Abstract
The NLRP3 inflammasome is a multi-molecular complex that acts as a molecular platform to mediate caspase-1 activation, leading to IL-1β/IL-18 maturation and release in cells stimulated by various pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs). This inflammasome plays an important role in the innate immunity as its activation can further promote the occurrence of inflammation, enhance the ability of host to remove pathogens, and thus facilitate the repair of injured tissues. But if the inflammasome activation is dysregulated, it will cause the development of various inflammatory diseases and metabolic disorders. Therefore, under normal conditions, the activation of inflammasome is tightly regulated by various positive and negative signaling pathways to respond to the stimuli without damaging the host itself while maintaining homeostasis. In this review, we summarize recent advances in the major signaling pathways (including TLRs, MAPK, mTOR, autophagy, PKA, AMPK, and IFNR) that regulate NLRP3 inflammasome activation, providing a brief view of the molecular network that regulates this inflammasome as a theoretical basis for therapeutic intervention of NLRP3 dysregulation-related diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The innate immune system is the first line of the host defense against infection and tissue damages, in which innate immune cells recognize various pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide (LPS) and alien nucleic acid from invaded bacteria, fungi, or viruses through their various pattern recognition receptors (PRRs) [1]. They also recognize various damage-associated molecular patterns (DAMPs) such as extracellular adenosine triphosphate (ATP), reactive oxygen species (ROS), high mobility group box-1 protein (HMGB1), and monosodium urate (MSU) crystals [2]. Upon the recognition of PAMPs or DAMPs by the PRRs, a series of signaling pathways are aroused in innate immune cells leading to gene expression of various inflammatory cytokines [3, 4]. Noticeably, one type of PRRs in the cytosol of a cell, including nucleotide-binding oligomerization domain (NOD) like receptors (NLR), rather than inducing the gene expression, can act differently by recruiting the apoptosis-associated speck-like protein containing a CARD (ASC) to form a large molecular complex named inflammasome, on which pro-caspase-1 is recruited and activated by autocatalytic cleavage. The active caspase-1 in turn cleaves downstream substrates pro-interleukin-1β (pro-IL-1β), pro-IL-18, and gasdermin D (GSDMD) to produce mature IL-1β, IL-18, and GSDMD N-terminal fragment (GSDMD-NT), respectively. Consequently, the perforating GSDMD-NT forms pores in the plasma membrane, leading to a lytic form of cell death named pyroptosis. Such a lytic cell death culminates in robust release of cellular components including mature IL-1β and IL-18, thus representing a form of inflammatory cell death [5,6,7].
The concept of inflammasome, with an apparent molecular weight of > 700 kDa, was first proposed by Tschopp in 2002 [8]. The past two decades have witnessed the continuous uncovering of different types of inflammasomes as well as the molecular mechanisms underlying their activation and regulation. So far, there are approximately 20 types of inflammasomes being discovered. Among them, the NLRP1 (NLR family pyrin domain containing 1), NLRP3 (NLR family pyrin domain containing 3), NLRC4 (NLR family CARD domain-containing protein 4), AIM2 (absent in melanoma 2), and pryin inflammasomes have been rigorously investigated, and their molecular mechanisms of activation have been relatively well elucidated [7, 9, 10].
It is worth noting that the NLRP3 inflammasome is currently the most extensively explored one and that the molecular mechanisms of its activation and regulation are progressively elucidated by mounting studies. Being distinct from other inflammasome sensors, NLRP3 can be activated by a wide spectrum of stimulators ranging from extracellular ATP and bacterial pore-forming toxins to microparticles including MSU crystals and asbestos. Upon pathogenic infection, innate immune cells including macrophages and neutrophils release mature IL-1β and IL-18 or neutrophil extracellular traps (NETs) upon NLRP3 inflammasome activation, which further induce innate immune responses resulting in a strong inflammatory status of the host [7, 11]. Concomitantly, pathogens that have invaded into the cells, if there were, are released out due to cell pyroptosis, followed by being killed and removed ultimately by other newly recruited phagocytes including neutrophils. The released pathogens may also be uptaken and processed by antigen presenting cells, thereby being presented to T lymphocytes, and thus activating the adaptive immunity [12]. NLRP3 inflammasome activation and pyroptosis therefore play a fundamental role in the innate immunity against the infections of bacteria, fungi, and viruses [12].
Despite the fact that the biological activities of IL-1β, IL-18, and other inflammatory cell contents released after pyroptosis are beneficial to the host in most cases of infections, endogenous danger signals including HMGB1 and ATP may trigger sterile inflammation, becoming risk factors for spontaneous inflammation and metabolic diseases [7, 13]. Such sterile inflammatory diseases include auto-inflammatory diseases such as cryopyrin-associated periodic syndrome (CAPS), auto-immune diseases such as gout and rheumatoid arthritis, as well as metabolic disorders like type 2 diabetes and atherosclerosis [14,15,16]. Therefore, under normal physiological conditions, invaded pathogens or metabolic wastes are eliminated by induction of inflammasome activation in the cells, while excessive activation of inflammasome is prevented through negative regulatory signaling pathways, so as to maintain an adequate host defense while avoiding damage to the normal tissues and organs of the host [17], highlighting the importance of regulatory signaling pathways for NLRP3 triggering.
In general, the signaling pathways that regulate NLRP3 inflammasome activation can be grouped into three types: one is those promoting NLRP3 activation, including Toll-like receptors (TLRs), mitogen-activated protein kinases (MAPKs), and mechanistic target of rapamycin (mTOR) signaling [7, 18, 19]; the second is those inhibiting NLRP3 activation, including protein kinase A (PKA), AMP-activated protein kinase (AMPK) signaling and autophagy [20, 21]; and the third is interferon (IFN) signaling pathways that may promote or inhibit NLRP3 dependently on the physiological conditions [22]. All these signaling pathways regulate NLRP3 inflammasome activation by interfering with its assembly, thus promoting or inhibiting its activation. Full understanding of these regulation mechanisms is a basis for therapeutic targeting to the inflammasome. We therefore in this review focus on current advances in the mechanisms of NLRP3 inflammasome activation and its regulatory signaling pathways.
ACTIVATION OF NLRP3 INFLAMMASOME
NLRP3 inflammasome is activated mainly through three pathways: the canonical pathway, the non-canonical pathway, and the alternative pathway.
The canonical pathway of NLRP3 activation generally requires two signals: the first one responsible for priming and the second for triggering [23]. The first signal regulates NLRP3 activation at both transcriptional and post-translational levels. It primes cells to express pro-IL-1β and NLRP3 at transcriptional level mainly through regulating TLRs-NF-κB signaling, which increases NLPR3 activation efficiently though some studies have indicated that the transcriptional regulation was dispensable for NLRP3 activation [7, 24], while post-translational modification is mainly through phosphorylation of NLRP3 protein at the Ser198 residue (human) which leads to its de-ubiquitination at Lys63 (Fig. 1), or ubiquitination of ASC protein at its Lys174 residue, and all these post-translational modifications promote the assembly of NLRP3-ASC inflammasome [25,26,27]. The second signal is mainly provided by K+ efflux which is induced by DAMPs including extracellular ATP, perforated toxins (such as nigericin), ROS produced by mitochondrial dysfunction, or released Ca2+ and cathepsin B due to lysosome rupture induced by microparticles (such as silica and MSU crystals) (Fig. 1) [7]. Yet the common second signaling still remains controversial. An early study reported that the rupture of lysosomes was dispensable for NLRP3 activation [28]. Subsequently Muñoz-Planillo and colleagues found that ROS was also dispensable, but K+ efflux was a common second signal for NLRP3 activation [29]. Later studies discovered that it is critical for NLRP3 activation to sense K+ efflux by NIMA-related kinase 7 (NEK7), which is bound to the leucine-rich repeat (LRR) domain of NLRP3 protein [30], thus considering K+ efflux as the common second signal for NLRP3 inflammasome activation. However, it has also been found that some small molecules, such as Imiquimod, can induce NLRP3 inflammasome activation independently of K+ efflux [31]. Therefore, although K+ efflux is believed to be a convergent point in activating the canonical NLRP3 inflammasome for a variety of inducers, the common second signal for the canonical NLRP3 inflammasome activation still awaits further clarification.
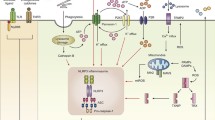
Current view of molecular mechanisms for canonical NLRP3 inflammasome activation. The activation of NLRP3 inflammasome requires two signals. The first signal activates the signaling pathways mediated by TLRs-NF-κB and IFN-γ and thereby up-regulates the expression of inflammasome-related components NLRP3 and pro-IL-1β at the transcriptional level. Potassium ion (K+) efflux and ROS, induced by mTORC1/2, and apoptosis signaling pathways, serve as the second signal to promote the assembly of inflammasome, thereby inducing the activation of caspase-1. By cleaving pro-IL-1β and GSDMD, active caspase-1 eventually converts pro-IL-1β into mature IL-1β and induces pyroptosis through pores formed in the plasma membrane by GSDMD-NT. ASC, apoptosis-associated speck-like protein containing a CARD; FADD, Fas-associated with death domain protein; Fas, CD95; FasL, Fas ligand; GSDMD, gasdermin D; GSDMD-FL, full length GSDMD; GSDMD-NT, GSDMD N-terminal; IκB, inhibitor of nuclear factor kappa B; IKK, IκB kinase; IRAK, interleukin-1 receptor related kinase; JAK, Janus Kinase; JNK, c-Jun N-terminal kinase; MAPKs, mitogen-activated protein kinases; mTORC, mammalian target of rapamycin complex; mtROS, mitochondrial reactive oxygen species; MyD88, myeloid differentiation factor 88; NEK7, NIMA-related kinase; NF-κB, nuclear factor kappa-B; NLRP3, NLR family pyrin domain containing 3; P—, phosphorylation; P2X7, purinergic ligand-gated ion channel 7 receptor; RIPK3, receptor interacting protein kinase; STAT1, signal transducer and activator of transcription; TRAF6, tumor necrosis factor receptor related kinase 6; TRIF, TIR-domain-containing adaptor inducing interferon-β; TXNIP, thioredoxin-interacting protein; Ub, Ubiquitin; ZBP1, Z-DNA binding protein 1.
