Abstract
Sjögren’s syndrome (SS) is a chronic autoimmune disease targeting salivary and lacrimal glands. C-X-C motif chemokine ligand 10 (CXCL10) expression is upregulated in lip salivary glands (LSGs) of primary SS (pSS) patients, and CXCL10 involved in SS pathogenesis via immune-cell accumulation. Moreover, interferon (IFN)-γ enhances CXCL10 production via the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway. We investigated the effects of baricitinib, a selective JAK1/2 inhibitor, on both IFN-γ-induced CXCL10 production and immune-cell chemotaxis. We used immunohistochemical staining to determine the expression levels and localization of JAK1 and JAK2 in LSGs of SS patients (n = 12) and healthy controls (n = 3). We then evaluated the effect of baricitinib in an immortalized normal human salivary gland ductal (NS-SV-DC) cell line. Immunohistochemical analysis of LSGs from pSS patients revealed strong JAK1 and JAK2 expression in ductal and acinar cells, respectively. Baricitinib significantly inhibited IFN-γ-induced CXCL10 expression as well as the protein levels in an immortalized human salivary gland ductal-cell clone in a dose-dependent manner. Additionally, western blot analysis showed that baricitinib suppressed the IFN-γ-induced phosphorylation of STAT1 and STAT3, with a stronger effect observed in the case of STAT1. It also inhibited IFN-γ-mediated chemotaxis of Jurkat T cells. These results suggested that baricitinib suppressed IFN-γ-induced CXCL10 expression and attenuated immune-cell chemotaxis by inhibiting JAK/STAT signaling, suggesting its potential as a therapeutic strategy for pSS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Sjögren’s syndrome (SS) is among the most common autoimmune diseases [1, 2] and is characterized by the destruction of acinar structure by marked lymphocytic infiltrates in the salivary and lacrimal glands, which results in sicca symptoms in affected patients [3,4,5]. SS pathogenesis is complicated and involves genetic contributions, frequency of the particular infection, and individual susceptibility to activating immune responses [6].
Recent data suggest central roles for interferon (IFN) types I (IFN-α and IFN-β) and II (IFN-γ) in SS pathogenesis based on the upregulation of their target genes in peripheral blood [7] and lip salivary glands (LSGs) [8, 9] from SS patients. In LSGs from SS patients, plasmacytoid dendritic cells are the main source of IFN-α, whereas CD4+ T cells and natural killer cells are the main IFN-γ producers [10]. Furthermore, the type I IFN signature is prevalent in the peripheral blood of SS patients, whereas the type II IFN signature predominates in LSGs [11].
C-X-C motif chemokine ligand 10 (CXCL10) is a chemokine induced by IFN-γ and produced by diverse cell types, including peripheral blood mononuclear cells, fibroblasts, and endothelial cells, during T helper 1 cell–mediated immune responses [12]. CXCL10 and its receptor, CXC receptor 3 (CXCR3), apparently contribute to the pathogenesis of many autoimmune diseases, including SS [13]. Gene-expression profiling of LSGs from healthy subjects and primary SS (pSS) patients demonstrated that CXCL10 is clearly upregulated in the latter [8]. Furthermore, CXCL10 is involved in the accumulation of infiltrating immune cells in the salivary glands of pSS patients [14, 15], and recent in vitro experiments showed that IFN-γ enhanced CXCL10 production via both the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) and nuclear factor-kappa B (NF-κB) pathways [16].
The JAK family of cytoplasmic protein tyrosine kinases comprises JAK1, JAK2, JAK3, and tyrosine kinase 2. JAKs bind to type 1 and type 2 cytokine receptors and transmit extracellular cytokine signals to activate STAT proteins (STAT1/2/3/4/5A/5B/6), which translocate to the nucleus and modulate transcription of effector genes [17]. JAK-STAT signaling is initiated by binding of cytokines to their respective receptors on the cell surface and utilized by > 50 cytokines [18]. Therefore, JAKs represent excellent targets for therapeutic interventions for various cytokine-mediated disorders. Baricitinib, a selective inhibitor of JAK1 and JAK2 [19], has been approved for the treatment of moderate-to-severe active rheumatoid arthritis. Although this drug is administered orally due to its small molecular weight, it has comparable efficacy with biological disease-modifying anti-rheumatic drugs, which require intravenous or subcutaneous injection [20].
