Abstract
Sepsis is an infectious disease that seriously endangers human health. It usually leads to myocardial injury which seriously endangers to the health of human beings. H19 has been confirmed to play key roles in various diseases, including sepsis. However, its function in the progression of sepsis-induced myocardial injury remains largely unknown. H9C2 cells were treated with lipopolysaccharide (LPS) to mimic sepsis-induced myocardial injury in vitro. Cell proliferation and apoptosis were detected by MTT assay and flow cytometry, respectively. In addition, gene and protein expression levels in H9C2 cells were measured by quantitative real-time PCR (qRT-PCR) and Western blotting. The levels of inflammatory cytokines in H9C2 cell supernatants were tested by ELISA. JC-1 staining was performed to observe the mitochondrial membrane potential level in H9C2 cells. H19 and SORBS2 were downregulated in H9C2 cells following LPS treatment, while miR-93-5p was upregulated. Moreover, LPS-induced cell growth inhibition and mitochondrial damage were significantly reversed by overexpression of H19. In addition, H19 upregulation notably suppressed LPS-induced inflammatory responses in H9C2 cells. Moreover, H19 sponged miR-93-5p to promote SORBS2 expression. Overall, H19 suppressed sepsis-induced myocardial injury via regulation of the miR-93-5p/SORBS2 axis. H19 attenuated the development of sepsis-induced myocardial injury in vitro via modulation of the miR-93-5p/SORBS2 axis. Thus, H19 could serve as a potential target for the treatment of sepsis-induced myocardial injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Sepsis has been considered one of the hidden public health diseases [1]. In the USA, the hospitalization of sepsis patients has dramatically increased for every 100,000 people [2]. Patients who survive sepsis suffer high incidence of cognitive impairment [3] and a high risk of death in the following 5 years compared to non-sepsis patients [4, 5]. Based on observations by the Centers for Disease Control and Prevention, in the twenty-first century, over 14 billion dollars were spent on sepsis in North America, and the high costs for treating this disease have increased every year from 1995 to 2005 [6]. Sepsis-induced myocardial injury is the most common complication of sepsis, which increases the morbidity and mortality of patients with sepsis [7]. Thus, it is urgent and necessary to find new strategies for the treatment of sepsis-induced myocardial injury.
Many reports have revealed that only a small portion of the human genome is transcribed into protein-coding RNAs, whereas most of the genome can be transcribed into noncoding RNAs (ncRNAs) [8]. Long noncoding RNAs (lncRNAs) are a class of ncRNAs that are more than 200 nucleotides long [9,10,11]. Recent reports have suggested that lncRNAs have key roles in many biological processes. In addition, they are commonly deregulated in many diseases, including cancers [12,13,14]. LncRNAs have also been reported to participate in the process of myocardial injury caused by sepsis [15]. For example, lncRNA MIAT could promote inflammation and oxidative stress in sepsis-induced cardiac injury [16]. Moreover, lncRNA CRNDE protected myocardial tissues from injury caused by sepsis [17]. A previous study confirmed that lncRNA H19 (H19) ameliorated myocardial infarction-induced myocardial injury [18]. H19 has also been verified to be a suppressor in sepsis-induced kidney and lung injury [19, 20]. Furthermore, a previous study found that H19 was downregulated in LPS-induced cardiomyocytes [21]. However, the role of H19 in sepsis myocardial injury remains largely unknown.
MicroRNAs (miRNAs) are endogenic small ncRNAs that are widely expressed in various tissues, and miRNAs regulate the expression of target genes by binding to the 3′-untranslated region (3’-UTR) [22]. It has been confirmed that miR-93-5p plays key roles in cancer and inflammation [23, 24]. In addition, miR-93-5p is abnormally expressed in a variety of diseases, including myocardial injury [25]. Liu J et al. revealed that miR-93-5p-containing exosomes could reverse acute myocardial infarction-induced myocardial damage [26]. However, the role of miR-93-5p in sepsis-induced myocardial injury remains unclear. Sorbin and SH3 domain containing 2 (SORBS2) has been reported to be a tumour suppressor and is involved in several types of malignancies [27, 28]. In addition, SORBS2 participates in left ventricular noncompaction cardiomyopathy [29]. Wang H et al. indicated that miR-21-3p suppressed sepsis-induced cardiac damage through regulation of SORBS2 [30]. In addition, bioinformatics software analysis showed that there were potential binding sites between miR-93-5p and H19, and miR-93-5p might bind to SORBS2 (http://starbase.sysu.edu.cn/). Based on this background, we sought to explore the correlation between miR-93-5p and H19 or SORBS2 and the regulation of the H19/miR-93-5p/SORBS2 axis in sepsis-induced myocardial injury.
In this study, we sought to confirm the biological function of H19 in sepsis-induced myocardial injury. As expected, we found that H19 and SORBS2 expression were reduced and miR-93-5p expression was increased in LPS-induced H9C2 cells. In addition, H19 could bind to miR-93-5p, and SORBS2 was a target gene of miR-93-5p. H19 suppressed cell proliferation and promoted apoptosis in H9C2 cells induced by LPS, indicating that H19 could inhibit the progression of sepsis-induced myocardial injury by sponging the miR-93-5p/SORBS2 signalling pathway. We hope this finding will provide a novel method for the treatment of sepsis-induced myocardial injury.
MATERIAL AND METHODS
Cell Culture and Treatment
Cardiomyocytes (H9C2 cells) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in Roswell Park Memorial Institute 1640 medium (RPMI 1640, Thermo Fischer Scientific, Waltham, MA, USA) with 10% foetal bovine serum (FBS, Thermo Fischer Scientific), 1% penicillin (Invitrogen, USA) and streptomycin (Invitrogen, USA) at 37 °C and 5% CO2. To mimic sepsis-induced myocardial injury in vitro, cells were subsequently diluted to 1 × 106 cells/mL and seeded into six-well plates followed by culturing for 48 h to 70% confluence and then incubation with 0.5 μg/mL lipopolysaccharide (LPS; L2880, Sigma-Aldrich, St. Louis, MA, USA) for 12 h according to previous references (16, 31).
