Abstract
Nemonoxacin is a novel non-fluorinated quinolone. The effect of nemonoxacin on modulation of host immune response is not known. We sought to determine whether nemonoxacin has immunoprotective effects on lipopolysaccharide (LPS)-induced mouse sepsis model. Therefore, mice were challenged with lethal dose LPS (12.5 mg/kg) only or LPS with multi-dose nemonoxacin (40 mg/kg q12h) by intraperitoneal injection, and the results showed nemonoxacin could significantly reduce mortality from 80 to 30% in this model. The effect of nemonoxacin on immune cells in vivo and in vitro was also investigated. Mice were treated with sublethal LPS (5 mg/kg) or LPS + nemonoxacin, the myeloid cell subsets in mouse spleen were analyzed by flow cytometry, and cytokines in mouse serum were measured by ELISA. Additionally, mouse macrophage RAW264.7 cells were treated with LPS or LPS + nemonoxacin to investigate the immune modulatory effect of nemonoxacin in vitro, and the level of cytokines in cell culture supernatant was determined by ELISA. Analysis of myeloid cell subsets in the spleen showed nemonoxacin pretreatment could significantly inhibit LPS-induced proliferation of macrophages and dendritic cells but have no effect on neutrophils. Nemonoxacin could significantly reduce the expression of pro-inflammatory cytokines IL-6 and TNF-α while increase anti-inflammatory cytokine IL-10 expression, which were induced by LPS in vivo and in vitro. Finally, the immunomodulation of nemonoxacin in macrophage phagocytosis was also examined. The results displayed nemonoxacin pretreatment could significantly enhance the phagocytic function of macrophage. In conclusion, nemonoxacin has immune modulatory and protective effect on LPS-induced inflammation in vivo and in vitro.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Fluoroquinolones have been widely used to treat a broad array of infectious diseases [1]. There was a growing evidence that fluoroquinolones could directly influence host physiology and alter immune response during its combating with pathogen infection [2]. In recent years, some new non-fluorinated quinolones including nemonoxacin, garenoxacin, and ozenoxacin have reached to the market [3, 4]. Nemonoxacin, a novel C-8-methoxy non-fluorinated quinolone, exhibits strong broad-spectrum antibacterial activity against Gram-positive and Gram-negative bacteria, anaerobes, and atypical pathogens [5, 6]. More importantly, nemonoxacin shows high antimicrobial activity against penicillin-resistant Streptococcus pneumoniae (PRSP) and methicillin-resistant Staphylococcus aureus (MRSA) [7, 8]. However, nemonoxacin still has the similar chemical structures and identical target enzymes (DNA gyrase and topoisomerase IV) compared with fluoroquinolones [9, 10]. Therefore, we speculate that nemonoxacin has some effect on host innate immune cells.
The innate immune cells and their released cytokines play an important role to control bacterial infection. Some previous studies showed that fluoroquinolones could influence the antimicrobial function of innate immune cells [11]. It has been shown that lower doses of ciprofloxacin enhance production of interleukin (IL)-1, IL-6, and tumor necrosis factor alpha (TNF-α) by human monocytes in vitro [12], while moxifloxacin has been found to significantly inhibit TNF-α and IL-10 production by human monocytes stimulated with lipopolysaccharide (LPS) [13]. In light of that, the effect of antibiotics on host immune system is dependent on the types of antibiotics used, and there is an urgent need to determine the immunomodulatory effect of nemonoxacin on host immune function.
Here we sought to determine whether nemonoxacin directly alters the innate immune cell function. In this study, by using LPS-induced sepsis and innate immune cell activation mouse model, we found that mouse pretreatment with nemonoxacin had reduced sepsis severity and mortality induced by LPS. Furthermore, we showed that nemonoxacin treatment could inhibit the activation of innate immune cells including macrophage and dendritic cells, subsequently reduce pro-inflammatory and increase anti-inflammatory cytokine expression, which were induced by LPS. Mechanically we demonstrated that nemonoxacin pretreatment could significantly enhance the phagocytic function of mouse macrophage.
MATERIALS AND METHODS
Reagents and Kits
Nemonoxacin standards were manufactured by Huayu (Wuxi) Pharmaceutical Co. Ltd. (Jiangsu, China). Drug concentration was adjusted by diluting with 5% dextrose water (D5W). RAW264.7 (mouse macrophage) was purchased from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China. Cell culture medium DMEM (including 10% FBS, 100 U/mL penicillin and 100 mg/L streptomycin) was purchased from Thermo Fisher Co. Ltd. (Shanghai, China). LPS (Escherichia coli O111:B4) and dimethylsulfoxide (DMSO) were purchased from Sigma Chemical Co. Ltd. (Shanghai, China). ELISA Kit and Western antibody were bought from Assay Biotechnology Co. Ltd. (CA, USA). Anti-CD16/anti-CD32, CD11b, CD11c, F4/80, and Ly6G used in flow cytometry were purchased from BD Biosciences Co. Ltd. (Shanghai, China). Bacterial strain: A wild-type Klebsiella pneumoniae (K. pneumoniae) isolate LS-256 was isolated from the blood culture of a ventilator-associated pneumonia (VAP) patient in Huashan Hospital, Fudan University, Shanghai, China. This isolate was resistant to nemonoxacin (MIC 64 mg/L). This K. pneumoniae isolate LS-256 was used to investigate the immunomodulation of nemonoxacin in macrophage phagocytosis.
