Abstract
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are associated with high morbidity and mortality. Mesenchymal stem cells (MSCs) have been shown to improve ALI, and the imbalance of regulatory T cells (Tregs) and Th17 cells is associated with mortality in ALI/ARDS patients. However, whether administration of lung-resident MSC (LRMSC) improves lung injury and regulates the balance of Tregs and Th17 cells remains unknown. An ALI animal model was induced by LPS, and PBS or LRMSC were administered via tail vein after 4 h. LRMSC were subsequently detected in the lungs by a live imaging system (Berthold LB983, Germany). Lung morphology; lung wet-to-dry weight ratio; and total protein concentration, inflammatory cells, and cytokines in bronchoalveolar lavage fluid (BALF) and plasma were determined. The percentage of Tregs in lung and spleen, and of Th17 cells in lung and blood, were also evaluated. The results showed that LRMSC not only attenuated histopathological damage but also mediated the downregulation of lung wet-to-dry weight ratio and the reduction of total protein concentration and inflammatory cells in BALF. LRMSC also decreased inflammatory cytokines in both BALF and plasma and increased KGF-2 and surfactant protein C (SPC) expression in the lung. Flow cytometry revealed the upregulation of Tregs and the downregulation of Th17 cells, and the increase in the ratio of Tregs and Th17 cells. The live imaging system showed that LRMSC migrated to and were retained in the injured area. In conclusion, the results indicated that administration of LRMSC attenuates LPS-induced ALI via upregulating the balance of Tregs and Th17 cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are characterized by hypoxemia and increased lung permeability [1, 2]. The current management is mainly supportive, with no effective therapies, and the mortality rate ranges from 22 to 40% [3,4,5]. More effective and safer therapies are therefore urgently needed.
Regulatory T cells (Tregs) play an important role in the suppression of the immune response [6]. Tregs could be activated in ALI, and transfer of Tregs into an ALI mice model may reduce the levels of alveolar proinflammatory cytokines and inhibit neutrophils apoptosis and fibrocyte recruitment [7, 8]. Th17 cells, which mediate the acute inflammatory response, play a detrimental role in experimental murine models of inflammatory disease [9]. A recent study found that a ratio of Th17 cells and Tregs > 0.79 is an independent predictor for 28-day mortality in patients with ARDS [10]. Thus, it is very important to maintain the balance of Tregs and Th17 cells in ALI/ARDS.
Recently, mesenchymal stem cell (MSC)-based therapies have been shown to be effective in preclinical models of ALI owing to their ability to secrete growth factors, anti-inflammatory cytokines, and antimicrobial peptides [11,12,13]. Studies have found that MSC prevents differentiation of naive CD4+ T cells into Th17 cells in vitro, inhibits the production of IL-17 and IL-22 by fully differentiated Th17 cells, and induces the Tregs phenotype [14, 15].
Lung-resident MSCs (LRMSCs) are highly proliferative stem cells that can be recruited into pulmonary alveoli by intratracheal administration of FGF-10 [16]. LRMSC can easily be collected from bronchoalveolar lavage fluid (BALF). After collection, LRMSCs are cultured and proliferated in vitro and can be delivered as cellular therapy with substantial beneficial effects. Our group has demonstrated that LRMSCs express surface markers typically associated with MSC, do not express hematopoietic stem cell markers, and are capable of differentiating into adipocytes, chondrocytes, and osteocytes. [16]. However, whether LRMSC injection can improve lung injury and regulate the balance of Tregs and Th17 cells remains unknown. The aim of our current study was to evaluate the application of LRMSC by insertion in the tail vein of rats and the effect of balancing Tregs and Th17 cells in ALI/ARDS.
METHODS
Animals
Male Sprague-Dawley rats aged 6–8 weeks old and male C57BL/6 mice aged 8–10 weeks and weighing 20–25 g were used and bred carefully in the Medicine School of Fudan University in accordance with the ethical guidelines of the National Institutes of Health on Animal Care. Mice were raised in specific pathogen-free cages and maintained at temperatures between 20 and 25 °C and relative humidity of 50–70%. The experimental protocols were approved by the Animal Care and Use Committee of Fudan University, Shanghai.