3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
MTT assay was used to detect the proliferation of H9C2 cells. H9C2 cells were seeded in 96-well plates, and then 10 μL of MTT solution was added to each well and incubated for 4 h. Then, 150 μL of dimethyl sulfoxide (DMSO) was added to each well. The absorbance at 490 nm was determined with a microplate reader.
Cell Transfection
The H19 sequence was synthesized and subcloned into an expression plasmid to generate the recombinant vector pcDNA-H19. Empty vector pcDNA3.1 was used as a negative control. Cells were transfected with the pcDNA3.1 vector or H19 overexpression plasmid (pcDNA-H19) using Lipofectamine 3000 (Invitrogen, USA) according to the manufacturer’s instructions. After 48 h of transfection, cells were collected for subsequent experiments.
For miRNA transfection, miR-93-5p mimics/inhibitor and NC duplex with a random sequence (denoted as mimics/inhibitor NC) were designed and synthesized by GenScript (Nanjing, China). H9C2 cells were transfected using Lipofectamine 2000 reagent (Invitrogen, USA) according to the manufacturer’s instructions. Subsequently, H9C2 cells were treated with LPS. RNA extraction and Western blot experiments were conducted after 48 h. The sequences were as follows: miR-93-5p mimics: sense 5’-CAAAGUGCUGUUCGUGCAGGUAG-3′, antisense: 5’-ACCUGCACGAACAGCACUUUGUU-3′; miR-93-5p inhibitor: 5’-CUACCUGCACGAACAGCACUUUG-3′.
Quantitative Real-Time PCR
Total RNA was extracted from H9C2 cells by using an RNA extraction kit (TaKaRa, Japan) according to the manufacturer’s instructions. The quantity and integrity of RNA were measured using a NanoDrop spectrophotometer (Thermo Scientific) and an Agilent Bioanalyzer RNA 6000 Nano kit (Agilent Technologies, Beijing, China), respectively. Two micrograms of RNA was reverse transcribed into cDNA using the PrimeScript RT reagent kit (TaKaRa, Japan). Subsequently, real-time PCR was performed by using SYBR™ Green Master Mixes (TaKaRa, Japan). The 2−ΔΔCt method was used to quantify the results. GAPDH or U6 was used as the internal control. All primers were obtained from GenScript. Primer sequences are listed as follows:
H19-F: 5’-CTGAGCTAGGGTTGGAGAGG-3′;
H19-R: 5’-TTAGAAGGTCAGTGCAGCGA-3′.
MiR-93-5p-F: 5’-AGCAGTCAGTAGTTGGTCCTTTG-3′;
MiR-93-5p-R: 5’-CCATCAGTCCCGTCTTGAAAC-3′.
SORBS2-F: 5’-TCCTCTACCCCACAGCTACT-3′;
SORBS2-R: 5′- CGAACCATCCGTCATCACAC -3′.
GAPDH-F: 5’-CATCATCCCTGCCTCTACTGG-3′;
GAPDH-F: 5’-GTGGGTGTCGCTGTTGAAGTC-3′.
U6-F: 5’-CTCGCTTCGGCAGCACA-3′;
U6-R: 5’-AACGCTTCACGAATTTGCGT-3′.
JC-1 Staining
The mitochondrial membrane potential level of H9C2 was monitored using JC-1 as described previously [32]. H9C2 cells from each group were incubated with JC-1 (7.5 μΜ) for 30 min at 37 °C away from light. The excess dye was then replaced with fresh medium, and images were obtained by confocal microscopy.
Flow Cytometry Analysis
For cell apoptosis detection, cells were harvested and then stained with annexin V-FITC and propidium iodide (PI). After that, cells were incubated in the dark at room temperature for 20 min. The result was quantified by BD LSRII Flow Cytometry System with FACSDiva (BD Bioscience, USA).
Enzyme-Linked Immunosorbent Assay
The levels of cardiac troponin T (cTnT), tumour necrosis factor-α (TNF-α), interleukin (IL-1β) and IL-6 in the supernatant of H9C2 cells were measured by using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s protocol. After coating with primary antibody overnight and blocking with 10% FBS for 1 h, the plate was filled with the supernatants of H9C2 cells. The supernatants of H9C2 cells were harvested by centrifugation (956×g, 5 min). Subsequently, the cells were incubated with secondary antibody for 1 h. Finally, after treatment with hydrochloric acid, the absorbance was measured by a microplate reader. cTnT, TNF-α, IL-1β and IL-6 ELISA kits were obtained from MultiSciences (Lianke) Biotech Co., Ltd. (Hangzhou, China).
Western Blotting
Total protein was isolated from cell lysates by using RIPA buffer and then quantified by a BCA protein assay kit (Beyotime, China). Proteins were separated by using a 10% SDS-PAGE gel, and proteins were transferred onto PVDF membranes (Millipore, USA). The PVDF membranes were blocked with 5% skim milk in TBST at room temperature for 1 h. Subsequently, PVDF membranes were incubated with the following primary antibodies: anti-cyt-C (Abcam; 1:1000), anti-Cox IV (1:1000), anti-SORBS2 (1:1000), anti-Bax (1:1000), anti-Bcl-2 (1:1000), anti-caspase-3 (1:1000), anti-caspase-9 (1:1000) and anti-GAPDH (1:1000) overnight at 4 °C. All antibodies were obtained from Sigma (USA). After that, the membrane was incubated with secondary anti-rabbit antibody (1:5000) for 1 h. Finally, the bands were analysed by an ECL detection kit (Pierce Biotechnology, USA).
