autoreactive B and T cells, has long been recognized as the underlying cause of a number of autoimmune diseases [34, 35]. The crucial role of early innate immune responses in the development of immune-mediated inflammation in general and autoimmunity in particular has received increasing attention [36,37,38]. The innate immune system is a complex network consisting of structured cells/proteins such as antigen-presenting cells (macrophages and dendritic cells), the complement cascade, and numerous receptors/cytokines/proteins [39,40,41]. Proinflammatory cytokines, including interleukin-6 (IL-6), IL-1β, C–C-motif chemokine ligand 5 (CCL5), and C-X-C-motif chemokine ligand 1 (CXCL1), have been found to exhibit daily rhythms in CIA mice [42]. Moreover, multiple types of innate immune cells have been shown to possess intrinsic clocks that regulate their biological rhythm [43,44,45,46,47,48]. These cell-specific internal oscillators participate in the immune response. In addition, B cell development, the BCR-signaling pathway, and C1q expression are regulated by circadian clock CRY proteins and that their dysregulation through loss of CRY contributes to autoimmunity [49]. Hirose et al. Found a treatment and cell-specific effect on the T cell clock function of chronic inflammation in a mouse model of autoimmune encephalomyelitis (EAE) [50]. Therefore, it is of great significance to study the relationship between biological clock and immune system for autoimmune diseases including rheumatoid arthritis.
The Circadian Clock in Innate Immunity
Natural Killer Cells
Natural killer (NK) cells are large granular lymphocytes derived from normal lymphoid progenitor cells [51, 52]. NK cell cytotoxicity and NK-derived cytokines play important roles in regulating immune responses and contributing to the pathogenesis of various immune-mediated diseases, including ankylosing spondylitis (AS), Behçet's disease (BD), multiple sclerosis (MS), rheumatoid arthritis (RA), psoriasis, systemic lupus erythematosus (SLE), and type 1 diabetes (T1D) [38]. During RA development, there is an increase in the NK cell population within inflamed joints, which is positively correlated with histopathological changes and bone destruction [53]. Arjona et al. demonstrated that the cytolytic activity of NK cells in the spleen exhibits a diurnal rhythm, synchronized with oscillations of biological clock genes [54, 55]. Period circadian protein homolog 1 (PER1) is essential for the optimal functioning of splenic NK cells. In the absence of PER1, there are alterations in the rhythm of IFNγ expression, as well as the expression of the cytotoxic factors perforin and granzyme B, which subsequently impact NK cell-mediated killing and immune regulatory functions [56]. Compared to wild-type mice, PER2-deficient mice exhibited enhanced resistance to LPS-induced endotoxic shock. The absence of the PER2 gene resulted in impaired functionality of NK and NKT cells [38].
Neutrophils
Neutrophils, as the primary cellular responders to acute pathogenic insults, possess a diverse array of antimicrobial mechanisms. They primarily recognize molecules classified as either pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) through various pattern recognition receptors, including Toll-like receptors (TLRs) [41]. The pathogen-fighting armory of neutrophils include phagocytosis, degranulation, oxidative bursts, and the formation of neutrophil extracellular traps (NETs). Neutrophils drive phagocytic function, produce superoxide, express cell adhesion molecules with definite rhythm changes, and express clock molecular oscillators in cells [57, 58]. Notably, the clock genes PER1, DBP and NR1D1 in neutrophils exhibit rhythmic expression patterns; meanwhile Bmal1 expression—an essential regulator of circadian clock genes is significantly increased. This finding suggested that neutrophils are capable of self-regulating their biological rhythm. Furthermore, In a human study, endotoxin administration decreases the expression of clock genes (PER3, REV-ERBs, CLOCK, CRY and ROR) in neutrophils [59]. Adrover et al. reported that the Bmal1 gene regulates CXCL2 chemokine expression to induce diurnal changes in transcriptional and migratory properties dependent on CXCR2 chemokine receptor signaling in circulating neutrophils [57]. By activating the CXCR2 signaling pathway in an autocrine manner, CXCL2 facilitates transcriptional and phenotypic alterations associated with neutrophil aging (neutrophils that have remained the longest in the circulation and are primed for clearance) [57, 60, 61]. Bmal1 and CXCR2 promoted diurnal neutrophil aging, but this effect was prevented by CXCR4 [57]. This process of neutrophil aging has been proposed to favor a proinflammatory phenotype that predisposes patients to vascular inflammation [61]. Additionally, the deletion of Bmal1 renders mice more susceptible to infection at night [57]. These findings demonstrated that the coordinated diurnal compartmentalization of neutrophils by an internal timer plays a crucial role in immune defense and vascular protection [50]. Consequently, Bmal1 regulates the molecular mechanisms underlying neutrophil aging to maintain the homeostasis of neutrophil number and phenotype in the circulatory system, thereby ensuring a delicate balance between antimicrobial defense and vascular health. Moreover, it was observed that the phagocytic activity of neutrophils shows low-amplitude oscillation throughout the day and peaks at the beginning of the resting phase, further suggesting that neutrophil responsiveness is governed by circadian rhythms [62]. Deletion of Bmal1 specifically in pulmonary airway epithelial cells results in enhanced infiltration of neutrophils, leading to impaired lung compliance, reduced elasticity, and fibrotic scar formation [56]. Notably, the bronchiole-specific ablation of Bmal1 disrupted the rhythmic expression of CXCL5, a key chemotactic factor for recruiting neutrophils, exacerbating the inflammatory response to lipopolysaccharide and compromising host defense against Streptococcus pneumoniae infection [63].
Macrophages
The presence of macrophages in synovial tissue is a crucial biomarker for RA disease activity, prognosis, and therapeutic response [64]. Huang et al.investigated that synovial tissue–resident macrophage niche in joint homeostasis played a vital role in repressing chronic joint inflammation in a mouse model of rheumatoid arthritis, in which Flip is deleted under the control of a CD11c promoter (HUPO mice) [65]. Distinct synovial tissue macrophage subsets may serve as pivotal factors in the progression and recovery of rheumatoid arthritis (RA), either by inducing an inflammatory response in synovial fibroblasts or promoting the conversion of fibroblast-like synoviocytes (FLSs) toward a reparative phenotype [66]. The immune functions of macrophages, including phagocytosis, cytokine production and antibacterial/antiviral activities, are tightly regulated by biological rhythms. Recently, studies have revealed that the proinflammatory factors suppresses PER2 rhythmicity in bone marrow-derived macrophages, while anti-inflammatory signals increase the amplitude of PER2 expression [67]. This suggests that the circadian clock responds to various immune-associated stimuli by regulating gene expression [66]. Furthermore, the deletion of Bmal1 in macrophages disrupts the activity of NRF2, a transcription factor crucial for regulating antioxidant proteins and protecting cells against oxidative damage caused by ROS-induced injury or inflammation. This disruption facilitates the accumulation of reactive oxygen species and IL-1β, triggering an immune response [11]. Similarly, Alexander et al. discovered that Bmal1 can serve as a checkpoint to integrate macrophage mitochondrial metabolism, mediate ROS production, and induce inflammatory damage [67]. Upon IFN-γ or LPS stimulation, myeloid-specific Bmal1 knockout renders macrophages unable to maintain immune function [67]. In addition to directly regulating the expression and activation of the NLRP3 gene, Bmal1 also controls the secretion of IL-1B and IL-18 within macrophages in an NLRP3-dependent manner [68]. Importantly, compared to that in normal synovial tissue samples, the synovial lining in RA patients is predominantly composed of increased numbers of macrophages [69]. Moreover, clinically effective therapies for rheumatoid arthritis also reduce macrophage populations within affected joints [70]. Anti-cytokine bDMARDs (anti-TNF agents) downregulated surface markers and cytokines associated with the inflammatory phenotype in macrophages, and promoted phagocytosis and negative feedback of inflammation [71]. The circadian rhythm governs the inflammatory response of macrophages, potentially influencing the progression of rheumatoid arthritis.
