Abstract
Although the E3 ubiquitin ligase Zinc and ring finger 3 (ZNRF3) negatively regulates the Wnt signaling pathway, its function in rheumatoid arthritis (RA) is elusive. Here, the effects and the mechanism of ZNRF3 on a mouse model of collagen-induced arthritis (CIA) and human fibroblast-like synoviocytes (FLS) obtained from RA patients were determined. Our results showed that ZNRF3 was highly expressed in tissues and FLSs compared to trauma patients. Lentivirus-mediated silencing of ZNRF3 induced apoptosis decreased cell viability and significantly attenuated inflammation in RA-FLSs via tumor necrosis-α (TNF-α). Additionally, silencing of ZNRF3 reduced knee joint damage and also decreased the level of TNF-α, IL-1β, and IL-6 in the CIA mouse model. These effects were mediated by the crosstalk between Wnt and NF-κB pathways in RA-FLS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Rheumatoid arthritis (RA) belongs to a group of chronic autoimmune conditions associated with invasive arthritis. Unlike healthy individuals, the synovium of RA patients is characterized by bone destruction, pannus formation, synovial inflammation, and joint cartilage erosion. Fibroblast-like synoviocytes (FLSs) are vital components of the synovium that contribute to the development of RA. RA-FLS can proliferate and secrete various immunoregulatory factors, vascular endothelial growth factor (VEGF), and matrix metalloproteinases (MMPs) [1,2,3,4].
In recent years, Wnt signaling pathways have been found to be associated with the activation of RA-FLS [5, 6]. Therefore, inhibition of the Wnt pathway may effectively treat RA.
The transmembrane E3 ubiquitin ligase Zinc and ring finger 3 (ZNRF3) is a member of Goliath and Godzilla families which inhibits the Wnt/β-catenin signaling cascades [7, 8]. The activation of Wnt signaling triggers the production of β-catenin which then translocates to the nucleus where it interacts with transcription factors of the TCF family leading to the expression of ZNRF3. When ZNRF3 translocates into the plasma membrane, it recognizes the Wnt receptor FZD through DVL. FZD is then ubiquitinated and degraded, resulting in the inhibition of the Wnt/β-catenin signaling. In the extramembrane region, ZNRF3 forms a complex with R-spondin and LGR4/5 and induce the ubiquitination and degradation of itself [7, 9]. In this study, we speculated that ZNRF3 may regulate the synovitis of RA via the Wnt pathway.
In this study, we examined the expression of ZNRF3 in the synovial tissues and FLSs, and the effect of the lentivirus-mediated silencing of ZNRF3 on apoptosis, proliferation, and inflammatory response in RA-FLS. The mechanisms of these effects were explored using a mouse model of arthritis.
MATERIALS AND PROTOCOLS
Patients Enrollment and Synovial Tissue Preparation
Synovial tissues were extracted from 10 patients with RA (3 men and 7 women; 49–68 years old) and 15 patients with trauma (3 women and 12 men; 21–32 years old). Synovial tissues of patients with trauma were used as normal controls. The American College of Rheumatology (ACR) criteria for RA were used to select patients [10]. Patients with trauma were diagnosed as having soft tissue injuries such as ligaments during exercise. Patients with other immune and musculoskeletal disorders were excluded. RA patients were treated with total knee replacement surgery. Arthroscopic examination was performed to examine patients with knee joint trauma. This study was approved by the Ethics Committee of the Shanghai Changhai Hospital.
Immunohistochemical
Synovial tissues were fixed in 4% paraformaldehyde for 48 h and then analyzed by immunohistochemistry using previously described protocols [11]. The scores were analyzed as reported in a previous study (0 = 0–9%, 1 = 10–24%, 2 = 25–49%, 3 = 50–74%, and 4 = 75–100%) [12].
Cell Isolation and Culture
FLS were isolated from the synovial tissues by enzymatic digestion [13]. The cells were cultured in a DMEM/F12 solution supplemented with 100 U/mL penicillin, 10% fetal bovine serum, and 100 μg/mL streptomycin (FBS; Gibco, Carlsbad, CA, USA) at 37 °C, 5% CO2 in a humidified incubator. Thereafter, they were harvested at 4–6 passages for use in experiments.