Animals
Male C57BL/6 mice weighing 21 to 25 g were purchased from Shanghai Slac Laboratory Animal Co. Ltd. (Shanghai, China). The mice were housed in ventilated microisolator cages to decrease the risk of infection from extraneous pathogens and had free access to food and water.
The Protective Effect of Nemonoxacin on Reducing Mouse Mortality Associated with Lethal LPS Challenge
To test whether nemonoxacin has immune protective effect on reducing mouse mortality in vivo, mice were challenged with lethal dose LPS [14] and administered nemonoxacin. A total of 40 mice were randomized into 4 groups, as 10 mice in each group. Group 1: LPS 12.5 mg/kg + nemonoxacin. All mice were administered 40 mg/kg nemonoxacin first dose by intraperitoneal injection (i.p.), then 12.5 mg/kg LPS 2 h later, after that, 40 mg/kg nemonoxacin q12h to 72 h, the observation time lasted to 96 h; group 2: LPS 12.5 mg/kg. Mice were administered PBS first and 12.5 mg/kg LPS 2 h later, then PBS q12h to 72 h; group 3: LPS 25 mg/kg + nemonoxacin; group 4: LPS 25 mg/kg. The survival rate of all mice was monitored for every 12 h.
The Effect of Nemonoxacin on Different Immune Cell Activation in LPS-Stimulated Mice
To investigate the effect of nemonoxacin on different immune cells in vivo, C57BL/6 mice were treated with LPS to induce immune stimulation with or without nemonoxacin pretreatment, and the myeloid cell subsets in spleen were analyzed by flow cytometry, described in detail as follows: Fifteen mice were randomized into 3 groups, with 5 mice in each group. Group 1: Control; group 2: LPS, mice were injected with PBS by i.p. first and then injected with 5 mg/kg LPS by i.p. 2 h later; group 3: LPS + nemonoxacin, mice were injected with 40 mg/kg nemonoxacin by i.p. and then injected with 5 mg/kg LPS by i.p. 2 h later. The mice were euthanized 6 h after LPS treatment, and the spleen cells were isolated and counted by the use of a hemocytometer. Aliquots of the immune cells (106/sample) were blocked with 10 mg/L anti-CD16/anti-CD32 (clone 2.4G2, ATCC) for 10 min in FACS buffer and then were stained on ice for 20 min with Pacific blue–conjugated, APC-, PE-, and FITC-conjugated mAbs to detect following surface Antigens including CD11b, CD11c, F4/80, and Ly6G. Stained cells were analyzed on a BD LSR II flow cytometer. Data analysis was carried out using FlowJo (TreeStar, Ashland, OR).
The Immune Modulation Effect of Nemonoxacin on Sublethal LPS-Induced Mouse Inflammation
To study the cytokine modulation and protecting effect on pathological damage of nemonoxacin in vivo, LPS was used to induce mouse inflammation model. A total of 40 mice were randomized into 4 groups, as 10 mice in each group. Group 1: LPS + nemonoxacin. All mice were administered 40 mg/kg nemonoxacin by i.p. first, then 5 mg/kg LPS 2 h later; group 2: Nemonoxacin. All mice were treated 40 mg/kg nemonoxacin by i.p., then PBS 2 h later; group 3: LPS. All mice were treated PBS first, then 5 mg/kg LPS 2 h later; group 4: Control. No nemonoxacin and LPS. Approximately 200 μL of blood of 5 mice in each group was drawn at 1 h, 3 h, 6 h, 12 h, and 24 h after LPS injection via the retro-orbital puncture. The level of IL-6, IL-10, and TNF-α at designated time point was determined by ELISA. The operating procedure followed ELISA kit instructions.
The Weight of Mouse Spleen
In the foregoing experiment, five mice in each group were sacrificed at 6 h after LPS treatment, and the spleens of the mice were aseptically removed and weighed to reflect the severity of inflammation in each group.