Dendritic Cells
Dendritic cells (DCs), professional antigen-presenting cells (APCs) uniquely capable of priming naïve T cells, have been identified as crucial mediators bridging innate and adaptive immunity [38, 39]. Through cytokine production and the presentation of arthritis-related antigens, DCs play a joint role in activating the transformation of naïve T cells into self-reactive Th17 cells and participate in the pathogenesis of RA [72,73,74]. Additionally, DCs contribute significantly to leukocyte infiltration into synovial tissue [75]. Deletion of Bmal1 reduces mitochondrial metabolism in DCs, resulting in decreased antigen processing by bone marrow-derived dendritic cells and a lack of rhythmicity [76]. This reduced antigen processing has a biological effect on T cells with significantly decreased IFN-γ production. Holtkamp et al. demonstrated that migration of CD11c + DCs to cutaneous lymphatic vessels is driven by endogenous circadian rhythm rather than just responding to the circadian rhythm environment [77]. The chemotactic factors CCL21, CCR7, and LYVE-1 are directly controlled by the circadian clock gene BMAL1, indicating that the clock plays an essential and broad regulatory role in DC migration [77].
The Circadian Clock in The Adaptive Immune System
B Cells
The activation of T cells and B cells, followed by the generation of specific autoantibodies and subsequent multiorgan damage resulting from the dysregulation of adaptive immune responses, constitute the primary mechanisms underlying autoimmune diseases [34, 36, 39]. B-cell targeted therapy has been expanded to encompass a wide range of autoimmune disorders, including pemphigus, multiple sclerosis, ANCA-associated vasculitis, RA, and systemic lupus erythematosus (SLE) [78, 79]. B-cell receptor (BCR) signals and costimulatory signals are two essential signals for B-cell activation. TLR and CD40 on B cells play crucial roles in providing costimulatory signals [80, 81]. The activation of T cell by CD40 ligands can enhance the generation of memory B cells and long-lived plasma cells. BCR signal activation only without the costimulatory signals results in the apoptosis of B cells [80, 81]. The functionality of BCRs is necessary for normal antibody production, and a deficiency in BCR signal transduction may lead to immune deficiency, autoimmune diseases, and malignant tumors involving B cells. The involvement of the BCR signaling pathway has been demonstrated in the development of self-reactive B cells in RA [82, 83]. The involvement of the BCR signaling pathway has been demonstrated in the development of self-reactive B cells in RA [82, 83]. Self-reactive B cells naturally generate high titers of anti-CII, C1-epitope specific antibodies to protected from arthritis [84]. Lymphoid/myeloid (dominated by the presence of B cells) gene expression of synovial tissue signatures correlate with RA disease activity [85]. Encouragingly, B cell depletion therapies(BCDTs) has been expanded to encompass a wide range of autoimmune disorders including RA [78, 79]. Indeed, the administration of rituximab targeting the CD20 receptor on the B cell membrane had exhibited remarkable efficacy [86]. The expression of the molecular clock genes PER2 and NR1D1 significantly oscillates in splenic B cells. Deletion of Bmal1 does not affect the number or distribution of B cells in the bone marrow, spleen, or lymph nodes; however, deletion of CRY alters B-cell development in mice by increasing the number of mature B cells and peritoneal B2 cells in the BM while decreasing the number of splenic marginal zone B cells. Notably, Cry knockout enhances various proximal signaling pathways related to the B-cell receptor. The compounds formed by CRY and PER negatively regulate the expression of Bmal1, which is a core component of the biological clock; therefore, the absence of Bmal1 should also affect the growth of B cells [87]. The regulation of B lymphocyte migration and function by the biological clock remains controversial. Hemmers et al. demonstrated that Bmal1 ablation did not have an impact on B-cell differentiation or function [88]. However, recent research has revealed that in DSS-treated BMAL1−/− mice of colitis models, Breg cells expressing PDL1 (a key immune checkpoint molecule on B cells) are activated in intestinal intraepithelial lymphocytes to protect mice from DSS [89]. Furthermore, IL-33, which contains an E-box site, is targeted by Bmal1-regulated PDL1 + Breg cells in the intestinal microenvironment [89].
T Cells
The migration and differentiation of T cells from the thymus are tightly regulated to maintain a delicate balance between central tolerance and immunity [90,91,92,93]. The expression of biological clock genes governs thymic egress genes, thereby exerting control over the migration and differentiation processes of T cells. Importantly, Bmal1 plays a pivotal role in coordinating the oscillatory behavior exhibited by these genes [94]. Loss of Bmal1 in myeloid cells promotes an augmented inflammatory response while concurrently suppressing the expression of the anti-inflammatory cytokine IL-10 [95]. In mice lacking myeloid Bmal1, enhanced EAE occurs due to the expansion and infiltration of CD11b + Ly6Chi monocytes into the CNS [96]. However, Hemmers et al. argued that there is no evident oscillation observed for the biological clock genes Bmal1 and NR1D1 in CD4 + T cells [88]. Further investigations have confirmed the rhythmic expression patterns of NR1D1 and Dbp in CD4 + T cells [97]. Notably, the regulation of NR1D1 rhythms does not involve Bmal1, suggesting the influence of extrinsic circadian factors driving NR1D1 oscillations. In a murine model of inflammatory arthritis (collagen-induced arthritis, CIA), there is a significant day‒night variation in the number of Treg cells, characterized by a notable increase when inflammation is mild, while Th1 and Th17 cell numbers remain unchanged. Per2, Bmal1, Cry1, and Per3 do not exhibit rhythmicity in Tregs from Bmal1 deletion mice; however, Nr1d1 expression differs significantly at different time points [98]. This further confirms the responsiveness of Nr1d1 to external signals. Although Treg cells lack a functional circadian clock, endogenous expression of the NR1D1 gene retains circadian rhythm regulation, which once again demonstrates its response to external signals [98]. Several studies have demonstrated that RORγt acts as a driver for Th17 cell differentiation [99,100,101]. Chang et al. identified NR1D1 as a transcriptional repressor that antagonizes RORγt function in Th17 cells [102]. NR1D1 binds to ROR response elements (RORE) in Th17 cells and inhibits the expression of RORγt-dependent genes including IL-17 [103]. In addition, Amir et al. demonstrated that REV-ERBα deficiency impairs Th17 cell differentiation. Additionally, a synthetic agonist of NR1D1 effectively suppressed the development and progression of Th17 cell-mediated encephalomyelitis (EAE) [103]. Although the rhythmic regulation of the core biological clock in T cells remains controversial, it is indisputable that peripheral clock genes exert regulatory control over immune activity in these cells.