Immunofluorescence
The distribution of ZNRF3 in Trauma-FLSs and RA-FLSs samples was assessed as described previously [14]. The cells were stained and then imaged with a fluorescence microscope (Olympus, Tokyo, Japan).
Cell Infection
Cells were added into 6-well plates at a density of 1 × 106 per well and incubated for 24 h. Next, a fresh medium containing lentivirus and 8 μg/mL Polybrene (Heyuan, Shanghai, China) was added. The amount of lentivirus added was calculated on the basis of the required multiplicity of infection (MOI). The optimal MOI for lentivirus of short hairpin (Sh)- ZNRF3 (Sh-ZNRF3) and the lentivirus of control (Sh-control) were 30 and 10, respectively, as determined by the preliminary experiments. The culture medium was changed within 24 h after infection, and 1‰Puromycin (Sigma, St. Louis, MO, USA) was added to the complete medium to kill the wide-type RA-FLSs. About 5 days later, the majority of the wild-type cells had died. The efficiency of infection was determined by western blot.
Cell Viability Assay
The impact of Sh-ZNRF3 on cell viability was assessed by the Cell Count Kit-8 (Dojindo, Kumamoto, Japan). The infected RA-FLSs were incubated in 96-well plates (8 × 103 cells/well) for 24 h in the presence or absence of 10 ng/mL TNF-α. Cells were washed with PBS, and then 10 μL CCK-8 solution and 90 μL DMEM/well were added. After incubation at 37 °C for 3 h, the absorbance of the supernatant of the culture medium was measured at 450 nm wavelength with a spectrophotometer.
Measurement of Apoptosis
RA-FLS often impairs apoptosis which leads to hyperplastic pannus [15]. Apoptosis was assessed by the Annexin V-APC/7-AAD kit (Multisciences, Hangzhou, China). The cells were divided into two groups, those infected with Sh-ctr or Sh-ZNRF3. Briefly, cells were washed with PBS and then reconstituted in a 1× binding buffer. This was followed by treatment with Annexin V-APC and 7-AAD for 15 min at RT in the dark and then analyzed by flow cytometric (BD, Frankin Lakes, NJ, USA).
Cell Migration
The effects of Sh-ZNRF3 on the migration ability of RA-FLSs were determined using the Transwell cell migration test and healing assay. After seeding cells on 6-well plates, a cell wound was created by scraping the surface with a sterile 1 mL pipette tip. The wound-healing process was analyzed at 0 h and 24 h by phase-contrast microscope (Leitz, Germany) as reported before [16]. The cell migration was calculated based on the healed area with image J at various time points. Transwell cell migration test was performed by the Transwell invasion chambers (Corning, USA) in 24-well plates according to a previous study [16]. The number of RA-FLSs was counted in five randomly selected fields per well. The cell migration rate was determined as the average number of invading cells compared to the Sh-ctr group.
qPCR Assays
RA-FLSs samples were divided into four groups, and two of which were infected by Sh-ctr and Sh-ZNRF3. The two groups infected by lentivirus and the negative control group were treated with 10 ng/mL TNF-α for 24 h. The total RNA was extracted with the RNA extraction kit (Fastagen, Shanghai, China) and transcribed to cDNA by PrimeScript reverse transcription kit (Takara, Shiga, Japan). qPCR was carried out by SYBR Green (Takara, Shiga, Japan) on a Fluorescence quantitative system (Roche, Basel, Switzerland). The following primer sequences were used: IL-1β forward, 5′- TTCGACACATGGGATAACGAGG-3′,and reverse, 5′- TTTTTGCTGTGAGTCCCGGAG-3′; IL-6 forward 5′- CCTTCGGTCCAGTTGCCTTCTC-3′, and reverse 5′- AGAGGTGAGTGGCTGTCTGTGT-3′; IL-8 forward 5′- ACTGAGAGTGATTGAGAGTGGAC-3′, and reverse 5′- AACCCTCTGCACCCAGTTTTC-3′; GAPDH forward, 5′- CATGAGAAGTATGACAACAGCCT-3′, and reverse, 5′- AGTCCTTCCACGATACCAAAGT-3′. The relative mRNA level was calculated by the 2−ΔΔCt method.
