Abstract
Disordered glucose and lipid metabolism contributes to the progression of several liver diseases, while the upregulation of phosphatase and tensin homology deleted on chromosome ten (PTEN), a well-known tumour suppressor gene, can improve the condition through metabolic programming. This study first characterized the metabolic profiles and the involvement of PTEN in the hepatic fibrosis induced by Schistosoma japonicum (S. japonicum) to provide a novel clue for metabolism-targeted treatment. Compared with control mice, infected mice showed infiltrated immune cells in their livers, increased levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) and decreased glucose levels in their sera. The expression of key enzymes in the glycolytic pathway was significantly increased, and the expression of gluconeogenic genes was distinctly decreased. Moreover, the infection upregulated the hepatic expression of enzymes involved in fatty acid oxidation, which was consistent with the decreased number of lipid droplets in livers and the lowered levels of triglyceride in sera. Consistently, PTEN and its downstream signalling were significantly inhibited. In vitro, soluble egg antigen (SEA) downregulated the expression of PTEN in both the macrophage RAW264.7 cell line and the murine hepatocellular carcinoma HEP1-6 cell line, and induced a metabolic phenotype similar to the in vivo results. Overall, this study showed that S. japonicum infection induced the reprogramming of glucose and lipid metabolism in mice during the period of liver fibrosis and that SEA could act as a modulator to trigger such a metabolic switch in macrophages and hepatocytes. PTEN might play an essential role in mediating these metabolic reprogramming events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Schistosomiasis is one of the neglected tropical disease (NTDS) caused by the infection of schistosomes, including Schistosoma japonicum (S. japonicum) [1]. It is still a serious public health problem, threatening the health of approximately 800 million people in 78 countries and regions [2]. The soluble egg antigen (SEA) secreted by insect eggs is the main pathogenic substance, and the most serious pathological effect of S. japonicum is liver fibrosis caused by the egg-induced granulomatous inflammatory response, which results in the development of liver cirrhosis and portal hypertension [3,4,5]. Praziquantel, a clinical drug for treating schistosomiasis, can effectively kill adult worms but has less of an impact on widespread liver fibrosis [6]. Therefore, it is urgent to explore new strategies for treating hepatic fibrosis caused by S. japonicum.
In recent years, a series of important advances have been made in studying the mechanism of hepatic fibrosis caused by S. japonicum. It has been demonstrated that hepatic stellate cells (HSCs), macrophages and hepatocytes are involved in the development of hepatic fibrosis [7,8,9,10]. Activated HSCs are responsible for the production of collagen and fibrogenesis within granuloma sites [7], and liver fibrosis can be improved by inhibiting the activation of HSCs [8]. Moreover, upregulated Wnt signalling in hepatocytes has been shown to be associated with liver fibrosis caused by S. japonicum [9]. In addition, the alternatively activated macrophage (M2) polarization of macrophages is classically believed to promote schistosomiasis hepatic fibrosis, and inhibiting notch signalling can attenuate progression by blocking macrophage M2 polarization [10].
Recently, immunology and metabolism have intersected to create the burgeoning field of immunometabolism [11]. This novel discipline has demonstrated that metabolic reprogramming events play an essential role in organ function and immune cell differentiation [12, 13], which provides a new direction for clarifying disease pathogenesis and developing intervention strategies. Metabolic alternation has been demonstrated to affect liver function and macrophage functional differentiation [14,15,16]. The liver is an important metabolic organ, and its metabolic disorder has been found to be associated with many diseases, such as chronic alcoholic fatty liver and liver cancers [14, 16]. As an important innate immune cell, M2-type macrophages were found to promote the progression of liver fibrosis induced by CCl4 [17]. Interestingly, M2 macrophages exhibit distinguishing metabolic characteristics that differentiate them from classically activated macrophage (M1) macrophages [18]. This suggests that targeting the metabolism of macrophages may be an attractive way to explore novel treatment strategies. In fact, it has been reported that S. japonicum infection increased whole-body and hepatic insulin sensitivity in mice while praziquantel chemotherapy significantly improved physiological status [19]. Thus, this study speculated that specific metabolic alternation may occur in hepatocytes or macrophages in the process of liver fibrosis induced by S. japonicum, thereby promoting the progression of the disease.
Phosphatase and tensin homology deleted on chromosome ten (PTEN), a member of the protein tyrosine phosphatase (PTP) gene family, is located at 10q23.3 [20]. As a tumour suppressor gene, the downregulation of PTEN is widely involved in the development and progression of cancer [21]. PTEN also participates in regulating glucose and lipid metabolism [22]. For example, PTEN liver-specific knockout (KO) mice are prone to developing fatty liver, with increased triglyceride content and reduced apolipoprotein B (ApoB) protein mass [23]. Moreover, elevated PTEN could inhibit the progression of tumour by inhibiting the glycolysis process, the characteristic metabolic mode of tumour cells [24], and PTEN could also regulate the M2 polarization of macrophages to participate in hepatic fibrosis induced by CCl4 [17]. Interestingly, PTEN expression was decreased in schistosomal urothelial and squamous cell carcinomas and might result in disease progression [25]. Overall, these studies imply that PTEN plays an essential role in regulating immune function and remodelling metabolism. However, whether PTEN and its downstream metabolic pathway are involved in the process of hepatic fibrosis induced by S. japonicum remains elusive.