In this study, we tested the hypothesis that baricitinib can suppress CXCL10 overexpression in LSGs of pSS patients. Additionally, we determined the in vitro effect of baricitinib on IFN-γ-induced CXCL10 expression and chemotaxis using a human salivary gland cell clone.
MATERIALS AND METHODS
Patients
Twelve female patients with pSS and three healthy female subjects were enrolled in this study. All individual participants enrolled in the study were treated at Tokushima University Hospital between 2011 and 2017. All pSS patients satisfied the revised Japanese Ministry of Health criteria for the diagnosis of SS [21] and the American College of Rheumatology classification criteria for SS [22]. Based on the biopsy scoring system described by Tarpley et al. [23], we categorized LSG biopsy samples from pSS patients into four groups according to the grade of infiltration: grade 1, Tarpley score 1 (1–2 lymphocytic aggregates/lobule), grade 2, Tarpley score 2 (2–3 lymphocytic aggregates/lobule); grade 3, Tarpley score 3 (diffuse infiltration through acini associated with partial destruction of acinar tissue); and grade 4, Tarpley score 4 (diffuse infiltration associated with complete loss of tissue architecture). Healthy controls were subjects who had experienced subjective symptoms of oral dryness but met none of the objective criteria for SS diagnosis.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board of Tokushima University Hospital (#2802) and with the 1964 Helsinki declaration and its later amendments.
Written informed consent was obtained from all individual participants included in the study, and this process was documented by the Institutional Review Board of Tokushima University Hospital. The informed consent procedure was approved by the Ethics Committee of Tokushima University Hospital.
Immunohistochemical Staining
Formalin-fixed paraffin-embedded LSG sections from pSS patients and healthy subjects were deparaffinized in xylene and rehydrated with graded ethanol (100%, 95%, 70%, and 50%). Antigen retrieval was performed by microwave treatment using a citrate-based antigen unmasking solution (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer’s instructions. Endogenous biotin was blocked using Blocking One reagent (Nacalai Tesque, Kyoto, Japan) according to the manufacturer’s instructions. Sections were then incubated overnight at 4 °C with rabbit polyclonal anti-human JAK1 (1:100; Cell Signaling Technology, Beverly, MA, USA) and rabbit polyclonal anti-human JAK2 (1:50; Cell Signaling Technology) primary antibodies. After three washes with phosphate-buffered saline, the sections were incubated for 30 min at 20 °C with horseradish peroxidase (HRP)–conjugated second antibody (Nichirei Biosciences Inc., Tokyo, Japan). HRP was reacted with the 3,3′-diaminobenzidine substrate using the Histofine Simple Stain MAX PO kit (Nichirei Biosciences Inc.), and the sections were observed using a light microscope equipped with a digital camera (BZ-X700; Keyence, Tokyo, Japan).
Cell Culture
The detailed characteristics of the immortalized normal human salivary gland ductal (NS-SV-DC) cell line have been described previously [24]. The cell line was cultured in keratinocyte serum-free medium (Gibco, Gaithersburg, MD, USA) in an incubator at 37 °C with a humidified atmosphere containing 5% CO2. Jurkat human leukemic T cells (Riken Cell Bank, Ibaraki, Japan) were maintained in RPMI-1640 medium (Gibco) supplemented with 10% fetal bovine serum (Gibco) in a 5% CO2-humidified incubator at 37 °C.
Reagents
Baricitinib was purchased from MedChemExpress (Monmouth Junction, NJ, USA), and recombinant human IFN-γ was purchased from R&D Systems (Minneapolis, MN, USA).
Cell Viability Assay
NS-SV-DC cells (1 × 104 cells/well) were seeded in 96-well plates (Falcon, Oxnard, CA, USA) in serum-free keratinocyte medium. After 24 h, the cells were treated with baricitinib (10, 100, 1000, 2500, or 5000 nM), and following an appropriate incubation period, an 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent (Roche, Basel, Switzerland) was added to each well for an additional 4-h incubation. The cells were then dissolved in solubilization solution (Roche) and analyzed at 570 nm using a Multiskan JX microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).
Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction
NS-SV-DC cells were treated for 6, 12, or 24 h with 10 ng/mL IFN-γ in the presence or absence of baricitinib (10, 100, or 1000 nM), followed by isolation of total RNA using TRIzol reagent (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. Total RNA was then converted into cDNA using the PrimeScript RT reagent kit (TaKaRa, Kusatsu, Japan) according to the manufacturer’s instructions. mRNA levels of CXCL10 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were analyzed using corresponding Assays-on-Demand Gene Expression products (Applied Biosystems, Tokyo, Japan) and TaqMan Universal PCR master mix (Applied Biosystems) with an ABI Prism 7000 sequence detection system (Applied Biosystems) according to the manufacturer’s instructions. The thermal cycler conditions were 95 °C for 10 min, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. Gene-expression data were analyzed using the 2−ΔΔCT method in Sequence Detection System software (v.1.0; Applied Biosystems). The relative quantification (RQ) of the fold change in gene expression was calculated using the formula RQ = 2−ΔΔCT and normalized using GAPDH as an internal reference. The relative mRNA levels were expressed as fold increases relative to GAPDH level.
Enzyme-Linked Immunosorbent Assay
NS-SV-DC cells were incubated for 6, 12, or 24 h with 10 ng/mL IFN-γ in the presence or absence of baricitinib (10, 100, or 1000 nM). The supernatants were collected, and secreted CXCL10 levels were analyzed using a human CXCL10 enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems) according to the manufacturer’s instructions. Absorbance at 450 nm was measured using a Multiskan JX microplate reader (Thermo Fisher Scientific), and CXCL10 levels were determined using a standard curve.
Protein Isolation and Western Blot Analysis
NS-SV-DC cells were treated for 5, 10, 30, or 60 min with 10 ng/mL IFN-γ in the presence or absence of 100 nM baricitinib, followed by preparation of cytoplasmic lysates using NE-PER nuclear and cytoplasmic extraction reagents (Thermo Fisher Scientific) according to the manufacturer’s instructions. Cytoplasmic lysate proteins (100 μg) were separated by electrophoresis using 10% sodium dodecyl sulfate polyacrylamide gels (Bio-Rad, Hercules, CA, USA), followed by transfer onto nitrocellulose membranes (Bio-Rad). The membranes were blocked with 3% bovine serum albumin in Tris-buffered saline containing 0.1% Tween 20 (TBS-T) and incubated for 1 h at 20 °C with anti-STAT1, anti-phospho-STAT1, anti-STAT3, anti-phospho-STAT3, and anti-β-actin primary antibodies (all from Cell Signaling Technology) diluted 1:1000 in Can Get Signal Solution 1 (TOYOBO, Osaka, Japan). After several washes with TBS-T, the membranes were incubated for 1 h at 20 °C with appropriate secondary antibodies (Cell Signaling Technology) diluted 1:1000 in Can Get Signal Solution 2 (TOYOBO). The immune complexes were visualized using an enhanced chemiluminescence western blotting detection reagent (GE Healthcare, Little Chalfont, UK). Densitometric analysis was performed using an Amersham Imager 600 (GE Healthcare) to determine the relative intensity of the immune complexes and using β-actin as an internal reference.
Cell Migration Assay
We analyzed Jurkat T cell directional migration, which was induced by conditioned medium (CM) derived from IFN-γ-treated NS-SV-DC cells, using a CytoSelect 96-well cell migration assay (5 μm; Fluorometric Format; Cell Biolabs, San Diego, CA, USA) according to the manufacturer’s instructions. Briefly, NS-SV-DC cells were treated with 10 ng/mL IFN-γ in the presence or absence of 100 nM baricitinib for 24 h, followed by transfer of 150 μL of CM into the bottom wells (feeder tray). Serum-free medium containing 5 × 105 Jurkat T cells (100 μL) was then placed in the migration chamber, and the chemotaxis plate was cultured at 37 °C for 24 h. After incubation, the cells that had migrated to the lower chambers were incubated for 20 min with 50 μL of lysis buffer/dye solution (Cell Biolabs). Fluorescence was read at 480/520 nm, and values were expressed as relative fluorescence units (RFUs). The experiments were performed in triplicate.