Cytokine Expression in Mouse Liver Was Determined by RT-qPCR
The livers of five mice in each group were separated and put into dry ice immediately, then stored on − 80 °C. The total RNA of mouse liver was extracted and the following protocol was followed: The mouse liver was put into Trazol and homogenized; then supernatant was removed, added chloroform, and centrifuged at 12,000g for 15 min; then isopropyl alcohol was added and centrifuged at 12,000g for 10 min; then supernatant was removed and added with 75% ethanol and RNA was dried; lastly, Depc H2O was added to dissolve RNA. The liver total RNA concentration was measured by Nanodrop (Thermo Fisher, MA, USA). cDNA synthesis and Realtime-PCR were performed using iTaq™ universal SYBR® Green one-step Kit (Bio-Rad, CA, USA). The mouse IL-6, TNF-α, IL-10, and 36B4 primer sequences were as follows: 36B4: 5′-TCCAGGCTTTGGGCATCA-3′ and 5′-CTTTATTCAGCTGCACATCACTCAGA-3′; IL-6: 5′-GTGGCTAAGGACCAAGACCA-3′ and 5′GGTTTGCCGAGTAGACCTCA-3′; TNF-α: 5 ′-CGAGTGACAAGCCTGTAGCC-3′ and 5′CATGCCGTTGGCCAGGA-3′. IL-10: 5′-CACTGCTATGCTGCCTGCTC3′ and 5′-TGGCCTTGTAGACACCTTGG-3′. Each gene expression was analyzed by the Ct method using 36B4 expression as an internal control.
Histopathological Findings in Mouse Spleen
In the same experiment, the other five mice in each group were sacrificed at 24 h, and the spleens were fixed immediately in 10% neutral buffered formalin and processed by standard paraffin embedding methods for histopathological examination. Sections were cut 4-mm thick, stained with hematoxylin-eosin (HE), and examined by light microscope.
The Cytokine Modulation of Nemonoxacin on LPS-Induced Macrophage Inflammation
Based on aforementioned experiment results, nemonoxacin could strongly inhibit LPS-induced mouse macrophage proliferation. Mouse macrophage RAW264.7 was used to examine the cytokine modulation of nemonoxacin on LPS-induced inflammation in vitro. RAW264.7 was cultured in DMEM medium and supplemented with 10% FBS and penicillin/streptomycin at 37 °C in a 5% CO2-humidified incubator. Assays with RAW264.7 cells were performed at a density of 5 × 105 cells/mL in 12-well plates. The cells were divided into four groups: group 1: LPS + nemonoxacin, nemonoxacin was added into RAW264.7 cell culture (final concentration was 4 mg/L), and then LPS was added 2 h later (final concentration was 10 ng/mL); group 2: LPS, just added LPS to cell culture, no nemonoxacin; group 3: Nemonoxacin, just added nemonoxacin to cell culture, no LPS; group 4: RAW264.7 cell culture as control. The cell culture supernatant of 4 groups was collected on 0 h, 3 h, 6 h, and 12 h after the addition of LPS, and then stored on − 80 °C. The level of IL-6, IL-10, and TNF-α at designated time point was determined by ELISA.
The Immunomodulation of Nemonoxacin on Macrophage Phagocytosis
To further characterize the interaction between nemonoxacin and macrophage, we examined the immunomodulation of nemonoxacin in macrophage phagocytosis using mouse macrophage RAW264.7 and K. pneumoniae isolate LS-256. RAW264.7 cells were performed at a density of 5 × 105 cells/mL in 12-well plate. The cells were divided into two groups: group 1: K. pneumoniae + nemonoxacin. Nemonoxacin was added into RAW264.7 cell culture (final concentration was 8 mg/L), and then K. pneumoniae was added 2 h later (final concentration was 2.8 × 106 CFU/mL); group 2: K. pneumoniae, just added K. pneumoniae to cell culture, no nemonoxacin. Two hours after the addition of K. pneumoniae, the cell culture medium was collected and the suspension was cultured on LB plates to quantify the extracellular number of K. pneumoniae. After that, the wells were washed using PBS three times, and then deionized sterile water was added to the wells to lysis macrophage and release K. pneumoniae. After 2 h later, the suspension was cultured on LB plates to quantify the intracellular number of K. pneumoniae.
Statistical Analysis
Differences between mean values of two groups normally distributed data were assessed by an unpaired Student’s t test. More than or equal to three groups, the mean value differences of any two groups were assessed by ANOVA with Tukey post-test. Differences in mortality of groups were assessed by the standard Mentel-Cox log-rank test. GraphPad Prism 7.0 was used to make figure and perform statistical analysis.
RESULTS
Nemonoxacin Treatment Decreases Sepsis Severity Induced by LPS
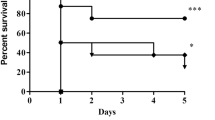
To investigate the effect of nemonoxacin on sepsis severity, C57BL/6 mice were pretreated with nemonoxacin and injected with lethal dose LPS (12.5 mg/kg and 25 mg/kg) intraperitoneally. We monitored the survival rate of these mice and found that in the LPS 12.5 mg/kg group, 2 mice died on 24 h, 5 mice died on 36 h, and one mouse died on 48 h, and the mortality rate was 80%. In contrast, 2 mice died on 24 h and one mouse died on 36 h, and the mortality rate was 30% in the LPS 12.5 mg/kg + nemonoxacin group. In LPS 25 mg/kg and LPS 25 mg/kg + nemonoxacin groups, all mice died within 36 h. Figure 1 shows the survival rate of LPS 12.5 mg/kg, LPS 12.5 mg/kg + nemonoxacin, LPS 25 mg/kg, and LPS 25 mg/kg + nemonoxacin groups. There was significant difference between the group LPS 12.5 mg/kg and group LPS 12.5 mg/kg + nemonoxacin (P = 0.037). Therefore, mice pretreated with nemonoxacin could significantly inhibit LPS (12.5 mg/kg)-induced sepsis and have better outcomes.