Apart from the canonical activation pathway for NLRP3, it has been found that intracellular (transfected) LPS-mediated caspase-11 activation can also lead to NLRP3 inflammasome assembly [32]. The recognition of caspase-11 with LPS, together with the action of IFN-induced guanylate-binding proteins (GBPs), leads to polymerization of caspase-11 and its autocatalytic activation, similarly to the auto-cleavage of pro-caspase-1 (Fig. 2). In contrast to caspase-1, activated caspase-11 cannot directly cleave pro-IL-1β and pro-IL-18 but can cleave GSDMD to generate GSDMD-NT, the latter of which forms pores in the plasma membrane, thereby mediating K+ efflux that is currently believed to induce the activation of non-canonical NLRP3 inflammasome [32,33,34].
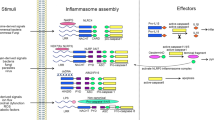
Mechanisms for non-canonical NLRP3 inflammasome activation. Gram-negative bacteria in vacuoles release LPS into the cytoplasm in the presence of type I IFN-induced GBPs and IRGs (immunity-related GTPases). After recognizing LPS directly and self-activation, caspase-11 (mouse) cleaves GSDMD to generate active N-terminal fragment (GSDMD-NT) which forms pores in the plasma membrane. Caspase-4 (the human analog of caspase-11) undergoes a similar process to that of caspase-11, except that it is recruited and activated by a platform formed by GBP1. The potassium ion (K+) efflux through the GSDMD-NT pores activates the NLRP3 inflammasome, which eventually induces the release of mature IL-1β and IL-18 as well as pyroptosis. GBPs, guanylate-binding proteins; IRF9, Interferon regulatory factor 9; IRGs, immunity-related GTPases.
Distinctly from both canonical and non-canonical activation pathways, the alternative activation of NLRP3 inflammasome is independent of K+ efflux but is induced through the TLR4-RIPK1-FADD-caspase-8 pathway by cell surface stimulation with LPS under certain circumstances (Fig. 1). FADD-caspase-8 represents an extrinsic pathway of apoptosis. Indeed, activation of the alternative NLRP3 inflammasome does not cause pyroptotic cell death [35].
It has been demonstrated that FADD-caspase-8 signaling pathway facilitates the activation of NLRP3 inflammasome by up-regulating the expression of NLRP3 and pro-IL-1β at transcriptional level through activating the NF-κB pathway; meanwhile, FADD-caspase-8 promotes the activation of pro-caspase-1 through directly binding to the NLRP3-ASC inflammasome [36]. Recently, Chen and colleagues found that caspase-8 and -9 can be activated via the intrinsic and extrinsic apoptosis pathways, respectively, leading to caspase-3 and -7 activation, the latter of which further cleaves the glycoprotein Pannexin-1 at its C-terminal, thus mediating cell membrane perforation and leakage of K+ from the porous channels (Fig. 1), thereby promoting the assembly of NLRP3 inflammasome [37]. Likewise, caspase-6 also plays a role in NLRP3 inflammasome activation. A latest study by Zheng et al. showed that in the cells infected with influenza A virus (IAV), caspase-6 binds with RIPK3 (receptor-interacting protein kinase 3) and serves as a scaffold to promote the interaction between RIPK3 and Z-DNA binding protein 1 (ZBP1), thus inducing the NLRP3 inflammasome activation (Fig. 1). But so far, such a pathway mediated by caspase-6 was only observed in the circumstance of IAV infection [38].
When NLRP3 inflammasome is activated, the cells may culminate in different outcomes including pyroptosis, apoptosis, secondary necrosis, or even hyperactivation, respectively, depending on their distinct statuses. In the cells with high levels of pro-caspase-1 and GSDMD proteins, activated NLRP3 inflammasome can recruit pro-caspase-1, leading to its polymerization and auto-cleavage at Asp316 residue in the linker between the large and small subunits of its protease domain to form an active p33/p10 complex [39]. The p33/p10 complex is the main form of active caspase-1, but it is also a transitional form as the complex may subsequently be auto-cleaved once more at the Asp119 residue in the connecting region between the large subunit and the CARD domain, producing a p20/p10 complex which is released from the cell while losing its protease activity [39]. The active caspase-1 can then cut pro-IL-1β at both Asp26 and Asp116 residues, thus producing an active p17 IL-1β fragment [40]. Active caspase-1 can also cleave GSDMD through its hydrophobic interface that is formed after the auto-cleavage of pro-caspase-1 to interact with the C-terminal domain of GSDMD. GSDMD is cleaved at the Asp275 residue (human) to produce an active p30 GSDMD-NT fragment, which can perforate the plasma membrane [41, 42]. So far, it is believed that when the density of GSDMD-NT pores in the cell membrane exceeds a certain threshold, lytic cell death will take place to release a large amount of inflammatory cellular contents. Such a phenomenon is called pyroptosis [43, 44]. But when the density of GSDMD-NT pores is lower than the threshold, they will allow the release of IL-1β while maintaining the integrity of the cell membrane. Such a status of the cells is called hyperactivation. Some researchers thought that the ESCRT-III (endosomal sorting complex required for transport III) repair mechanism played an important role in maintaining the hyperactivation through reducing the amount of GSDMD-NT pores and preventing the cell from lysis [44, 45].
In caspase-1-deficient cells, however, NLRP3 inflammasome activation may lead to apoptosis through ASC-caspase-8-mediated caspase-3/-7 activation [46], or secondary necrosis through cleavage of GSDME by caspase-3/-7 when the cells expressing a high level of GSDME [47]. Apoptosis and secondary necrosis may also be induced in GSDMD-deficient cells upon the activation of NLRP3 inflammasome, but the underlying mechanism is different from that of caspase-1-deficient cells: the apoptosis is induced directly by caspase-1-mediated Bid-caspase-9-caspase-3 axis, instead of by caspase-8 activation [48]. Interestingly, a recent study has revealed that caspase-3 is activated through the caspase-1/caspase-8-Bid-SMAC (second mitochondria-derived activator of caspases)-caspase-9 pathway to induce secondary necrosis in a GSDME-independent manner, but which member of the gasdermin family or other cell membrane perforating proteins is targeted to mediate the secondary necrosis is currently unclear [49].
SIGNALING PATHWAYS THAT PROMOTE NLRP3 INFLAMMASOME ACTIVATION
Toll-Like Signaling Pathway
TLRs are the most widely expressed PRRs in innate immune cells, which play an important role in the innate immunity [3]. The TLR pathway is composed of various adaptors such as myeloid differentiation factor 88 (MyD88) and other TIR domain adaptor protein (TIRAP), which respectively mediates sub-pathways to induce the expression of multiple pro-inflammatory cytokines including pro-IL-1β, TNF-ɑ, and IL-6, via various downstream kinases such as the tumor necrosis factor receptor-related kinase 6 (TRAF6) and interleukin-1 receptor-related kinase 1/4 (IRAK1/4), and thus promoting the inflammatory responses [50, 51].
TLR pathways also play a critical role in promoting the activation of NLRP3 inflammasome. They do so mainly at the transcriptional and post-translational levels. At the transcriptional level, triggering TLRs up-regulate the expression of NLRP3 inflammasome components such as NLRP3 and pro-IL-1β through the canonical MyD88-IRAK1/4-TRAF6-TAK1-IKK-NF-κB pathway [50, 52,53,54]; however, Juliana et al. revealed that when the first and second signals are present simultaneously, 10 min is enough for NLRP3 activation, suggesting that the transcriptional regulation in this process may be dispensable [55]. In view of post-translational modifications, triggering of TLRs leads to the activation of the MyD88/TRIF-TARF6-TAK1-JNKs signaling pathway, which in turn targets NLRP3, phosphorylating it at Ser198 residue, and only by phosphorylation at this residue, the NLRP3 will undergo de-ubiquitination, thereby allowing it to be assembled with ASC to form the NLRP3 inflammasome [25, 56, 57] (Fig. 1). Therefore, NLRP3 phosphorylation through TLR pathways is a switch for NLRP3 inflammasome activation, and the threshold of NLRP3 activation will be reduced when its expression is transcriptionally up-regulated. But only when both the transcriptional up-regulation and the post-translational modification act together, the NLRP3 inflammasome can be fully activated to ensure its physiological function of killing and clearing pathogens [7]. Moreover, as mentioned above, the alternative pathway of NLRP3 activation can also be mediated by TLR signaling, specifically the TLR4-TRIF-RIPK1-FADD-caspase-8 pathway upon LPS stimulation, which is independent of a second signal like K+ efflux (Fig. 1). That is to say, a single TLR signal is sufficient for NLRP3 inflammasome activation in certain cells (such as human monocytes) [35].
mTOR Signaling Pathway
mTOR can be activated by multiple environmental input signals, including nutrients and growth factors, to coordinate the growth and metabolism of eukaryotic cells [58]. mTOR is a serine/threonine protein kinase member of the phosphatidylinositol 3-kinase (PI3K)-related kinase family. It is the catalytic subunit of two different protein complexes, named mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) [58, 59]. mTORC1/2 are able to sense the changes of intracellular energy and the stimulation of growth factors; promote biosynthesis, cell growth, or division; and play an important role in various immune responses [58, 59].