EFFECTS OF THE CIRCADIAN RHYTHM ON SYNOVIAL TISSUE IN RHEUMATOID ARTHRITIS
The synovium, which surrounds the joint, plays a crucial role in facilitating joint surface lubrication and providing nourishment for articular cartilage. Synovial fluid is rich in nutrients, including collagen, fibrin, and other matrix proteins that diffuse through the serum. Moreover, it contains a significant population of synovial cells. These synoviocytes are further classified as macrophage-like synoviocytes and fibroblast-like synoviocytes due to their ability to perform functions resembling those of macrophages and fibrocytes [104,105,106]. Macrophage-like synoviocytes exhibit a phenotype and phagocytic activity comparable to that of other macrophages. In contrast, fibroblast-like synoviocytes possess distinctive characteristics that distinguish them from other fibroblast lines, such as the secretion of cadherin-11, Uridine diphosphoglucose dehydrogenase (UDPGD), and vascular adhesion molecule 1 (VCAM-1). Additionally, they express type IV and V collagens and vimentin [107, 108]. Macrophage-like synoviocytes exhibit a highly activated phenotype in the abnormal synovial environment and produce a large number of inflammatory factors, chemokines and growth factors [108]. These products further stimulate fibroblasts and immune cells to secrete IL-6, TNF-α and other factors, leading to the cascade reaction of immune cells. TNF-α stimulation enhances Bmal1 expression through calcium signaling, but does not alter its rhythmic oscillation in the synovial cells of RA patients [109]. Interestingly, BMAL1 protein levels are higher in the synovial fluid of RA patients than in OA patients while the expression levels of CLOCK and Period 2 are similar between RA and OA patients [110]. Loss of Bmal1 in FLSs significantly affects joint structure leading to posterior paw thickness and exacerbating inflammatory arthritis [111]. Consistent with this observation, the levels of the proinflammatory cytokines Il6, Cxcl1, Ccl2, Cxcl5 and Rankl are significantly elevated [111]. In the synovial cells of patients with arthritis, the circadian rhythm regulator PER2 exhibited significant disruption and loss of oscillation [112]. The arthritis score of CAIA mice is higher after Cry gene knockout due to biological clock dysfunction [113]. Conversely, Per was not expressed in synovial intimal cells, lymphocytes, or chondrocytes within the joint cavity of rheumatoid arthritis mice; however, the serum TNF-α concentration was significantly increased by twofold [109]. Notably, transfection of the Cry gene markedly reduced TNFα levels, indicating that Cry can mitigate inflammatory responses and ameliorate symptoms associated with rheumatoid arthritis by inhibiting TNFα production [112]. Furthermore, ectopic expression of Cry1 in Cry1−/-Cry2−/− MEFs not only improved joint inflammation but also downregulated the expression of Wee-1, a crucial cell cycle regulator, potentially accounting for its ability to suppress tumor-like invasion by synovial cells [113]. The promoter region of the Wee-1 gene contains three E boxes that serve as binding sites for the CLOCK–BMAL1 complex [114]. Wood et al. demonstrate that the circadian rhythm protein Bmal1 and the alteration in Wee-1 protein content within tumor cells exhibit synchronized frequencies. Furthermore, they observe a correlation between tumor growth, growth factor levels, and mitotic index [115]. CRY is suggested to inhibit Wee-1 expression in synovial cells by negatively regulating the biological clock Bmal1, thereby impeding synovial cell proliferation and growth. Suzuki et al. reported that PER2 and BIK were specifically overexpressed on apoptotic synovial fibroblasts but not in normal-appearing cells after treated with MTX (10 nM for 24 h) [116]. As a member of the BCL-2 family, BIK acts as a crucial upstream signaling molecule for both the Bcl-2 and Bax subfamilies, binding to them to induce apoptosis [117]. Interestingly, simultaneous inhibition of PER2 and BIK significantly attenuated the effects of MTX on cell viability and apoptosis induction [116, 118]. Researchers have proposed that PER2 plays a fundamental role in mtx-dependent synovial cell death. Notably, BMAL1 also contributes to the transcriptional regulation of p21 (a cell cycle regulator) and Wee1 kinase, key factors involved in controlling the proliferation and “tumor-like” overgrowth of rheumatoid synovial cells.
THE CIRCADIAN RHYTHM GOVERNS THE NEUROENDOCRINE FACTORS IMPLICATED IN RHEUMATOID ARTHRITIS
The effects of the circadian rhythm on the neuroendocrine system play a crucial role in the time-dependent changes in joint inflammation in patients with RA. The GLORIA trial provides compelling evidence for the benefits of low-dose glucocorticoids (GC) on disease activity and slowing the progression of RA joint damage [119]. GC exerts immunosuppressive effects by inhibiting cytokine secretion and acting on immune cells [120,121,122]. Furthermore, circadian GC rhythms regulate chemokine recruitment to neutrophils, thereby impacting the overall immune response [63, 123]. As a critical regulator of various aspects of mammalian physiology, including glucose homeostasis and immune function, glucocorticoids exhibit a robust circadian rhythm in blood circulation [124, 125]. Circulating glucocorticoid fluctuate throughout the day, peaking shortly before activity begins [126,127,128,129]. This diurnal variation partially accounts for the alleviation of typical morning stiffness symptoms observed in patients with rheumatoid arthritis following exercise. Time therapy with glucocorticoid is more effective in relieving joint inflammation in RA patients (Fig. 2B). A double-blind, randomised controlled trial showed that the administration of modified release (delayed-release) prednisone at 22 o'clock (releasing prednisone around 2–3 o'clock) significantly reduces the duration of joint stiffness in patients with chronic conditions compared to administering immediate-release prednisone at 6–8 o'clock in the morning [130]. Moreover, modified-release prednisone exhibits a favorable safety and high tolerability, and it has a prolonged therapeutic efficacy for a minimum duration of 12 months [131]. Prior to this, multiple studies have demonstrated that administering glucocorticoids at night significantly reduces the duration of morning stiffness [132, 133]. Arvidson NG et al. further confirmed that administration of low-dose glucocorticoid prior to the peak of IL-6 inflammatory factor, specifically during nighttime, appears to ameliorate acute symptoms associated with rheumatoid arthritis [134]. The central clock located in the suprachiasmatic nucleus controls the diurnal release pattern of hormones through direct neural connections. Glucocorticoids are downstream products of the hypothalamic‒pituitary‒adrenal (HPA) axis [135]. It exerts its effect through binding to the intracellular GC receptor (GR). The dimer formed by GC and GR translocates to the nucleus, where it regulates the transcription of target genes via GC response elements (GREs) [121, 136]. Notably, GRE-binding domains are present at clock gene sites Per1, Per2, and Nfil3, suggesting that GCs can modulate biological rhythms to some extent [137]. Han et al. reported that CLOCK/BMAL1 reduces maximal GR transactivation and efficacy through GRE without affecting E-box-dependent transactivation [138]. BMAL1 plays a crucial role in influencing GR signaling, while CLOCK itself does not directly impact GR signaling; instead, it likely facilitates BMAL1 binding [138]. In NIH 3T3 cells, the abundance of GR protein exhibited a negative correlation with the expression level of NR1D1 protein; however, NR1D1 did not exert any influence on the transcriptional activity of GR. The impact of NR1D1 on GR is mediated through its interaction with HSP90, a chaperone protein that facilitates the stabilization of GR, thereby leading to a reduction in the half-life of GR protein [139]. Moreover, NR1D1 affects NF-κB, an inflammatory pathway downstream of GR. In NR1D1 knockout hepatocytes, the accumulation of nonphosphorylated IκBα increased, potentially attributed to the upregulation of GR levels resulting from NR1D1 knockout [139]. Conversely, GR directly associates with the BMAL1/CLOCK complex and exerts its inhibitory effect on NR1D1 transcription by targeting the E-Box element [140]. By regulating GR activity, NR1D1 appears to link biological rhythms with immune function (Fig. 3). Intermittent treatment with exogenous glucocorticoids improves nutrient oxidation in dystrophic muscle via a functional circadian clock since these effects are disrupted upon Bmal1 deletion [141]. Using RNAscope, Bering et al. demonstrated the colocalization of glucocorticoid receptors and PER2 transcripts in the cerebellar cortex [142]. In SCN-lesioned rats, the rhythmic administration of exogenous corticosterone restored the daily rhythms of PER1 and PER2, indicating the direct action of corticosterone on neurons harboring a molecular oscillator in the cerebellum [142]. CRY interact with glucocorticoid receptors, binding to GRE on gene promoters such as phosphoenolpyruvate carboxykinase 1 (PCK1) in the liver and inhibit glucocorticoid receptor-dependent transcription, thereby regulating gluconeogenesis. However, this physiological rhythm can autonomously fluctuate without being influenced by the BMAL1 and CLOCK [143] (Fig. 3).
GR are modulated by biological clock (by Figdraw). The interaction between REV-ERB and HSP90 protein attenuates the stability of GR, thereby facilitating the activation of the NF-κB inflammatory signaling pathway GR can also interact with the Bmal1/CLOCK dimer, thereby facilitating the transcriptional activation of REV-ERB. Furthermore, CRY exerts regulatory control over gluconeogenesis-related genes through its interaction with GR and subsequent binding to GREs elements, thereby attenuating the stimulatory effects of GC on gluconeogenesis. BMAL1: Brain and muscle ARNT-like 1; CLOCK: Circadian locomotor output cycles kaput; CRY: Cryptochrome; REV-ERB: Reverse-erythroblastosis virus; GC: Glucocorticoids; GR: Glucocorticoids receptor; GREs: GC-response elements. HSP90: Heat shock protein 90; PCK1:phosphoenolpyruvate carboxykinase 1. NR1D: nuclear receptor subfamily 1 group D member.