This study first characterized the metabolic phenotype profiles and the involvement of PTEN in hepatic fibrosis induced by S. japonicum. The results showed that parasitic infection induced the reprogramming of glucose and lipid metabolism in the livers of mice. Consistently, the expression of PTEN was downregulated in infected mice. Notably, SEA could act as a modulator to induce the metabolic switch in macrophages and hepatocytes, which was similar to the in vivo results. These findings might provide novel information for elaborating the mechanism of liver fibrosis caused by the parasite and developing a metabolism-targeted treatment strategy.
MATERIALS AND METHODS
Mice and Parasites
Female BALB/c mice were obtained from Shanghai Laboratory Animal Center (SLAC, Shanghai, China) and were bred in the University facilities. All mice were housed in an air-conditioned room at 24 °C with a 12 h dark/light cycle and permitted free access to standard laboratory food and water. Oncomelania hupensis snails were provided by the National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention.
Animal Treatment
Female BALB/c mice were sacrificed in all experiments. They were randomly divided into 2 groups: a control group and an infected group. For the infected group, mice were percutaneously infected with 20 cercariae of S. japonicum. Nine weeks after infection, the liver fibrosis model was successfully established according to Zhu et al.’s study [26]. The liver tissues and sera were collected and stored at − 80 °C. Parts of the liver tissues were fixed in 4% formaldehyde solution for pathological analysis.
Quantitative Real-Time PCR
Total RNA was extracted from pulverized mouse liver tissues with TRIzol reagent, and cDNA was synthesized from the RNA using PrimeScript™ RT Master Mix. Quantitative PCR analyses were performed in a LightCycler® 480II detection system (Roche Applied Science, Penzberg, Germany) under the following thermal cycler conditions: one cycle of 5 min denaturation at 95 °C and then 30 s at 95 °C, 30 s at 60 °C and 30 s at 72 °C for 45 cycles using the primers listed in Table 1. mRNA levels for specific genes were normalized by β-actin mRNA levels.
Western Blot Analysis
Total protein was extracted from mouse liver tissues, and the concentration was determined with a bicinchoninic acid protein concentration assay kit (Beyotime Biotech, Beijing, China). Sample protein was separated by electrophoresis in 10% SDS-PAGE and separated proteins were transferred to polyvinylidene difluoride membranes with a Bio-Rad electrophoresis system (Hercules, CA, USA). The indicated primary antibodies used are listed in Table 2. The bands were scanned and analysed using Quantity ONE software (Bio-Rad, Hercules, CA, USA). The expression of protein in each sample was normalized to β-actin.
Histopathological Examination
The sections of liver tissues were fixed in 4% paraformaldehyde, embedded in paraffin and sectioned (5 mm). Haematoxylin and eosin (H&E) staining and Masson staining were performed according to the standard procedure. For lipid deposition, liver tissues were embedded, frozen and sectioned (15 mm), and the sections were stained with Oil-Red O solution.
Preparation of SEA
Eggs were obtained from the livers of mice infected with S. japonicum for 9 weeks, and the preparation method of SEA was described by Zhu et al. [26]. The infected mice were perfused, and their livers were collected and rinsed with LPS-free phosphate-buffered saline (PBS). Liver homogenate was filtered, washed and centrifuged at 12,000 rpm for 15 min. Eggs were suspended in PBS containing 1 mM phenylmethylsulfonyl fluoride (PMSF) (Roche Diagnostics) and 2 μg/ml leupeptin (Sigma) and homogenized on ice using a homogenizer (VirTis Co.). The suspension was frozen/thawed several times and centrifuged at 12000 rpm for 30 min at 4 °C. The supernatant was passed through a 0.22-μm filter and used as SEA. The protein concentration was determined by a bicinchoninic acid (BCA) protein assay kit (Beyotime Biotech, Beijing, China).
Cell Culture and Stimulation
The mouse hepatocarcinoma cell line HEP1-6 and the macrophage cell line RAW264.7 were purchased from Shanghai Cell Bank, Chinese Academy of Sciences, and were maintained in our laboratory. Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (glutamine, high glucose) supplemented with penicillin (100 units/mL), streptomycin (100 μg/mL) and 10% heat-inactivated foetal bovine serum at 37 °C with 5% CO2. For HEP1-6 cells, cells were incubated with SEA (20 μg/mL) or its vehicle for 24 h; RAW264.7 cells were challenged with SEA (6 μg/ml) alone, the PTEN activator polyene phosphatidyl choline (PPC) (15 μg/ml) alone or co-treatment with both of them for 24 h.
Biochemical Analyses of Serum Parameters
The levels of triacylglycerol (TG), AST and ALT in the sera were measured using commercial assay kits according to the instructions (Jiancheng Biological Engineering Institute, Nanjing, China). The plasma glucose concentrations from control and infected mice were measured with a Glucometer Elite monitor.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism software 5.0. The results are presented as the mean ± standard error of mean (SEM). Data were analysed using SPSS 16.0, and all data of the control and infected groups were consistent with the features of a normal distribution. Differences between two groups were examined for statistical significance using the independent sample t test. Differences for multiple comparisons were compared using one-way analysis of variance (ANOVA) (Tukey’s test or LSD test). P values < 0.05 were considered statistically significant.