Animal Treatment
Rats were anesthetized with an intraperitoneal injection of 25 mg/kg Avertin (Sigma-Aldrich, St Louis, MO, USA). KGF-2 (provided by Newsummit, Shanghai, China) at a dose of 5 mg/kg was instilled intratracheally. Three days after KGF-2 instillation, the rats were sacrificed with an intraperitoneal injection of chloral hydrate and exsanguinated through the femoral artery. BALF was then collected immediately with medium consisting of DMEM/F12 supplemented with 5% fetal bovine serum (Invitrogen, CA, USA), 100 U/mL penicillin/streptomycin (Invitrogen), 2.5 μg/mL amphotericin B (Invitrogen), and 0.02 wt% EDTA (Invitrogen).
For the LPS-induced ALI model, mice were intratracheally injected with either 5 mg/kg LPS (Pseudomonas aeruginosa 10, Sigma-Aldrich, St Louis, MO, USA) dissolved in PBS or an equal volume of vehicle (PBS). After 4 h, PBS or 5 × 105 LRMSC (dissolved in 0.2 mL PBS) was injected into mice via the tail vein. The mice were sacrificed 48 h after LPS instillation with an intraperitoneal injection of chloral hydrate.
Isolation and Culture of LRMSC
LRMSCs were isolated from BALF of rats as previously described [16]. Briefly, BALF was centrifuged at 800 rpm for 10 min at 4 °C and the cells were resuspended, seeded at a density of 1 × 105 in T25 cell culture flasks, and incubated at 37 °C in an atmosphere of 5% CO2 with saturated humidity. An inverted microscope was used to monitor cell morphology. Cells were maintained in MSC complete medium, which was changed after 24 h to remove residual non-adherent cells and then every 3 days. Single separated fibroblastoid colonies termed LRMSC were identified 14 days after initial plating. A homogeneous population of mesenchymal cells was obtained from individual rats after 3–5 passages. The 4th and 5th passages of LRMSC were used for the experiments.
Total and Differential Cell Count
BALF was centrifuged at 1500 rpm for 10 min at 4 °C, and the supernatant was stored at − 80 °C for further analysis whereas the cell pellet was used for cell counting. The total number of nucleated cells in BALF was counted with a hemocytometer. The resuspended BALF cells were centrifuged, transferred to slides, and stained with the Wright–Giemsa stain. Neutrophils and macrophages on slides were quantified by counting a total of 200 cells/slide at × 40 magnification.
Wet-To-Dry Weight Ratio
The right lower lobe of the lung was used to calculate the lung wet-to-dry (W/D) weight ratio. Weight was determined immediately after isolation and after being oven dried at 60 °C for 72 h.
BALF Protein Concentration
BALF protein concentration was measured using a bicinchoninic acid (BCA) protein assay kit according to the manufacturer’s instruction (Beyotime, China).
Cytokines in BALF
The levels of IL-1β, IL-6, TNF-a, IL-17, IL-22, IL-10, and TGF- β (eBioscience, San Diego, CA, USA) in BALF supernatant were determined using enzyme-linked immunosorbent assays (ELISA) according to the manufacturer’s protocol.
Lung Morphometry Analyses
The right upper lobe of the lung was subjected to histological analysis. The lung sections were stained with hematoxylin and eosin (H&E), and the histological score of lung injury was determined in a blind manner.
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted using the TRIzol reagent (Invitrogen). RNA was reverse-transcribed into cDNA using the cDNA kit (TOYOBO, Japan). The primers were designed and synthesized by Shenggong (Shanghai, China). For amplification, the SYBR-Green I Real-Time PCR kit (TOYOBO) was used. Each reaction was run in triplicate and normalized to the housekeeping gene β-actin transcripts.