Statistical Analyses
All statistical analyses were performed using SPSS (v.15.0; SPSS Inc., Chicago, IL USA). Data were analyzed using the non-parametric two-tailed Mann–Whitney U test, and statistical significance was defined as a p < 0.05.
RESULTS
Expression of JAK1 and JAK2 in LSG Sections from pSS Patients and Healthy Controls
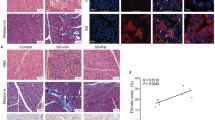
To assess the efficacy of a highly selective JAK1 and JAK2 inhibitor (baricitinib) as a therapeutic agent for pSS patients, we used immunohistochemical staining to examine expression levels and localization of JAK1 and JAK2 in LSGs from SS patients and healthy controls. Representative data from both groups are shown in Fig. 1. In both healthy controls and pSS patients, JAK1 showed particularly intense staining in ductal cells and to a lesser extent in acinar cells. By contrast, although JAK2 showed intense staining in acinar cells from healthy controls, it was also observed in ductal cells. In grade 1 pSS patients showing slight lymphocytic infiltration, JAK2 expression appeared similar to that in healthy controls, whereas grades 2 through 4 pSS patients with moderate-to-severe lymphocytic infiltration showed diminished JAK2 expression in acinar cells. Additionally, we observed enhanced JAK2 levels at a lesion displaying immune-cell infiltration around the ductal structure. These results indicated that JAK1 and JAK2 were present in both healthy controls and pSS patients.
Expression of JAK1 and JAK2 in LSG sections from pSS patients and healthy controls. LSG biopsy samples from 12 SS patients categorized into four groups according to the grade of infiltration. Healthy controls included subjects who had experienced subjective symptoms of oral dryness but met none of the objective criteria for SS diagnosis. Representative images are shown. Scale bars, 100 μm
Effects of Baricitinib on NS-SV-DC Cell Viability
We then examined the effects of baricitinib on NS-SV-DC cell viability by MTT assay. Following treatment of NS-SV-DC cells with various concentrations of baricitinib for up to 3 days, we observed no effect on NS-SV-DC viability and no significant difference in viability at doses ranging from 10 to 5000 nM (Fig. 2).
Effects of Baricitinib on CXCL10 Expression and Protein Secretion in IFN-γ-Stimulated NS-SV-DC Cells
We previously determined whether IFN-α, IFN-γ, tumor necrosis factor-α, and interleukin-1β could regulate CXCL10 expression in human salivary gland cell lines, revealing that IFN-γ-stimulated ductal cells were mainly responsible for CXCL10 overexpression in the salivary glands of pSS patients [16]. In the present study, we performed real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR) analyses to explore the potential involvement of baricitinib in regulation of IFN-γ-induced CXCL10 expression. Following treatment of NS-SV-DC cells with 10 ng/mL IFN-γ in the presence or absence of baricitinib (10, 100, or 1000 nM), we observed significant suppression of CXCL10 mRNA levels (p < 0.05) in IFN-γ-stimulated NS-SV-DC cells in a dose-dependent manner (Fig. 3a).
Effects of baricitinib on CXCL10 expression and protein secretion in IFN-γ-stimulated NS-SV-DC cells. a Histogram showing relative changes in CXCL10 mRNA levels in NS-SV-DC cells treated for 6, 12, or 24 h with 10 ng/mL IFN-γ in the presence or absence of baricitinib (10, 100, or 1000 nM). Untreated cells were used as a control. Fold changes in mRNA levels were evaluated by RT-qPCR using GAPDH mRNA as an internal reference. Data represent the mean ± SD of three independent experiments. *p < 0.05, two-tailed Mann–Whitney U test. b Histogram showing the concentration of CXCL10 measured by ELISA in the supernatants of NS-SV-DC cells treated for 6, 12, or 24 h with 10 ng/mL IFN-γ in the presence or absence of baricitinib (10, 100, or 1000 nM). Untreated cells were used as a control. Data represent the mean ± SD of three independent experiments. *p < 0.05, two-tailed Mann–Whitney U test
To further evaluate the effects of baricitinib, we performed an ELISA to measure secreted CXCL10 levels in supernatants of NS-SV-DC cells treated with IFN-γ in the presence or absence of baricitinib. As expected, baricitinib induced in a significant decrease in secreted CXCL10 levels (p < 0.05) in a dose-dependent manner (Fig. 3b), suggesting that JAKs were involved in IFN-γ-induced CXCL10 production in salivary gland ductal cells.