Luciferase Reporter Assay
The dual-luciferase reporter assay was conducted as previously described, with a slight modification [30]. Partial sequences of H19 and the SORBS2 3’-UTR containing the putative binding sites of miR-93-5p were synthetized and obtained from Sangon Biotech Co., Ltd. The aforementioned sequences were cloned into the pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega Corporation) to construct wild-type (WT) H19 and SORBS2 reporter vectors. The mutant (MUT) H19 and SORBS2 3’-UTR sequences containing the putative binding sites of miR-93-5p were generated using the Q5 Site-Directed Mutagenesis kit (New England Biolabs, Inc.) according to the manufacturer’s protocol. The aforementioned MUT sequences were cloned into the pmirGLO vector to construct MUT H19 and SORBS2 reporter vectors. The WT or MUT H19 vector was transfected into H9C2 cells together with NC mimics or miR-93-5p mimic using Lipofectamine® 2000 reagent (Thermo Fisher Scientific, Inc.) according to the manufacturer’s instructions. Similarly, the WT and MUT SORBS2 vectors were transfected into H9C2 cells together with NC mimics or miR-93-5p mimics using Lipofectamine 2000. Relative luciferase activities were detected using a Dual-Glo Luciferase Assay System (Promega Corporation). The data were quantified by normalizing to Renilla luciferase activity.
Statistical Analysis
GraphPad Prism 5.0 was used for statistical analysis. Data are represented as the mean ± standard deviation (SD). All experiments were repeated at least three times. The comparison was analysed by Student’s t test or one-way analysis of variance (ANOVA) followed by Tukey’s test. P < 0.05 was considered a statistically significant difference.
RESULTS
H19 and SORBS2 Were Downregulated in an LPS-Induced Sepsis Cell Model, while miR-93-5p Was Upregulated
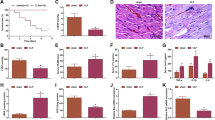
To mimic sepsis-induced myocardial injury in vitro, H9C2 cells were treated with LPS. As indicated in Fig. 1 a, the proliferation of H9C2 cells was inhibited by LPS in a time-dependent manner. Thus, a 12-h time point was used in subsequent experiments. Moreover, ELISA data demonstrated that the levels of cTnT, TNF-α, IL-1β and IL-6 in the supernatant of H9C2 cells were significantly increased in the presence of LPS, indicating that an in vitro model of sepsis myocardial injury was successfully established (Fig. 1b). Additionally, the levels of H19 and SORBS2 in LPS-treated H9C2 cells were notably downregulated, while miR-93-5p was upregulated (Fig. 1c). Furthermore, compared with the control, LPS treatment significantly promoted H9C2 cell apoptosis (Fig. 1d). Moreover, the expression levels of pro-apoptotic proteins in H9C2 cells were increased by LPS, while anti-apoptotic proteins were decreased (Fig. 1e). In addition, as shown in Fig. 1f, JC-1 staining results showed that the mitochondrial membrane potential in H9C2 cells was obviously decreased after LPS treatment. Subsequently, we used a Western blot assay to detect cyt-C expression in H9C2 cells. As expected, the expression of cyt-C protein in mitochondria was decreased, while it was increased in the cytoplasm (Fig. 1g). Altogether, these results suggested that H19 and SORBS2 expression levels were downregulated in LPS-treated H9C2 cells, while miR-93-5p was upregulated.
H19 and SORBS2 were downregulated in LPS-induced sepsis in vitro, while miR-93-5p was upregulated. H9C2 cells were treated with LPS at different concentrations. Then, a the OD value was tested by MTT assay. b the levels of inflammatory cytokines and cTnT in the supernatant of H9C2 cells were detected by ELISA. c H9C2 cells were transfected with H19, miR-93-5p or SORBS2 for 48 h. then, the gene expression of H19, miR-93-5p or SORBS2 in H9C2 cells was measured by qRT-PCR. d the apoptosis of H9C2 cells was tested by flow cytometry. e protein expression was detected by Western blotting. f the JC-1 staining results were presented. g the protein expression of cyt-C was detected by Western blotting. The relative expression was quantified by normalizing to GAPDH expression. Each experiment was performed 3 times. The comparison was analysed by Student’s t test or one-way ANOVA followed by Tukey’s test. *P < 0.05, **P < 0.01, *** P < 0.001.
Overexpression of H19 Inhibited Inflammatory Responses and Mitochondrial Damage in LPS-Treated Cardiomyocytes
To explore the role of H19 in sepsis-induced myocardial injury, qRT-PCR and Western blotting were used. As expected, overexpression of H19 notably increased the expression of H19 and SORBS2 and decreased the expression of miR-93-5p in LPS-treated H9C2 cells (Fig. 2a). In addition, the levels of inflammatory cytokines (TNF-α, IL-1β and IL-6) in the supernatant of LPS-treated H9C2 cells were significantly downregulated by H19 overexpression (Fig. 2b). Moreover, LPS significantly induced mitochondrial damage in cardiomyocytes. However, H19 upregulation significantly reversed the mitochondrial membrane potential in LPS-treated H9C2 cells (Fig. 2c). In addition, the protein level of cyt-C in the mitochondria of LPS-treated H9C2 cells was notably increased by upregulation of H19, while it was decreased in the cytoplasm (Fig. 2d). All these data concluded that overexpression of H19 inhibited the inflammatory responses and mitochondrial damage in LPS-treated H9C2 cells.
Overexpression of H19 inhibited the inflammatory responses and mitochondrial damage in LPS-treated cardiomyocytes. H9C2 cells were treated with LPS and then transfected with an H19 overexpression plasmid. Then, a the expression of H19, miR-93-5p and SORBS2 in H9C2 cells was detected by qRT-PCR. b the levels of TNF-α, IL-1β, IL-6 and cTnT were detected by ELISA. c the JC-1 staining results were presented. d the protein expression of cyt-C in LPS-treated H9C2 cells was tested by Western blotting. The relative expression was quantified by normalizing to GAPDH expression. All experiments were performed in triplicate. The comparisons were analysed with one-way ANOVA followed by Tukey’s test. *P < 0.05, **P < 0.01.