Melatonin secretion is dependent on a fully intact connection between the SCN, paraventricular nucleus, and pineal neurons [144]. The SCN, acting as a biological clock, actively participates in regulating melatonin secretion and rhythmicity. In addition to being influenced by light, exercise, food, smell, and the sympathetic nervous system, melatonin rhythm is also regulated by peripheral biological clocks [144, 145]. Furthermore, melatonin plays a pivotal role in modulating circadian rhythms and exerting antioxidant and anti-inflammatory effects. Hansson et al. found that disruption of melatonin production due to constant darkness triggers arthritis onset while promoting collagen production [146]. Subsequently, Hansson et al. demonstrated that administration of low-dose melatonin (1 mg/kg) within 1–10 days after collagen induction can exacerbate arthritis in DBA/1 mice, potentially through immune system activation [147] It indicates that melatonin significantly reduces the gene and protein expression of Cry1, potentially implicating the weakened Cry1 in the exacerbation of CAIA [148], Cry-deficient mice exhibit more pronounced joint inflammation upon stimulation by a mixture of anti-CII mAb and LPS [113]. Furthermore, synovial tissue showed undetectable levels of the PER protein, likely due to rapid degradation caused by the absence of the accompanying CRY protein [149]. While other research has demonstrated that melatonin effectively mitigates paw swelling, cartilage degeneration, and bone erosion in arthritic mice by suppressing the PI3K/AKT signaling pathway along with the ERK and NF-κB pathways [150]. These contradictory observations may be related to the different timing of melatonin administration. Melatonin can induce inflammation during the initial 1–10 days following collagen induction; however, it does not exacerbate joint inflammation during the subsequent 30–39 days after collagen induction [147]. On the other hand, the administration of melatonin during the induction phase of arthritis may stimulate immunocyte and elicit an inflammatory response [151, 152]. It is worth noting that the circadian rhythm signal may play a role in modulating the function of melatonin in rheumatoid arthritis. The nuclear signal transduction pathway is one mechanism through which melatonin exerts its effects [153]. RZR/ROR serves as a crucial transcription activator complex in the biological clock system [154]. Although it does not directly bind to ROR receptors, melatonin can induce anti-allergic and anti-inflammatory effects by inhibiting 5-lipoxygenase (5-LOX) via transcriptional activation of RZR/ROR [155, 156]. ROR acts as a negative regulator of inflammation through the NF-κB signaling pathway and plays an essential role in both melatonin activity and Bmal1 clock gene function for maintaining 24-h rhythms and regulating immune responses [30, 31, 111]. The proinflammatory and anti-inflammatory effects of melatonin on rheumatoid arthritis remain controversial but are closely intertwined with the biological clock.
CONCLUSION
From the discovery of diurnal variation in symptoms of rheumatoid arthritis (RA) to research on the role of biological clock genes in regulating the immune system and neuroendocrine functions, an increasing body of evidence highlights the crucial involvement of the circadian rhythm in disease progression. Immune cells are not only influenced by peripheral clocks but also precisely regulated by intracellular clock genes. Disruption of the biological clock network leads to uncontrolled activation of the immune system and inflammatory reactions in patients with RA. Deletion of either the PER1 or PER2 gene can result in NK cell dysfunction, while downregulation of PER2 gene expression in macrophages promotes a proinflammatory effect. The circadian rhythm governing neutrophil transcription and migration is regulated by BMAL1, which maintains a balance between neutrophil number and phenotype within the circulatory system. Additionally, BMAL1 deficiency reduces mitochondrial metabolism in dendritic cells (DCs), impairing their antigen presentation ability. However, there is controversy regarding the impact of Bmal1 on B-cell differentiation and function. T-cell regulation involves not only core biological clocks but also secondary circuits; furthermore, the influence of NR1D1 on T cells is independent of the main clock mechanism. Moreover, biological clock-mediated regulation extends to neuroendocrine processes that complement inflammatory responses. Rhythmic oscillations in glucocorticoids and melatonin play a critical role in the time-dependent changes observed during joint inflammation among RA patients. Although most studies on biology clocks have been conducted in the fields of cytology and zoology thus far, conducting an extensive analysis of the complex interactions between circadian rhythms, the immune system, and the neuroendocrine system among RA patients would provide valuable insights for developing effective treatments.
Data Availability
No datasets were generated or analysed during the current study.
Abbreviations
- RA :
-
Rheumatoid arthritis
- SCN :
-
The hypothalamic suprachiasmatic nucleus
- CLOCK :
-
Circadian locomotor output cycles kaput
- BMAL1 :
-
Brain and muscle ARNT-like 1
- PER :
-
Clock genes period
- CRY :
-
Cryptochrome
- NR1D :
-
Nuclear receptor subfamily 1 group D member
- REV-ERB :
-
Reverse-erythroblastosis virus
- ROR :
-
Retinoic acid-related orphan nuclear receptor
- NK cells :
-
Natural killer cells
- RORE :
-
ROR response elements
- DCs :
-
Dendritic cells
- GC :
-
Glucocorticoids
- GR :
-
Glucocorticoids receptor
- GREs :
-
GC-response elements
References
Conigliaro, P., A. D’Antonio, S. Pinto, et al. 2020. Autoimmune thyroid disorders and rheumatoid arthritis: A bidirectional interplay. Autoimmunity Reviews 19 (6): 102529.
Aletaha, D., and J.S. Smolen. 2018. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 320: 1360–1372.
Smolen, J.S., D. Aletaha, and I.B. McInnes. 2016. Rheumatoid arthritis. Lancet 388: 2023–2038.
Guo, Q., Y. Wang, D. Xu, J. Nossent, N.J. Pavlos, and J. Xu. 2018. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res 6: 15.
Deane, K.D., M.K. Demoruelle, L.B. Kelmenson, K.A. Kuhn, J.M. Norris, and V.M. Holers. 2017. Genetic and environmental risk factors for rheumatoid arthritis. Best Practice & Research Clinical Rheumatology 31 (1): 3–18.
Van der Woude, D., J.J. Houwing-Duistermaat, R.E. Toes, et al. 2009. Quantitative heritability of anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis. Arthritis and Rheumatism 60 (4): 916–923.
Messemaker, T.C., T.W. Huizinga, and F. Kurreeman. 2015. Immunogenetics of rheumatoid arthritis: Understanding functional implications. Journal of Autoimmunity 64: 74–81.
Pivovarova-Ramich, O., H.G. Zimmermann, and F. Paul. 2023. Multiple sclerosis and circadian rhythms: Can diet act as a treatment? Acta Psychologica 237 (4): e13939.
Downton, P., J.O. Early, and J.E. Gibbs. 2020. Circadian rhythms in adaptive immunity. Immunology 161 (4): 268–277.
Angelousi, A., N. Nasiri-Ansari, E. Spilioti, et al. 2018. Altered expression of circadian clock genes in polyglandular autoimmune syndrome type III. Endocrine 59 (1): 109–119.
Early, J.O., D. Menon, C.A. Wyse, et al. 2018. Circadian clock protein BMAL1 regulates IL-1β in macrophages via NRF2. Proc Natl Acad Sci U S A 115 (36): E8460–E8468.
Wu, Y., B. Tao, T. Zhang, Y. Fan, and R. Mao. 2019. Pan-Cancer Analysis Reveals Disrupted Circadian Clock Associates With T Cell Exhaustion. Frontiers in Immunology 10: 2451.
Gray, K.J., and J.E. Gibbs. 2022. Adaptive immunity, chronic inflammation and the clock. Semin Immunopathol 44 (2): 209–224.