The survival rate of 4 groups mice injected with lethal dose LPS with or without nemonoxacin. Mice (n = 10 per group) were pretreated with PBS (control) or 40 mg/kg nemonoxacin before injecting with 12.5 mg/kg or 25 mg/kg LPS by i.p. These mice were monitored for their survival to 72 h after LPS injection.
Nemonoxacin Treatment Attenuates LPS-Induced Innate Immune Cell Activation in Mouse Model
Bacterial lipopolysaccharides (LPS) are the major outer surface membrane components present in almost all Gram-negative bacteria and act as extremely strong stimulators of innate or natural immunity. Analysis of innate immune cells in the spleen by flow cytometry showed that total number of immune cells in the LPS + nemonoxacin group was significantly lower than that in the LPS alone group (6.40 ± 0.24 × 108 in the LPS group vs 2.87 ± 0.57 × 108 in the LPS + nemonoxacin group). Using the ANOVA with Tukey post-test, P value was 0.0122. Analysis of the myeloid cells subsets showed that the number of macrophage in the LPS and LPS + nemonoxacin groups was 3.25 ± 1.51 × 106 and 1.20 ± 0.14 × 106, and the LPS + nemonoxacin group was significantly lower than the LPS group (P = 0.0176). The number of dendritic cells (DC) in the LPS and LPS + nemonoxacin groups was 2.76 ± 1.20 × 107 and 9.36 ± 1.63 × 106, and the LPS + nemonoxacin group was significantly lower than the LPS group (P = 0.0096). However, the number of neutrophils in LPS and LPS + nemonoxacin groups was 5.46 ± 1.77 × 106 and 5.13 ± 0.96 × 106 respectively and no statistical difference between two groups (P = 0.9090) (Fig. 2). The results implied that nemonoxacin pretreatment could significantly inhibit LPS-induced proliferation of macrophage and have a mild effect on DC, but does not have effect on neutrophil during LPS treatment.
Innate immune cells in mouse spleen analyzed by flow cytometry. The mice (n = 5 per group) were treated with LPS (5 mg/kg LPS) or LPS plus nemonoxacin (40 mg/kg nemonoxacin + 5 mg/kg LPS) and euthanized 6 h after LPS treatment. a The spleen cells were isolated and analyzed by flow cytometry. b Total number of immune cells. c Number of macrophages (CD11b+CD11c−F4/80+). d Number of neutrophils (CD11b+CD11c−Ly6G+). e Number of dendritic cell (CD11c+); *P ≤ 0.05; **P ≤ 0.01.
Effect of Nemonoxacin on Cytokine Responses Induced by Sublethal Doses of LPS In Vivo
The activation of innate immune cells by LPS will lead to increasing expression of pro-inflammatory cytokines. The cytokine levels in mouse blood were measured by ELISA. The average IL-6 concentration in mouse blood was elevated and reached peak on 3 h after LPS treatment, and then decreased and returned to the level before LPS treatment on 12 h. Compared with the LPS-treated group, the level of IL-6 in the group LPS + nemonoxacin was significantly lower at 6 h after LPS treatment, reduced by 47.1% at 6 h (Fig. 3a). Using the ANOVA with Tukey post-test, P value was 0.0004. The TNF-α concentration in the serum of groups LPS and LPS + nemonoxacin reached peak on 1 h and then decreased to undetected level at 6 h post-LPS treatment, and the mean TNF-α level in the group LPS + nemonoxacin was significantly lower than in the group LPS on 1 h (P = 0.0255) and 3 h (P = 0.0119), which was decreased by 38.3% on 1 h and 99.6% on 3 h, respectively (Fig. 3b). For anti-inflammatory cytokine IL-10, the mean IL-10 level in the group LPS + nemonoxacin was significantly higher than in the group LPS at 1 h (P = 0.0016), increasing by 72.4% (Fig. 3c).
Analysis of the expression of cytokines in mouse serum and the liver after LPS treatment. The mice (n = 5 per group) were treated with LPS (5 mg/kg LPS) or LPS plus nemonoxacin (40 mg/kg nemonoxacin + 5 mg/kg LPS). The serum was collected at 1 h, 3 h, 6 h, 12 h, and 24 h and the level of IL-6, IL-10, and TNF-α was determined by ELISA. Five mice in each group were sacrificed at 6 h, and the relative expression of cytokines in mouse liver was analyzed by RT-qPCR. a IL-6 in mouse serum. b TNF-α in mouse serum. c IL-10 in mouse serum. d IL-6 in mouse liver. e TNF-α in mouse liver. f IL-10 in mouse liver *P ≤ 0.05; **P ≤ 0.01.