Measurement of Inflammatory Cytokines Release
The supernatants containing cell-infected RA-FLSs were treated in the presence or absence of 10 ng/mL TNF-α(Propotec, USA) for 24 h and then analyzed with ELISA to determine the levels of IL-1β, IL-6, and IL-8. The serums obtained from CIA mice were examined by the Multi-Analyte Flow Assay Kit (Biolegend, USA).
Western Blot
Proteins isolated from the synovial tissues and infected RA-FLSs were resolved by the SDS-PAGE gels electrophoresis and then electrotransferred to PVDF membranes. Then the membranes were blocked with 5% fat-free milk and then treated with corresponding primary and secondary antibodies. Next, the blots were exposed to the enhanced chemiluminescence detection system (GE, USA). The following antibodies were used: HRP-conjugated secondary antibody and GAPDH antibodies (Servicebio, Wuhan, China); antibody of p65, p-p65, IκB, p-IκB, IKK-α (Cell Signaling (Beverly, MA, USA)); antibody of C-myc (Millipore, Boston, MA, USA); antibody of β-catenin (Cambridge, MA, USA); and antibody of ZNRF3 (Biorbyt, Cambridge, England).
Animal Studies
Twenty-two SPF male 6 weeks old DBA/1 mice were purchased from SLRC Laboratory Animals (Shanghai, China). All animal procedures and handling were approved by the Animal Care and Use Committee of Changhai hospital. Six mice were assigned to the normal group, two mice were infected with lentivirus, and 14 were used to establish the collagen-induced arthritis (CIA) as previously described [17]. The Cri Maestro in-vivo Imaging System (Caliper Life Sciences) was employed to determine the efficiency of the lentiviral transfection into the knee joint of mice. Clinical arthritis was observed about a week after the second immunization. Twelve typical arthritic mice were selected from the mice with CIA according to the severity score of arthritis [17] and categorized into 2 groups on the 28th day. Sh-ZNRF3 and Sh-ctr were administered separately into the knee joints of mice of Sh-ZNRF3 and Sh-ctr groups once a week for 3 weeks. Finally, the mice were euthanized on the 49th.
Histopathology of Mice
Knee joints were fixed in 4% paraformaldehyde for 48 h followed by decalcification in 14% EDTA glycerol at 37 °C for 30 days in couveuse. H&E staining was performed to examine the changes and extent of synovitis according to the reports [18, 19].
Data Analysis
Data are shown as mean ± SD and were analyzed with SPSS 19.0. Differences between two and three groups were assessed by t-test and one-way analysis of variance (ANOVA), respectively. P-values < 0.05 were considered statistically significant *P < 0.05, **P < 0.01.
ZNRF3 Expression Is Increased in Synovial Tissues and FLSs of RA Patients
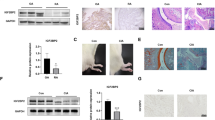
The immunohistochemical (Fig. 1a,b) and western blotting (Fig. 1c,d) assays were employed to measure the ZNRF3 expression in the synovial specimen from RA patients to Trauma patients. Further, western blotting (Fig. 1e,f) and immunofluorescence (Fig. 1g) were performed to quantify the expression of ZNRF3 in FLSs of the two groups. The results indicated that ZNRF3 was localized in the plasma membrane, and it was elevated in the synovial tissues and FLSs of RA group compared to the trauma group.
The expression of ZNRF3 in synovial and FLSs samples from RA patient. a Typical images of immunohistochemistry which was used to detect the expression of ZNRF3 in synovial extracts from trauma (n = 15) and RA (n = 10) patients. b The scores of immunohistochemistry in trauma group was lower in RA group. c Western blot results for GAPDH, ZNRF3 of synovial tissues from trauma(n = 6) and RA (n = 6) patients. d Analysis of protein levels shown above. e Western blot results for GAPDH, ZNRF3 of FLSs from trauma (n = 4) and RA (n = 4) patients. f the protein expression levels shown above. g Immunofluorescence was used to observe the expression of ZNRF3 in FLSs of RA and trauma patients. (n = 3).
Infection of RA-FLSs with Sh-ZNRF3
The infection rate of RA-FLSs with Sh-ZNRF3 was approximately 60% when infected at the MOI of 30 for 48 h (Fig. 2a). Five days after infection, the effect of lentivirus transduction was examined by the western blot. Results showed that the expression of ZNRF3 was significantly decreased in RA-FLSs compared with the NC group and Sh-ctr group (Fig. 2b,c).