RESULTS
The Establishment and Evaluation of Hepatic Fibrosis Induced by S. japonicum
To explore the mechanism of liver fibrosis induced by S. japonicum, we established models in BALB/c mice and evaluated their hepatic fibrosis. As shown in Supplementary Fig. 1, compared with control mice, the ratio of liver/body weight in infected mice was significantly elevated 9 weeks after infection (t = − 5.021, P < 0.05, Supplementary Fig. 1a). The livers of the infected group were slightly enlarged, obviously blackened and slightly hard (Supplementary Fig. 1b). H&E staining showed that a large number of inflammatory cells were infiltrated in the livers of the infected group, and hepatocytes underwent varying degrees of denaturation and necrosis and were arranged in disorder (Supplementary Fig. 1c). Masson staining showed that large amounts of collagen and eggs were deposited in the hepatic catchment area (Supplementary Fig. 1d). Concomitantly, the levels of ALT and AST in the sera of infected mice were significantly elevated (t = − 3.593, − 3.139, P < 0.01, Supplementary Fig. 1e). The above results suggested that the liver fibrosis model was successfully established, which laid a basis for further evaluating the metabolic profiles of livers.
The Glycolysis Pathway Was Significantly Activated in Hepatic Fibrosis Induced by S. japonicum
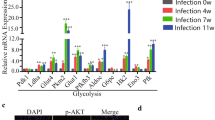
Glucose metabolism is an important function of the liver and can provide energy for life activities. This study speculated that several pathways of glucose metabolism in livers might be disordered in the liver due to serious lesions caused by S. japonicum. To address this question, this study detected the expression of key enzymes involved in the glucose transporter, glycolysis and gluconeogenesis pathways and the tricarboxylic acid cycle (TCA). Glucose transporter 4 (GLUT4) is the main protein assisting in the glucose transport in adipocytes and skeletal muscle cells [27]. We found that the expression of GLUT4 became much higher in the infected group (t = − 4.055, − 4.564, P < 0.05, Fig. 1a and b). Consistently, the expression of glycolytic enzymes (M2 subtype pyruvate kinase, PKM2; 6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase 3, PFKFB3; pyruvate kinase, PK) in the infected group was significantly higher than that in the control group (t = − 4.639, − 2.142, − 4.451, P < 0.05, Fig. 1a and b). However, the gluconeogenic genes (peroxisome proliferator–activated receptor-γ coactivator-1α, PGC-1α; glucose-6-phosphatase, G6PC) were significantly downregulated in the infected group (t = − 6.926, − 4.400, P < 0.05, Fig. 1c and d). These results indicated that the glucose transporter and glycolysis pathways were activated while gluconeogenesis was inactivated during liver fibrosis induced by S. japonicum.
Glucose metabolism was enhanced in the hepatic fibrosis induced by S. japonicum. Livers were collected from control and infected mice, and glucose metabolism–related genes were determined by western blot and real-time RT-PCR. a The relative protein levels of GLUT4, PKM2 and PFKFB3 (n = 4). b The relative mRNA levels of GLUT4, PK and PFK (n = 6). c The protein expression level of PGC-1α (n = 4). d The relative mRNA levels of G6PC (n = 6). e The relative mRNA levels of CS and IDH3 (n = 6). The differences were analysed using the independent sample t test. Asterisks indicate statistically significant differences between groups. *P < 0.05; **P < 0.01; ***P < 0.001.
On the other hand, citrate synthase (CS) and isocitrate dehydrogenase 3 (IDH3) (the key enzymes controlling the tricarboxylic acid cycle) in the infected group were found to be distinctly lower in comparison to levels in the control group (t = 4.148, 10.848, P < 0.001, Fig. 1e). In combination with the downregulation of PGC-1α, these results suggest that oxidative phosphorylation was inhibited in the progression of hepatic fibrosis.
Fatty Acid Oxidation Was Significantly Enhanced in Hepatic Fibrosis Induced by S. japonicum
Lipid metabolism is another function of the liver in the maintenance of life activities. This study further characterized the profiles of lipid metabolism in S. japonicum–induced liver fibrosis by determining the expression of enzymes related to mitochondrial and peroxisomal fatty acid oxidation or lipogenic pathways. As shown in Fig. 2 a, compared with the control group, infected mice showed a higher expression of fatty acid oxidases (cytochrome P450 proteins 4, CYP4; medium-chain acyl-CoA dehydrogenase, MCAD) (t = − 3.583, − 4.838, P < 0.01) and a much lower expression of liver-fatty acid binding protein 10 (L-FABP10) (t = 4.662, P < 0.001). However, the expression of PPAR-α (peroxisome proliferator–activated receptor alpha) had no obvious alteration, and carnitine palmitoyl transferase 1a (CTP-1a) was significantly downregulated (t = 3.908, P < 0.05) (Fig. 2b and c). On the other hand, the expression of lipogenic genes, such as sterol regulatory element-binding protein-1c (SREBP-1c), was downregulated, while acetyl coenzyme A carboxylase 1 (ACC1) and stearoyl-CoA desaturase 1 (SCD1) were significantly upregulated (t = 2.950, − 2.260, − 10.608, P < 0.05) (Fig. 2d and e).