Effects of Baricitinib on IFN-γ-Induced STAT1 and STAT3 Phosphorylation in NS-SV-DC Cells
IFN-γ binds to its receptor (IFNγR) and activates JAK1 and JAK2, leading to primary phosphorylation of STAT1 and secondarily of STAT3, resulting in their translocation to the nucleus in order to bind conserved DNA elements. We previously reported that IFN-γ stimulated the production of CXCL10 via the JAK/STAT pathway in salivary gland ductal cells [16]. To evaluate the effects of baricitinib on IFN-γ-induced STAT1 and STAT3 phosphorylation in NS-SV-DC cells, we performed western blot analyses of extracts from NS-SV-DC cells treated with 10 ng/mL of IFN-γ in the presence or absence of baricitinib (100 nM). The results demonstrated that baricitinib suppressed IFN-γ-induced phosphorylation of STAT1 and STAT3 in NS-SV-DC cells (Fig. 4a), with quantitative data revealing stronger suppression of STAT1 phosphorylation by baricitinib relative to STAT3 phosphorylation (Fig. 4b).
Effects of baricitinib on IFN-γ-induced STAT1 and STAT3 phosphorylation in NS-SV-DC cells. a Representative western blot analysis showing STAT1, phospho-STAT1 (P-STAT1), STAT3, and phospho-STAT3 (P-STAT3) levels in NS-SV-DC cells treated for various time periods (5, 10, 30, or 60 min) with 10 ng/mL IFN-γ in the presence or absence of 100 nM baricitinib. β-Actin was used as an internal control. b Histogram showing relative protein levels normalized against that of β-actin
Effects of Baricitinib on the Chemotaxis of Jurkat T Cells
Because a previous report indicated that CXCL10 promotes accumulation of CXCR3+ T cells in LSGs of SS patients [14], we assessed the ability of baricitinib to promote recruitment of Jurkat T cells by IFN-γ-treated NS-SV-DC cells. Migration assays demonstrated that the chemotaxis of Jurkat T cells increased in the presence of IFN-γ-treated NS-SV-DC cells as compared with untreated NS-SV-DC cells. Additionally, baricitinib treatment significantly inhibited the observed IFN-γ-mediated chemotaxis of Jurkat T cells (p < 0.05) (Fig. 5). These results indicated that baricitinib inhibited the chemotaxis of CXCR3+ T cells by attenuating CXCL10 levels.
Effects of baricitinib on the chemotaxis of Jurkat T cells. NS-SV-DC cells were treated with 10 ng/mL IFN-γ in the presence or absence of baricitinib (100 nM) for 24 h. Serum-free Jurkat T cells were placed in the migration chamber, and the chemotaxis plate was incubated at 37 °C for 24 h. Fluorescence values (480/520 nm) are expressed as RFUs. Data represent the mean ± SD of three independent experiments. *p < 0.05, two-tailed Mann–Whitney U test
DISCUSSION
This is the first study to elucidate the effect of baricitinib, a selective inhibitor of JAK1 and JAK2, on IFN-induced CXCL10 expression in salivary gland cell clones. Although SS is a chronic autoimmune disease that affects exocrine glands, such as salivary and lacrimal glands, it also causes systemic autoimmune lesions [1,2,3]. The clinical symptoms of SS include both extraglandular (systemic) and glandular symptoms, such as dryness and swelling [2]. Histologically, SS patients exhibit selective and progressive destruction of the acinar structures in LSGs, resulting in infiltration of various immune cells [4], with destruction of the acinar structures resulting in reduced salivary flow. Gene-expression profiling of LSGs shows that CXCL10 expression is upregulated in pSS patients [8]. CXCL10 is involved in the accumulation of infiltrating CXCR3+ immune cells and SS pathogenesis in the salivary glands of pSS patients [14, 15]. We recently reported that IFN-γ is responsible for CXCL10 overexpression in salivary glands, and that IFN-γ-stimulated ductal cells, but not acinar cells, significantly secrete CXCL10 [16]. Furthermore, in vitro results showed that IFN-γ enhanced CXCL10 production via both the JAK/STAT and NF-κB pathways [16]. These findings suggested that JAK inhibitors might inhibit CXCL10 production in salivary glands and suppress destruction of acinar cells.