Upregulation of H19 Inhibited Apoptosis and Promoted the Proliferation of H9C2 Cells Treated with LPS
Next, flow cytometry was used to verify the function of H19 on the apoptosis of LPS-treated H9C2 cells. The data showed that overexpression of H19 greatly inhibited apoptosis in LPS-induced H9C2 cells (Fig. 3a). In addition, the protein levels of Bax, cleaved caspase9 and cleaved caspase3 in LPS-treated H9C2 cells were obviously inhibited by upregulation of H19 (Fig. 3b). In contrast, Bcl-2 expression in LPS-induced H9C2 cells was activated by H19 overexpression (Fig. 3b). Moreover, the OD value of LPS-treated H9C2 cells was significantly increased in the presence of H19 overexpression (Fig. 3c). Overall, the overexpression of H19 reversed the inhibitory effect of LPS treatment on H9C2 cell proliferation.
Upregulation of H19 promoted the growth of H9C2 cells treated with LPS. a cell apoptosis was tested by flow cytometry. b the protein expression levels of Bax, Bcl-2, cleaved caspase9 and cleaved caspase3 in LPS-treated H9C2 cells were detected by Western blotting. The relative expression was calculated via normalization to GAPDH expression. c cell viability was tested by MTT assay. All experiments were performed in triplicate. The comparisons were analysed by one-way ANOVA followed by Tukey’s test. *P < 0.05, **P < 0.01.
H19 Regulated the Expression of SORBS2 by Sponging miR-93-5p
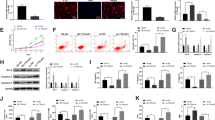
To determine the relationship between H19, miR-93-5p and SORBS2, a bioinformatics method was used. The data suggested that miR-93-5p might be the downstream target of H19. SORBS2 might be a direct target of miR-93-5p, and the binding sequences were shown in Fig. 4 a. Furthermore, the results of the dual luciferase reporter assay further confirmed this hypothesis (Fig. 4b). Moreover, as expected, the expression of miR-93-5p in LPS-induced H9C2 cells was upregulated by overexpression of miR-93-5p, but it was inhibited by the miR-93-5p inhibitor (Fig. 4c). In contrast, overexpression of miR-93-5p significantly decreased the expression of SORBS2 and H19 in LPS-treated H9C2 cells (Fig. 4c). Conversely, the miR-93-5p inhibitor exhibited the opposite effect on H19 and SORBS2 expression (Fig. 4c). Furthermore, the protein expression of SORBS2 in LPS-treated H9C2 cells was significantly increased after H19 overexpression or miR-93-5p inhibitor treatment (Fig. 4d, e). In summary, H19 regulated the expression of SORBS2 by sponging miR-93-5p.
H19 regulated SORBS2 expression by directly targeting miR-93-5p. a the potential binding site between H19 and miR-93-5p was predicted by StarBase v2.0 software. b the luciferase activity was measured by using the dual luciferase reporter assay. c the expression of H19, miR-93-5p and SORBS2 was detected by qRT-PCR. d, e the protein expression of SORBS2 was detected by Western blotting. The relative expression was quantified via normalization to GAPDH expression. All experiments were performed in triplicate. The comparisons were analysed by one-way ANOVA followed by Tukey’s test. *P < 0.05, **P < 0.01, ***P < 0.001.
H19 Decreased the Mitochondrial Membrane Potential in LPS-Treated H9C2 Cells via Regulation of the miR-93-5p/SORBS2 Axis
To explore the mechanism by which H19 modulates the process of sepsis-induced myocardial injury, qRT-PCR was used. The data showed that overexpression of H19 notably inhibited the expression of miR-93-5p but increased SORBS2 in LPS-treated H9C2 cells (Fig. 5a). In contrast, miR-93-5p mimics notably decreased the level of SORBS2 (Fig. 5a), suggesting that the inhibitory effect of H19 was significantly reversed by miR-93-5p mimics (Fig. 5a). In addition, the secretion of inflammatory cytokines by LPS-treated H9C2 cells was notably inhibited in the presence of H19 overexpression, which was partially restored by miR-93-5p mimics (Fig. 5b). Moreover, overexpression of miR-93-5p significantly reversed the therapeutic effect of H19 upregulation on mitochondrial damage in LPS-treated H9C2 cells (Fig. 5c). Furthermore, H19 overexpression notably inhibited the protein expression of cyt-C in the cytoplasm of LPS-treated H9C2 cells but activated cyt-C in mitochondria. However, miR-93-5p mimics alone exhibited the opposite effect of H19. In addition, the inhibitory effect of H19 overexpression on septic myocardial injury in vitro was partially restored by miR-93-5p overexpression (Fig. 5d). Taken together, H19 significantly reversed mitochondrial damage in vitro through modulation of the miR-93-5p/SORBS2 axis.
H19 reversed mitochondrial damage in LPS-induced H9C2 cells via the miR-93-5p/SORBS2 axis. H9C2 cells were treated as follows: LPS, LPS + H19 overexpression, NC + LPS, miR-93-5p mimics+LPS or miR-93-5p mimics+LPS + H19 overexpression plasmid. Then, a the gene expression of miR-93-5p and SORBS2 in H9C2 cells was detected by qRT-PCR. b the levels of TNF-α, IL-1β, IL-6 and cTnT in the supernatant of H9C2 cells were detected by ELISA. c the JC-1 staining results were presented. d the protein expression of cyt-C in H9C2 cells following LPS treatment was tested by Western blotting. The relative expression was quantified by normalizing to GAPDH expression. All experiments were performed in triplicate. The comparisons were analysed by one-way ANOVA followed by Tukey’s test. *P < 0.05, **P < 0.01, ***P < 0.001.
Overexpression of H19 Significantly Inhibited Apoptosis of LPS-Treated H9C2 Cells via Modulation of the miR-93-5p/SORBS2 Axis
As expected, upregulation of H19 notably suppressed the apoptosis of LPS-induced H9C2 cells, while miR-93-5p mimics induced apoptosis. However, miR-93-5p mimics significantly reversed the anti-apoptotic effect of H19 (Fig. 6a). Additionally, the results of Western blotting further confirmed that H19 notably inhibited the apoptosis of LPS-treated H9C2 cells, which was partially reversed by miR-93-5p mimics. Overexpression of H19 inhibited the expression of Bax, cleaved caspase9 and cleaved caspase3 in LPS-treated H9C2 cells while increased Bcl-2 expression, and miR-93-5p mimics reversed the effect of H19 (Fig. 6b). Moreover, the proliferation of LPS-treated H9C2 cells was obviously promoted by H19 upregulation (Fig. 6c). In addition, miR-93-5p mimics notably reversed the effect of H19 on cell proliferation (Fig. 6c). In summary, overexpression of H19 significantly suppressed the apoptosis of LPS-treated H9C2 cells via modulation of the miR-93-5p/SORBS2 axis.