Lipid metabolism was disordered and lipid droplets were decreased in the hepatic fibrosis induced by S. japonicum. Livers were collected from control and infected mice and lipid metabolism–related genes were determined by western blot and real-time RT-PCR. a The mRNA expression levels of CYP4, MCAD and L-FABP10 (n = 6). b The protein levels of PPARα and CPT-1a (n = 4). c The protein and mRNA levels of SREBP-1c, ACC1, FAS and SCD1 (n = 6). d The mRNA levels of SREBP-1c (n = 6). e Representative liver images of Oil-Red O staining. f The altered levels of triglyceride in the sera (n = 15). The differences were analysed using the independent sample t test. Asterisks indicate statistically significant differences between groups. *P < 0.05; **P < 0.01; ***P < 0.001.
Collectively, the above results showed that both the pathways of fatty acid oxidation and lipid synthesis might be activated in S. japonicum–infected livers. However, fatty acid oxidation might be more dominant because there was a decrease in lipid droplet numbers as shown by Oil-Red O staining (Fig. 2f) and a reduced level of triacylglycerol (TG) in the infected sera (t = 6.403, P < 0.001, Fig. 2g).
The PTEN/PI3K/AKT Pathway Was Inactivated in the Hepatic Fibrosis Induced by S. japonicum
PTEN is a well-known tumour suppressor gene regulating the phosphoinositide 3-kinase (PI3K)/protein-serine-threonine kinase (AKT) pathway [28] and has been shown to be associated with the condition of liver fibrosis [29, 30]. There is accumulating evidence showing that PTEN can regulate the metabolism of glucose and lipids in cancer [23, 24]. This study further investigated whether PTEN and its downstream signalling were associated with the abovementioned metabolic pathways in the infected group.
As shown in Fig. 3 a and b, the expression levels of PTEN and PI3K (p85α and p110β subunits) were significantly downregulated in the infected group (t = 8.119, 3.898, 3.203, P < 0.05), while the expression of total AKT was slightly upregulated in comparison to those in the control mice. Nevertheless, p-AKT (Ser473), the activated form of AKT, was apparently inhibited (t = 5.064, P < 0.05), while p-AKT (Ser129) had no obvious change (Fig. 3a). Overall, the PTEN/PI3K/AKT pathway was inhibited in the progression of hepatic fibrosis induced by S. japonicum.
The PTEN/PI3K/AKT pathway was inactivated in the hepatic fibrosis induced by S. japonicum. Livers were collected from control and infected mice, and the signalling molecules were determined by western blot and real-time RT-PCR (n = 4~6 mice for each group). a The relative protein levels of PTEN, PI3K-p85α, PI3K-p110β, p-AKT (Ser129), p-AKT (Ser473), total AKT and c-Myc. c The relative mRNA expression of PTEN. d The concentration of glucose in sera. e The relative mRNA expression of HIF-1α. The differences were analysed using the independent sample t test. Asterisks indicate statistically significant differences between groups. *P < 0.05; **P < 0.01; ***P < 0.001.
Previous studies have reported that PTEN and the proto-oncogene cancer-Myc (c-Myc) are antagonistic to each other [31, 32] and that PTEN could regulate glucose metabolism [24]. Consistently, our study showed that the expression of c-Myc was greatly increased (t = − 8.170, P < 0.05, Fig. 3a). Moreover, the blood glucose levels of the infected group became much lower (t = 9.319, P < 0.05, Fig. 3c), showing the glucose metabolism disorder. These changes may force hepatocytes or immune cells to gain energy through the glycolysis pathway. Thus, this study further determined the expression level of hypoxia inducible factor-1α (HIF-1α), a factor that modulates key enzymes involved in aerobic glycolysis [33]. The results showed that HIF-1α expression was significantly elevated in the infected group (t = − 7.030, P < 0.001, Fig. 3c), suggesting a hypoxia microenvironment in the liver of infected mice.
SEA Induced a Similar Metabolic Phenotype in Macrophages and Hepatocytes
It is well known that SEA is a main pathogenic reagent that induces liver fibrosis by inducing M2 polarization of macrophages [10]. This study further determined whether SEA promotes such a specific metabolic switch in macrophages (RAW264.7 cells) in vitro, and several genes were chosen to confirm the results. As shown in Fig. 4 a, the expression of GLUT4, MCAD, ACC1, FAS and CYP4 was significantly upregulated (t = − 4.346, − 7.484, − 5.010, − 9.977, − 8.330, P < 0.05) while PTEN, CS and IDH3 were significantly downregulated after exposure to SEA compared with expression in the PBS group (t = 12.944, 5.094, 8.384, P < 0.05), which was consistent with our in vivo results.