Sh-ZNRF3 Increases Apoptosis and Decreases the Proliferation of RA-FLS
The effect of Sh-ZNRF3 on RA-FLS viability was determined by the CCK-8 assay. The results showed that, compared to the Sh-ctr group, the cell viability of Sh-ZNRF3 group was decreased. This inhibitory effect exists after treatment with 10 ng/ml TNF-α (Fig. 3a). The apoptosis was examined by flow cytometry. It was found that the proportion of apoptotic cells in Sh-ZNRF3 group exceeded that of the V-ctr group (Fig. 3b,c).
Impact of Sh-ZNRF3 on apoptosis, viability, and inflammation of RA-FLS. a The results of CCK-8 assay revealed that the cell viability in Sh-ZNRF3 group was lower compared with Sh-ctr group. b The typical image of apoptosis in RA-FLSs. c Results of apoptosis rate showed that Sh-ZNRF3 induced apoptosis in RA-FLSs. The relative mRNA d and protein levels e of IL-1β, IL-6 and IL-8. (n = 3).
TNF-α-Stimulated Sh-ZNRF3 Inhibited the Release of Inflammatory Cytokines from RA-FLSs
The release of inflammatory cytokines from RA-FLSs promotes the development of RA [20]. ELISA and qPCR assays were performed to assess the impact of Sh-ZNRF3 on the proteins and genes associated with inflammation, respectively. Data analysis revealed that the level of IL-8, IL-1β, and IL-6 in the TNF-α-stimulated Sh-ZNRF3 cells was lower than those in the TNF-α-stimulated Sh-ctr group (Fig. 3d).
Sh-ZNRF3 Inhibits the Cell Migration of RA-FLSs
The migration of RA-FLSs cells promotes tissue destruction [21]. Moreover, RA-FLS migration and invasion are important characteristics that distinguish it from FLS. The effects of ZNRF3 on cell migration were determined using the wound healing assay (Fig. 4a,b) and the Transwell cell migration assays (Fig. 4c,d), respectively, at two-dimensional and three-dimensional levels. The results showed that cell migration was decreased in Sh-ZNRF3 compared to Sh-ctr group.
Sh-ZNRF3 Alleviates Inflammation and Prevents Knee Joint Destruction in CIA Mice
In this study, a CIA mouse model was developed to explore the therapeutic effect of Sh-ZNRF3 in vivo. As shown in Fig. 5a, the lentivirus (3.19 × 108 IU/mL) successfully infected mice knee joint as evidenced by the high expression of ZNRF3 in the synovium (Fig.5b). Figure 5c shows the typical histopathological lesions in the knee joints. Results revealed that the number of infiltrating inflammatory cells, the level of synovial hyperplasia, pannus formation, and the narrowing of the joint space were smaller in the CIA mice injected with Sh-ZNRF3 compared to CIA mice injected with Sh-ctr. Finally, the Multi-Analyte Flow Assay revealed that the serum levels of TNF-α, IL-6, and IL-1β were significantly decreased in Sh-ZNRF3 group compared to the Sh-ctr group. (Fig. 5d).
The effect of Sh-ZNRF3 on CIA mice. a The expression of Sh-ZNRF3 in the knee joint in vivo imaging(right knee injected with Sh-ZNRF3, left knee injected with PBS). b The expression of ZNRF3 in the knee joint detected by WB. c The typical H&E images of knee joint (yellow arrow stands for inflammatory cell infiltration, green arrow stands for joint space, and blue arrow for synovial hyperplasia and pannus). d The protein levels of IL-1β, IL-6, and IL-8 in the serum were tested by Multi-Analyte Flow Assay Kit. (n = 3).
Sh-ZNRF3 Plays a Role in RA-FLSs by Regulating Wnt/β-Catenin and NF-κB Pathways
Having found that ZNRF3 knockdown eliminates inflammation both in vivo and in vitro, the impact of Sh-ZNRF3 on NF-κB and Wnt signaling in RA-FLS was examined. It was found that in the Wnt pathway, Sh-ZNRF3 upregulated the expression of p-GSK3β but downregulated the expression of β-catenin and C-myc (Fig. 6a,b). In the NF-κB pathway, Sh-ZNRF3 downregulated the phosphorylated IκBα, phosphorylated p65, and IKKα protein expression (Fig. 6c,d).