Preparation of Lung Tissue and Spleen Lymphocytes and Flow Cytometry Analysis
Mouse lungs were dissected into single lobes and rinsed in a petri dish containing PBS to remove the thymus, heart, trachea, connective tissue, and efferent and afferent blood vessels. Lobes were digested with an enzyme mix (buffer S, enzyme D, and enzyme A) (Miltenyi, Bergisch Gladbach, Germany). After filtration and centrifugation, the collected single-cell suspensions were subjected to density gradient centrifugation using Ficoll-Paque to isolate lymphocytes. Mouse splenocytes were harvested and triturated with sterile syringes, and the resulting cell suspension was filtered through a 70-μm nylon mesh. The RBCs were lysed using cell lysis solution (BD Biosciences).
Collected cells were incubated with Fc Block-2.4G2 (BD Biosciences PharMingen, San Diego, CA, USA) antibody to block Fcγ III/II receptors before staining with a specific antibody. The following antibodies (eBioscience) were used for surface staining: FITC-labeled anti-CD3, FITC-labeled anti-CD4, APC-labeled anti-CD8, APC-labeled anti-CD25, PE-labeled anti-Foxp3, and PE-labeled anti-IL-17A. For the analysis of Tregs, the collected cells were placed into tubes without stimulation with phorbol 12-myristate 13-acetate (PMA), ionomycin, or brefeldin A (BD Biosciences). Cells were incubated with surface marker antibodies FITC-anti-CD4 and APC-anti-CD25, followed by fixation and permeabilization with Foxp3 staining buffer (eBioscience) and intracellular staining with PE-anti-Foxp3. To detect the phenotypes of Th17 in whole blood and lung, a mixture of PMA, ionomycin, and brefeldin A was used to stimulate cytokine expression in Th17 cells for 6 h. The surface marker antibodies FITC-anti-CD3 and APC-anti-CD8 were used to determine the number of CD4+ T cells (CD3+CD8− T cells), as CD4 is downregulated when stimulated by PMA. After staining with FITC-anti-CD3 and APC-anti-CD8, the cells were fixed and permeabilized using Fix/Perm Reagent (BD Biosciences), followed by intracellular staining with anti-IL-17A-PE antibody. Isotype controls of IL-17A and Foxp3 were used to correct for compensation and confirm antibody specificity.
In Vivo Tracing of LRMSC
C57BL/6 mice were divided into control and LPS groups, and LPS (5 mg/kg) and the same volume of PBS were instilled intratracheally into the animals. After 4 h, 5 × 105 LRMSCs containing the luciferase gene were injected into the mice tail vein, and the migration of LRMSC was traced using a live imaging system (Berthold LB983, Germany) for 4 days.
Statistics
SPSS v17.0 statistical software for Windows was used for all statistical analyses. Differences between groups were examined for statistical significance using the Kruskal–Wallis H test. Data are expressed as mean ± standard deviation (SD). p < 0.05 was considered statistically significant.
RESULTS
LRMSC Ameliorates LPS-Induced Inflammatory Response
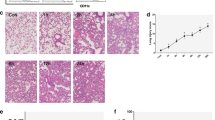
Intratracheal instillation of LPS induced a robust inflammatory response in the lung and alveolus of mice after 2 days (Fig. 1a, b). Histologic examination revealed that administration of LRMSC reduced infiltration of inflammatory cells and interstitial thickening after LPS-induced ALI (Fig. 1c). To quantify the differences, lung injury was scored according to the degree of alveolar congestion, hemorrhage, neutrophil infiltration, and wall thickening. We found that administration of LRMSC significantly decreased the lung injury score (Fig. 1d). Moreover, LRMSC administration reduced the influx of inflammatory cells (Fig. 2a–c, e) and decreased the secretion of the inflammatory cytokines IL-1β, IL-6, and TNF-α in the injured alveolus (Fig. 3).
Measurement of lung injury parameters. a Total cell count. b, c Differential cell count. d Total protein concentration in bronchoalveolar lavage fluid (BALF). e Representative images of Giemsa-stained cells from the BALF samples (× 400 magnification). f Lung wet-to-dry weight ratio. **P < 0.01 vs. PBS + PBS, ##P < 0.05 vs. LPS + PBS.
LRMSC Restores LPS-Induced Increase in Vascular Permeability
The W/D weight ratio of the lung and the protein concentration in BALF are markers of vascular permeability. Lung W/D weight ratio and total protein levels in BALF were increased in the LPS-induced ALI. Administration of LRMSC significantly reduced the lung W/D ratio (Fig. 2f) and total protein concentration (Fig. 2d) in BALF.