SEA induced a similar metabolic reprogramming in macrophages and liver cells in vitro. The macrophage cell line RAW264.7 cells (a) and the hepatocarcinoma cell line HEP1-6 cells (b) were exposed to SEA for 24 h, and the mRNA expression of enzymes in metabolic pathways was determined by real-time RT-PCR. The differences were analysed using the independent sample t test. Asterisks indicate statistically significant differences between groups. *P < 0.05; **P < 0.01; ***P < 0.001.
Hepatocytes also participate in the process of hepatic fibrosis in S. japonicum. This study investigated whether SEA can induce the reprogramming of glucose and lipid metabolism in hepatocarcinoma cells (HEP1-6) in vitro. Interestingly, SEA could induce metabolic characterization in liver cells similar to the results in macrophages (Fig. 4b), except for CS expression. Therefore, these data supported that the in vivo metabolic phenotype might be induced by SEA.
DISCUSSION
This study investigated the potential metabolic reprogramming events in the progression of hepatic fibrosis induced by S. japonicum. The results showed that the glycolysis and fatty acid oxidation pathways were both activated. Interestingly, in vitro SEA could induce the metabolic phenotype in macrophages and hepatocytes, similar to the in vivo results. Consistently, PTEN, a negative regulator of glucose and lipid metabolism, was significantly inhibited both in vitro and in vivo. These results suggested the occurance of metabolic reprogramming in S. japonicum–induced hepatic fibrosis (Fig. 5).
Overview of metabolic reprogramming in the liver fibrosis induced by S. japonicum infection in mice. Fatty acid oxidation and glycolysis were activated while gluconeogenesis and oxidative phosphorylation were inhibited during fibrosis progression induced by S. japonicum. This specific metabolic reprogramming could be stimulated in both macrophages and liver cells by SEA. PTEN might play a central role in regulating this metabolic phenotype.
As an emerging field, immunometabolism has received increasing attention, as it mainly connects glucose and lipid metabolism to immunology [16] and is widely involved in the occurrence and development of diseases such as obesity and diabetes [34, 35]. However, the discipline was not highly appreciated in parasite infection. Since the liver is the central organ of metabolism, and is also an important pathological site of schistosomiasis, this study focused on the metabolic alternation of livers. Both the levels of ALT and AST were elevated, suggesting damage to the infected liver. Both glucose and lipid metabolism were enhanced based on the expression of rate-limiting enzymes in the infected group. In particular, the glycolysis and fatty acid oxidation pathways were significantly activated while oxidative phosphorylation was inhibited, which was consistent with the infiltrated immune cells, the decreased number of lipid droplets and the lowered level of triglyceride in infected livers. However, there were some divergences with regard to the enzyme expression of lipogenic genes, reflecting the feature of compensatory metabolism against liver damage. Overall, these results supported the metabolic disorder in S. japonicum–induced liver fibrosis.
Previous studies have shown that hepatocytes and macrophages are both involved in the progression of hepatic fibrosis [7,8,9,10]. In particular, it is well known that SEA secreted by eggs triggers the progression of liver fibrosis induced by S. japonicum through the M2 polarization of macrophages [10, 26]. We were therefore interested in whether the metabolic phenotype is induced by SEA or which type of cells is involved in the metabolic switch in infected liver. The results showed that the expression tendency of most genes could be induced by SEA in RAW264.7 macrophages and HEP1-6 hepatocytes (Fig. 4), although several other genes were not consistent with the in vivo results. This finding supported that SEA acts as a metabolic modulator during the process of liver fibrosis. Moreover, it was reported that M2 macrophages are characterized by an enhanced lipid oxidation pathway [36]. This study found that macrophages exposed to SEA exhibit the upregulation of lipid oxidation genes (MCAD, CYP4), which was consistent with the capability of SEA to induce M2 polarization [26].
As a classical tumour suppressor, PTEN has not only been involved in hepatic fibrosis [17, 37,38,39], but can also regulate glucose and lipid metabolism in cancer [24]. This study found that the PTEN/PI3K/AKT pathway was inhibited in the progression of hepatic fibrosis induced by S. japonicum. Classically, PTEN negatively regulates PI3K activity, and AKT is a key downstream effector molecule of PI3K, which can be phosphorylated and activated by PI3K [31, 40]. However, this study showed that the expression levels of both PI3K (p85α) and p-AKT (Ser473) were significantly decreased. The result seems to be beyond our expectations, but it is rational due to the serious damage in S. japonicum–induced liver fibrosis. In addition, given the fact that PTEN and c-Myc are antagonistic to each other [31], this study found upregulated expression of c-Myc in infected livers (Fig. 4a).
In general, this suggested that the downregulation of PTEN was closely associated with the disordered glucose and lipid metabolism. To further reveal the relationship, polyene phosphatidylcholine (PPC), a hepatica widely used in the clinic to treat liver disease and previously proven to upregulate the expression of PTEN in macrophages (data not published), was added to the RAW264.7 cell culture system. The results showed a reversal tendency of the glycolysis and fatty acid oxidation pathways between upregulation of PTEN (PPC group) and downregulation of PTEN (SEA group) (Supplementary Fig. 2). Therefore, this study proposed that PTEN plays an essential role in liver fibrosis most likely through regulating metabolism, but the specific mechanism needs to be further investigated.