JAK1 and JAK2 are ubiquitously expressed in various tissues [25]; however, to the best of our knowledge, their expression in LSGs has not been investigated. In the present study, immunohistochemical analyses using LSG sections from control subjects showed that JAK1 and JAK2 localization differed (i.e., JAK1 was strongly expressed in ductal cells, whereas JAK2 was strongly expressed in acinar cells). In the LSGs of pSS patients, the JAK1-expression pattern was similar to that of control subjects; however, JAK2 expression in acinar cells appeared to diminish along with increasing lymphocytic infiltration, possibly indicating that JAK2 phosphorylation increases along with infiltration grade. These findings suggested that JAK1 and JAK2 inhibition by baricitinib could represent a potential treatment for pSS.
Clinical trials for pSS have generally failed, because the majority of enrolled patients showed no extraglandular manifestations at the time of enrollment but rather suffered from fatigue, dryness, and pain that did not significantly respond to the study medication [26]. Improving xerostomia (dry mouth) and keratoconjunctivitis sicca (dry eye) are mostly required for patients with pSS. Therefore, we concluded that treatment of pSS is important to prevent the destruction of glandular tissues. Based on previous studies, we predicted that blockade of JAK-STAT signaling might inhibit both IFN-γ-induced CXCL10 expression and chemotaxis in the salivary glands of pSS patients. Therefore, we investigated the in vitro pharmacological profile of baricitinib, a novel selective inhibitor of JAK1 and JAK2, using a human salivary gland cell line in order to evaluate its therapeutic potential for treating pSS.
In vitro experiments using immortalized human salivary gland ductal cells, RT-qPCR, and ELISA demonstrated that baricitinib treatment significantly suppressed IFN-γ-induced CXCL10 expression in a dose-dependent manner. Additionally, IFN-γ induced rapid phosphorylation of both STAT1 and STAT3, whereas these activities were inhibited by baricitinib treatment. These results suggested that baricitinib inhibited IFN-γ-induced CXCL10 expression in NS-SV-DC cells by suppressing the activation of JAK/STAT signaling.
Previous studies reported that CXCL10 is expressed in the salivary ductal glands of SS patients and accumulates in CXCR3+ immune cells in the LSGs of SS patients [14, 15]. In the present study, we performed migration assays to investigate whether baricitinib can inhibit immune-cell accumulation. We found that the migration of Jurkat T cells was stimulated in response to CM obtained from IFN-γ-treated NS-SV-DC cells. This finding is consistent with the histopathology of LSGs from SS patients (i.e., showing periductal infiltration of T cells). Notably, in the presence of baricitinib, we observed significant suppression of Jurkat T cell migration. This result suggests that baricitinib contributed to inhibition of T cell chemotaxis through downregulation of IFN-γ-stimulated CXCL10 secretion from salivary gland ductal cells.
In conclusion, this study provides new insight into the underlying molecular mechanism of baricitinib by demonstrating its ability to suppress IFN-γ-induced CXCL10 expression via inhibition of JAK/STAT signaling in human salivary gland ductal cells. Furthermore, baricitinib inhibited the chemotaxis of Jurkat T cells by reducing CXCL10 production and secretion by salivary gland ductal cells. These findings suggest baricitinib as a potential therapeutic strategy for pSS patients.
Data Availability
Not applicable.
References
Alspaugh, M.A., and K. Whaley. 1981. Sjögren’s syndrome. In Textbook of Rheumatology, ed. W.N. Kelley, E.D. Harris, S. Ruddy, and C.B. Sledge, 971–999. Philadelphia: Saunders (imprint).
Vivino, F.B., V.Y. Bunya, G. Massaro-Giordano, C.R. Johr, S.L. Giattino, A. Schorpion, B. Shafer, A. Peck, K. Sivils, A. Rasmussen, J.A. Chiorini, J. He, and J.L. Ambrus Jr. 2019. Sjögren’s syndrome: An update on disease pathogenesis, clinical manifestations and treatment. Clinical Immunology 203: 81–121.