LRMSC Restores LPS-Induced Imbalance of Tregs and Th17 Cells
Tregs and Th17 cell imbalance has been described in ALI, and the 28-day mortality in patients with ARDS significantly increased when the Th17/Treg ratio was greater than 0.79. The Ghannam group found that MSCs inhibit Th17 cell differentiation and function and induce a Tregs phenotype [14]. In our study, to evaluate the effect of LRMSC on the balance of Tregs and Th17 cells, we determined the percentage of Tregs in lung and spleen and Th17 cells in lung and peripheral blood. We found that intratracheal instillation of LPS significantly increased the percentage of Tregs in lung and spleen (Fig. 4a, b) and the levels of the Tregs-related cytokine IL-10 (Fig. 4c), but not of TGF-β (Fig. 4d). LPS instillation also elevated the percentage of Th17 cells in lung and peripheral blood (Fig. 5a, b) and the levels of the Th17-related cytokines IL-17 (Fig. 5c) and IL-22 (Fig. 5d). Administration of LRMSC increased the percentage of Tregs and IL-10 levels (Fig. 4) and reduced the percentage of Th17 cells and the levels of IL-17 and IL-22 (Fig. 5).
Percentage of CD4+CD25+Foxp3+ Tregs after injection of lung-resident mesenchymal stem cells (LRMSCs). The percentage of CD4+CD25+Foxp3+ Tregs in the lungs (a) and spleen (b) was determined using flow cytometry. The relative levels of IL-10 (c) and TGF-beta (d) in the bronchoalveolar lavage fluid (BALF) are shown in bar graphs. *P < 0.05 vs. PBS + PBS, **P < 0.01 vs. PBS + PBS, #P < 0.05 vs. LPS + PBS, ##P < 0.01 vs. LPS + PBS.
Percentage of CD3+CD8−IL-17+ Th17 cells after injection of lung-resident mesenchymal stem cells (LRMSCs). The percentage of CD3+CD8−IL-17+ Th17 cells in the lungs (a) and peripheral blood (b) was determined using flow cytometry. The relative levels of IL-17 (c) and IL-22 (d) in the bronchoalveolar lavage fluid (BALF) are shown in bar graphs. **P < 0.01 vs. PBS + PBS, #P < 0.05 vs. LPS + PBS, ##P < 0.01 vs. LPS + PBS.
We further analyzed the ratio of Tregs and Th17 cells in the lung and found that the ratio decreased after LPS instillation. Treatment with LRMSC increased the ratio of Tregs and Th17 cells compared with LPS-induced mice (Fig. 6a).
Changes in the Tregs/Th17 cell ratio and the levels of interstitial collagen, KGF-2, and SPC after injection of lung-resident mesenchymal stem cells (LRMSCs). a Ratio of Tregs and Th17 cells. KGF-2 (b) and SPC (c) mRNA levels were determined using RT-PCR. d Masson staining was used to detected the deposition of interstitial collagen.
LRMSC Increases the Expression of KGF-2 and SPC in Lung Tissue
Keratinocyte growth factor (KGF-2) is a member of the fibroblast growth factor family, facilitating differentiation and proliferation of epithelial cells and angiogenesis and barrier function of capillary monolayers. Instillation of LPS decreased KGF-2 mRNA levels in mice, whereas administration of LRMSC significantly increased its levels (Fig. 6b). LRMSC treatment also significantly increased SPC mRNA levels (Fig. 6c).
LRMSC Decreases Deposition of Interstitial Collagen
Masson staining revealed an increased amount of interstitial collagen in LPS-induced ALI. In contrast, injection of LRMSC decreased interstitial collagen deposition in LPS-induced ALI mice (Fig. 6d).