Emerging evidence indicates that mTOR signaling is involved in NLRP3 inflammasome activation. Rapamycin (an inhibitor of mTORC1) can inhibit the activity of NF-κB signaling through targeting mTOR, suggesting that mTOR can regulate gene transcription via the NF-κB pathway [60, 61]. It has also been shown that the activation of mTORC1 can promote the expression and maturation of IL-1β through HIF-1α (hypoxia-inducible factor-1α), though these studies had not specifically linked mTORC1 to NLRP3 inflammasome activation [62,63,64]. Recently, one study revealed that activated mTOR could promote NLRP3 inflammasome activation by increasing mitochondrial ROS (mtROS) production [65]. All these studies have shown that mTOR may act at both the first and second steps of NLRP3 inflammasome activation, but which of the mTOR complexes (mTORC1/2 or both) is involved in these processes remains unclear. Some studies revealed that mTORC2 could promote the development of inflammation and had been involved in skin aging through the AKT1-IKK-NF-κB pathway [66]. Another recent study have pointed out that mTORC2 catalyzes the phosphorylation of serum- and glucocorticoid-inducible kinase 1 (SGK1) in renal tubule cells, which in turn activates the epithelial Na+ channel (ENaC), and promotes Na+ influx and K+ efflux [67], suggestive of a potential role for mTORC2 to regulate NLRP3 activation considering that K+ efflux is sufficient for NLRP3 inflammasome activation [7]. However, whether mTORC2 regulates Na+/K+ currents in innate immune cells as it does in renal tubule cells is worthy of further investigation. Together, these findings suggest that the mTOR signaling may facilitate the activation of NLRP3 inflammasome by up-regulating the expression of NLRP3, pro-IL-1β, and other components through the AKT1-IKK-NF-κB pathway, or inducing the production of ROS and K+ efflux (Fig. 1), but how it works in this process requires more investigations.
MAPK Signaling Pathway
MAPKs are another class of serine/threonine kinases, which transmit extracellular growth-stimulating signals to the nucleus, thus promoting cell proliferation. The mammalian MAPK family is composed of several kinases such as ERKs (extracellular signal-regulated kinases), p38, and JNKs (c-Jun N-terminal kinases), which are activated through MKKK (MAP 3K/MEKK)-MAPKK (MAP 2 K/MEK)-MAPKs pathways, respectively [68, 69]. MAPKs participate not only in biological responses to growth factor and cytokine stimulations but also in sensing non-biological stimuli such as oxidative stress, DNA damage, and osmotic imbalance, suggesting the association of MAPK signaling with inflammatory responses [70, 71].
Early studies have shown that ROS, a second signal for NLRP3 activation, could induce signaling in the MAP 3K-MAP 2K-MAPK pathway [7, 72, 73]. A later study by Wang et al. shows that over-expressed microRNA-377 in cells induced the production of ROS, which further induced MAPK signaling to activate thioredoxin-interacting protein (TXIP), thus facilitating NLRP3 inflammasome activation [74] (Fig. 1). In resting cells, NLRP3 is located at the endoplasmic reticulum (ER), and ASC is evenly distributed in the cytoplasm; upon activation, however, NLRP3 and ASC are redistributed around the nucleus, co-localized with the ER and mitochondrial clusters. TXNIP is transported to the mitochondria-associated ER membranes where it interacts with NLRP3, thus linking the oxidative stress to inflammasome activation [75, 76]. Besides, TXNIP also acts as a positive factor for NLRP3 activation by inhibiting LPS-induced production of nitric oxide (NO), a negative regulator of NLRP3 inflammasome activation [77, 78]. Recent studies have shown that ROS can also activate NF-κB signaling through MAPKs (JNK/ERK/p38) pathways to promote the expression of NLRP3 and pro-IL-1β, thereby promoting the activation of NLRP3 inflammasome [79, 80]. All these findings support the notion that MAPKs, together with their upstream ROS and downstream TXNIP and NF-κB, play a pivotal role in regulating NLRP3 activation (Fig. 1).
SIGNALING PATHWAYS THAT INHIBIT THE ACTIVATION OF NLRP3 INFLAMMASOME
Autophagy
Under some conditions such as amino acid starvation, pathogenic infection, and organelle damage, various autophagy-related (ATG) protein complexes mediate phagophores to form autophagosomes that contain targets (such as misfolded proteins and damaged organelles) for degradation by fusing with lysosomes to become autolysosomes, thereby recycling of nutrients and removing harmful substances [81, 82] (Fig. 3). Over the past decade, autophagy has been shown to be critically linked to immune responses. Deficiency of autophagy is associated with various inflammatory diseases such as Crohn’s disease (DC) and systemic lupus erythematosus (SLE) [83, 84], while abnormal activation of NLRP3 inflammasome boosts the development of these diseases [85].
Signaling pathways that inhibit the activation of NLRP3 inflammasome. The signaling pathways that inhibit the activation of NLRP3 inflammasome mainly include autophagy, cAMP-PKA, and AMPK. These signaling pathways work primarily by inhibiting NLRP3 phosphorylation, ubiquitination, and down-regulating pro-IL-1β and NLRP3 expression, suppressing the co-localization of NLRP3 and ASC, as well as clearing ROS and inflammasome component ASC, thereby inhibiting the assembly and activation of inflammasome. AC, adenylate cyclase; AMPK, AMP-activated protein kinase; ATF4, activating transcription factor 4; ATG, autophagy-related protein; cAMP, cyclic adenosine monophosphate; NOD2, nucleotide-binding oligomerization domain 2; elF2a, eukaryotic translation initiation factor 2α; EP4, prostaglandin E2 receptor 4; ER, endoplasmic reticulum; Nrf2, NF-E2-related factor 2; PEG, prostaglandin E; PERK, protein kinase R (PKR)-like endoplasmic reticulum kinase; PKA, protein kinase A; Trx, thioredoxin.
Early studies have shown that ATG16L1-deficient macrophages produce much higher levels of IL-1β than normal cells upon the activation of NLRP3 inflammasome [86, 87], indicating the importance of autophagy in maintaining the homeostasis of the innate immunity. In step with this, many studies have indicated that autophagy can negatively regulate the activation of inflammasome through a variety of mechanisms [88]. When bone marrow-derived dendritic cells (BMDCs) are infected by IAV, cytoplasmic nucleotide-binding oligomerization domain-containing protein 2 (NOD2) and its downstream regulator RIPK2 (receptor interacting protein kinase 2) elicit mitochondrial autophagy (mitophagy) by inducing the phosphorylation of Unc-51 like autophagy activating kinase 1 (ULK1) at Ser555 residue, when the damaged mitochondria produce a mass of ROS [76, 89]. In addition, upon ER stress, PKR-like eukaryotic initiation factor 2a kinase (PERK) is activated by misfolded proteins from the ER and phosphorylates eukaryotic translation initiation factor 2 (eIF2a ) at the Ser51 residue on its α subunit. Activated eIF2a in turn up-regulates the expression of activating transcription factor 4 (ATF4), which is a transcription factor that promotes the expression of various ATG proteins, thus promoting autophagy and alleviating the ER stress [90]. As ER stress is able to activate NLRP3 inflammasome, autophagy-mediated alleviation of ER stress can dampen NLRP3 inflammasome activation [91]. Moreover, autophagy can also inhibit inflammasome activation by reducing ASC [92]. In sum, autophagy induced by mitochondrial damage (via the NOD2-RIPK2-ULK1 pathway) or ER stress (via the PERK-eIF2α-ATF4-ATGs pathway) can clear damaged organelles to prevent generation of second signals such as ROS or degradation of ASC, thereby negatively regulating NLRP3 activation.
Increasing evidence makes the mechanism by which autophagy regulates NLRP3 inflammasome more and more clear. Various NLRP3 stimuli induce the disassembly of the trans-Golgi network (dTGN), which serves as a scaffold for NLRP3 aggregation and ASC speck formation [93]; however, other reports including ours have revealed that NLRP3 inflammasome and ASC speck are formed near the microtubule organization center (MTOC) [94, 95]. But another question comes, how is the dTGN-localized NLRP3 aggregation delivered to the MTOC during its activation? Recently, an excellent study has indicated that it is the histone deacetylase 6 (HDAC6) together with dynein, the retrograde transport motor, that undertakes the delivery of NLRP3 aggregate to the MTOC, thus facilitating the association of centrosome-localized kinase NEK7 with the NLRP3 inflammasome [96]. Interestingly, the NLRP3 inflammasome puncta can be degraded by autophagy as they colocalize with LC3B, an autophagy marker, thus suppressing its activation [96, 97]. Consistent with a previous report [98], however, the delivery function of HDAC6 does not depend on its microtubule deacetylation activity but depends on its ubiquitin-binding domain [96]. Noticeably, our study demonstrates that knockdown of αTAT1, the α-tubulin N-acetyltransferase, to reduce microtubule acetylation significantly suppresses IL-1β secretion and cell death [95], indicating the fundamental role of microtubule acetylation in NLRP3 inflammasome activation though it is unclear how HDAC6 acts synergistically with α-TAT1 in this process.
cAMP-PKA Signaling Pathway
PKA is a cyclic adenosine monophosphate (cAMP)-activated serine/threonine kinase. The cAMP-PKA pathway has long been found to play an important role in negative regulation of inflammatory responses. Early studies showed that induction of cAMP could suppress the release of IL-1β and alleviate the development of inflammation [99]. Subsequently it was found that the cAMP-PKA signaling regulates transcription and post-translational modification by phosphorylating specific proteins, thus being an important negative regulator for the immune system [100].
Emerging evidence demonstrates that the cAMP-PKA signaling can directly regulate both canonical and non-canonical NLRP3 inflammasome activation. It was found that some physiological products such as prostaglandin and dopamine, through binding with their respective receptors, induce the generation of cAMP, leading to the ubiquitination and degradation of NLRP3 and thus preventing its activation [101, 102]. Another study has identified a more specific mechanism by which cAMP-PKA signaling inhibits the activation of NLRP3 inflammasome: prostaglandin E2 (PGE2) binds to its receptor and activates the adenylate cyclase (AC), the latter of which converts ATP into cAMP; cAMP then binds to and activate PKA, which in turn phosphorylates the Ser295 residue (human) of NLRP3, thereby inhibiting the assembly of NLRP3 inflammasome [15] (Fig. 3). Similarly, through binding with the transmembrane G-protein-coupled receptor 5 (TGR5), bile acid induces the production of cAMP, which further activates PKA to phosphorylate the Ser295 residue of NLRP3, allowing its ubiquitination, thus negatively regulating the activation of NLRP3 inflammasome [103]. Pharmacological studies revealed that scutellarin or wedelolactone can facilitate Ser/Thr phosphorylation of NLRP3 via PKA to suppress the inflammasome activation and pyroptosis [104,105,106].