Overexpression of H19 suppressed the apoptosis of LPS-treated H9C2 cells via the miR-93-5p/SORBS2 axis. a cell apoptosis was tested by flow cytometry. b the protein expression levels of Bax, Bcl-2, cleaved caspase9 and cleaved caspase3 in H9C2 cells after LPS treatment were detected by Western blotting. The relative expression was calculated via normalization to GAPDH expression. b cell viability was measured by MTT assay. All experiments were performed in triplicate. The comparisons were analysed by one-way ANOVA followed by Tukey’s test. *P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
Sepsis-related myocardial injury remains a major cause of death in critically ill patients and lacks effective therapy [33]. Recent studies have confirmed that mitochondrial damage is the major cause of sepsis-induced myocardial injury [34,35,36]. Although some studies have indicated that lncRNAs are involved in sepsis [37, 38], the effect of H19 on sepsis-induced myocardial injury and its underlying mechanisms remain unclear. In this study, we first found that overexpression of H19 could suppress the progression of sepsis-induced myocardial injury by promoting cell growth and inhibiting mitochondrial damage. In addition, our results showed that the miR-93-5p/SORBS2 axis could be regulated by H19 in sepsis-induced myocardial injury, which supplemented the mechanism of H19-mediated septic myocardial injury development.
H19 has been reported to participate in multiple diseases [39, 40]. H19 has been shown to promote the progression of various malignant tumours [40,41,42]. Similarly, our study indicated that H19 could inhibit apoptosis and reverse mitochondrial injury in LPS-induced H9C2 cells. Furthermore, IL-6, TNF-α and IL-1β are key markers in inflammatory responses [43, 44]. In our study, we found that H19 inhibited the secretion of IL-6, TNF-α and IL-1β in H9C2 cells induced by LPS treatment. Overall, H19 expression was reduced in H9C2 cells induced by LPS, and it could act as an inflammation inhibitor in sepsis-induced myocardial injury.
Previous studies demonstrated that miR-93-5p was overexpressed in multiple diseases [45,46,47]. However, the relationship between miR-93-5p and sepsis-induced myocardial injury remains unclear. Our study first found that miR-93-5p could enhance the progression of sepsis-induced myocardial injury. Liu J et al. confirmed that miR-93-5p is involved in myocardial injury [26]. Similar to this previous report, our study further characterized the effect of miR-93-5p on myocardial injury caused by sepsis. Our study confirmed that miR-93-5p mimics could aggravate sepsis-induced myocardial injury by promoting mitochondrial damage in H9C2 cells. Moreover, miR-93-5p was sponged by H19 in H9C2 cells. Li JP et al. found that H19 competitively bound miR-93-5p to regulate STAT3 expression in breast cancer [48]. Our result was consistent with this report, and we found that miR-93-5p mimics could reverse the regulatory effect of H19 in H9C2 cells induced by LPS. This consistency may be due to the various functions of miR-93-5p. In short, our study showed that miR-93-5p expression was upregulated in LPS-induced H9C2 cells. Furthermore, H19 regulated apoptosis and inflammation in LPS-induced H9C2 cells by targeting miR-93-5p expression.
SORBS2 is a key member of a three-protein family that contains CAP (SORBS1) and vinexin (SORBS3) [49]. It has been previously reported in many types of diseases [50, 51]. In addition, SORBS2 could promote the proliferation of various cell types [28, 52, 53]. Similarly, we found that SORBS2 was downregulated in LPS-induced H9C2 cells, confirming that SORBS2 could promote cell growth in sepsis-induced myocardial injury. However, Yan B et al. found that SORBS2 could inhibit HCC cell growth [27]. This discrepancy may result from different types of diseases investigated in these reports. Moreover, our results demonstrated that miR-93-5p directly targeted SORBS2. Since the relationship between miR-93-5p and SORBS2 has not been reported before, we further explored the miRNA-mRNA interaction. In addition, TNF-α has been confirmed to impair damage to the human heart [54]. Moreover, there is also evidence for the presence of related factors that affect cardiomyocytes in vitro [55]. Recent studies have also demonstrated that the decrease in these cytokines is beneficial for sepsis treatment [56]. Wang H et al. revealed that SORBS2 could modulate the levels of IL-6, TNF-α and IL-1β in sepsis-associated cardiac dysfunction [30]. Our data were similar to this previous result, indicating that SORBS2 could act as an inhibitor of inflammatory responses.
It is important to note that this study had some limitations. For example, we focused only on the miR-93-5p/SORBS2 axis. Since there is a close relationship between PI3K/Akt and inflammatory cytokines [57], we will further confirm the effect of H19 on the PI3K/Akt signalling pathway. In addition, our research did not include animal studies. Thus, we will investigate the effect of H19 on sepsis-induced myocardial injury in vivo in the future.
In conclusion, H19 promoted cell proliferation and inhibited apoptosis and the expression of inflammatory factors in LPS-induced H9C2 cells, suggesting that H19 inhibited the development of sepsis-induced myocardial injury in vitro via regulation of the miR-93-5p/SORBS2 axis. Therefore, H19 might serve as a new target for sepsis-induced myocardial injury treatment.
Data Availability
All data generated or analysed during this study are included in this published article.