Effects of Sh-ZNRF3 on Wnt and NF-κB pathways activation in RA-FLS. All the cells were treated in the presence or absence of 10 ng/mL TNF-α for 24 h before Western blotting. a The protein level of β-catenin, GAPDH, and C-myc. b Quantitative data of the protein levels of GAPDH, β-catenin, and C-myc. c The protein expression of phospho-p65, phospho-IκBα, IκBα, GAPDH, p65, and IKKα. d the protein levels of phospho-p65, phospho-IκBα, and IKKα (n = 3).
DISCUSSION
ZNRF3 is a E3 ubiquitin ligase that contains an intracellular RING domain, a transmembrane domain, an extracellular domain, and a signal peptide [22]. ZNRF3 and its homolog RNF43 have a sequence similarity of up to 39% [8]. Previous studies have demonstrated that ZNRF3 inhibits Wnt/β-catenin signaling cascade [7, 8]. When the Wnt pathway is activated, it enhances the expression of β-catenin and promotes the transcription of TCF/LEF-1. ZNRF3 is released in the cytoplasm where it binds FZD on the plasma membrane, thereby inhibiting Wnt signaling [7, 9].
Wnt signaling pathway is a conserved pathway that modulates the mature tissue homeostasis and embryonic development in all large animals. It is also involved in cell differentiation, migration, adhesion, and proliferation. A number of studies have shown that β-catenin is highly expressed in the synovial tissues and FLSs of RA patients, implying that Wnt pathways may be abnormally activated in the synovitis of RA [23, 24]. Therefore, we selected ZNRF3 as a target gene of this pathway in this study.
Results showed that ZNRF3 was highly expressed in the synovial tissues and FLS of RA patient. ZNRF3 inhibited the Wnt/β-catenin pathway, whereas Wnt pathway was overactivated in RA synovitis. Hence, we speculated that the increase in ZNRF3 expression occurred via a negative feedback mechanism. Unexpectedly, we observed that the knocked down of ZNRF3 (Sh-ZNRF3) decreased cells proliferation, increased apoptosis, inhibited migration, and alleviated inflammation in RA-FLS, indicating its therapeutic effect on CIA mice. Mechanistically, Sh-ZNRF3 increased the expression of p-GSK3β but decreased the expression of c-Myc and β-catenin. Further tests showed that Sh-ZNRF3 inhibited the NF-κB signaling cascade in RA-FLS.
NF-κB plays a role in inflammation, proliferation, and apoptosis of RA and is stimulated by TNF-α [25, 26]. Suppressing the activation of NF-κB alleviates inflammation both in FLSs and animal models of RA [13, 16, 27]. Several studies have demonstrated that there is a crosstalk between the Wnt/β-catenin and the NF-κB pathway [28]. NF-κB signal pathway activates Wnt/ β-catenin pathway by fine-tuning the expression of genes related to this pathway and the activity of IKK-α [29,30,31]. IKK-α increases the turnover of β-catenin [29] and up-regulates the expression of beta-catenin/TCF [30]. Here, we speculated that in RA-FLSs, Sh-ZNRF3 promoted the ubiquitination mediated β-catenin breakdown by suppressing IKKα and down-regulating the expression of β-catenin and its target genes. In addition, Sh-ZNRF3 inhibited the degradation of phosphorylated IκB and p65 by inhibiting the activity of IKK-α, leading to the suppression of NF-κB signaling. This may explain the protective role of Sh-ZNRF3 observed in vivo and in vitro.
Taken together, this study shows that ZNRF3 protects against RA by regulating the crosstalk between Wnt/ β-catenin and NF-κB signaling pathways. Therefore, Sh-ZNRF3 might be a potential therapeutic target for RA patients. Other unidentified mechanisms that orchestrate the regulatory role of ZNRF3 on RA remain to be elucidated in future studies.
References
Firestein, G.S. 1996. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors. Arthritis and Rheumatism 39 (11): 1781–1790.
Müller-Ladner, U., J. Kriegsmann, B.N. Franklin, S. Matsumoto, T. Geiler, R.E. Gay, and S. Gay. 1996. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. The American Journal of Pathology 149 (5): 1607–1615.