LRMSCs Migrate to and Are Retained in the Injured Area
The migration of LRMSC was monitored for 4 days in vivo. LRMSCs in both LPS and PBS groups were detected in the lungs of mice and displayed a strong signal after 2 h of LRMSC administration. Over the following 48 h, the signals in the LPS group significantly increased, and the signals disappeared after 96 h. Signals in the PBS group gradually decreased after 24 h and disappeared after 72 h. The LPS group showed a larger retention of cells in the lung compared with the PBS group (Fig. 7). These results suggest that LRMSCs migrate to and are retained in the injured area, and these cells may play an important role in the treatment of ALI.
DISCUSSION
LRMSCs are mesenchymal stem cells isolated from the distal airways of rats treated with KGF-2. Our group demonstrated that LRMSCs exhibit several characteristics similar to classical bone marrow-derived MSC [16]. First, they can be isolated by adherence purification and form distinct CFU-Fs during cell culture. Second, they express surface markers typically associated with MSC. Third, they are multipotent and can differentiate into adipocytes, chondrocytes, and osteocytes. These characteristics make LRMSC a candidate for cell therapy.
There have been reports that the administration of MSC improves lung inflammation in LPS-mediated ALI animal models [11, 17,18,19,20,21] and bleomycin-induced lung injury [22]. In this study, we found that the inflammatory cells and total protein in BALF decreased after LRMSC administration. Most importantly, LRMSC therapy attenuated the observed histopathological impairments in lung texture and deposition of interstitial collagen. The expression of KGF-2 and SPC was also upregulated after LRMSC administration. These results indicate the potential advantages of LRMSC in repairing lung injury.
The retention of injected LRMSC is one of the foremost advantages of cell-based therapies. In our study, we found that LRMSCs migrated to the inflammation site after injury. In addition, we inserted the luciferase gene in LRMSC to better detect the cells in vivo. Using a live imaging system, we found that LRMSCs were efficiently delivered to and retained in the injured tissues compared to normal lungs, which is consistent with previous reports [11, 17, 23].
Although beneficial effects of the LRMSC administration were observed, the precise mechanisms remain unclear. Differentiation, paracrine function, and cell-cell contact have all been implicated as possible mechanisms. In this study, we found that LRMSC partially restored SPC expression. The increased mRNA expression of SPC might be attributed to the differentiation of LRMSC into, or to proliferation of, the alveolar type II epithelial cell. However, some authors have suggested that MSC differentiation may not be the main contributor because of poor MSC engraftment and survival at the site of lung injury, as well as the low probability of differentiation in a short time [24, 25]. Paracrine function has been proposed as the main mechanism accounting for the beneficial effects in injury repair. KGF is an important growth factor secreted by MSC in the repair of the damaged lung, and its effects in the injured lung have been confirmed. KGF can also promote the proliferation of AT2 and inhibit apoptosis in the injured lung [26, 27]. Cell-cell contact is also a key factor for cell-based therapy. Studies have suggested that cell-cell contact may enhance the protective effect of MSC in the injury environment through activation of the programmed death 1 pathway [28]. Recent studies indicated that, in vitro, MSCs repress the Th17 molecular program through the PD-1 pathway, prevent the differentiation of naive CD4+ T cells into Th17 cells, inhibit the production of inflammatory cytokines by Th17 cells, and induce the Tregs phenotype [14, 15, 29]. In this study, we found that the administration of LRMSC upregulated Tregs and downregulated Th17 cells, increasing the Tregs/Th17 cell ratio in the lung. Thus, we propose that maintaining the balance of Tregs and Th17 cells may be a mechanism by which LRMSCs improve lung injury. Further work is needed to investigate the mechanism underlying the regulation of Tregs and Th17 cells by LRMSC in ALI.
This study has several limitations. First, we induced ALI by LPS in mice. This mouse model of ALI is focused on inflammation and cannot fully reflect the complexity of clinical ARDS in human patients. Second, we only administered LRMSC once and sacrificed the mice 48 h later, which may also not fully reflect the clinical application of such a therapy.
In conclusion, our findings indicate that LRMSC administration improves lung injury and inhibits the inflammatory response mainly by upregulating the Tregs/Th17 cells ratio.
References
Ware, L.B., and M.A. Matthay. 2000. The acute respiratory distress syndrome. The New England Journal of Medicine 342: 1334–1349.