Ye, H., H. Weng, Y. Xu, et al. 2022. Efectiveness and safety of aerobic exercise for rheumatoid arthritis: A systematic review and meta-analysis of randomized controlled trials. BMC Sports Science, Medicine and Rehabilitation 14 (1): 17.
Aletaha, D., T. Neogi, A.J. Silman, et al. 2010. Rheumatoid arthritis classifcation criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Annals of the Rheumatic Diseases 69: 1580–1588.
Arvidson, N.G., B. Gudbjörnsson, L. Elfman, A.C. Rydén, T.H. Tötterman, and R. Hällgren. 1994. Circadian rhythm of serum interleukin-6 in rheumatoid arthritis. Annals of the Rheumatic Diseases 53 (8): 521–524.
Perry, M.G., J.R. Kirwan, D.S. Jessop, and L.P. Hunt. 2009. Overnight variations in cortisol, interleukin 6, tumour necrosis factor alpha and other cytokines in people with rheumatoid arthritis. Annals of the Rheumatic Diseases 68 (1): 63–68.
Sulli, A., G.J. Maestroni, B. Villaggio, E. Hertens, C. Craviotto, C. Pizzorni, M. Briata, B. Seriolo, and M. Cutolo. 2002. Melatonin serum levels in rheumatoid arthritis. Annals. New York Academy of Sciences 966: 276–283.
Butler, T., J.R. Maidstone, K.M. Rutter, T.J. McLaughlin, W.D. Ray, and E.J. Gibbs. 2023. The Associations of Chronotype and Shift Work With Rheumatoid Arthritis. Journal of Biological Rhythms 38 (5): 510–518.
Puttonen, S., T. Oksanen, J. Vahtera, J. Pentti, M. Virtanen, P. Salo, and M. Kivimaki. 2010. Is shift work a risk factor for rheumatoid arthritis? The Finnish Public Sector study. Annals of the Rheumatic Diseases 69: 779–780.
Gibbs, J.E., and D.W. Ray. 2013. The role of the circadian clock in rheumatoid arthritis. Arthritis Research & Therapy 15 (1): 205.
Ralph, M.R., R.G. Foster, F.C. Davis, and M. Menaker. 1990. Transplanted suprachiasmatic nucleus determines circadian period. Science 247: 975–978.
Paul, J.R., J.A. Davis, L.K. Goode, et al. 2020. Circadian regulation of membrane physiology in neural oscillators throughout the brain. European Journal of Neuroscience 51: 109–138.
Meredith, A.L., S.W. Wiler, B.H. Miller, et al. 2006. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nature Neuroscience 9: 1041–1049.
Bass, J., and M.A. Lazar. 2016. Circadian time signatures of fitness and disease. Science 354: 994–999.
Cermakian, N., and P. Sassone-Corsi. 2000. Multilevel regulation of the circadian clock. Nature Reviews Molecular Cell Biology 1 (1): 59–67.
Gamble, K.L., R. Berry, S.J. Frank, et al. 2014. Circadian clock control of endocrine factors. Nature Reviews. Endocrinology 10 (8): 466–475.
Kizaki, T., S. Sato, K. Shirato, et al. 2015. Effect of circadian rhythm on clinical and pathophysiological conditions and inflammation. Critical Reviews in Immunology 35 (4): 261–275.
Albrecht, U. 2012. Timing to perfection: The biology of central and peripheral circadian clocks. Neuron 74 (2): 246–260.
Olkkonen, J., V.P. Kouri, E. Kuusela, et al. 2017. DEC2 blocks the effect of the ARNTL2/ NPAS2 dimer on the expression of PER3 and DBP. Journal of Circadian Rhythms 15: 6.
King, D.P., Y. Zhao, A.M. Sangoram, et al. 1997. Positional cloning of the mouse circadian clock gene. Cell 89: 641–653.
Patke, A., M.W. Young, and S. Axelrod. 2020. Molecular mechanisms and physiological importance of circadian rhythms. Nature Reviews Molecular Cell Biology 21: 67–84.
Koike, N., S.-H. Yoo, H.-C. Huang, et al. 2012. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 338: 349–354.
Place, D.E., and T.D. Kanneganti. 2020. The innate immune system and cell death in autoinflammatory and autoimmune disease. Current Opinion in Immunology 67: 95–105.
Szekanecz, Z., I.B. McInnes, G. Schett, S. Szamosi, S. Benkő, and G. Szűcs. 2021. Autoinflammation and autoimmunity across rheumatic and musculoskeletal diseases. Nature Reviews Rheumatology 17 (10): 585–595.
Scherer, H.U., T. Häupl, and G.R. Burmester. 2020. The etiology of rheumatoid arthritis. Journal of Autoimmunity 110: 102400.
Hemmer, B., M. Kerschensteiner, and T. Korn. 2015. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurology 14 (4): 406–419.
Kucuksezer, U.C., E. Aktas Cetin, F. Esen, et al. 2021. The Role of Natural Killer Cells in Autoimmune Diseases. Frontiers in Immunology 12: 622306.
Toubi, E., and Z. Vadasz. 2019. Innate immune-responses and their role in driving autoimmunity. Autoimmunity Reviews 18 (3): 306–311.
Litman, G.W., and M.D. Cooper. 2007. Why study the evolution of immunity? Nature Immunology 8 (6): 547–548.
Janeway, C.A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annual Review of Immunology 20: 197–216.
Hand, L.E., T.W. Hopwood, S.H. Dickson, et al. 2016. The circadian clock regulates inflammatory arthritis. The FASEB Journal 30 (11): 3759–3770.
Pham, L., L. Baiocchi, L. Kennedy, et al. 2021. The interplay between mast cells, pineal gland, and circadian rhythm: Links between histamine, melatonin, and inflammatory mediators. Journal of Pineal Research 70 (2): e12699.
Christ, P., A.S. Sowa, O. Froy, and A. Lorentz. 2018. The Circadian Clock Drives Mast Cell Functions in Allergic Reactions. Frontiers in Immunology 9: 7.
Martínez de Toda, I., C. Vida, E. Díaz-Del Cerro, and M. De la Fuente. 2021. The Immunity Clock. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 76 (11): 1939–1945.
Arjona, A., and D.K. Sarkar. 2006. Evidence supporting a circadian control of natural killer cell function. Brain, Behavior, and Immunity 20 (5): 469–476.
Gibbs, J.E., J. Blaikley, S. Beesley, et al. 2012. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proceedings of the National Academy of Sciences U S A 109 (2): 582–587.
Keller, M., J. Mazuch, U. Abraham, et al. 2009. A circadian clock in macrophages controls inflammatory immune responses. Proceedings of the National Academy of Sciences U S A 106 (50): 21407–21412.
Cao, Q., X. Zhao, J. Bai, et al. 2017. Circadian clock cryptochrome proteins regulate autoimmunity. Proceedings of the National Academy of Sciences U S A 114 (47): 12548–12553.
Hirose, M., A. Leliavski, L.V.M. de Assis, et al. 2024. Chronic Inflammation Disrupts Circadian Rhythms in Splenic CD4+ and CD8+ T Cells in Mice. Cells 13 (2): 151.
Constantinides, M.G., B.D. McDonald, P.A. Verhoef, and A. Bendelac. 2014. A committed precursor to innate lymphoid cells. Nature 508 (7496): 397–401.
Lim, A.I., Y. Li, S. Lopez-Lastra, et al. 2017. Systemic Human ILC Precursors Provide a Substrate for Tissue ILC Differentiation. Cell 168 (6): 1086-1100.e10.
Yamin, R., O. Berhani, H. Peleg, et al. 2019. High percentages and activity of synovial fluid NK cells present in patients with advanced stage active Rheumatoid Arthritis. Science and Reports 9 (1): 1351.
Arjona, A., N. Boyadjieva, and D.K. Sarkar. 2004. Circadian rhythms of granzyme B, perforin, IFN-gamma and NK cell cytolytic activity in the spleen: Effects of chronic ethanol. The Journal of Immunology 172 (5): 2811–2817.