In conclusion, the present study showed that glycolysis and fatty acid oxidation were significantly activated in liver fibrosis induced by S. japonicum. SEA, the most serious pathogenic factor, could mock the metabolic effect in macrophages and hepatocytes. PTEN seemed to be the essential modulator in such metabolic reprogramming. Overall, this study provide novel insight into the pathogenesis of S. japonicum–induced liver fibrosis, and modulation of PTEN might be a novel target to improve the condition.
CONCLUSION
Our study showed that the downregulation of PTEN expression is associated with the active metabolic phenotype of glycolysis, fatty acid oxidation and lipid synthesis in S. japonicum–induced liver fibrosis. Together with other results, we conclude that PTEN has an essential role in regulating glucose and lipid metabolic reprogramming that links with disease progression. Thus, modulation of PTEN may provide a novel insight into understanding the pathogenic mechanism of the parasite.
Abbreviations
- PTEN:
-
Phosphatase and tensin homology deleted on chromosome ten
- PI3K-p85α:
-
p85α subunit of Phosphatidylinositol 3-kinase
- PI3K-p110:
-
p110 subunit of Phosphatidylinositol 3-kinase
- AKT:
-
Protein kinase B
- p-AKT:
-
Phosphorylated AKT
- c-Myc:
-
Cancer-Myc
- β-actin:
-
Beta-actin
- HIF-1α:
-
Hypoxia-inducible factor 1α
- GLUT4:
-
Glucose transporter 4
- PKM2:
-
Pyruvate kinase isozyme type M2
- PFKFB3:
-
Phosphofructo-1-kinase/fructose-2, 6-bisphosphatase isoform 3
- PGC-1α:
-
Peroxisome proliferator–activated receptor-γ coactivator-1α
- SREBP-1c:
-
Sterol regulatory element-binding protein-1c
- CPT-1a:
-
Carnitine palmitoyl transferase 1a
- PPARα:
-
Peroxisome proliferator–activated receptor alpha
- CS:
-
Citrate synthase
- IDH3G:
-
Isocitrate dehydrogenase 3
- CYP4:
-
Cytochrome P450 proteins 4
- MCAD:
-
Medium-chain acyl-CoA dehydrogenase
- SCD1:
-
Stearoyl-CoA desaturase 1
- L-FABP10:
-
Liver-fatty acid binding protein 10
- G6PC:
-
Glucose-6 phosphatase
- FAS:
-
Fatty acid synthase
- ACC1:
-
Acetyl coenzyme A carboxylase 1
- SEA:
-
Soluble egg antigen
- PPC:
-
Polyene phosphatidylcholine
References
Song, L.G., X.Y. Wu, M. Sacko, and Z.D. Wu. 2016. History of schistosomiasis epidemiology, current status, and challenges in China: On the road to schistosomiasis elimination. Parasitology Research 115: 4071–4081.
Mutapi, F., R. Maizels, A. Fenwick, and M. Woolhouse. 2017. Human schistosomiasis in the post mass drug administration era. The Lancet Infectious Diseases 17: e42–e48.
Wilson, M.S., M.M. Mentink-Kane, J.T. Pesce, T.R. Ramalingam, R. Thompson, and T.A. Wynn. 2007. Immunopathology of schistosomiasis. Immunology and Cell Biology 85 (2): 148–154.
Kamdem, S.D., R. Moyou-Somo, F. Brombacher, and J.K. Nono. 2018. Host regulators of liver fibrosis during human schistosomiasis. Frontiers in Immunology 9: 2781.
McManus, D.P., and A. Loukas. 2008. Current status of vaccines for schistosomiasis. Clinical Microbiology Reviews 21 (1): 225–242.
Vale, N., M.J. Gouveia, G. Rinaldi, P.J. Brindley, F. Gärtner, and J.M. Correia da Costa. 2017. Praziquantel for schistosomiasis: Single-drug metabolism revisited, mode of action, and resistance. Antimicrobial Agents and Chemotherapy 61 (5).
Anthony, B.J., G.A. Ramm, and D.P. McManus. 2012. Role of resident liver cells in the pathogenesis of schistosomiasis. Trends in Parasitology 28 (12): 572–579.
Friedman, S.L. 2008. Mechanisms of hepatic fibrogenesis. Gastroenterology. 134 (6): 1655–1669.
Wang, Q., X. Chou, F. Guan, Z. Fang, S. Lu, J. Lei, Y. Li, and W. Liu. 2017. Enhanced Wnt signalling in hepatocytes is associated with Schistosoma japonicum infection and contributes to liver fibrosis. Scientific Reports 7 (1): 230.
Zheng, S., P. Zhang, Y. Chen, S. Zheng, L. Zheng, and Z. Weng. 2016. Inhibition of notch signaling attenuates schistosomiasis hepatic fibrosis via blocking macrophage M2 polarization. PLoS One 11 (11): e0166808.
Murray, P.J., J. Rathmell, and E. Pearce. 2015. SnapShot: Immunometabolism. Cell Metabolism 22 (1): 190–190.
Newton, R., B. Priyadharshini, and L.A. Turka. 2016. Immunometabolism of regulatory T cells. Nature Immunology 17 (6): 618–625.