Rubenfeld, G.D., E. Caldwell, E. Peabody, J. Weaver, D.P. Martin, M. Neff, E.J. Stern, and L.D. Hudson. 2005. Incidence and outcomes of acute lung injury. The New England Journal of Medicine 353: 1685–1693.
Matthay, M.A., L.B. Ware, and G.A. Zimmerman. 2012. The acute respiratory distress syndrome. The Journal of Clinical Investigation 122 (8): 2731–2740.
Calfee, C.S., and M.A. Matthay. 2007. Nonventilatory treatments for acute lung injury and ARDS. Chest 131 (3): 913–920.
Lee, J.W., X. Fang, N. Gupta, V. Serikov, and M.A. Matthay. 2009. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proceedings of the National Academy of Sciences of the United States of America 106 (38): 16357–16362.
Sakaguchi, S. 2004. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annual Review of Immunology 22: 531–562.
D'Alessio, F.R., K. Tsushima, N.R. Aggarwal, E.E. West, M.H. Willett, M.F. Britos, M.R. Pipeling, R.G. Brower, R.M. Tuder, J.F. McDyer, and L.S. King. 2009. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. The Journal of Clinical Investigation 119: 2898–2913.
Garibaldi, B.T., F.R. D'Alessio, J.R. Mock, D.C. Files, E. Chau, Y. Eto, M.B. Drummond, N.R. Aggarwal, V. Sidhaye, and L.S. King. 2013. Regulatory T cells reduce acute lung injury fibroproliferation by decreasing fibrocyte recruitment. American Journal of Respiratory Cell and Molecular Biology 48: 35–43.
Ji, Y.Q., and W.G. Zhang. 2010. Th17 cells: positive or negative role in tumor? Cancer Immunol Immun 59 (7): 979–987.
Yu, Z.X., M.S. Ji, J. Yan, Y. Cai, J. Liu, H.F. Yang, Y. Li, Z.C. Jin, and J.X. Zheng. 2015. The ratio of Th17/Treg cells as a risk indicator in early acute respiratory distress syndrome. Critical Care 19: 82.
Mei, S.H., S.D. McCarter, Y. Deng, C.H. Parker, W.C. Liles, and D.J. Stewart. 2007. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Medicine 4: e269.
Ortiz, L.A., M. Dutreil, C. Fattman, A.C. Pandey, G. Torres, K. Go, and D.G. Phinney. 2007. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proceedings of the National Academy of Sciences of the United States of America 104: 11002–11007.
Mei, S.H., J.J. Haitsma, C.C. Dos Santos, Y. Deng, P.F.H. Lai, A.S. Slutsky, W.C. Liles, and D.J. Stewart. 2010. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. American Journal of Respiratory and Critical Care Medicine 182: 1047–1057.
Ghannam, S., J. Pène, G. Moquet-Torcy, C. Jorgensen, and H. Yssel. 2010. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. Journal of Immunology 185 (1): 302–312.
Luz-Crawford, P., M. Kurte, J. Bravo-Alegría, R. Contreras, E. Nova-Lamperti, G. Tejedor, D. Noël, C. Jorgensen, F. Figueroa, F. Djouad, and F. Carrión. 2013. Mesenchymal stem cells generate a CD4+CD25+Foxp3+regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Research & Therapy 4 (3): 65.
Tong, L., J. Zhou, L. Rong, E.J. Seeley, J. Pan, X. Zhu, J. Liu, Q. Wang, X. Tang, J. Qu, C. Bai, and Y. Song. 2016. Fibroblast growth factor-10 (FGF-10) mobilizes lung-resident mesenchymal stem cells and protects against acute lung injury. Scientific Reports 6: 21642.
Gupta, N., X. Su, B. Popov, J.W. Lee, V. Serikov, and M.A. Matthay. 2007. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. Journal of Immunology 179 (3): 1855–1863.
Xu, J., C.R. Woods, A.L. Mora, R. Joodi, K.L. Brigham, S. Iyer, and M. Rojas. 2007. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. American Journal of Physiology. Lung Cellular and Molecular Physiology 293 (1): L131–L141.