Arjona, A., and D.K. Sarkar. 2005. Circadian oscillations of clock genes, cytolytic factors, and cytokines in rat NK cells. The Journal of Immunology 174 (12): 7618–7624.
Logan, R.W., O. Wynne, D. Levitt, D. Price, and D.K. Sarkar. 2013. Altered circadian expression of cytokines and cytolytic factors in splenic natural killer cells of Per1(-/-) mutant mice. Journal of Interferon and Cytokine Research 33 (3): 108–114.
Adrover, J.M., C. Del Fresno, G. Crainiciuc, et al. 2019. A Neutrophil Timer Coordinates Immune Defense and Vascular Protection. Immunity 50 (2): 390-402.e10.
Aroca-Crevillén, A., J.M. Adrover, and A. Hidalgo. 2020. Circadian Features of Neutrophil Biology. Frontiers in Immunology 11: 576.
Haimovich, B., J. Calvano, A.D. Haimovich, et al. 2010. In vivo endotoxin synchronizes and suppresses clock gene expression in human peripheral blood leukocytes. Critical Care Medicine 38 (3): 751–758.
Casanova-Acebes, M., C. Pitaval, L.A. Weiss, et al. 2013. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 153 (5): 1025–1035.
Zhang, D., G. Chen, D. Manwani, et al. 2015. Neutrophil ageing is regulated by the microbiome. Nature 525 (7570): 528–532.
Sennels, H.P., H.L. Jørgensen, A.L. Hansen, et al. 2011. Diurnal variation of hematology parameters in healthy young males: The Bispebjerg study of diurnal variations. Scandinavian Journal of Clinical and Laboratory Investigation 71 (7): 532–541.
Gibbs, J., L. Ince, L. Matthews, et al. 2014. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nature Medicine 20 (8): 919–926.
Wijbrandts, C.A., C.E. Vergunst, J.J. Haringman, et al. 2007. Absence of changes in the number of synovial sublining macrophages after ineffective treatment for rheumatoid arthritis: Implications for use of synovial sublining macrophages as a biomarker. Arthritis and Rheumatism 56 (11): 3869–3871.
Huang, Q.Q., R. Doyle, S.Y. Chen, et al. 2021. Critical role of synovial tissue-resident macrophage niche in joint homeostasis and suppression of chronic inflammation. Science Advances 7 (2): eabd0515.
Alivernini, S., L. MacDonald, A. Elmesmari, et al. 2020. Distinct synovial tissue macrophage subsets regulate inflammation and remission in rheumatoid arthritis. Nature Medicine 26 (8): 1295–1306.
Chen, S., K.K. Fuller, J.C. Dunlap, and J.J. Loros. 2020. A Pro- and Anti-inflammatory Axis Modulates the Macrophage Circadian Clock. Frontiers in Immunology 11: 867.
Hong, H., Y.M. Cheung, X. Cao, Y. Wu, C. Li, and X.Y. Tian. 2021. REV-ERBα agonist SR9009 suppresses IL-1β production in macrophages through BMAL1-dependent inhibition of inflammasome. Biochemical Pharmacology 192: 114701.
Croft, A.P., J. Campos, K. Jansen, et al. 2019. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 570 (7760): 246–251.
Haringman, J.J., D.M. Gerlag, A.H. Zwinderman, et al. 2005. Synovial tissue macrophages: A sensitive biomarker for response to treatment in patients with rheumatoid arthritis. Annals of the Rheumatic Diseases 64 (6): 834–838.
Degboé, Y., B. Rauwel, M. Baron, et al. 2019. Polarization of Rheumatoid Macrophages by TNF Targeting Through an IL-10/STAT3 Mechanism. Frontiers in Immunology 10: 3.
Prendergast, C.T., A. Patakas, S. Al-Khabouri, et al. 2018. Visualising the interaction of CD4 T cells and DCs in the evolution of inflammatory arthritis. Annals of the Rheumatic Diseases 77 (4): 579–588.
Wehr, P., H. Purvis, S.C. Law, and R. Thomas. 2019. Dendritic cells, T cells and their interaction in rheumatoid arthritis. Clinical and Experimental Immunology 196 (1): 12–27.
Hu, X.X., Y.J. Wu, J. Zhang, and W. Wei. 2019. T-cells interact with B cells, dendritic cells, and fibroblast-like synoviocytes as hub-like key cells in rheumatoid arthritis. International Immunopharmacology 70: 428–434.
Suwa, Y., Y. Nagafuchi, S. Yamada, and K. Fujio. 2023. The role of dendritic cells and their immunometabolism in rheumatoid arthritis. Frontiers in Immunology 14: 1161148.
Nobis, C.C., G. Dubeau Laramée, L. Kervezee, D. Maurice De Sousa, N. Labrecque, and N. Cermakian. 2019. The circadian clock of CD8 T cells modulates their early response to vaccination and the rhythmicity of related signaling pathways. Proc Natl Acad Sci U S A 116 (40): 20077–20086.
Holtkamp, S.J., L.M. Ince, C. Barnoud, et al. 2021. Circadian clocks guide dendritic cells into skin lymphatics. Nature Immunology 22 (11): 1375–1381.
Lee, D.S.W., O.L. Rojas, and J.L. Gommerman. 2021. B cell depletion therapies in autoimmune disease: Advances and mechanistic insights. Nature Reviews. Drug Discovery 20 (3): 179–199.
Dang, V.D., A.L. Stefanski, A.C. Lino, and T. Dörner. 2022. B- and Plasma Cell Subsets in Autoimmune Diseases: Translational Perspectives. Journal of Investigative Dermatology 142 (3 Pt B): 811–822.
Barnas, J.L., R.J. Looney, and J.H. Anolik. 2019. B cell targeted therapies in autoimmune disease. Current Opinion in Immunology 61: 92–99.
Li, J., M. Zhao, W. Luo, J. Huang, B. Zhao, and Z. Zhou. 2023. B cell metabolism in autoimmune diseases: Signaling pathways and interventions. Frontiers in Immunology 14: 1232820.
Haselmayer, P., M. Camps, L. Liu-Bujalski, et al. 2019. Efficacy and Pharmacodynamic Modeling of the BTK Inhibitor Evobrutinib in Autoimmune Disease Models. The Journal of Immunology 202 (10): 2888–2906.
Nakken, B., L.A. Munthe, Y.T. Konttinen, et al. 2011. B-cells and their targeting in rheumatoid arthritis–current concepts and future perspectives. Autoimmunity Reviews 11 (1): 28–34.
Ia, K., A. Saxena, K.S. Nandakumar, et al. 2015. B-cell epitope spreading and inflammation in a mouse model of arthritis is associated with a deficiency in reactive oxygen species production. European Journal of Immunology 45 (8): 2243–2251.
Humby, F., M. Lewis, N. Ramamoorthi, et al. 2019. Synovial cellular and molecular signatures stratify clinical response to csDMARD therapy and predict radiographic progression in early rheumatoid arthritis patients. Annals of the Rheumatic Diseases 78 (6): 761–772.
Kavanaugh, A., S. Rosengren, S.J. Lee, et al. 2008. Assessment of rituximab’s immunomodulatory synovial effects (ARISE trial). 1: clinical and synovial biomarker results. Annals of the Rheumatic Diseases 67 (3): 402–408.
Silver, A.C., A. Arjona, M.E. Hughes, M.N. Nitabach, and E. Fikrig. 2012. Circadian expression of clock genes in mouse macrophages, dendritic cells, and B cells. Brain, Behavior, and Immunity 26 (3): 407–413.
Hemmers, S., and A.Y. Rudensky. 2015. The Cell-Intrinsic Circadian Clock Is Dispensable for Lymphocyte Differentiation and Function. Cell Reports 11 (9): 1339–1349.
Liu, J.L., C.Y. Wang, T.Y. Cheng, et al. 2021. Circadian Clock Disruption Suppresses PDL1+ Intraepithelial B Cells in Experimental Colitis and Colitis-Associated Colorectal Cancer. Cell Mol Gastroenterol Hepatolx 12 (1): 251–276.