Analysis of cytokine expression in mouse liver by RT-PCR at 6 h post-LPS treatment showed nemonoxacin pretreatment could also reduce the expression of pro-inflammatory cytokines including IL-6 (Fig. 3d, P = 0.0058) and TNF-α (Fig. 3e, P = 0.0842). However, there was no significant difference just borderline difference between group LPS and group LPS + nemonoxacin. Meanwhile, nemonoxacin pretreatment could significantly increase the level of anti-inflammatory cytokine IL-10 (Fig. 3f, P = 0.0009). Therefore, these results implied nemonoxacin had an inhibitory effect on pro-inflammatory cytokines and stimulatory effect on anti-inflammatory cytokine in LPS-induced inflammation mouse model.
Effect of Nemonoxacin on Weight Gain and the Pathology of the Spleen Induced by LPS
LPS treatment significantly increased the weight of the spleen; however, nemonoxacin pretreatment significantly inhibited LPS-induced weight gain of the spleen. The results showed that the mean weight of the spleen in the LPS + nemonoxacin group was 93.4 ± 3.1 mg, while in the LPS group was 104.6 ± 3.5 mg, and there was statistical difference between two groups (P = 0.0382) (Fig. 4a). Analysis of spleen histopathology indicated that mice in the LPS + nemonoxacin group showed obviously less spleen pathologic changes than mice in the LPS group. In the LPS group, the mouse spleen histopathology showed lymphocytes were decreased distinctly and lymphoid depletion occurred seriously in lymph nodes (Fig. 4c). While in the LPS + nemonoxacin group, the lymph node structure was almost integrate and lymphoid depletion occurred mildly in the spleen (Fig. 4d). Therefore, the results implied nemonoxacin pretreatment could significantly alleviate pathological damage of mouse spleen in LPS-induced inflammation mouse model.
Effect of nemonoxacin on the pathology of the spleen induced by LPS. Five mice in each group were sacrificed at 6 h and 24 h after LPS treatment with or without nemonoxacin pretreatment, and the spleens were weighted (6 h) and processed pathological examination (24 h). a The weight of mouse spleen at 6 h after LPS treatment. b Control mice. c Mice of the group LPS. Lymph nodes destructed, lymphoid depletion occurred seriously, and lymphocytes reduced distinctly (arrow). d Mice of the group LPS + nemonoxacin. Lymph node structure was almost integrate and lymphoid depletion occurred mildly (arrow). * P ≤ 0.05.
Nemonoxacin Suppresses LPS-Induced Cytokine Response in Macrophage In Vitro
To investigate whether nemonoxacin could directly inhibit LPS-induced cytokine expression, RAW264.7 cells were pretreated with nemonoxacin before stimulating with LPS, and the supernatant was collected to measure cytokines by ELISA. Compared with the group LPS + nemonoxacin, the average level of pro-inflammatory cytokine IL-6 in the group LPS was decreased by 59.4% at 3 h and 50.9% at 6 h after LPS treatment, and there was highly significant difference between two groups on 3 h (P < 0.0001) and 6 h (P = 0.0001). Moreover, the average TNF-α level in the group LPS + nemonoxacin was significantly lower than in the group LPS at 3 h (P = 0.0005), which was decreased by 39.5%. Analysis of anti-inflammatory cytokine IL-10 showed that there was a higher expression in the LPS + nemonoxacin group compared with that in the LPS group. The mean level in the group LPS + nemonoxacin was significantly higher than in the group LPS at 6 h (P = 0.0002) and 12 h (P = 0.0089), increasing by 39.4% at 6 h and 35.8% at 12 h respectively (Fig. 5). These results suggested that the modulation of innate immune response by nemonoxacin may be via its direct effect.
The cytokine modulation of nemonoxacin on LPS-induced macrophage inflammation in vitro. Raw264.7 cells were pretreated with nemonoxacin before stimulating with LPS, and the supernatant was collected at 0 h, 3 h, 6 h, and 12 h before LPS treatment, and then cytokines were measured by ELISA. Four groups, group LPS + nemonoxacin (4 μg/mL nemonoxacin + 10 ng/mL LPS), group LPS (10 ng/mL LPS), group nemonoxacin (4 μg/mL nemonoxacin), and group control. a IL-6. b TNF-α. c IL-10. **P ≤ 0.01.
The Effect of Nemonoxacin on the Phagocytosis of Macrophage
To investigate the effect of nemonoxacin on the phagocytosis of macrophage, RAW264.7 macrophage cells were pretreated with nemonoxacin and then incubated with bacteria K. pneumoniae for 2 h before removal of the extracellular bacteria by three washes. The counting of intracellular K. pneumoniae showed that in the group K. pneumoniae + nemonoxacin and group K. pneumoniae was 4.12 ± 0.86 × 103 CFU/mL and 2.08 ± 0.45 × 103 CFU/mL, respectively. The bacterial counting of the K. pneumoniae + nemonoxacin group was significantly higher than in the K. pneumoniae group (P = 0.03) (Fig. 6), while the extracellular bacterial counting of two groups was almost the same (1.67 ± 0.67 × 106 CFU/mL and 1.82 ± 0.74 × 106 CFU/mL, respectively). The results implied that nemonoxacin pretreatment could significantly enhance the phagocytic function of mouse macrophage.