It was recently found that L-adrenaline activates AC through binding to the adrenoceptor α 2B (ADRA2B) to produce cAMP and thus activating PKA, but interestingly, the activated PKA directly bind to and phosphorylate caspase-11, thereby suppressing caspase-11-mediated non-canonical NLRP3 inflammasome activation [107] (Fig. 3). Besides, the cAMP-PKA signaling may also inhibit the activation of IKK and NF-κB, preventing the latter from translocating into the nucleus and thereby suppressing the expression of inflammatory genes. This suggests that cAMP-PKA signaling may inhibit the expression of NLRP3 and pro-IL-β, thus negatively regulating NLRP3 activation at the transcriptional level through dampening NF-κB signaling [108, 109]. Together, cAMP-PKA signaling regulates NLRP3 inflammasome activation both at the transcriptional level and at the post-translational level (Fig. 3).
AMPK Signaling Pathway
AMP-activated protein kinase (AMPK) is another serine/threonine kinase, which is allosterically activated by AMP when the intracellular AMP:ATP ratio rises. It regulates energy homeostasis and metabolic stresses and plays an important role in the development of neurodegeneration, inflammation, and oxidative stress [110, 111]. In response to anti-inflammatory cytokines such as IL-10 and TGF-β, AMPK is activated to negatively regulate the IKK/IκB/NF-κB signaling while converting the macrophages into an anti-inflammatory type, thus inhibiting the development of inflammation [112]. These studies indicate that AMPK plays a fundamental role in suppressing inflammation.
AMPK signaling may attenuate the activation of NLRP3 inflammasome by decreasing cellular ROS levels. An early study by Ouslimani et al. has manifested that AMPK agonist metformin could reduce ROS production from the mitochondrial respiratory chain of aortic endothelial cells, though the underlying molecular mechanism was not clearly revealed [113]. Later, it was found that AICAR, an AMP analog, up-regulated the expression of IκB and inhibited its phosphorylation through activating AMPK, thus preventing NF-κB activation and NAD(P) H oxidase synthesis. As NAD(P) H oxidase is vital for mtROS production, AMPK activation is proposed to inhibit the production of mtROS [114]. Based on these studies, other investigators have showed that AMPK activation inhibited NLRP3 inflammasome by suppressing the production of ROS in BMDMs [115]. Further studies confirm that AMPK activation not only down-regulates the expression of NAD(P) H oxidase and the production of mtROS thus inhibiting NLRP3 inflammasome activation but also prevents the assembly of the inflammasome through inducing the AMPK-GSK3β-Nrf2 pathway (Fig. 3). In the lung cells of patients with acute lung injury, xanthohumol can induce the phosphorylation (activation) of AMPK, which in turn phosphorylates and inactivates glycogen synthase kinase 3β (GSK3β), thereby up-regulating the activated p62 transcription factor NF-E2-related factors (p62-Nrf2) [116]. Nrf2 plays a pivotal role in reducing various diseases caused by inflammation and oxidative stress, and can induce the expression of NADPH and thioredoxin (Trx), among which Trx inhibits TXNIP-mediated inflammasome assembly and activation [117].
Although AMPK signaling is generally regarded to inhibit NLRP3 inflammasome activation, it is worth noting that, however, metformin- and berberine-mediated AMPK signaling could greatly enhance ATP-induced NLRP3 inflammasome activation and pyroptosis in macrophages, respectively [118, 119]. The discrepancy between these studies and previous ones may be due to different cellular models used in respective studies, which should be clarified in further research.
IFN SIGNALING PATHWAY
The interferon (IFN) family is composed of type I IFNs (IFN-α/β), type II IFN (IFN-γ), and type III IFN (IFN-λ), among which type I IFN and type II IFN function in various innate and adaptive immune cells, while the receptor of type III IFN is currently only found in endothelial cells [120, 121]. Therefore, herein, we mainly introduce the roles of the type I and type II IFNs in regulating NLRP3 inflammasome activation. Through binding to IFN-α/β receptor (IFNAR) and INF-γ receptor (IFNγR), respectively, type I and II IFN activate signal transducer and activator of transcription (STATs), and induce the expression of interferon-stimulated genes (ISGs) such as GBPs and iNOS (inducible nitric oxide synthase), to regulate innate immune responses especially anti-viral infection [120, 122]. One important mechanism by which IFN signaling regulates innate immunity is that it regulates the activation of inflammasomes especially NLRP3 inflammasome [22]; however, whether IFN signaling promote or inhibit NLPR3 inflammasome activation depends on the status of the cell [17, 22]. For example, if the cells are infected with different viruses or at different stages of infection, they may produce anti-inflammatory IL-10 or pro-inflammatory TNF-α [123, 124]. The following continues to review the role of IFN signaling in regulating NLRP3 inflammasome activation, respectively (Figs. 2 and 3).
IFN Signaling Pathways Promote the Activation of NLRP3 Inflammasome
The expression of caspase-11 in mouse macrophages was previously thought to be induced by IFN-β, and only when the IFNR signaling pathway was intact, could the invaded Escherichia coli induce the activation of caspase-1 and caspase-11, leading to the release of IL-1β [125, 126]. However, the basal level of intracellular caspase-11 is adequate, and there are reports indicated that transfected LPS can directly activate caspase-11, suggesting that the IFN pathway is dispensable for caspase-11 activation [127, 128]. Later studies have found that under the treatment of type I IFN, STAT1/2, and IRF9 forms a transcription factor complex, which induces the expression of caspase-11 and GBPs. After lysis by GBPs, vacuoles containing invaded bacteria release the bacteria into the cytosol, and then, GBPs and IRG10B bind to the bacteria and release their LPS into the cytosol for caspase-11 recognition [33, 129,130,131]. Since humans lack the IRG family, a recent study showed that GBP1 directly binds to LPS on the surface of cytoplasmic bacteria to form a platform to recruit and induce caspase-4 (human analog of caspase-11) activation, thus further mediating the activation of non-canonical NLRP3 inflammasome [131] (Fig. 2). Moreover, type I IFN can induce the expression of Z-DNA binding protein 1 (ZBP1), which promotes NLRP3 inflammasome activation by recognizing the components of IAV [132]. On the other hand, IFN-γ together with STAT1 up-regulates the expression of NLRP3, pro-caspase-1, and GSDMD (Figs. 2 and 3), and such a pathway is NF-κB-independent, indicating that IFN-γ positively regulates the activation of NLRP3 inflammasome by up-regulating the expression of its components [133]. Thus, IFN signaling promotes both canonical and non-canonical NLRP3 inflammasome activation under the above-mentioned circumstances.
IFN Signaling Pathways Inhibit the Activation of NLRP3 Inflammasome
On the contrary, IFN signaling may inhibit NLRP3 inflammasome activation under following circumstances. In macrophages, IFN-γ-STAT1 signaling induces the expression of CD40, which inhibits the production of ROS and the phosphorylation of ERK1/2, thus preventing TLR4 and ATP signaling to activate NLRP3 inflammasome [134, 135]. In monocytes from patients of multiple sclerosis, IFN-β induces the expression of IL-10 through IFNR-STAT1 pathway, and in turn, IL-10 down-regulates the expression of pro-IL-1β through the IL-10R-STAT3 pathway (Fig. 3), thus mitigating NLRP3 inflammasome activation [136]. In mouse lung cells intracellularly infected with Mycobacterium tuberculosis, IFN-γ could induce the expression of inducible NO synthase (iNOS), which produces NO to make NLRP3 thiol nitrosylated, thus inhibiting the assembly of NLRP3 inflammasome to alleviate tuberculosis caused by M. tuberculosis infection [78].
CROSS-TALK AMONG THE SIGNALING PATHWAYS
We in this review have narrated several signaling pathways, inhibitory or promotional, that regulate NLRP3 inflammasome activation. But their actions are quite complicated as these pathways do not act independently but cross-talk with each other.
For example, TLRs are at the upstream of MAPKs in innate immune responses, and TLR signaling induces the activation of MAPKs through the TRAF6-TAK pathway [50]. In addition, the TLR signaling may also induce autophagy: mTOR senses intracellular energy levels and regulates metabolism through the RTK-PI3K-AKT-mTOR pathway, and if such a pathway is blocked, autophagy is to take place [137, 138]. This was corroborated by Yang and his colleagues, who found that in A549 cells, TLR-MyD88-TRAF6 signaling induces the activation of the PI3K-AKT-mTOR pathway, thus indirectly inhibited autophagy and promoted the development of inflammation [139]. Among the many NLRP3 signaling pathways, the cross-talking between AMPK, mTORC, and autophagy is commonly observed. AMPK directly or indirectly induces autophagy, in which AMPK promotes the autophagosome assembly by phosphorylating the Ser555 of ATG1/ULK1, or by promoting the nucleus translocation of transcription factor EB (TFEB ) [140,141,142]. TFEB is a key positive regulator of autophagy and lysosome biogenesis, which induces the expression of autophagy-related LC3 and ATGs. AMPK can also phosphorylate the Ser1345 residue of the tumor suppressor TSC2 to enhance the ability of TSC2, or phosphorylate the Ser792 residue of Raptor of mTORC1, thus inhibiting the mTORC1 signaling [143, 144]. In addition to inducing autophagy, AMPK may also inhibit the activity of NF-κB, thus to certain extent inhibiting the TLR signaling pathway [145]. Moreover, MAPK activation can also inhibit autophagy in that MAPK1/ERK regulates the phosphorylation of TFEB to prevent it from entering the nucleus, thus inhibiting the occurrence of autophagy [142].