Levine, A.G., A. Mendoza, S. Hemmers, et al. 2017. Stability and function of regulatory T cells expressing the transcription factor T-bet. Nature 546 (7658): 421–425.
Sawant, D.V., H. Yano, M. Chikina, et al. 2019. Adaptive plasticity of IL-10+ and IL-35+ Treg cells cooperatively promotes tumor T cell exhaustion. Nature Immunology 20 (6): 724–735.
Almeida, A.R., B. Rocha, A.A. Freitas, and C. Tanchot. 2005. Homeostasis of T cell numbers: From thymus production to peripheral compartmentalization and the indexation of regulatory T cells. Seminars in Immunology 17 (3): 239–249.
Maggi, E., L. Cosmi, F. Liotta, P. Romagnani, S. Romagnani, and F. Annunziato. 2005. Thymic regulatory T cells. Autoimmunity Reviews 4 (8): 579–586.
Minaduola, M., A. Aili, Y. Bao, Z. Peng, Q. Ge, and R. Jin. 2022. The circadian clock sets a spatial-temporal window for recent thymic emigrants. Immunology and Cell Biology 100 (9): 731–741.
Lang, V., S. Ferencik, B. Ananthasubramaniam, A. Kramer, and B. Maier. 2021. Susceptibility rhythm to bacterial endotoxin in myeloid clock-knockout mice. eLife 10: e62469.
Sutton, C.E., C.M. Finlay, M. Raverdeau, et al. 2017. Loss of the molecular clock in myeloid cells exacerbates T cell-mediated CNS autoimmune disease. Nature Communications 8 (1): 1923.
Druzd, D., O. Matveeva, L. Ince, et al. 2017. Lymphocyte Circadian Clocks Control Lymph Node Trafficking and Adaptive Immune Responses. Immunity 46 (1): 120–132.
Hand, L.E., K.J. Gray, S.H. Dickson, et al. 2020. Regulatory T cells confer a circadian signature on inflammatory arthritis. Nature Communications 11 (1): 1658.
Yang, J., M.S. Sundrud, J. Skepner, and T. Yamagata. 2014. Targeting Th17 cells in autoimmune diseases. Trends in Pharmacological Sciences 35 (10): 493–500.
Hall, J.A., M. Pokrovskii, L. Kroehling, et al. 2022. Transcription factor RORα enforces stability of the Th17 cell effector program by binding to a Rorc cis-regulatory element. Immunity 55 (11): 2027-2043.e9.
Kumar, R., A.L. Theiss, and K. Venuprasad. 2021. RORγt protein modifications and IL-17-mediated inflammation. Trends in Immunology 42 (11): 1037–1050.
Chang, C., C.S. Loo, X. Zhao, et al. 2019. The nuclear receptor REV-ERBα modulates Th17 cell-mediated autoimmune disease. Proc Natl Acad Sci U S A 116 (37): 18528–18536.
Amir, M., S. Chaudhari, R. Wang, et al. 2018. REV-ERBα Regulates TH17 Cell Development and Autoimmunity. Cell Reports 25 (13): 3733-3749.e8.
Bartok, B., and G.S. Firestein. 2010. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunological Reviews 233 (1): 233–255.
Falconer, J., A.N. Murphy, S.P. Young, et al. 2018. Review: Synovial Cell Metabolism and Chronic Inflammation in Rheumatoid Arthritis. Arthritis & Rhematology 70 (7): 984–999.
Chijimatsu, R., and T. Saito. 2019. Mechanisms of synovial joint and articular cartilage development. Cellular and Molecular Life Sciences 76 (20): 3939–3952.
Tu, J., W. Hong, P. Zhang, X. Wang, H. Körner, and W. Wei. 2018. Ontology and Function of Fibroblast-Like and Macrophage-Like Synoviocytes: How Do They Talk to Each Other and Can They Be Targeted for Rheumatoid Arthritis Therapy? Frontiers in Immunology 9: 1467.
Feng, L.J., T.C. Jiang, C.Y. Zhou, et al. 2014. Activated macrophage-like synoviocytes are resistant to endoplasmic reticulum stress-induced apoptosis in antigen-induced arthritis. Inflammation Research 63 (5): 335–346.
Yoshida, K., A. Nakai, K. Kaneshiro, et al. 2018. TNF-α induces expression of the circadian clock gene Bmal1 via dual calcium-dependent pathways in rheumatoid synovial cells. Biochemical and Biophysical Research Communications 495 (2): 1675–1680.
Haas, S., and R.H. Straub. 2012. Disruption of rhythms of molecular clocks in primary synovial fibroblasts of patients with osteoarthritis and rheumatoid arthritis, role of IL-1β/TNF. Arthritis Research & Therapy 14 (3): R122.
Hand, L.E., S.H. Dickson, A.J. Freemont, D.W. Ray, and J.E. Gibbs. 2019. The circadian regulator Bmal1 in joint mesenchymal cells regulates both joint development and inflammatory arthritis. Arthritis Research & Therapy 21 (1): 5.
Yoshida, K., A. Hashiramoto, T. Okano, et al. 2013. TNF-α modulates expression of the circadian clock gene Per2 in rheumatoid synovial cells. Scandinavian Journal of Rheumatology 42 (4): 276–280.
Hashiramoto, A., T. Yamane, K. Tsumiyama, et al. 2010. Mammalian clock gene Cryptochrome regulates arthritis via proinflammatory cytokine TNF-alpha. The Journal of Immunology 184 (3): 1560–1565.
Obst, R. 2015. The Timing of T Cell Priming and Cycling. Frontiers in Immunology 6: 563.
Wood, P.A., J. Du-Quiton, S. You, and W.J. Hrushesky. 2006. Circadian clock coordinates cancer cell cycle progression, thymidylate synthase, and 5-fluorouracil therapeutic index. Molecular Cancer Therapeutics 5 (8): 2023–2033.
Suzuki, K., K. Yoshida, T. Ueha, et al. 2018. Methotrexate upregulates circadian transcriptional factors PAR bZIP to induce apoptosis on rheumatoid arthritis synovial fibroblasts. Arthritis Research & Therapy 20 (1): 55.
Hafezi, S., and M. Rahmani. 2021. Targeting BCL-2 in Cancer: Advances, Challenges, and Perspectives. Cancers (Basel) 13 (6): 1292.
Okamoto, K., A. Zaanan, H. Kawakami, S. Huang, and F.A. Sinicrope. 2015. Reversal of Mutant KRAS-Mediated Apoptosis Resistance by Concurrent Noxa/Bik Induction and Bcl-2/Bcl-xL Antagonism in Colon Cancer Cells. Molecular Cancer Research 13 (4): 659–669.
Boers, M., L. Hartman, D. Opris-Belinski, et al. 2022. Low dose, add-on prednisolone in patients with rheumatoid arthritis aged 65+: The pragmatic randomised, double-blind placebo-controlled GLORIA trial. Annals of the Rheumatic Diseases 81 (7): 925–936.
De Bosscher, K., I.M. Beck, L. Dejager, et al. 2014. Selective modulation of the glucocorticoid receptor can distinguish between transrepression of NF-κB and AP-1. Cellular and Molecular Life Sciences 71 (1): 143–163.
Olejniczak, I., H. Oster, and D.W. Ray. 2014. Glucocorticoid circadian rhythms in immune function. Semin Immunopathol 44 (2): 153–163.
Shimba, A., and K. Ikuta. 2020. Control of immunity by glucocorticoids in health and disease. Semin Immunopathol 42 (6): 669–680.
Taves, M.D., and J.D. Ashwell. 2021. Glucocorticoids in T cell development, differentiation and function. Nature Reviews Immunology 21 (4): 233–243.
Kokkinopoulou, I., A. Diakoumi, and P. Moutsatsou. 2021. Glucocorticoid Receptor Signaling in Diabetes. International Journal of Molecular Sciences 22 (20): 11173.