Hao, Q., Y.G. Zhu, A. Monsel, S. Gennai, T. Lee, F. Xu, and J.W. Lee. 2015. Study of bone marrow and embryonic stem cell-derived human mesenchymal stem cells for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells Translational Medicine 4 (7): 832–840.
Kim, E.S., Y.S. Chang, S.J. Choi, J.K. Kim, H.S. Yoo, S.Y. Ahn, D.K. Sung, S.Y. Kim, Y.R. Park, and W.S. Park. 2011. Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells attenuate Escherichia coli-induced acute lung injury in mice. Respiratory Research 12: 108.
Xiang B, Chen L, Wang X, Zhao Y, Wang Y, Xiang C. 2017. Transplantation of menstrual blood-derived mesenchymal stem cells promotes the repair of LPS-induced acute lung injury. International Journal of Molecular Sciences 18(4).
Ortiz, L.A., F. Gambelli, C. McBride, D. Gaupp, M. Baddoo, N. Kaminski, and D.G. Phinney. 2003. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proceedings of the National Academy of Sciences of the United States of America 100 (14): 8407–84011.
Xu, J., J. Qu, L. Cao, Y. Sai, C. Chen, L. He, and L. Yu. 2008. Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. The Journal of Pathology 214 (4): 472–481.
Psaltis, P.J., A.C. Zannettino, S.G. Worthley, and S. Gronthos. 2008. Mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells 26 (9): 2201–2210.
Hocking, A.M., and N.S. Gibran. 2010. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Experimental Cell Research 316 (14): 2213–2219.
Baba, Y., T. Yazawa, Y. Kanegae, S. Sakamoto, I. Saito, N. Morimura, T. Goto, Y. Yamada, and K. Kurahashi. 2007. Keratinocyte growth factor gene transduction ameliorates acute lung injury and mortality in mice. Human Gene Therapy 18 (2): 130–141.
Bao, S., Y. Wang, P. Sweeney, A. Chaudhuri, A.I. Doseff, C.B. Marsh, and D.L. Knoell. 2005. Keratinocyte growth factor induces Akt kinase activity and inhibits Fas-mediated apoptosis in A549 lung epithelial cells. American Journal of Physiology. Lung Cellular and Molecular Physiology 288 (1): L36–L42.
Augello, A., R. Tasso, S.M. Negrini, A. Amateis, F. Indiveri, R. Cancedda, and G. Pennesi. 2005. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. European Journal of Immunology 35 (5): 1482–1490.
Luz-Crawford, P., D. Noël, X. Fernandez, M. Khoury, F. Figueroa, F. Carrión, C. Jorgensen, and F. Djouad. 2012. Mesenchymal stem cells repress Th17 molecular program through the PD-1 pathway. PLoS One 7 (9): e45272.
Funding
This study was supported by The National Natural Science Foundation of China key grant (81630001, 81490533) and grant (81770075, 81500026, 81570028, 81600056, 81870062), The State Key Basic Research Program project (2015CB553404), Shanghai Science and Technology Committee grant (15DZ1930600/15DZ1930602/16ZR1405700, 18140-903400) and Shanghai Municipal Commission of Health and Family Planning (201540370), Shanghai Science and Technology Committee Grant (15DZ1941103), Fudan University (IAH6281420/058).
Author information
Authors and Affiliations
Contributions
Linlin Wang performed the experiments, analyzed the data, and wrote the manuscript. Meng Shi, Xiaocen Wang, Jian Wang, Shimeng Ji, Jing Bi, Cuicui Chen, Lin Tong, and Dong Yang analyzed the data. Yuanlin Song, Jian Zhou, and Chunxue Bai designed the study and wrote and revised the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no conflict of interest.
Additional information
Linlin Wang and Meng Shi are Co-first authors.
Rights and permissions
About this article
Cite this article
Wang, L., Shi, M., Tong, L. et al. Lung-Resident Mesenchymal Stem Cells Promote Repair of LPS-Induced Acute Lung Injury via Regulating the Balance of Regulatory T cells and Th17 cells. Inflammation 42, 199–210 (2019). https://doi.org/10.1007/s10753-018-0884-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0884-6