In the process of inflammasome activation, the induction of intracellular IFNs is generally TLRs-dependent, through the TRIF-TRAF3-IKK-TBK1 pathway to promote the nucleus translocation of interferon regulatory factor 3/7 (IRF3/7) to induce the expression of IFNs [146,147,148]. As induction of intracellular IFN is TRIF-dependent, autophagy may suppress the IFN signaling by directly targeting TRIF through autophagy-related molecules sequestosome 1 (SQSTM1) and TAX1-binding protein 1 (TAX1BP1) to reduce its intracellular levels [149]. Besides autophagy, AMPK also inhibits IFN signaling by preventing STAT1 from entering the nucleus, thus inhibiting the expression of inflammatory cytokines such as CCL2 and CXCL10 through the IFNγ-STAT1 signaling [150].
Therefore, induction of one pathway may activate or inhibit another one, and they synergistically regulate the physiological status of cells or decide their fate. Therefore, the related signaling pathways are multi-dimensional and interconnected with each other to orchestrate NLRP3 inflammasome activation in different types of cells.
CONCLUSION
NLRP3 inflammasome stimuli may simultaneously or sequentially activate several pathways as mentioned above, and these pathways may have cross-talk with each other. With the hint of the cross-talk between the known signaling pathways, experiments can be designed to study the as-yet-unknown mechanisms of NLRP3 inflammasome activation, thus more comprehensively understanding the mechanism(s) of the inflammasome activation, which provides a theoretical basis for developing therapeutic drugs targeting NLRP3 inflammasome.
Data Availability
All data and materials can be freely obtained from the authors through correspondence.
References
Kagan, J.C. 2012. Signaling organelles of the innate immune system. Cell 151: 1168–1178.
Patel, S. 2018. Danger-associated molecular patterns (DAMPs): the derivatives and triggers of inflammation. Current Allergy and Asthma Reports 18: 63.
Medzhitov, R. 2007. Recognition of microorganisms and activation of the immune response. Nature 449: 819–826.
Satoh, T., and S. Akira. 2016. Toll-like receptor signaling and its inducible proteins. Microbiology Spectrum 4. https://doi.org/10.1128/microbiolspec.MCHD-0040-2016.
Xue, Y., D. Enosi Tuipulotu, W.H. Tan, C. Kay, and S.M. Man. 2019. Emerging activators and regulators of inflammasomes and pyroptosis. Trends in Immunology 40: 1035–1052.
Platnich, J.M., and D.A. Muruve. 2019. NOD-like receptors and inflammasomes: a review of their canonical and non-canonical signaling pathways. Archives of Biochemistry and Biophysics 670: 4–14.
Swanson, K.V., M. Deng, and J.P. Ting. 2019. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nature Reviews. Immunology 19: 477–489.
Martinon, F., K. Burns, and J. Tschopp. 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Molecular Cell 10: 417–426.
Tweedell, R.E., and T.D. Kanneganti. 2020. Advances in inflammasome research: recent breakthroughs and future hurdles. Trends in Molecular Medicine 26: 969–971.
Place, D.E., and T.D. Kanneganti. 2018. Recent advances in inflammasome biology. Current Opinion in Immunology 50: 32–38.
Liu, L., and B. Sun. 2019. Neutrophil pyroptosis: new perspectives on sepsis. Cellular and Molecular Life Sciences: CMLS 76: 2031–2042.
Jorgensen, I., J.P. Lopez, S.A. Laufer, and E.A. Miao. 2016. IL-1β, IL-18, and eicosanoids promote neutrophil recruitment to pore-induced intracellular traps following pyroptosis. European Journal of Immunology 46: 2761–2766.
Christgen, S., and T.D. Kanneganti. 2020. Inflammasomes and the fine line between defense and disease. Current Opinion in Immunology 62: 39–44.
Guo, H., J.B. Callaway, and J.P. Ting. 2015. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nature Medicine 21: 677–687.
Mortimer, L., F. Moreau, J.A. MacDonald, and K. Chadee. 2016. NLRP3 inflammasome inhibition is disrupted in a group of auto-inflammatory disease CAPS mutations. Nature Immunology 17: 1176–1186.
Kaneko, N., M. Kurata, T. Yamamoto, S. Morikawa, and J. Masumoto. 2019. The role of interleukin-1 in general pathology. Inflammation and Regeneration 39: 12.
Pedraza-Alva, G., L. Pérez-Martínez, L. Valdez-Hernández, K.F. Meza-Sosa, and M. Ando-Kuri. 2015. Negative regulation of the inflammasome: keeping inflammation under control. Immunological Reviews 265: 231–257.
Rajamäki, K., M.I. Mäyränpää, A. Risco, J. Tuimala, K. Nurmi, A. Cuenda, K.K. Eklund, K. Öörni, and P.T. Kovanen. 2016. p38δ MAPK: A novel regulator of NLRP3 inflammasome activation with increased expression in coronary atherogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology 36: 1937–1946.
Dai, J., C. Jiang, H. Chen, and Y. Chai. 2019. Rapamycin attenuates high glucose-induced inflammation through modulation of mTOR/NF-κB pathways in macrophages. Frontiers in Pharmacology 10: 1292.
Yang, F., Y. Qin, Y. Wang, S. Meng, H. Xian, H. Che, J. Lv, Y. Li, Y. Yu, Y. Bai, and L. Wang. 2019. Metformin inhibits the NLRP3 inflammasome via AMPK/mTOR-dependent effects in diabetic cardiomyopathy. International Journal of Biological Sciences 15: 1010–1019.
Saitoh, T., and S. Akira. 2016. Regulation of inflammasomes by autophagy. The Journal of Allergy and Clinical Immunology 138: 28–36.
Labzin, L.I., M.A. Lauterbach, and E. Latz. 2016. Interferons and inflammasomes: cooperation and counterregulation in disease. The Journal of Allergy and Clinical Immunology 138: 37–46.
He, Y., H. Hara, and G. Núñez. 2016. Mechanism and regulation of NLRP3 inflammasome activation. Trends in Biochemical Sciences 41: 1012–1021.
Xing, Y., X. Yao, H. Li, G. Xue, Q. Guo, G. Yang, L. An, Y. Zhang, and G. Meng. 2017. Cutting edge: TRAF6 mediates TLR/IL-1R signaling-induced nontranscriptional priming of the NLRP3 inflammasome. Journal of Immunology (Baltimore, Md. : 1950) 199: 1561–1566.
Song, N., Z.S. Liu, W. Xue, Z.F. Bai, Q.Y. Wang, J. Dai, X. Liu, Y.J. Huang, H. Cai, X.Y. Zhan, Q.Y. Han, H. Wang, Y. Chen, H.Y. Li, A.L. Li, X.M. Zhang, T. Zhou, and T. Li. 2017. NLRP3 Phosphorylation is an essential priming event for inflammasome activation. Molecular Cell 68: 185–197.e186.
Guan, K., C. Wei, Z. Zheng, T. Song, F. Wu, Y. Zhang, Y. Cao, S. Ma, W. Chen, Q. Xu, W. Xia, J. Gu, X. He, and H. Zhong. 2015. MAVS promotes inflammasome activation by targeting ASC for K63-linked ubiquitination via the E3 ligase TRAF3. Journal of Immunology (Baltimore, Md. : 1950) 194: 4880–4890.
Py, B.F., M.S. Kim, H. Vakifahmetoglu-Norberg, and J. Yuan. 2013. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Molecular Cell 49: 331–338.
Lima, H., Jr., L.S. Jacobson, M.F. Goldberg, K. Chandran, F. Diaz-Griffero, M.P. Lisanti, and J. Brojatsch. 2013. Role of lysosome rupture in controlling Nlrp3 signaling and necrotic cell death. Cell Cycle (Georgetown, Texas) 12: 1868–1878.
Muñoz-Planillo, R., P. Kuffa, G. Martínez-Colón, B.L. Smith, T.M. Rajendiran, and G. Núñez. 2013. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38: 1142–1153.
He, Y., M.Y. Zeng, D. Yang, B. Motro, and G. Núñez. 2016. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 530: 354–357.
Groß, C.J., R. Mishra, K.S. Schneider, G. Médard, J. Wettmarshausen, D.C. Dittlein, H. Shi, O. Gorka, P.A. Koenig, S. Fromm, G. Magnani, T. Ćiković, L. Hartjes, J. Smollich, A.A.B. Robertson, M.A. Cooper, M. Schmidt-Supprian, M. Schuster, K. Schroder, P. Broz, C. Traidl-Hoffmann, B. Beutler, B. Kuster, J. Ruland, S. Schneider, F. Perocchi, and O. Groß. 2016. K(+) Efflux-independent NLRP3 inflammasome activation by small molecules targeting mitochondria. Immunity 45: 761–773.
Rühl, S., and P. Broz. 2015. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. European Journal of Immunology 45: 2927–2936.
Meunier, E., M.S. Dick, R.F. Dreier, N. Schürmann, D. Kenzelmann Broz, S. Warming, M. Roose-Girma, D. Bumann, N. Kayagaki, K. Takeda, M. Yamamoto, and P. Broz. 2014. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature 509: 366–370.
Shi, J., Y. Zhao, Y. Wang, W. Gao, J. Ding, P. Li, L. Hu, and F. Shao. 2014. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514: 187–192.
Gaidt, M.M., T.S. Ebert, D. Chauhan, T. Schmidt, J.L. Schmid-Burgk, F. Rapino, A.A. Robertson, M.A. Cooper, T. Graf, and V. Hornung. 2016. Human monocytes engage an alternative inflammasome pathway. Immunity 44: 833–846.
Gurung, P., P.K. Anand, R.K. Malireddi, L. Vande Walle, N. Van Opdenbosch, C.P. Dillon, R. Weinlich, D.R. Green, M. Lamkanfi, and T.D. Kanneganti. 2014. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. Journal of Immunology (Baltimore, Md. : 1950) 192: 1835–1846.
Chen, K.W., B. Demarco, R. Heilig, K. Shkarina, A. Boettcher, C.J. Farady, P. Pelczar, and P. Broz. 2019. Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP3 inflammasome assembly. The EMBO Journal 38: e101638.