Waeber, G., T. Calandra, C. Bonny, and R. Bucala. 1999. A role for the endocrine and pro-inflammatory mediator MIF in the control of insulin secretion during stress. Diabetes/Metabolism Research and Reviews 15 (1): 47–54.
Cheifetz, P.N. 1971. The daily rhythm of the secretion of corticotrophin and corticosterone in rats and mice. Journal of Endocrinology 49 (3): xi–xii.
Irvine, C.H., and S.L. Alexander. 1994. Factors affecting the circadian rhythm in plasma cortisol concentrations in the horse. Domestic Animal Endocrinology 11 (2): 227–238.
Minnetti, M., V. Hasenmajer, R. Pofi, et al. 2020. Fixing the broken clock in adrenal disorders: Focus on glucocorticoids and chronotherapy. Journal of Endocrinology 246 (2): R13–R31.
Nader, N., G.P. Chrousos, and T. Kino. 2010. Interactions of the circadian CLOCK system and the HPA axis. Trends in Endocrinology and Metabolism 21 (5): 277–286.
Buttgereit, F., G. Doering, A. Schaeffler, et al. 2008. Efficacy of modified-release versus standard prednisone to reduce duration of morning stiffness of the joints in rheumatoid arthritis (CAPRA-1): A double-blind, randomised controlled trial. Lancet 371 (9608): 205–214.
Buttgereit, F., G. Doering, A. Schaeffler, et al. 2010. Targeting pathophysiological rhythms: Prednisone chronotherapy shows sustained efficacy in rheumatoid arthritis. Annals of the Rheumatic Diseases 69 (7): 1275–1280.
De Silva, M., A. Binder, and B.L. Hazleman. 1984. The timing of prednisolone dosage and its effect on morning stiffness in rheumatoid arthritis. Annals of the Rheumatic Diseases 43 (6): 790–793.
Deandrade, J.R., J.N. McCormick, and A.G. Hill. 1964. SMALL DOSES OF PREDNISOLONE IN THE MANAGEMENT OF RHEUMATOID ARTHRITIS. Annals of the Rheumatic Diseases 23 (2): 158–162.
Arvidson, N.G., B. Gudbjörnsson, A. Larsson, and R. Hällgren. 1997. The timing of glucocorticoid administration in rheumatoid arthritis. Annals of the Rheumatic Diseases 56 (1): 27–31.
Spiga, F., J.J. Walker, J.R. Terry, and S.L. Lightman. 1999. HPA axis-rhythms. Comprehensive Physiology 4 (3): 1273–1298.
Son, Y.L., T. Ubuka, M. Narihiro, et al. 2014. Molecular basis for the activation of gonadotropin-inhibitory hormone gene transcription by corticosterone. Endocrinology 155 (5): 1817–1826.
Kennedy, C.L.M., S.D. Carter, K.R. Mifsud, and J.M.H.M. Reul. 2014. Unexpected effects of metyrapone on corticosteroid receptor interaction with the genome and subsequent gene transcription in the hippocampus of male rats. Journal of Neuroendocrinology 32 (2): e12820.
Han, D.H., Y.J. Lee, K. Kim, C.J. Kim, and S. Cho. 2014. Modulation of glucocorticoid receptor induction properties by core circadian clock proteins. Molecular and Cellular Endocrinology 383 (1–2): 170–180.
Okabe, T., R. Chavan, S.S. Fonseca Costa, A. Brenna, J.A. Ripperger, and U. Albrecht. 2016. REV-ERBα influences the stability and nuclear localization of the glucocorticoid receptor. Journal of Cell Science 129 (21): 4143–4154.
Murayama, Y., N. Yahagi, Y. Takeuchi, et al. 2019. Glucocorticoid receptor suppresses gene expression of Rev-erbα (Nr1d1) through interaction with the CLOCK complex. FEBS Letters 593 (4): 423–432.
Quattrocelli, M., M. Wintzinger, K. Miz, et al. 2022. Muscle mitochondrial remodeling by intermittent glucocorticoid drugs requires an intact circadian clock and muscle PGC1α. Science Advances 8 (7): eabm1189.
Bering, T., H. Hertz, and M.F. Rath. 2022. Rhythmic Release of Corticosterone Induces Circadian Clock Gene Expression in the Cerebellum. Neuroendocrinology 110 (7–8): 604–615.
Lamia, K.A., S.J. Papp, R.T. Yu, et al. 2011. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 480 (7378): 552–556.
Claustrat, B., J. Brun, and G. Chazot. 2005. The basic physiology and pathophysiology of melatonin. Sleep Medicine Reviews 9 (1): 11–24.
Pevet, P., and E. Challet. 2011. Melatonin: Both master clock output and internal time-giver in the circadian clocks network. Journal of Physiology - Paris 105 (4–6): 170–182.
Hansson, I., R. Holmdahl, and R. Mattsson. 1990. Constant darkness enhances autoimmunity to type II collagen and exaggerates development of collagen-induced arthritis in DBA/1 mice. Journal of Neuroimmunology 27 (1): 79–84.
Hansson, I., R. Holmdahl, and R. Mattsson. 1992. The pineal hormone melatonin exaggerates development of collagen-induced arthritis in mice. Journal of Neuroimmunology 39 (1–2): 23–30.
Bang, J., H.W. Chang, H.R. Jung, et al. 2012. Melatonin attenuates clock gene cryptochrome1, which may aggravate mouse anti-type II collagen antibody-induced arthritis. Rheumatology International 32 (2): 379–385.
Shigeyoshi, Y., K. Taguchi, S. Yamamoto, et al. 1997. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell 91 (7): 1043–1053.
Huang, C.C., C.H. Chiou, S.C. Liu, et al. 2019. Melatonin attenuates TNF-α and IL-1β expression in synovial fibroblasts and diminishes cartilage degradation: Implications for the treatment of rheumatoid arthritis. Journal of Pineal Research 66 (3): e12560.
Jiménez-Caliani, A.J., S. Jiménez-Jorge, P. Molinero, et al. 2005. Dual effect of melatonin as proinflammatory and antioxidant in collagen-induced arthritis in rats. Journal of Pineal Research 38 (2): 93–99.
Garcia-Mauriño, S., M.G. Gonzalez-Haba, J.R. Calvo, R. Goberna, and J.M. Guerrero. 1998. Involvement of nuclear binding sites for melatonin in the regulation of IL-2 and IL-6 production by human blood mononuclear cells. Journal of Neuroimmunology 92 (1–2): 76–84.
Barrett, P., M. Morris, W.S. Choi, A. Ross, and P.J. Morgan. 1999. Melatonin receptors and signal transduction mechanisms. Biological Signals and Receptors 8 (1–2): 6–14.
Carlberg, C., and I. Wiesenberg. 1995. The orphan receptor family RZR/ROR, melatonin and 5-lipoxygenase: An unexpected relationship. Journal of Pineal Research 18 (4): 171–178.
Ma, H., J. Kang, W. Fan, H. He, and F. Huang. 2021. ROR: Nuclear Receptor for Melatonin or Not? Molecules 26 (9): 2693.
Jahanban-Esfahlan, R., S. Mehrzadi, R.J. Reiter, et al. 2018. Melatonin in regulation of inflammatory pathways in rheumatoid arthritis and osteoarthritis: Involvement of circadian clock genes. British Journal of Pharmacology 175 (16): 3230–3238.
Acknowledgements
We thank the National Natural Science Foundation of China for its support for our manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (82102533, Y.H.Z.).
Author information
Authors and Affiliations
Contributions
Z designed and supervised the experiment. L analysis the literatures concerned and wrote manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent for Publication
All authors of the manuscript have read and agreed to its content and are accountable for all aspects of the accuracy and integrity of the manuscript. The article is original, has not already been published in a journal, and is not currently under consideration by another.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Q., Zhang, Y. Biological Clock Perspective in Rheumatoid Arthritis. Inflammation (2024). https://doi.org/10.1007/s10753-024-02120-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10753-024-02120-4