The effect of nemonoxacin on the phagocytosis of macrophage. Raw264.7 cells were pretreated with or without nemonoxacin (8 μg/mL) for 2 h, and then Raw264.7 immediately infected with K. pneumoniae (2.8 × 106 CFU/mL) for 2 h. The extracellular bacteria were removed after 2-h incubation with macrophage, the cells were lysed with deionized sterile water, and the number of released intracellular K. pneumoniae was quantified by plate counting. *P ≤ 0.05.
DISCUSSION
Pathogen infection will elicit the activation of host immune cells, and subsequently to eliminate these invaders. The intensity of host innate immune is important for host to control the pathogen infection, but if uncontrolled, it will lead to tissue damage [15]. Non-fluorinated quinolones are now widely used to kill bacteria during their infection, but the contribution of non-fluorinated quinolones to regulation of host innate immune response is poorly understood. Only one article investigated the immunomodulatory effect of garenoxacin in vitro [16]. LPS is a major component of the outer membrane of Gram-negative bacteria and it is widely used to mimic bacterial infection to induce inflammatory response. In the present study, by using LPS-induced mouse endotoxin and inflammatory response in vitro and in vivo model, we, for the first time, demonstrated that nemonoxacin could modulate host innate immune response, which may benefit to reduce inflammatory-induced tissue damage during the bacterial infection. Additionally, to our knowledge, it is the first time to investigate the immune protective effects of non-fluorinated quinolones including garenoxacin and ozenoxacin in vivo.
In our previous nemonoxacin pharmacokinetics (PK) study, when nemonoxacin was administered orally to healthy volunteers with single dose 500 mg, the peak plasma concentration (Cmax) was 5.91 mg/L [17, 18]. Our article displayed the Cmax of nemonoxacin in a modified infection model in vitro was similar to the human data [19]. Furthermore, our recent study showed Cmax was nearly 4 mg/L when mice were administered 40 mg/kg nemonoxacin (data not published). Therefore, in the present study, 4 mg/L nemonoxacin was used in vitro study and 40 mg/kg in mouse endotoxin model.
Consistent with the results that the inhibition of LPS induced cytokine secretion by nemonoxacin, we observed that nemonoxacin partially prevents the weight gain and immune cell expansion in the spleen induced by LPS. Further analysis of myeloid population in the spleen by flow cytometry suggested that nemonoxacin specifically inhibited macrophage proliferation rather than neutrophils, and the mechanism of this cell type–specific inhibition needs to be further investigated.
In the present study, the results also showed multi-dose of nemonoxacin can prevent mortality associated with lethal LPS challenge. The mouse mortality of the LPS 12.5 mg/kg group and LPS 12.5 mg/kg + nemonoxacin group was 80% and 30%, respectively. It suggested that multi-dose of nemonoxacin had immune protective effect and improved survival rate 50% in LPS-induced sepsis mouse model. It has been published that ciprofloxacin has protective effects on reducing mortality in LPS-induced mouse sepsis and lung injury model [20].
In this study, the results showed pretreatment with nemonoxacin had an inhibitory effect on pro-inflammatory cytokine IL-6 and TNF-α and stimulatory effect on anti-inflammatory cytokine IL-10 in LPS-induced inflammation mouse model. Meanwhile, nemonoxacin pretreatment could obviously reduce pathological damage of mouse spleen in this model. The results also displayed nemonoxacin pretreatment could significantly inhibit LPS-induced IL-6 and TNF-α expression, and stimulate IL-10 expression in vitro. It has been confirmed that endotoxin-mediated death can be reversed either by decreasing TNF-α or by increasing IL-10 in animal models [21,22,23]. Therefore, it is proposed that nemonoxacin’s effect on cytokine responses is a major factor causing improved survival in this sepsis mouse model. The immunomodulatory effect on altering early host cytokine responses in vitro can also be observed in another non-fluorinated quinolone, garenoxacin. Treatment with garenoxacin significantly inhibited the transcription and secretion of IL-8 induced by LPS-stimulated A549 and THP-1 cells [16]. Since NF-κB and MAPKs play an import role in regulation of inflammatory response during pathogen infection [24, 25], so further study needs to investigate the effects of nemonoxacin on activation of these major signal transduction pathways.
Even though our data demonstrated nemonoxacin pretreatment could reduce LPS-induced pro-inflammatory cytokine secretion by macrophage, it enhanced macrophage phagocytic capacity. We speculated that the significant increase in phagocytosis function is due to that nemonoxacin treatment influenced the expression level or the binding efficiency of phagocytosis relevant receptors on macrophage. Phagocytosis of invading pathogen by macrophage is important to clear infection, so enhanced phagocytosis by nemonoxacin treatment could shorten inflammation and reduced tissue damage during the infection. LPS is an endotoxin and important pathogenic factor of Gram-negative bacteria. It is considered to play a central role in mediating sepsis caused by Gram-negative bacteria. In this study, we testified that nemonoxacin had ability to reduce LPS-mediated fatality in mouse model. Therefore, nemonoxacin may have potential application in treating patients with LPS-induced sepsis. The effects of nemonoxacin on sepsis, inflammation, and tissue injury protection should be further studied in the clinical setting.