Zheng, M., R. Karki, P. Vogel, and T.D. Kanneganti. 2020. Caspase-6 is a key regulator of innate immunity, inflammasome activation, and host defense. Cell 181: 674–687.e613.
Boucher, D., M. Monteleone, R.C. Coll, K.W. Chen, C.M. Ross, J.L. Teo, G.A. Gomez, C.L. Holley, D. Bierschenk, K.J. Stacey, A.S. Yap, J.S. Bezbradica, and K. Schroder. 2018. Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. The Journal of Experimental Medicine 215: 827–840.
Chan, A.H., and K. Schroder. 2020. Inflammasome signaling and regulation of interleukin-1 family cytokines. The Journal of Experimental Medicine 217: e20190314.
Wang, K., Q. Sun, X. Zhong, M. Zeng, H. Zeng, X. Shi, Z. Li, Y. Wang, Q. Zhao, F. Shao, and J. Ding. 2020. Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis. Cell 180: 941–955.e920.
Orning, P., E. Lien, and K.A. Fitzgerald. 2019. Gasdermins and their role in immunity and inflammation. The Journal of Experimental Medicine 216: 2453–2465.
Mulvihill, E., L. Sborgi, S.A. Mari, M. Pfreundschuh, S. Hiller, and D.J. Müller. 2018. Mechanism of membrane pore formation by human gasdermin-D. The EMBO Journal 37: e98321.
Evavold, C.L., J. Ruan, Y. Tan, S. Xia, H. Wu, and J.C. Kagan. 2018. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity 48: 35–44.e36.
Rühl, S., K. Shkarina, B. Demarco, R. Heilig, J.C. Santos, and P. Broz. 2018. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 362: 956–960.
Sagulenko, V., S.J. Thygesen, D.P. Sester, A. Idris, J.A. Cridland, P.R. Vajjhala, T.L. Roberts, K. Schroder, J.E. Vince, J.M. Hill, J. Silke, and K.J. Stacey. 2013. AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death and Differentiation 20: 1149–1160.
Rogers, C., T. Fernandes-Alnemri, L. Mayes, D. Alnemri, G. Cingolani, and E.S. Alnemri. 2017. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nature Communications 8: 14128.
Tsuchiya, K., S. Nakajima, S. Hosojima, D. Thi Nguyen, T. Hattori, T. Manh Le, O. Hori, M.R. Mahib, Y. Yamaguchi, M. Miura, T. Kinoshita, H. Kushiyama, M. Sakurai, T. Shiroishi, and T. Suda. 2019. Caspase-1 initiates apoptosis in the absence of gasdermin D. Nature Communications 10: 2091.
Heilig, R., M. Dilucca, D. Boucher, K.W. Chen, D. Hancz, B. Demarco, K. Shkarina, and P. Broz. 2020. Caspase-1 cleaves Bid to release mitochondrial SMAC and drive secondary necrosis in the absence of GSDMD. Life Science Alliance 3: e202000735.
Kawai, T., and S. Akira. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature Immunology 11: 373–384.
Kawai, T., and S. Akira. 2011. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34: 637–650.
Kang, L.L., D.M. Zhang, C.H. Ma, J.H. Zhang, K.K. Jia, J.H. Liu, R. Wang, and L.D. Kong. 2016. Cinnamaldehyde and allopurinol reduce fructose-induced cardiac inflammation and fibrosis by attenuating CD36-mediated TLR4/6-IRAK4/1 signaling to suppress NLRP3 inflammasome activation. Scientific Reports 6: 27460.
Su, Q., L. Li, Y. Sun, H. Yang, Z. Ye, and J. Zhao. 2018. Effects of the TLR4/Myd88/NF-κB signaling pathway on NLRP3 inflammasome in coronary microembolization-induced myocardial injury. Cellular Physiology and Biochemistry : International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology 47: 1497–1508.
Hu, X., Q. Chi, Q. Liu, D. Wang, Y. Zhang, and S. Li. 2019. Atmospheric H(2) S triggers immune damage by activating the TLR-7/MyD88/NF-κB pathway and NLRP3 inflammasome in broiler thymus. Chemosphere 237: 124427.
Juliana, C., T. Fernandes-Alnemri, S. Kang, A. Farias, F. Qin, and E.S. Alnemri. 2012. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. The Journal of Biological Chemistry 287: 36617–36622.
Fernandes-Alnemri, T., S. Kang, C. Anderson, J. Sagara, K.A. Fitzgerald, and E.S. Alnemri. 2013. Cutting edge: TLR signaling licenses IRAK1 for rapid activation of the NLRP3 inflammasome. Journal of Immunology (Baltimore, Md. : 1950) 191: 3995–3999.
Okada, M., A. Matsuzawa, A. Yoshimura, and H. Ichijo. 2014. The lysosome rupture-activated TAK1-JNK pathway regulates NLRP3 inflammasome activation. The Journal of Biological Chemistry 289: 32926–32936.
Saxton, R.A., and D.M. Sabatini. 2017. mTOR signaling in growth, metabolism, and disease. Cell 168: 960–976.
Kim, J., and K.L. Guan. 2019. mTOR as a central hub of nutrient signalling and cell growth. Nature Cell Biology 21: 63–71.
Dhingra, R., H. Gang, Y. Wang, A.K. Biala, Y. Aviv, V. Margulets, A. Tee, and L.A. Kirshenbaum. 2013. Bidirectional regulation of nuclear factor-κB and mammalian target of rapamycin signaling functionally links Bnip3 gene repression and cell survival of ventricular myocytes. Circulation. Heart Failure 6: 335–343.
Temiz-Resitoglu, M., S.P. Kucukkavruk, D.S. Guden, P. Cecen, A.N. Sari, B. Tunctan, A. Gorur, L. Tamer-Gumus, C.K. Buharalioglu, K.U. Malik, and S. Sahan-Firat. 2017. Activation of mTOR/IκB-α/NF-κB pathway contributes to LPS-induced hypotension and inflammation in rats. European Journal of Pharmacology 802: 7–19.
Tannahill, G.M., A.M. Curtis, J. Adamik, E.M. Palsson-McDermott, A.F. McGettrick, G. Goel, C. Frezza, N.J. Bernard, B. Kelly, N.H. Foley, L. Zheng, A. Gardet, Z. Tong, S.S. Jany, S.C. Corr, M. Haneklaus, B.E. Caffrey, K. Pierce, S. Walmsley, F.C. Beasley, E. Cummins, V. Nizet, M. Whyte, C.T. Taylor, H. Lin, S.L. Masters, E. Gottlieb, V.P. Kelly, C. Clish, P.E. Auron, R.J. Xavier, and L.A. O'Neill. 2013. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496: 238–242.
Covarrubias, A.J., H.I. Aksoylar, and T. Horng. 2015. Control of macrophage metabolism and activation by mTOR and Akt signaling. Seminars in Immunology 27: 286–296.
Woo, Y., H. Kim, K.C. Kim, J.A. Han, and Y.J. Jung. 2018. Tumor-secreted factors induce IL-1β maturation via the glucose-mediated synergistic axis of mTOR and NF-κB pathways in mouse macrophages. PLoS One 13: e0209653.
Li, X., X. Zhang, Y. Pan, G. Shi, J. Ren, H. Fan, H. Dou, and Y. Hou. 2018. mTOR regulates NLRP3 inflammasome activation via reactive oxygen species in murine lupus. Acta Biochimica et Biophysica Sinica 50: 888–896.
Choi, Y.J., K.M. Moon, K.W. Chung, J.W. Jeong, D. Park, D.H. Kim, B.P. Yu, and H.Y. Chung. 2016. The underlying mechanism of proinflammatory NF-κB activation by the mTORC2/Akt/IKKα pathway during skin aging. Oncotarget 7: 52685–52694.
Sørensen, M.V., B. Saha, I.S. Jensen, P. Wu, N. Ayasse, C.E. Gleason, S.L. Svendsen, W.H. Wang, and D. Pearce. 2019. Potassium acts through mTOR to regulate its own secretion. JCI Insight 5: e126910.
Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410: 37–40.
Arthur, J.S., and S.C. Ley. 2013. Mitogen-activated protein kinases in innate immunity. Nature Reviews. Immunology 13: 679–692.
Rezatabar, S., A. Karimian, V. Rameshknia, H. Parsian, M. Majidinia, T.A. Kopi, A. Bishayee, A. Sadeghinia, M. Yousefi, M. Monirialamdari, and B. Yousefi. 2019. RAS/MAPK signaling functions in oxidative stress, DNA damage response and cancer progression. Journal of Cellular Physiology 234: 14951–14965.
Morrison, D.K. 2012. MAP kinase pathways. Cold Spring Harbor Perspectives in Biology 4: a011254.
Kovtun, Y., W.L. Chiu, G. Tena, and J. Sheen. 2000. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proceedings of the National Academy of Sciences of the United States of America 97: 2940–2945.
McCubrey, J.A., M.M. Lahair, and R.A. Franklin. 2006. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxidants & Redox Signaling 8: 1775–1789.
Wang, W., X.Q. Ding, T.T. Gu, L. Song, J.M. Li, Q.C. Xue, and L.D. Kong. 2015. Pterostilbene and allopurinol reduce fructose-induced podocyte oxidative stress and inflammation via microRNA-377. Free Radical Biology & Medicine 83: 214–226.
Zhou, R., A. Tardivel, B. Thorens, I. Choi, and J. Tschopp. 2010. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nature Immunology 11: 136–140.
Zhou, R., A.S. Yazdi, P. Menu, and J. Tschopp. 2011. A role for mitochondria in NLRP3 inflammasome activation. Nature 469: 221–225.
Park, Y.J., S.J. Yoon, H.W. Suh, D.O. Kim, J.R. Park, H. Jung, T.D. Kim, S.R. Yoon, J.K. Min, H.J. Na, S.J. Lee, H.G. Lee, Y.H. Lee, H.B. Lee, and I. Choi. 2013. TXNIP deficiency exacerbates endotoxic shock via the induction of excessive nitric oxide synthesis. PLoS Pathogens 9: e1003646.