O'Neill, L.A., R.J. Kishton, and J. Rathmell. 2016. A guide to immunometabolism for immunologists. Nature Reviews Immunology 16 (9): 553–565.
Krenkel, O., and F. Tacke. 2017. Macrophages in nonalcoholic fatty liver disease: A role model of pathogenic Immunometabolism. Seminars in Liver Disease 37 (3): 189–197.
Kumar, V. 2018. Targeting macrophage immunometabolism: Dawn in the darkness of sepsis. International Immunopharmacology 58: 173–185.
Zhang, Q., Y. Lou, X.L. Bai, and T.B. Liang. 2018. Immunometabolism: A novel perspective of liver cancer microenvironment and its influence on tumor progression. World Journal of Gastroenterology 24 (31): 3500–3512.
Cheng, Y., Y. Tian, J. Xia, X. Wu, Y. Yang, X. Li, C. Huang, X. Meng, T. Ma, and J. Li. 2017. The role of PTEN in regulation of hepatic macrophages activation and function in progression and reversal of liver fibrosis. Toxicology and Applied Pharmacology 317: 51–62.
Saha, S., I.N. Shalova, and S.K. Biswas. 2017. Metabolic regulation of macrophage phenotype and function. Immunological Reviews 280 (1): 102–111.
Luo, X., Y. Zhu, R. Liu, J. Song, F. Zhang, W. Zhang, Z. Xu, M. Hou, B. Yang, L. Chen, and M. Ji. 2017. Praziquantel treatment after Schistosoma japonicum infection maintains hepatic insulin sensitivity and improves glucose metabolism in mice. Parasites & Vectors 10: 453.
Phin, S., M.W. Moore, and P.D. Cotter. 2013. Genomic rearrangements of PTEN in prostate cancer. Frontiers in Oncology 3: 240.
Lee, Y.R., M. Chen, and P.P. Pandolfi. 2013. The functions and regulation of the PTEN tumour suppressor: New modes and prospects. Nature Reviews. Molecular Cell Biology 19: 547–562.
Smith, U. 2012. PTEN—Linking metabolism, cell growth, and cancer. The New England Journal of Medicine 367: 1061–1063.
Qiu, W., L. Federico, M. Naples, R.K. Avramoglu, R. Meshkani, J. Zhang, J. Tsai, M. Hussain, K. Dai, J. Iqbal, C.D. Kontos, and Y. Horie. 2008. Phosphatase and tensin homolog (PTEN) regulates hepatic lipogenesis, microsomal triglyceride transfer protein, and the secretion of apolipoprotein B-containing lipoproteins. Hepatology. 48 (6): 1799–1809.
Garcia-Cao, I., M.S. Song, R.M. Hobbs, G. Laurent, C. Giorgi, V.C. de Boer, D. Anastasiou, K. Ito, A.T. Sasaki, L. Rameh, and A. Carracedo. 2012. Systemic elevation of PTEN induces a tumor-suppressive metabolic state. Cell. 149 (1): 49–62.
Makboul, R., A. Refaiy, I.F. Abdelkawi, D.A. Hameed, A.A. Elderwy, M.M. Shalaby, A.S. Merseburger, and M.R. Hussein. 2016. Alterations of mTOR and PTEN protein expression in schistosomal squamous cell carcinoma and urothelial carcinoma. Pathology, Research and Practice 212 (5): 385–392.
Zhu, J., Z. Xu, X. Chen, S. Zhou, W. Zhang, Y. Chi, W. Li, X. Song, F. Liu, and C. Su. 2014. Parasitic antigens alter macrophage polarization during Schistosoma japonicum infection in mice. Parasites & Vectors. 7: 122.
Moltke, I., N. Grarup, M.E. Jørgensen, P. Bjerregaard, J.T. Treebak, M. Fumagalli, T.S. Korneliussen, M.A. Andersen, T.S. Nielsen, N.T. Krarup, A.P. Gjesing, J.R. Zierath, A. Linneberg, X. Wu, G. Sun, X. Jin, J. Al-Aama, J. Wang, K. Borch-Johnsen, O. Pedersen, R. Nielsen, A. Albrechtsen, and T. Hansen. 2014. A common Greenlandic TBC1D4 variant confers muscle insulin resistance and type 2 diabetes. Nature. 512 (7513): 190–193.
Wise, H.M., and M.A. Hermida. 2017. Prostate cancer, PI3K, PTEN and prognosis. Clinical Science (London) 131: 197–210.
Tian, Y., H. Li, T. Qiu, J. Dai, Y. Zhang, J. Chen, and H. Cai. 2018. Loss of PTEN induces lung fibrosis via alveolar epithelial cell senescence depending on NF-κB activation. Aging Cell: e12858.
Zhang, X., T. Jin, X. Huang, X. Liu, Z. Liu, Y. Jia, and J. Hao. 2018. Effects of the tumor suppressor PTEN on biological behaviors of activated pancreatic stellate cells in pancreatic fibrosis. Experimental Cell Research 373 (1–2): 132–144.
Kim, J., I.E. Eltoum, M. Roh, J. Wang, and S.A. Abdulkadir. 2009. Interactions between cells with distinct mutations in c-MYC and Pten in prostate cancer. PLoS Genetics 5: e1000542.