In summary, this is the first time to demonstrate the immunomodulatory effect of nemonoxacin on host immune function. Nemonoxacin can reduce severity and mortality on LPS-induced mouse sepsis model. Nemonoxacin pretreatment could significantly inhibit LPS-induced IL-6 and TNF-α expression, and stimulate IL-10 expression in vivo and in vitro. This observation suggests that nemonoxacin can inhibit excessive inflammation and have a protective effect on host.
Abbreviations
- LPS:
-
lipopolysaccharide
- ELISA:
-
enzyme-linked immunosorbent assay
- IL-1:
-
interleukin-1
- IL-6:
-
interleukin-6
- IL-10:
-
interleukin-10
- TNF-α:
-
tumor necrosis factor alpha
- i.p.:
-
intraperitoneal injection
- RAW264.7:
-
macrophage; Abelson murine leukemia virus transformed
References
Ezelarab, H.A.A., S.H. Abbas, H.A. Hassan, and G.E.A. Abuo-Rahma. 2018. Recent updates of fluoroquinolones as antibacterial agents. Archiv der Pharmazie (Weinheim) 351 (9): e1800141.
Dalhoff, A., and I. Shalit. 2003. Immunomodulatory effects of quinolones. The Lancet Infectious Diseases 3 (6): 359–371.
Takagi, H., K. Tanaka, H. Tsuda, and H. Kobayashi. 2008. Clinical studies of garenoxacin. International Journal of Antimicrobial Agents 32 (6): 468–474.
Vila, J., A.A. Hebert, A. Torrelo, Y. Lopez, M. Tato, M. Garcia-Castillo, and R. Canton. 2019. Ozenoxacin: a review of preclinical and clinical efficacy. Expert Review of Anti-Infective Therapy 17 (3): 159–168.
Chen, Y.H., C.Y. Liu, J.J. Lu, C.H. King, and P.R. Hsueh. 2009. In vitro activity of nemonoxacin (TG-873870), a novel non-fluorinated quinolone, against clinical isolates of Staphylococcus aureus, enterococci and Streptococcus pneumoniae with various resistance phenotypes in Taiwan. The Journal of Antimicrobial Chemotherapy 64 (6): 1226–1229.
Lauderdale, T.L., Y.R. Shiau, J.F. Lai, H.C. Chen, and C.H. King. 2010. Comparative in vitro activities of nemonoxacin (TG-873870), a novel nonfluorinated quinolone, and other quinolones against clinical isolates. Antimicrobial Agents and Chemotherapy 54 (3): 1338–1342.
Li, C.R., Y. Li, G.Q. Li, X.Y. Yang, W.X. Zhang, R.H. Lou, J.F. Liu, M. Yuan, P. Huang, S. Cen, L.Y. Yu, L.X. Zhao, J.D. Jiang, and X.F. You. 2010. In vivo antibacterial activity of nemonoxacin, a novel non-fluorinated quinolone. The Journal of Antimicrobial Chemotherapy 65 (11): 2411–2415.
Li, Z., Y. Liu, R. Wang, and A. Li. 2014. Antibacterial activities of nemonoxacin against clinical isolates of Staphylococcus aureus: an in vitro comparison with three fluoroquinolones. World Journal of Microbiology and Biotechnology 30 (11): 2927–2932.
Roychoudhury, S., T.L. Twinem, K.M. Makin, E.J. McIntosh, B. Ledoussal, and C.E. Catrenich. 2001. Activity of non-fluorinated quinolones (NFQs) against quinolone-resistant Escherichia coli and Streptococcus pneumoniae. Journal of Antimicrobial Chemotherapy 48 (1): 29–36.
Adam, H.J., N.M. Laing, C.R. King, B. Lulashnyk, D.J. Hoban, and G.G. Zhanel. 2009. In vitro activity of nemonoxacin, a novel nonfluorinated quinolone, against 2,440 clinical isolates. Antimicrobial Agents and Chemotherapy 53 (11): 4915–4920.
Dalhoff, A. 2005. Immunomodulatory activities of fluoroquinolones. Infection 33 (Suppl 2): 55–70.
Bailly, S., M. Fay, B. Ferrua, and M.A. Gougerot-Pocidalo. 1991. Ciprofloxacin treatment in vivo increases the ex vivo capacity of lipopolysaccharide-stimulated human monocytes to produce IL-1, IL-6 and tumour necrosis factor-alpha. Clinical and Experimental Immunology 85 (2): 331–334.
Araujo, F.G., T.L. Slifer, and J.S. Remington. 2002. Effect of moxifloxacin on secretion of cytokines by human monocytes stimulated with lipopolysaccharide. Clinical Microbiology and Infection 8 (1): 26–30.