Mishra, B.B., V.A. Rathinam, G.W. Martens, A.J. Martinot, H. Kornfeld, K.A. Fitzgerald, and C.M. Sassetti. 2013. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1β. Nature Immunology 14: 52–60.
Gan, P., Z. Gao, X. Zhao, and G. Qi. 2016. Surfactin inducing mitochondria-dependent ROS to activate MAPKs, NF-κB and inflammasomes in macrophages for adjuvant activity. Scientific Reports 6: 39303.
Zhao, W., L. Ma, C. Cai, and X. Gong. 2019. Caffeine inhibits NLRP3 inflammasome activation by suppressing MAPK/NF-κB and A2aR signaling in LPS-induced THP-1 macrophages. International Journal of Biological sciences 15: 1571–1581.
Shibutani, S.T., T. Saitoh, H. Nowag, C. Münz, and T. Yoshimori. 2015. Autophagy and autophagy-related proteins in the immune system. Nature Immunology 16: 1014–1024.
Levine, B., and G. Kroemer. 2019. Biological functions of autophagy genes: a disease perspective. Cell 176: 11–42.
Nguyen, H.T., P. Lapaquette, M.A. Bringer, and A. Darfeuille-Michaud. 2013. Autophagy and Crohn’s disease. Journal of Innate Immunity 5: 434–443.
Allison, S.J. 2016. Systemic lupus erythematosus: defective noncanonical autophagy in SLE-like disease. Nature Reviews. Nephrology 12: 375.
Spalinger, M.R., S. Lang, C. Gottier, X. Dai, D.J. Rawlings, A.C. Chan, G. Rogler, and M. Scharl. 2017. PTPN22 regulates NLRP3-mediated IL1B secretion in an autophagy-dependent manner. Autophagy 13: 1590–1601.
Saitoh, T., N. Fujita, M.H. Jang, S. Uematsu, B.G. Yang, T. Satoh, H. Omori, T. Noda, N. Yamamoto, M. Komatsu, K. Tanaka, T. Kawai, T. Tsujimura, O. Takeuchi, T. Yoshimori, and S. Akira. 2008. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 456: 264–268.
Lee, J., H.R. Kim, C. Quinley, J. Kim, J. Gonzalez-Navajas, R. Xavier, and E. Raz. 2012. Autophagy suppresses interleukin-1β (IL-1β) signaling by activation of p62 degradation via lysosomal and proteasomal pathways. The Journal of Biological Chemistry 287: 4033–4040.
Seveau, S., J. Turner, M.A. Gavrilin, J.B. Torrelles, L. Hall-Stoodley, J.S. Yount, and A.O. Amer. 2018. Checks and balances between autophagy and inflammasomes during infection. Journal of Molecular Biology 430: 174–192.
Lupfer, C., P.G. Thomas, P.K. Anand, P. Vogel, S. Milasta, J. Martinez, G. Huang, M. Green, M. Kundu, H. Chi, R.J. Xavier, D.R. Green, M. Lamkanfi, C.A. Dinarello, P.C. Doherty, and T.D. Kanneganti. 2013. Receptor interacting protein kinase 2-mediated mitophagy regulates inflammasome activation during virus infection. Nature Immunology 14: 480–488.
B'Chir, W., A.C. Maurin, V. Carraro, J. Averous, C. Jousse, Y. Muranishi, L. Parry, G. Stepien, P. Fafournoux, and A. Bruhat. 2013. The eIF2α/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Research 41: 7683–7699.
Chen, X., X. Guo, Q. Ge, Y. Zhao, H. Mu, and J. Zhang. 2019. ER stress activates the NLRP3 inflammasome: a novel mechanism of atherosclerosis. Oxidative Medicine and Cellular Longevity 2019: 3462530.
Shi, C.S., K. Shenderov, N.N. Huang, J. Kabat, M. Abu-Asab, K.A. Fitzgerald, A. Sher, and J.H. Kehrl. 2012. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nature Immunology 13: 255–263.
Chen, J., and Z.J. Chen. 2018. PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature 564: 71–76.
Li, X., S. Thome, X. Ma, M. Amrute-Nayak, A. Finigan, L. Kitt, L. Masters, J.R. James, Y. Shi, G. Meng, and Z. Mallat. 2017. MARK4 regulates NLRP3 positioning and inflammasome activation through a microtubule-dependent mechanism. Nature Communications 8: 15986.
Li, C.G., Q.Z. Zeng, M.Y. Chen, L.H. Xu, C.C. Zhang, F.Y. Mai, C.Y. Zeng, X.H. He, and D.Y. Ouyang. 2019. Evodiamine augments nlrp3 inflammasome activation and anti-bacterial responses through inducing α-tubulin acetylation. Frontiers in Pharmacology 10: 290.
Magupalli, V.G., R. Negro, Y. Tian, A.V. Hauenstein, G. Di Caprio, W. Skillern, Q. Deng, P. Orning, H.B. Alam, Z. Maliga, H. Sharif, J.J. Hu, C.L. Evavold, J.C. Kagan, F.I. Schmidt, K.A. Fitzgerald, T. Kirchhausen, Y. Li, and H. Wu. 2020. HDAC6 mediates an aggresome-like mechanism for NLRP3 and pyrin inflammasome activation. Science 369: eaas8995.
Cao, Z., Y. Wang, Z. Long, and G. He. 2019. Interaction between autophagy and the NLRP3 inflammasome. Acta Biochimica et Biophysica Sinica 51: 1087–1095.
Misawa, T., M. Takahama, T. Kozaki, H. Lee, J. Zou, T. Saitoh, and S. Akira. 2013. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nature Immunology 14: 454–460.
Brandwein, S.R. 1986. Regulation of interleukin 1 production by mouse peritoneal macrophages. Effects of arachidonic acid metabolites, cyclic nucleotides, and interferons. The Journal of Biological Chemistry 261: 8624–8632.
Taskén, K., and E.M. Aandahl. 2004. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiological Reviews 84: 137–167.
Sokolowska, M., L.Y. Chen, Y. Liu, A. Martinez-Anton, H.Y. Qi, C. Logun, S. Alsaaty, Y.H. Park, D.L. Kastner, J.J. Chae, and J.H. Shelhamer. 2015. Prostaglandin E2 inhibits NLRP3 inflammasome activation through EP4 receptor and intracellular cyclic AMP in human macrophages. Journal of Immunology (Baltimore, Md. : 1950) 194: 5472–5487.
Yan, Y., W. Jiang, L. Liu, X. Wang, C. Ding, Z. Tian, and R. Zhou. 2015. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 160: 62–73.
Guo, C., S. Xie, Z. Chi, J. Zhang, Y. Liu, L. Zhang, M. Zheng, X. Zhang, D. Xia, Y. Ke, L. Lu, and D. Wang. 2016. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity 45: 802–816.
Liu, Y., Y.Y. Jing, C.Y. Zeng, C.G. Li, L.H. Xu, L. Yan, W.J. Bai, Q.B. Zha, D.Y. Ouyang, and X.H. He. 2017. Scutellarin suppresses NLRP3 inflammasome activation in macrophages and protects mice against bacterial sepsis. Frontiers in Pharmacology 8: 975.
Pan, H., Y. Lin, J. Dou, Z. Fu, Y. Yao, S. Ye, S. Zhang, N. Wang, A. Liu, X. Li, F. Zhang, and D. Chen. 2020. Wedelolactone facilitates Ser/Thr phosphorylation of NLRP3 dependent on PKA signalling to block inflammasome activation and pyroptosis. Cell Proliferation 53: e12868.
Ye J., B. Zeng, M. Zhong, H. Li, L. Xu, J. Shu, Y. Wang, F. Yang, C. Zhong, X. Ye, D. Ouyang, X. He. 2020. Scutellarin inhibits caspase-11 activation and pyroptosis in macrophages via regulating PKA signaling. Acta Pharmaceutica Sinica B 11: 112–126.
Chen, R., L. Zeng, S. Zhu, J. Liu, H.J. Zeh, G. Kroemer, H. Wang, T.R. Billiar, J. Jiang, D. Tang, and R. Kang. 2019. cAMP metabolism controls caspase-11 inflammasome activation and pyroptosis in sepsis. Science Advances 5: eaav5562.
Ollivier, V., G.C. Parry, R.R. Cobb, D. de Prost, and N. Mackman. 1996. Elevated cyclic AMP inhibits NF-kappaB-mediated transcription in human monocytic cells and endothelial cells. The Journal of Biological Chemistry 271: 20828–20835.
Chen, B.C., C.C. Liao, M.J. Hsu, Y.T. Liao, C.C. Lin, J.R. Sheu, and C.H. Lin. 2006. Peptidoglycan-induced IL-6 production in RAW 264.7 macrophages is mediated by cyclooxygenase-2, PGE2/PGE4 receptors, protein kinase A, I kappa B kinase, and NF-kappa B. Journal of Immunology (Baltimore, Md. : 1950) 177: 681–693.
Steinberg, G.R., and B.E. Kemp. 2009. AMPK in Health and Disease. Physiological Reviews 89: 1025–1078.
Hardie, D.G., F.A. Ross, and S.A. Hawley. 2012. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature Reviews. Molecular Cell Biology 13: 251–262.
Sag, D., D. Carling, R.D. Stout, and J. Suttles. 2008. Adenosine 5'-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. Journal of Immunology (Baltimore, Md. : 1950) 181: 8633–8641.
Ouslimani, N., J. Peynet, D. Bonnefont-Rousselot, P. Thérond, A. Legrand, and J.L. Beaudeux. 2005. Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells. Metabolism, Clinical and Experimental 54: 829–834.
Wang, S., M. Zhang, B. Liang, J. Xu, Z. Xie, C. Liu, B. Viollet, D. Yan, and M.H. Zou. 2010. AMPKalpha2 deletion causes aberrant expression and activation of NAD(P) H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes. Circulation Research 106: 1117–1128.