Daniel, T.E. 1984. Labial salivary gland biopsy in Sjögren’s syndrome. Assessment as a diagnostic criterion in 362 suspected cases. Arthritis and Rheumatism 27: 147–156.
Christodoulou, M.I., E.K. Kapsogeorgou, and H.M. Moutsopoulos. 2010. Characteristics of the minor salivary gland infiltrates in Sjögren’s syndrome. Journal of Autoimmunity 34: 400–407.
Kamiński, B. 2019. Laryngological manifestations of Sjögren’s syndrome. Reumatologia 57: 37–44.
Nocturne, G., and X. Mariette. 2013. Advances in understanding the pathogenesis of primary Sjögren’s syndrome. Nature Reviews. Rheumatology 9: 544–556.
Brkic, Z., N.I. Maria, C.G. van Helden-Meeuwsen, J.P. van de Merwe, P.L. van Daele, V.A. Dalm, M.E. Wildenberg, W. Beumer, H.A. Drexhage, and M.A. Versnel. 2013. Prevalence of interferon type I signature in CD14 monocytes of patients with Sjögren’s syndrome and association with disease activity and BAFF gene expression. Annals of the Rheumatic Diseases 72: 728–735.
Hjelmervik, T.O., K. Petersen, I. Jonassen, R. Jonsson, and A.I. Bolstad. 2005. Gene expression profiling of minor salivary glands clearly distinguishes primary Sjögren’s syndrome patients from healthy control subjects. Arthritis and Rheumatism 52: 1534–1544.
Wakamatsu, E., Y. Nakamura, I. Matsumoto, D. Goto, S. Ito, A. Tsutsumi, and T. Sumida. 2007. DNA Microarray Analysis of Labial Salivary Glands of Patients With Sjögren’s Syndrome. Annals of the Rheumatic Diseases 66: 844–845.
Imgenberg-Kreuz, J., J.K. Sandling, J.C. Almlöf, J. Nordlund, L. Signér, K.B. Norheim, R. Omdal, L. Rönnblom, M.L. Eloranta, A.C. Syvänen, and G. Nordmark. 2016. Genome-wide DNA methylation analysis in multiple tissues in primary Sjögren’s syndrome reveals regulatory effects at interferon-induced genes. Annals of the Rheumatic Diseases 75: 2029–2036.
Hall, J.C., A.N. Baer, A.A. Shah, L.A. Criswell, C.H. Shiboski, A. Rosen, and L. Casciola-Rosen. 2015. Molecular subsetting of interferon pathways in Sjögren’s syndrome. Arthritis and Rheumatology 67: 2437–2446.
Luster, A.D., J.C. Unkeless, and J.V. Ravetch. 1985. γ-Interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature 315: 672–676.
Antonelli, A., S.M. Ferrari, D. Giuggioli, E. Ferrannini, C. Ferri, and P. Fallahi. 2014. Chemokine (C-X-C motif) ligand (CXCL)10 in autoimmune diseases. Autoimmunity Reviews 13: 272–280.
Ogawa, N., L. Ping, L. Zhenjun, Y. Takada, and S. Sugai. 2002. Involvement of the interferon-γ-induced T cell-attracting chemokines, interferon-γ-inducible 10-kd protein (CXCL10) and monokine induced by interferon-γ (CXCL9), in the salivary gland lesions of patients with Sjögren’s syndrome. Arthritis and Rheumatism 46: 2730–2741.
Aota, K., T. Yamanoi, K. Kani, K.I. Nakashiro, N. Ishimaru, and M. Azuma. 2018. Inverse correlation between the number of CXCR3+ macrophages and the severity of inflammatory lesions in Sjögren’s syndrome salivary glands: A pilot study. Journal of Oral Pathology and Medicine 47: 710–718.
Aota, K., K. Kani, T. Yamanoi, K.I. Nakashiro, N. Ishimaru, and M. Azuma. 2018. Distinct regulation of CXCL10 production by cytokines in human salivary gland ductal and acinar cells. Inflammation 41: 1172–1181.
Leonard, W.J., and J.J. O’Shea. 1998. Jaks and STATs: Biological implications. Annual Review of Immunology 16: 293–322.