Benhamou, D., V. Labi, A. Getahun, E. Benchetrit, R. Dowery, K. Rajewsky, J.C. Cambier, and D. Melamed. 2018. The c-Myc/miR17-92/PTEN axis tunes PI3K activity to control expression of recombination activating genes in early B cell development. Frontiers in Immunology 9: 2715.
Cummins, E.P., C.E. Keogh, and D. Crean. 2016. The role of HIF in immunity and inflammation. Molecular Aspects of Medicine 47-48: 24–34.
Hotamisligil, G.S. 2017. Foundations of immunometabolism and implications for metabolic health and disease. Immunity. 47 (3): 406–420.
McPhee, J.B., and J.D. Schertzer. 2015. Immunometabolism of obesity and diabetes: Microbiota link compartmentalized immunity in the gut to metabolic tissue inflammation. Clinical Science (London, England) 129 (12): 1083–1096.
Rodríguez-Prados, J.C., P.G. Través, J. Cuenca, D. Rico, J. Aragonés, P. Martín-Sanz, M. Cascante, and L. Boscá. 2010. Substrate fate in activated macrophages: A comparison between innate, classic, and alternative activation. Immunology. 185 (1): 605–614.
An, J., L. Zheng, S. Xie, F. Yin, X. Huo, J. Guo, and X. Zhang. 2016. Regulatory effects and mechanism of adenovirus-mediated PTEN gene on hepatic stellate cells. Digestive Diseases and Sciences 61 (4): 1107–1120.
Hao, L.S., X.L. Zhang, J.Y. An, J. Karlin, X.P. Tian, Z.N. Dun, S.R. Xie, and S. Chen. 2009. PTEN expression is down-regulated in liver tissues of rats with hepatic fibrosis induced by biliary stenosis. APMIS. 117 (9): 681–691.
Chen, Y.L., X. Zhang, J. Bai, L. Gai, X.L. Ye, L. Zhang, Q. Xu, Y.X. Zhang, L. Xu, H.P. Li, and X. Ding. 2013. Sorafenib ameliorates bleomycin-induced pulmonary fibrosis: Potential roles in the inhibition of epithelial-mesenchymal transition and fibroblast activation. Cell Death & Disease 4: e665.
Matsuda, S., M. Kobayashi, and Y. Kitagishi. 2013. Roles for PI3K/AKT/PTEN pathway in cell signaling of nonalcoholic fatty liver disease. ISRN Endocrinology 2013: 472432.
Funding
Project support was provided in part by the National Natural Science Foundation of China (Nos. 81871670, 81800718), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (No. 18KJB310015), the Jiangsu Planned Projects for Postdoctoral Research Funds (No. 2019K063), the Training Programs of Innovation and Entrepreneurship for College Students in Jiangsu Province (No. 201810313063X), the Key Project of “Challenge Cup/Youth creation” in Xuzhou Medical University (No. 18ZK09) and Xuzhou Science and Technology Bureau Project (No. KC17098).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: WP, XYY, FFS. Performed the experiments: WMD, XYQ, XZ, QQC. Analysed the data: WP, XYQ. Contributed reagents/materials/analysis tools: WP, XYY, FFS, XYL. Wrote the manuscript: XZ, QQC, WP.
Corresponding authors
Ethics declarations
Disclaimer
The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Ethics Statement
This study was carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the Ministry of Health, China. The protocol was approved by the Laboratory Animal Welfare and Ethics Committee (LAWEC) of Xuzhou Medical University, China. All surgery was performed under sodium pentobarbital anaesthesia, and all efforts were made to minimize suffering.
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Supplementary Figure 1
The establishment and evaluation of hepatic fibrosis induced by S. japonicum. BALB/c mice were sacrificed at the 9th week post infection. (a) The ratio of liver/body weight of BALB/c mice (n = 15). (b) Gross appearance of the livers. (c) Representative liver images of H&E staining. (d) Representative liver images of Masson staining. (e) The altered levels of AST and ALT in the sera (n = 15). The differences were analszed using the independent samplet-test. Asterisks indicate statistically significant differences between groups. P < 0.01. (JPG 1102 kb)
Supplementary Figure 2
The relationship between PTEN and glucose and lipid metabolism in macrophages. RAW264.7 cells were challenged with SEA (6 μg/ml) alone, the PTEN activator polyene phosphatidylcholine (PPC, 15 μg/ml) alone or co-treatment with both of them for 24 h. The mRNA expression of enzymes in metabolic pathways was determined by real-time RT-PCR. Differences between means were compared using one-way analysis of variance (ANOVA) (Tukey’s test or LSD test) for multiple comparisons. vs the PBS group, *P < 0.05; **P < 0.01; ***P < 0.001. vs the SEA group, #P < 0.05; ##P < 0.01; ###P < 0.001. (JPG 715 kb)
Rights and permissions
About this article
Cite this article
Qian, Xy., Ding, Wm., Chen, Qq. et al. The Metabolic Reprogramming Profiles in the Liver Fibrosis of Mice Infected with Schistosoma japonicum. Inflammation 43, 731–743 (2020). https://doi.org/10.1007/s10753-019-01160-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-01160-5