Alsaleh, G., A. François, A.M. Knapp, J.N. Schickel, J. Sibilia, J.L. Pasquali, J.E. Gottenberg, D. Wachsmann, and P. Soulas-Sprauel. 2011. Synovial fibroblasts promote immunoglobulin class switching by a mechanism involving BAFF. European Journal of Immunology 41 (7): 2113–2122.
Quirke, A.M., B.A. Fisher, A.J. Kinloch, and P.J. Venables. 2011. Citrullination of autoantigens: Upstream of TNFα in the pathogenesis of rheumatoid arthritis. FEBS Letters 585 (23): 3681–3688.
Sen, M. 2005. Wnt signalling in rheumatoid arthritis. Rheumatology (Oxford, England) 44 (6): 708–713.
Miao, C.G., Y.Y. Yang, X. He, X.F. Li, C. Huang, Y. Huang, L. Zhang, X.W. Lv, Y. Jin, and J. Li. 2013. Wnt signaling pathway in rheumatoid arthritis, with special emphasis on the different roles in synovial inflammation and bone remodeling. Cellular Signalling 25 (10): 2069–2078.
Hao, H.X., Y. Xie, Y. Zhang, et al. 2012. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 485 (7397): 195–200.
Serra, S., and R. Chetty. 2018. Rnf43. Journal of Clinical Pathology 71 (1): 1–6.
Hao, H.X., X. Jiang, and F. Cong. 2016. Control of Wnt Receptor Turnover by R-spondin-ZNRF3/RNF43 Signaling Module and Its Dysregulation in Cancer. Cancers (Basel) 8 (6).
Aletaha, D., T. Neogi, A.J. Silman, et al. 2010. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European league against rheumatism collaborative initiative. Arthritis and Rheumatism 62 (9): 2569–2581.
Yamada, S., S. Itai, T. Nakamura, et al. 2019. Immunohistochemical analysis of the harbor porpoise using Antipodoplanin antibody PMab-237. Monoclonal Antibodies in Immunodiagnosis and Immunotherapy.
Liu, X.Z., J. Fan, K. Qi, S.P. Liu, W.D. Xu, Y. Gao, X.D. Gu, J. Li, C.G. Bai, Y.Q. Shi, L.L. Zhang, and D.B. Zhao. 2017. Dishevelled2 promotes apoptosis and inhibits inflammatory cytokine secretion in rheumatoid arthritis fibroblast-like synoviocytes through crosstalk with the NF-κB pathway. Oncotarget. 8 (8): 12649–12663.
Liang, J.J., H.R. Li, Y. Chen, C. Zhang, D.G. Chen, Z.C. Liang, Y.Q. Shi, L.L. Zhang, L. Xin, and D.B. Zhao. 2019. Diallyl Trisulfide can induce fibroblast-like synovial apoptosis and has a therapeutic effect on collagen-induced arthritis in mice via blocking NF-κB and Wnt pathways. International Immunopharmacology 71: 132–138.
Greven, D.E., E.S. Cohen, D.M. Gerlag, J. Campbell, J. Woods, N. Davis, A. van Nieuwenhuijze, A. Lewis, S. Heasmen, M. McCourt, D. Corkill, A. Dodd, J. Elvin, G. Statache, I.P. Wicks, I.K. Anderson, A. Nash, M.A. Sleeman, and P.P. Tak. 2015. Preclinical characterisation of the GM-CSF receptor as a therapeutic target in rheumatoid arthritis. Annals of the Rheumatic Diseases 74 (10): 1924–1930.
Baier, A., I. Meineckel, S. Gay, and T. Pap. 2003. Apoptosis in rheumatoid arthritis. Current Opinion in Rheumatology 15 (3): 274–279.
Li, G., Z. Xia, Y. Liu, et al. 2018. SIRT1 inhibits rheumatoid arthritis fibroblast-like synoviocyte aggressiveness and inflammatory response via suppressing NF-κB pathway. Bioscience Reports 38 (3).
Brand, D.D., K.A. Latham, and E.F. Rosloniec. 2007. Collagen-induced arthritis. Nature Protocols 2 (5): 1269–1275.