Purswani, M.U., S.J. Eckert, H.K. Arora, and G.J. Noel. 2002. Effect of ciprofloxacin on lethal and sublethal challenge with endotoxin and on early cytokine responses in a murine in vivo model. The Journal of Antimicrobial Chemotherapy 50 (1): 51–58.
Kumar, S., H. Ingle, D.V. Prasad, and H. Kumar. 2013. Recognition of bacterial infection by innate immune sensors. Critical Reviews in Microbiology 39 (3): 229–246.
Hara, S., Y. Ishimatsu, H. Mukae, N. Sakamoto, T. Kakugawa, H. Fujita, A. Hara, and S. Kohno. 2011. Anti-inflammatory effects of garenoxacin on IL-8 production and ERK1/2 activation induced by lipopolysaccharides in A549 and THP-1 cells. European Journal of Pharmacology 668 (1–2): 264–270.
Cao, G.Y., J. Zhang, Y.Y. Zhang, B.N. Guo, J.C. Yu, X.J. Wu, Y.C. Chen, J.F. Wu, and Y.G. Shi. 2014. Safety, tolerability, and pharmacokinetics of intravenous nemonoxacin in healthy chinese volunteers. Antimicrobial Agents and Chemotherapy 58 (10): 6116–6121.
Guo, B., X. Wu, Y. Zhang, Y. Shi, J. Yu, G. Cao, and J. Zhang. 2012. Safety and clinical pharmacokinetics of nemonoxacin, a novel non-fluorinated quinolone, in healthy Chinese volunteers following single and multiple oral doses. Clinical Drug Investigation 32 (7): 475–486.
Liang, W., Y.C. Chen, Y.R. Cao, X.F. Liu, J. Huang, J.L. Hu, M. Zhao, Q.L. Guo, S.J. Zhang, X.J. Wu, D.M. Zhu, Y.Y. Zhang, and J. Zhang. 2013. Pharmacokinetics and pharmacodynamics of nemonoxacin against Streptococcus pneumoniae in an in vitro infection model. Antimicrobial Agents and Chemotherapy 57 (7): 2942–2947.
Huang, H.C., C.C. Shieh, W.L. Yu, K.C. Cheng, C.C. Chen, S.T. Chang, and Y.C. Chuang. 2008. Comparing the protective effects of ciprofloxacin, moxifloxacin and levofloxacin in mice with lipopolysaccharide-induced acute lung injuries. Respirology 13 (1): 47–52.
Dharmana, E., M. Keuter, M.G. Netea, I.C. Verschueren, and B.J. Kullberg. 2002. Divergent effects of tumor necrosis factor-alpha and lymphotoxin-alpha on lethal endotoxemia and infection with live Salmonella typhimurium in mice. European Cytokine Network 13 (1): 104–109.
Howard, M., T. Muchamuel, S. Andrade, and S. Menon. 1993. Interleukin 10 protects mice from lethal endotoxemia. The Journal of Experimental Medicine 177 (4): 1205–1208.
Murphey, E.D., and D.L. Traber. 2001. Protective effect of tumor necrosis factor-alpha against subsequent endotoxemia in mice is mediated, in part, by interleukin-10. Critical Care Medicine 29 (9): 1761–1766.
Weiss, T., I. Shalit, H. Blau, S. Werber, D. Halperin, A. Levitov, and I. Fabian. 2004. Anti-inflammatory effects of moxifloxacin on activated human monocytic cells: inhibition of NF-kappaB and mitogen-activated protein kinase activation and of synthesis of proinflammatory cytokines. Antimicrobial Agents and Chemotherapy 48 (6): 1974–1982.
Werber, S., I. Shalit, I. Fabian, G. Steuer, T. Weiss, and H. Blau. 2005. Moxifloxacin inhibits cytokine-induced MAP kinase and NF-kappaB activation as well as nitric oxide synthesis in a human respiratory epithelial cell line. The Journal of Antimicrobial Chemotherapy 55 (3): 293–300.
Acknowledgments
We would like to thank Zhenda Shi, a Post-Doctor from Georgia State University, Atlanta, GA, USA, for his valuable help in animal model construction.
Funding
This study was financially supported by the Major Research and Development Project of Innovative Drugs, Ministry of Science and Technology of China (2017ZX09304005) and the Shanghai Natural Science Fund (No. 17ZR1425100).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no conflict of interest.
Ethical Approval
The experimental protocol was approved by the Animal Research Committee of School of pharmacy, Fudan University, Shanghai, China. (No. 2019-03-HSYY-ZX-01) The care and handling of the animals were carried out according to the institutional and national guidelines.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 1970 kb)
Rights and permissions
About this article
Cite this article
Chen, N., Li, X., Guo, B. et al. Nemonoxacin Has Immunoprotective Effects on Reducing Mortality in Lipopolysaccharide-Induced Mouse Sepsis Model. Inflammation 43, 2276–2286 (2020). https://doi.org/10.1007/s10753-020-01296-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01296-9