Abbreviations
- SORBS2:
-
sorbin and SH3 domain-containing 3
- MiR-93-5p:
-
microRNA-93-5p
- LncRNA H19:
-
long noncoding RNA H19
- FBS:
-
foetal bovine serum
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- DMSO:
-
dimethyl sulfoxide
- cTnT:
-
cardiac troponin T
- TNF-α:
-
tumour necrosis factor-α
- IL-1β:
-
interleukin-1β
References
Candel, F.J., M. Borges Sa, S. Belda, G. Bou, J.L. Del Pozo, O. Estrada, et al. 2018. Current aspects in sepsis approach. Turning things around. Revista espanola de quimioterapia : publicacion oficial de la Sociedad Espanola de Quimioterapia. 31 (4): 298–315.
Rhee, C., M.V. Murphy, L. Li, R. Platt, and M. Klompas. 2015. Centers for disease C, et al. comparison of trends in sepsis incidence and coding using administrative claims versus objective clinical data. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 60 (1): 88–95.
Prescott, H.C., T.M. Cope, F.C. Gesten, T.A. Ledneva, M.E. Friedrich, T.J. Iwashyna, T.M. Osborn, C.W. Seymour, and M.M. Levy. 2018. Reporting of sepsis cases for performance measurement versus for reimbursement in New York state. Critical care medicine. 46 (5): 666–673.
Faix, J.D. 2013. Biomarkers of sepsis. Critical reviews in clinical laboratory sciences. 50 (1): 23–36.
Souto FO, Castanheira FVS, Trevelin SC, Lima BHF, Cebinelli GCM, Turato WM, et al. Liver X receptor activation impairs neutrophil functions and aggravates sepsis. The Journal of infectious diseases. 2019.
Li D, Cheng Y, Yu J, Jia Y, Liu B, Xia Y, et al. Thrombo-inflammatory prognostic score improves qSOFA for risk stratification in patients with sepsis: A retrospective cohort study. Clinical chemistry and laboratory medicine. 2019.
Rello, J., F. Valenzuela-Sanchez, M. Ruiz-Rodriguez, and S. Moyano. 2017. Sepsis: A review of advances in management. Advances in therapy. 34 (11): 2393–2411.
Cui, C., D. Zhai, L. Cai, Q. Duan, L. Xie, and J. Yu. 2018. Long noncoding RNA HEIH promotes colorectal cancer tumorigenesis via counteracting miR-939 mediated transcriptional repression of Bcl-xL. Cancer research and treatment : official journal of Korean Cancer Association. 50 (3): 992–1008.
Muret, K., C. Klopp, V. Wucher, D. Esquerre, F. Legeai, F. Lecerf, et al. 2017. Long noncoding RNA repertoire in chicken liver and adipose tissue. Genetics, Selection, Evolution: GSE 49 (1): 6.
Wu, Z.Y., M. Trenner, R.A. Boon, J.M. Spin, and L. Maegdefessel. 2019. Long noncoding RNAs in key cellular processes involved in aortic aneurysms. Atherosclerosis. 292: 112–118.
Fang, K., C. Hu, X. Zhang, Y. Hou, D. Gao, Z. Guo, and L. Li. 2019. LncRNA ST8SIA6-AS1 promotes proliferation, migration and invasion in breast cancer through the p38 MAPK ssignalling pathway. Carcinogenesis.
Rao, N., X. Wang, J. Xie, J. Li, Y. Zhai, X. Li, et al. 2019. Stem cells from human exfoliated deciduous teeth ameliorate diabetic nephropathy in vivo and in vitro by inhibiting advanced glycation end product-activated epithelial-mesenchymal transition. Stem Cells International 2019: 2751475.
Hu, H., J. Wu, X. Yu, J. Zhou, H. Yu, and L. Ma. 2019. Long non-coding RNA MALAT1 enhances the apoptosis of cardiomyocytes through autophagy inhibition by regulating TSC2-mTOR signaling. Biological Research 52 (1): 58.
Luo, Y., L. Huang, W. Luo, S. Ye, and Q. Hu. 2019. Genomic analysis of lncRNA and mRNA profiles in circulating exosomes of patients with rheumatic heart disease. Biology open. 8: bio045633.
Xue, P., J. Zhao, A. Zheng, L. Li, H. Chen, W. Tu, N. Zhang, Z. Yu, Q. Wang, and M. Gu. 2019. Chrysophanol alleviates myocardial injury in diabetic db/db mice by regulating the SIRT1/HMGB1/NF-kappaB signaling pathway. Experimental and Therapeutic Medicine 18 (6): 4406–4412.
Xing, P.C., P. An, G.Y. Hu, D.L. Wang, and M.J. Zhou. 2020. LncRNA MIAT promotes inflammation and oxidative stress in sepsis-induced cardiac injury by targeting miR-330-5p/TRAF6/NF-kappaB axis. Biochemical Genetics 58: 783–800.
Zhu, Y., A. Sun, T. Meng, and H. Li. 2020. Protective role of long noncoding RNA CRNDE in myocardial tissues from injury caused by sepsis through the microRNA-29a/SIRT1 axis. Life Sciences 255: 117849.
Zhang, B.F., H. Jiang, J. Chen, Q. Hu, S. Yang, X.P. Liu, et al. 2019. LncRNA H19 ameliorates myocardial infarction-induced myocardial injury and maladaptive cardiac remodelling by regulating KDM3A. Journal of cellular and molecular medicine.
Cui, C., X. Chen, W. Du, L. Jing, L. Shi, D. Xia, et al. 2020. Correlations of inflammation, oxidative stress and prognosis with expression of LncRNA H19 in rats with sepsis-evoked lung injury. Panminerva Medica.
Wang, X., Y. Zhang, S. Han, Y. Yin, C. Chen, H. Chen, et al. 2019. LncRNA H19 inhibits kidney injury in sepsis rats through MAPK pathway. Minerva Medica.
Fang, Y., J. Hu, Z. Wang, H. Zong, L. Zhang, R. Zhang, et al. 2018. LncRNA H19 functions as an aquaporin 1 competitive endogenous RNA to regulate microRNA-874 expression in LPS sepsis. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 105: 1183–1191.
Valera, V.A., R. Parra-Medina, B.A. Walter, P. Pinto, and M.J. Merino. 2020. microRNA expression profiling in young prostate cancer patients. Journal of Cancer 11 (14): 4106–4114.