Camps, M., T. Rückle, H. Ji, V. Ardissone, F. Rintelen, J. Shaw, C. Ferrandi, C. Chabert, C. Gillieron, B. Françon, T. Martin, D. Gretener, D. Perrin, D. Leroy, P.A. Vitte, E. Hirsch, M.P. Wymann, R. Cirillo, M.K. Schwarz, and C. Rommel. 2005. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nature Medicine 11 (9): 936–943.
Yan, X., Y. Cen, and Q. Wang. 2016. Mesenchymal stem cells alleviate experimental rheumatoid arthritis through microRNA-regulated IκB expression. Scientific Reports 6: 28915.
McInnes, I.B., and G. Schett. 2007. Cytokines in the pathogenesis of rheumatoid arthritis. Nature Reviews. Immunology 7 (6): 429–442.
Bustamante, M.F., R. Garcia-Carbonell, K.D. Whisenant, and M. Guma. 2017. Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Research & Therapy 19 (1): 110.
Zebisch, M., Y. Xu, C. Krastev, et al. 2013. Structural and molecular basis of ZNRF3/RNF43 transmembrane ubiquitin ligase inhibition by the Wnt agonist R-spondin. Nature Communications 4: 2787.
Sen, M., K. Lauterbach, H. El-Gabalawy, G.S. Firestein, M. Corr, and D.A. Carson. 2000. Expression and function of wingless and frizzled homologs in rheumatoid arthritis. Proceedings of the National Academy of Sciences of the United States of America 97 (6): 2791–2796.
Xiao, C.Y., Y.F. Pan, X.H. Guo, Y.Q. Wu, J.R. Gu, and D.Z. Cai. 2011. Expression of β-catenin in rheumatoid arthritis fibroblast-like synoviocytes. Scandinavian Journal of Rheumatology 40 (1): 26–33.
Xu, H., Y. He, X. Yang, et al. 2007. Anti-malarial agent artesunate inhibits TNF-alpha-induced production of proinflammatory cytokines via inhibition of NF-kappaB and PI3 kinase/Akt signal pathway in human rheumatoid arthritis fibroblast-like synoviocytes. Rheumatology (Oxford) 46 (6): 920–926.
Guo, X., Y. Pan, C. Xiao, Y. Wu, D. Cai, and J. Gu. 2012. Fractalkine stimulates cell growth and increases its expression via NF-κB pathway in RA-FLS. International Journal of Rheumatic Diseases 15 (3): 322–329.
Wu, S., Y. Li, Y. Li, L. Yao, T. Lin, S. Jiang, H. Shen, L. Xia, and J. Lu. 2016. Interleukin-35 attenuates collagen-induced arthritis through suppression of vascular endothelial growth factor and its receptors. International Immunopharmacology 34: 71–77.
Ma, B., and M.O. Hottiger. 2016. Crosstalk between Wnt/β-catenin and NF-κB signaling pathway during inflammation. Frontiers in Immunology 7: 378.
Lamberti, C., K.M. Lin, Y. Yamamoto, U. Verma, I.M. Verma, S. Byers, and R.B. Gaynor. 2001. Regulation of beta-catenin function by the IkappaB kinases. The Journal of Biological Chemistry 276 (45): 42276–42286.
Albanese, C., K. Wu, M. D'Amico, et al. 2003. IKKalpha regulates mitogenic signaling through transcriptional induction of cyclin D1 via Tcf. Molecular Biology of the Cell 14 (2): 585–599.
Carayol, N., and C.Y. Wang. 2006. IKKalpha stabilizes cytosolic beta-catenin by inhibiting both canonical and non-canonical degradation pathways. Cellular Signalling 18 (11): 1941–1946.
Acknowledgements
The authors thank Guo Xian and all the teachers in Experimental Center, Changhai Hospital for their valuable assistance.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81671595 and 81471607).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing and Conflicting Interests
The authors declare that there is no conflict of interest associated with this work.
Disclosures
None
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
All procedures performed in the studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (Changhai hospital+ No. CHEC2017159).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liang, J.J., Li, H.R., Chen, Y. et al. ZNRF3 Regulates Collagen-Induced Arthritis Through NF-kB and Wnt Pathways. Inflammation 43, 1077–1087 (2020). https://doi.org/10.1007/s10753-020-01193-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01193-1