Pringle, S., X. Wang, H. Bootsma, F.K.L. Spijkervet, A. Vissink, and F.G.M. Kroese. 2019. Small-molecule inhibitors and the salivary gland epithelium in Sjögren’s syndrome. Expert Opinion on Investigational Drugs 28: 605–616.
Kubo, S., S. Nakayamada, and Y. Tanaka. 2016. Baricitinib for the treatment of rheumatoid arthritis. Expert Review of Clinical Immunology 12: 911–919.
Taylor, P.C., E.C. Keystone, D. van der Heijde, M.E. Weinblatt, L. Del Carmen MoralesL, J. Reyes Gonzaga, S. Yakushin, T. Ishii, K. Emoto, S. Beattie, V. Arora, C. Gaich, T. Rooney, D. Schlichting, W.L. Macias, S. de Bono, and Y. Tanaka. 2017. Baricitinib versus Placebo or adalimumab in rheumatoid arthritis. The New England Journal of Medicine 16 (376): 652–662.
Fujibayashi, T., S. Sugai, N. Miyasaka, Y. Hayashi, and K. Tsubota. 2004. Revised Japanese criteria for Sjögren’s syndrome (1999): Availability and validity. Modern Rheumatology 14: 425–434.
Shiboski, S.C., C.H. Shiboski, L.A. Criswell, A.N. Baer, S. Challacombe, H. Lanfranchi, M. Schiødt, H. Umehara, F. Vivino, Y. Zhao, Y. Dong, D. Greenspan, A.M. Heidenreich, P. Helin, B. Kirkham, K. Kitagawa, G. Larkin, M. Li, T. Lietman, J. Lindegaard, N. McNamara, K. Sack, P. Shirlaw, S. Sugai, C. Vollenweider, J. Whitcher, A. Wu, S. Zhang, W. Zhang, J.S. Greenspan, and T.E. Daniels for the Sjögren’s International Collaborative Clinical Alliance (SICCA) Research Groups. 2012. American College of Rheumatology classification criteria for Sjögren’s syndrome: A data-driven, expert consensus approach in the Sjögren’s International Collaborative Clinical Alliance cohort. Arthritis Care and Research 64: 475–487.
Tarpley, T.M., Jr., L.G. Anderson, and C.L. White. 1974. Minor salivary gland involvement in Sjögren’s syndrome. Oral Surgery, Oral Medicine, and Oral Pathology 37: 64–74.
Azuma, M., T. Tamatani, Y. Kasai, and M. Sato. 1993. Immortalization of normal human salivary gland cells with duct-, myoepithelial-, acinar-, or squamous phenotype by transfection with SV40 ori- mutant deoxyribonucleic acid. Laboratory Investigation 69: 24–42.
Yamaoka, K., P. Saharinen, M. Pesu, V.E. Holt 3rd, O.J. Silvennoinen, and J.J. O’Shea. 2004. The Janus Kinases (Jaks). Genome Biology 5: 253.
Fox, R.I., C.M. Fox, J.E. Gottenberg, and T. Dörner. 2019. Treatment of Sjögren’s syndrome: current therapy and future directions. Rheumatology (Oxford). https://doi.org/10.1093/rheumatology/kez142.
Funding
This work was supported by the Grants-in-Aid for Scientific Research program of the Japanese Ministry of Education, Culture, Sports, Science, and Technology (No. 19K10311).
Author information
Authors and Affiliations
Contributions
Keiko Aota and Masayuki Azuma contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Keiko Aota, Tomoko Yamanoi, Koichi Kani, Shinji Ono, and Yukihiro Momota. The first draft of the manuscript was written by Keiko Aota and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board of Tokushima University Hospital (#2802) and with the 1964 Helsinki declaration and its later amendments.
Consent to Participate
Written informed consent was obtained from all individual participants included in the study, and this process was documented by the Institutional Review Board of Tokushima University Hospital. The informed consent procedure was approved by the Ethics Committee of Tokushima University Hospital.
Consent for Publication
Authorization has been given from all authors to use unpublished data.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aota, K., Yamanoi, T., Kani, K. et al. Inhibition of JAK-STAT Signaling by Baricitinib Reduces Interferon-γ-Induced CXCL10 Production in Human Salivary Gland Ductal Cells. Inflammation 44, 206–216 (2021). https://doi.org/10.1007/s10753-020-01322-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01322-w