Hua, Q., Y. Chen, Y. Liu, M. Li, Q. Diao, H. Xue, H. Zeng, L. Huang, and Y. Jiang. 2019. Circular RNA 0039411 is involved in neodymium oxide-induced inflammation and antiproliferation in a human bronchial epithelial cell line via sponging miR-93-5p. Toxicological Sciences 170 (1): 69–81.
Zhang, S., Y. He, C. Liu, G. Li, S. Lu, Q. Jing, X. Chen, H. Ma, D. Zhang, Y. Wang, D. Huang, P. Tan, J. Chen, X. Zhang, Y. Liu, and Y. Qiu. 2020. miR-93-5p enhances migration and invasion by targeting RGMB in squamous cell carcinoma of the head and neck. Journal of Cancer 11 (13): 3871–3881.
JF OS, Neylon A, McGorrian C, Blake GJ. 2016. miRNA-93-5p and other miRNAs as predictors of coronary artery disease and STEMI. International Journal of Cardiology 224: 310–316.
Liu, J., M. Jiang, S. Deng, J. Lu, H. Huang, Y. Zhang, et al. 2018. miR-93-5p-containing exosomes treatment attenuates acute myocardial infarction-induced myocardial damage. Mol Ther Nucleic Acids 11: 103–115.
Yan, B., Z. Peng, and C. Xing. 2019. SORBS2, mediated by MEF2D, suppresses the metastasis of human hepatocellular carcinoma by inhibitiing the c-Abl-ERK signaling pathway. American Journal of Cancer Research 9 (12): 2706–2718.
Zhao, L., W. Wang, S. Huang, Z. Yang, L. Xu, Q. Yang, X. Zhou, J. Wang, Q. Shen, C. Wang, X. le, M. Feng, N. Zhou, W.B. Lau, B. Lau, S. Yao, T. Yi, X. Wang, X. Zhao, Y. Wei, and S. Zhou. 2018. The RNA binding protein SORBS2 suppresses metastatic colonization of ovarian cancer by stabilizing tumor-suppressive immunomodulatory transcripts. Genome Biology 19 (1): 35.
Li, C., F. Liu, S. Liu, H. Pan, H. Du, J. Huang, et al. 2020. Elevated myocardial SORBS2 and the underlying implications in left ventricular noncompaction cardiomyopathy. EBioMedicine. 53: 102695.
Wang, H., Y. Bei, S. Shen, P. Huang, J. Shi, J. Zhang, et al. 2016. miR-21-3p controls sepsis-associated cardiac dysfunction via regulating SORBS2. Journal of Molecular and Cellular Cardiology 94: 43–53.
Sun, F., W. Yuan, H. Wu, G. Chen, Y. Sun, L. Yuan, W. Zhang, and M. Lei. 2020. LncRNA KCNQ1OT1 attenuates sepsis-induced myocardial injury via regulating miR-192-5p/XIAP axis. Experimental Biology and Medicine (Maywood, N.J.) 245 (7): 620–630.
Guo, Y., J. Ni, S. Chen, M. Bai, J. Lin, G. Ding, Y. Zhang, P. Sun, Z. Jia, S. Huang, L. Yang, and A. Zhang. 2018. MicroRNA-709 mediates acute tubular injury through effects on mitochondrial function. Journal of the American Society of Nephrology : JASN. 29 (2): 449–461.
Essandoh, K., X. Wang, W. Huang, S. Deng, G. Gardner, X. Mu, Y. Li, E.G. Kranias, Y. Wang, and G.C. Fan. 2019. Tumor susceptibility gene 101 ameliorates endotoxin-induced cardiac dysfunction by enhancing Parkin-mediated mitophagy. The Journal of biological chemistry. 294 (48): 18057–18068.
Zhang J, Wang L, Xie W, Hu S, Zhou H, Zhu P, et al. Melatonin attenuates ER stress and mitochondrial damage in septic cardiomyopathy: A new mechanism involving BAP31 upregulation and MAPK-ERK pathway. Journal of Cellular Physiology. 2019.
Denning, N.L., M. Aziz, S.D. Gurien, and P. Wang. 2019. DAMPs and NETs in sepsis. Frontiers in immunology. 10: 2536.
Harrington, J.S., A.M.K. Choi, and K. Nakahira. 2017. Mitochondrial DNA in sepsis. Current opinion in critical care. 23 (4): 284–290.
Supinski GS, Schroder EA, Callahan LA. Mitochondria and critical illness. Chest. 2019.
Liu, Y., W. Yang, X. Sun, L. Xie, Y. Yang, M. Sang, and R. Jiao. 2019. SS31 ameliorates sepsis-induced heart injury by inhibiting oxidative stress and inflammation. Inflammation. 42 (6): 2170–2180.
Wang, Y.P., J. Liu, D. Liu, X.D. Wang, A.M. Bian, D.Z. Fang, and X.B. Hui. 2019. MiR-532-5p acts as a tumor suppressor and inhibits glioma cell proliferation by targeting CSF1. European Review for Medical and Pharmacological Sciences 23 (20): 8964–8970.
Huang, M.C., Y.H. Chou, H.P. Shen, S.C. Ng, Y.C. Lee, Y.H. Sun, C.F. Hsu, S.F. Yang, and P.H. Wang. 2019. The clinicopathological characteristic associations of long non-coding RNA gene H19 polymorphisms with uterine cervical cancer. Journal of Cancer. 10 (25): 6191–6198.
Liu ZZ, Tian YF, Wu H, Ouyang SY, Kuang WL. LncRNA H19 promotes glioma angiogenesis through miR-138/HIF-1alpha/VEGF axis. Neoplasma. 2019.
Ye, Y., A. Shen, and A. Liu. 2019. Long non-coding RNA H19 and cancer: A competing endogenous RNA. Bulletin du cancer. 106: 1152–1159.