Wen, H., D. Gris, Y. Lei, S. Jha, L. Zhang, M.T. Huang, W.J. Brickey, and J.P. Ting. 2011. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nature Immunology 12: 408–415.
Lv, H., Q. Liu, Z. Wen, H. Feng, X. Deng, and X. Ci. 2017. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biology 12: 311–324.
Walters, D.M., H.Y. Cho, and S.R. Kleeberger. 2008. Oxidative stress and antioxidants in the pathogenesis of pulmonary fibrosis: a potential role for Nrf2. Antioxidants & Redox Signaling 10: 321–332.
Zha, Q.B., H.X. Wei, C.G. Li, Y.D. Liang, L.H. Xu, W.J. Bai, H. Pan, X.H. He, and D.Y. Ouyang. 2016. ATP-induced inflammasome activation and pyroptosis is regulated by AMP-activated protein kinase in macrophages. Frontiers in Immunology 7: 597.
Li, C.G., L. Yan, Y.Y. Jing, L.H. Xu, Y.D. Liang, H.X. Wei, B. Hu, H. Pan, Q.B. Zha, D.Y. Ouyang, and X.H. He. 2017. Berberine augments ATP-induced inflammasome activation in macrophages by enhancing AMPK signaling. Oncotarget 8: 95–109.
Sadler, A.J., and B.R. Williams. 2008. Interferon-inducible antiviral effectors. Nature Reviews. Immunology 8: 559–568.
Robb, R.J., and G.R. Hill. 2012. The interferon-dependent orchestration of innate and adaptive immunity after transplantation. Blood 119: 5351–5358.
Schneider, W.M., M.D. Chevillotte, and C.M. Rice. 2014. Interferon-stimulated genes: a complex web of host defenses. Annual Review of Immunology 32: 513–545.
McNab, F., K. Mayer-Barber, A. Sher, A. Wack, and A. O'Garra. 2015. Type I interferons in infectious disease. Nature Reviews. Immunology 15: 87–103.
Negishi, H., T. Taniguchi, and H. Yanai. 2018. The interferon (IFN) class of cytokines and the IFN regulatory factor (IRF) transcription factor family. Cold Spring Harbor Perspectives in Biology 10: a028423.
Schauvliege, R., J. Vanrobaeys, P. Schotte, and R. Beyaert. 2002. Caspase-11 gene expression in response to lipopolysaccharide and interferon-gamma requires nuclear factor-kappa B and signal transducer and activator of transcription (STAT) 1. The Journal of Biological Chemistry 277: 41624–41630.
Rathinam, V.A., S.K. Vanaja, L. Waggoner, A. Sokolovska, C. Becker, L.M. Stuart, J.M. Leong, and K.A. Fitzgerald. 2012. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 150: 606–619.
Kayagaki, N., M.T. Wong, I.B. Stowe, S.R. Ramani, L.C. Gonzalez, S. Akashi-Takamura, K. Miyake, J. Zhang, W.P. Lee, A. Muszyński, L.S. Forsberg, R.W. Carlson, and V.M. Dixit. 2013. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science (New York, N.Y.) 341: 1246–1249.
Py, B.F., M. Jin, B.N. Desai, A. Penumaka, H. Zhu, M. Kober, A. Dietrich, M.M. Lipinski, T. Henry, D.E. Clapham, and J. Yuan. 2014. Caspase-11 controls interleukin-1β release through degradation of TRPC1. Cell Reports 6: 1122–1128.
Man, S.M., R. Karki, M. Sasai, D.E. Place, S. Kesavardhana, J. Temirov, S. Frase, Q. Zhu, R.K.S. Malireddi, T. Kuriakose, J.L. Peters, G. Neale, S.A. Brown, M. Yamamoto, and T.D. Kanneganti. 2016. IRGB10 liberates bacterial ligands for sensing by the AIM2 and caspase-11-NLRP3 inflammasomes. Cell 167: 382–396.e317.
Santos, J.C., M.S. Dick, B. Lagrange, D. Degrandi, K. Pfeffer, M. Yamamoto, E. Meunier, P. Pelczar, T. Henry, and P. Broz. 2018. LPS targets host guanylate-binding proteins to the bacterial outer membrane for non-canonical inflammasome activation. The EMBO Journal 37: e98089.
Santos, J.C., D. Boucher, L.K. Schneider, B. Demarco, M. Dilucca, K. Shkarina, R. Heilig, K.W. Chen, R.Y.H. Lim, and P. Broz. 2020. Human GBP1 binds LPS to initiate assembly of a caspase-4 activating platform on cytosolic bacteria. Nature Communications 11: 3276.
Kuriakose, T., S.M. Man, R.K. Malireddi, R. Karki, S. Kesavardhana, D.E. Place, G. Neale, P. Vogel, and T.D. Kanneganti. 2016. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Science Immunology 1: aag2045.
Xie, C.B., L. Qin, G. Li, C. Fang, N.C. Kirkiles-Smith, G. Tellides, J.S. Pober, and D. Jane-Wit. 2019. Complement membrane attack complexes assemble NLRP3 inflammasomes triggering IL-1 activation of IFN-γ-primed human endothelium. Circulation Research 124: 1747–1759.
Nguyen, V.T., and E.N. Benveniste. 2000. Involvement of STAT-1 and ets family members in interferon-gamma induction of CD40 transcription in microglia/macrophages. The Journal of Biological Chemistry 275: 23674–23684.
Gaikwad, S., D. Patel, and R. Agrawal-Rajput. 2017. CD40 negatively regulates ATP-TLR4-activated inflammasome in microglia. Cellular and Molecular Neurobiology 37: 351–359.
Guarda, G., M. Braun, F. Staehli, A. Tardivel, C. Mattmann, I. Förster, M. Farlik, T. Decker, R.A. Du Pasquier, P. Romero, and J. Tschopp. 2011. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34: 213–223.
Manning, B.D., and L.C. Cantley. 2007. AKT/PKB signaling: navigating downstream. Cell 129: 1261–1274.
Petrulea, M.S., T.S. Plantinga, J.W. Smit, C.E. Georgescu, and R.T. Netea-Maier. 2015. PI3K/Akt/mTOR: A promising therapeutic target for non-medullary thyroid carcinoma. Cancer Treatment Reviews 41: 707–713.
Yang, Y., Y. Sun, J. Xu, K. Bao, M. Luo, X. Liu, and Y. Wang. 2018. Epithelial cells attenuate toll-like receptor-mediated inflammatory responses in monocyte-derived macrophage-like cells to mycobacterium tuberculosis by modulating the PI3K/Akt/mTOR signaling pathway. Mediators of Inflammation 2018: 3685948.
Settembre, C., C. Di Malta, V.A. Polito, M. Garcia Arencibia, F. Vetrini, S. Erdin, S.U. Erdin, T. Huynh, D. Medina, P. Colella, M. Sardiello, D.C. Rubinsztein, and A. Ballabio. 2011. TFEB links autophagy to lysosomal biogenesis. Science 332: 1429–33.
Krieg, S., B. Lüscher, J. Vervoorts, and M. Dohmen. 2018. Studying the role of AMPK in autophagy. Methods in Molecular Biology (Clifton, N.J.) 1732: 373–391.
Kim, S.H., G. Kim, D.H. Han, M. Lee, I. Kim, B. Kim, K.H. Kim, Y.M. Song, J.E. Yoo, H.J. Wang, S.H. Bae, Y.H. Lee, B.W. Lee, E.S. Kang, B.S. Cha, and M.S. Lee. 2017. Ezetimibe ameliorates steatohepatitis via AMP activated protein kinase-TFEB-mediated activation of autophagy and NLRP3 inflammasome inhibition. Autophagy 13: 1767–1781.
Inoki, K., T. Zhu, and K.L. Guan. 2003. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577–590.
Gwinn, D.M., D.B. Shackelford, D.F. Egan, M.M. Mihaylova, A. Mery, D.S. Vasquez, B.E. Turk, and R.J. Shaw. 2008. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Molecular Cell 30: 214–226.
Salminen, A., and K. Kaarniranta. 2012. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Research Reviews 11: 230–241.
Sato, M., H. Suemori, N. Hata, M. Asagiri, K. Ogasawara, K. Nakao, T. Nakaya, M. Katsuki, S. Noguchi, N. Tanaka, and T. Taniguchi. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13: 539–548.
Fitzgerald, K.A., S.M. McWhirter, K.L. Faia, D.C. Rowe, E. Latz, D.T. Golenbock, A.J. Coyle, S.M. Liao, and T. Maniatis. 2003. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nature Immunology 4: 491–496.
Tamura, T., H. Yanai, D. Savitsky, and T. Taniguchi. 2008. The IRF family transcription factors in immunity and oncogenesis. Annual Review of Immunology 26: 535–584.
Samie, M., J. Lim, E. Verschueren, J.M. Baughman, I. Peng, A. Wong, Y. Kwon, Y. Senbabaoglu, J.A. Hackney, M. Keir, B. McKenzie, D.S. Kirkpatrick, M. van Lookeren Campagne, and A. Murthy. 2018. Selective autophagy of the adaptor TRIF regulates innate inflammatory signaling. Nature Immunology 19: 246–254.
Meares, G.P., H. Qin, Y. Liu, A.T. Holdbrooks, and E.N. Benveniste. 2013. AMP-activated protein kinase restricts IFN-γ signaling. Journal of Immunology (Baltimore, Md. : 1950) 190: 372–380.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81873064, No. 81773965 and No. 81673664).
Author information
Authors and Affiliations
Contributions
Chen M and Ye X wrote this manuscript. He X and Ouyang D conceived and supervised this review.
Corresponding authors
Ethics declarations
Consent for Publication
All the authors have approved to publish this manuscript.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, My., Ye, Xj., He, Xh. et al. The Signaling Pathways Regulating NLRP3 Inflammasome Activation. Inflammation 44, 1229–1245 (2021). https://doi.org/10.1007/s10753-021-01439-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01439-6