Bi, Y., Y. Fu, S. Wang, X. Chen, and X. Cai. 2019. Schizandrin A exerts anti-tumor effects on A375 cells by down-regulating H19. Brazilian Journal of Medical and Biological Research = Revista brasileira de pesquisas medicas e biologicas 52 (10): e8385.
Safi, R., T. Mohsen-Kanson, G. Nemer, B. Dekmak, N. Rubeiz, M. El-Sabban, et al. 2019. Loss of ferrochelatase is protective against colon cancer cells: Ferrochelatase a possible regulator of the long noncoding RNA H19. Journal of gastrointestinal oncology. 10 (5): 859–868.
Wu, H., L. Liu, and J.M. Zhu. 2019. MiR-93-5p inhibited proliferation and metastasis of glioma cells by targeting MMP2. European review for medical and pharmacological sciences. 23 (21): 9517–9524.
Liu, Z.M., X.L. Yang, F. Jiang, Y.C. Pan, and L. Zhang. 2019. Matrine involves in the progression of gastric cancer through inhibiting miR-93-5p and upregulating the expression of target gene AHNAK. Journal of Cellular Biochemistry.
Huang, W., Y. Yang, J. Wu, Y. Niu, Y. Yao, J. Zhang, et al. 2019. Circular RNA cESRP1 sensitises small cell lung cancer cells to chemotherapy by sponging miR-93-5p to inhibit TGF-beta signalling. Cell death and differentiation.
Li, J.P., Y. Xiang, L.J. Fan, A. Yao, H. Li, and X.H. Liao. 2019. Long noncoding RNA H19 competitively binds miR-93-5p to regulate STAT3 expression in breast cancer. Journal of Cellular Biochemistry 120 (3): 3137–3148.
Han, L., C. Huang, and S. Zhang. 2019. The RNA-binding protein SORBS2 suppresses hepatocellular carcinoma tumourigenesis and metastasis by stabilizing RORA mRNA. Liver international : official journal of the International Association for the Study of the Liver. 39 (11): 2190–2203.
Ma, N., J. Pan, X. Ye, B. Yu, W. Zhang, and J. Wan. 2019. Whole-transcriptome analysis of APP/PS1 mouse brain and identification of circRNA-miRNA-mRNA networks to investigate AD pathogenesis. Molecular therapy Nucleic acids. 18: 1049–1062.
Wang, L., L.F. Song, X.Y. Chen, Y.L. Ma, J.F. Suo, J.H. Shi, and G.H. Chen. 2019. MiR-181b inhibits P38/JNK signaling pathway to attenuate autophagy and apoptosis in juvenile rats with kainic acid-induced epilepsy via targeting TLR4. CNS Neuroscience & Therapeutics 25 (1): 112–122.
Fredriksson-Lidman, K., C.M. Van Itallie, A.J. Tietgens, and J.M. Anderson. 2017. Sorbin and SH3 domain-containing protein 2 (SORBS2) is a component of the acto-myosin ring at the apical junctional complex in epithelial cells. PLoS One 12 (9): e0185448.
Ichikawa, T., M. Kita, T.S. Matsui, A.I. Nagasato, T. Araki, S.H. Chiang, T. Sezaki, Y. Kimura, K. Ueda, S. Deguchi, A.R. Saltiel, and N. Kioka. 2017. Vinexin family (SORBS) proteins play different roles in stiffness-sensing and contractile force generation. Journal of cell science. 130 (20): 3517–3531.
Teyra, J., H. Huang, S. Jain, X. Guan, A. Dong, Y. Liu, W. Tempel, J. Min, Y. Tong, P.M. Kim, G.D. Bader, and S.S. Sidhu. 2017. Comprehensive analysis of the human SH3 domain family reveals a wide variety of non-canonical specificities. Structure. 25 (10): 1598–1610 e3.
Fan, J., Y.C. Zhang, D.F. Zheng, M. Zhang, H. Liu, M. He, et al. 2019. IL-27 is elevated in sepsis with acute hepatic injury and promotes hepatic damage and inflammation in the CLP model. Cytokine. 127: 154936.
Zhuang, Y.T., D.Y. Xu, G.Y. Wang, J.L. Sun, Y. Huang, and S.Z. Wang. 2017. IL-6 induced lncRNA MALAT1 enhances TNF-alpha expression in LPS-induced septic cardiomyocytes via activation of SAA3. European review for medical and pharmacological sciences. 21 (2): 302–309.
Qu, Y., Q. Sun, X. Song, Y. Jiang, H. Dong, W. Zhao, et al. 2019. Helix B surfacepeptide reduces sepsis-induced kidney injury via PI3K/Akt pathway. Nephrology.
Funding
We thank the support of the funding obtained from Chenzhou Bureau of Science and Technology (ZDYF2020095 and ZDYF201941).
Author information
Authors and Affiliations
Contributions
Guarantor of integrity of the entire study: Bin Shan, Liang-Xian Luo.
Study concepts: Bin Shan.
Study design: Bin Shan, Liang-Xian Luo.
Definition of intellectual content: Bin Shan, Jia-Yan Li.
Literature research: Bin Shan, Jia-Yan Li, Ya-Jiang Liu.
Experimental studies: Ya-Jiang Liu, Xiao-Bin Tang, Zheng Zhou.
Data acquisition: Bin Shan, Zheng Zhou.
Data analysis: Xiao-Bin Tang, Zheng Zhou.
Statistical analysis: Xiao-Bin Tang, Zheng Zhou.
Manuscript preparation: Bin Shan, Liang-Xian Luo.
Manuscript editing: Bin Shan, Liang-Xian Luo.
Manuscript review: Bin Shan, Liang-Xian Luo.
All the authors approved for the final version.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Ethics Approval and Consent to Participate
Not applicable. This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for Publication
The informed consent was obtained from the study participants.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shan, B., Li, JY., Liu, YJ. et al. LncRNA H19 Inhibits the Progression of Sepsis-Induced Myocardial Injury via Regulation of the miR-93-5p/SORBS2 Axis. Inflammation 44, 344–357 (2021). https://doi.org/10.1007/s10753-020-01340-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01340-8