Abstract
Mer receptor tyrosine kinase (MerTK) is key for efficient phagocytosis of apoptotic neutrophils (ANs) and homeostasis of IL-10 production by human anti-inflammatory M2c monocytes/macrophages. We asked whether stimulation of M2c surface receptors contributes in turn to MerTK activation. For this purpose, human monocytes/macrophages were differentiated under M1, M2a, and M2c polarizing conditions. The effects of antibody-mediated cross-linking of M2c receptors (i.e., CD14, CD16, CD32, CD163, CD204) on MerTK phosphorylation and phagocytosis of ANs were tested. MerTK expression was also studied by flow cytometry and western blot in the presence of LPS and in M2c-derived microvesicles (MVs). Antibody cross-linking of either CD14 or CD32/FcγRII led to Syk activation and MerTK phosphorylation in its two distinct glycoforms (175–205 and 135–155 kDa). Cross-linked CD14 enhanced efferocytosis by M2c macrophages and enabled M1 and M2a cells to clear ANs efficiently. In M1 conditions, LPS abolished surface MerTK expression on CD14bright cell subsets, so disrupting the anti-inflammatory pathway. In M2c cells, instead, MerTK was diffusely and brightly co-expressed with CD14, and was also detected in M2c macrophage-derived MVs; in these conditions, LPS only partially downregulated MerTK on cell surfaces, while the smaller MerTK glycoform contained in MVs remained intact. Altogether, cooperation between CD14 and MerTK may foster the clearance of ANs by human monocytes/macrophages. CD14 stands between M1-related LPS co-receptor activity and M2c-related MerTK-dependent response. MerTK interaction with CD32/FcγRII, its detection in M2c MVs, and the differential localization and LPS susceptibility of MerTK glycoforms add further new elements to the complexity of the MerTK network.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Dying neutrophils, if not rapidly removed, can release large amounts of danger signals, nucleic acids, and other antigenic material, so triggering the development of chronic inflammation and autoimmunity [1]. Macrophage-expressed Mer receptor tyrosine kinase (MerTK), through its ligands growth arrest-specific 6 (Gas6) and protein S, is of key importance for the prompt clearance of apoptotic neutrophils (ANs) and resolution of innate inflammation. In humans, MerTK is preferentially expressed on discrete populations of anti-inflammatory macrophages called M2c cells, phenotypically characterized as CD14+/brightCD16+CD163+ CD204+/−CD206+CD209−, as well as in minor M2c-like cell subsets differentiated in M1 and M2a conditions, and in circulating CD14brightCD16+ M2c-like monocytes [2]. M2c cells are elicited in vitro under anti-inflammatory conditions (e.g., in the presence of M-CSF plus IL-10, M-CSF plus serum, M-CSF plus glucocorticoids, or glucorticoids alone), or upon intervention of anti-inflammatory stimuli onto inflammatory contexts (e.g., addition of IL-10 or glucocorticoids to pre-existing GM-CSF or IL-17) [1, 2]. These specialized cells utilize MerTK to promote efficient phagocytosis of ANs in the presence of protein S or protein S-containing serum [2, 3]. Moreover, the MerTK ligand Gas6 is produced in an autocrine fashion by M2c cells and stimulates, in turn, macrophage production of IL-10, thereby tracing a positive loop in which MerTK further amplifies M2c polarization and is closely involved in M2c cell homeostasis [2].
In murine macrophages, MerTK was shown to synergize and interact, either physically or functionally, with other receptors and ligands involved in macrophage phagocytosis of apoptotic cells (ACs), such as αvβ5 integrin, T cell immunoglobulin mucin protein 4 (Tim-4), scavenger receptor A (SR-A)/CD204, and C1q [4,5,6,7]. However, these synergistic interactions have not been confirmed in humans [8, 9] or, at least, do not occur in human glucocorticoid-treated (i.e., M2c) macrophages [10].
In the present study, conducted on human monocytes/macrophages, we asked whether, in parallel with MerTK’s contribution to M2c anti-inflammatory responses, the engagement of M2c receptors themselves may in turn contribute to MerTK activation. We identified MerTK interactions with either CD14 or CD32 receptors as two novel mechanisms that may facilitate phagocytosis of ANs and restoration of anti-inflammatory conditions, following LPS removal in bacterial infections or upon glucocorticoid therapy in immune-complex diseases, respectively. We also discovered that, whereas LPS downregulates MerTK while preserving its co-receptor CD14 on the surface of proinflammatory M1 cells, regulatory M2c cells can release an LPS-resistant MerTK glycoform through their microvesicles (MVs).
MATERIALS AND METHODS
Cell Cultures
Human monocytes from buffy coats of healthy blood donors were isolated by Ficoll-Paque™ Plus gradient (GE Healthcare Life Sciences, Pittsburgh, PA, USA) and magnetic separation, using a kit for human monocyte enrichment by negative selection (EasySep™, StemCell Technologies, Vancouver, BC, Canada), according to the manufacturer’s instructions. Monocytes were then cultured in non-tissue culture treated 24-well plates at 0.8 × 106 cells/ml, in serum-free X-Vivo™15 medium formulated with L-glutamine, gentamicin, and phenol red (Lonza, Walkersville, MD, USA) for 48 h, in the presence of dexamethasone (100 nM, ex-Sigma-Aldrich, EMD Millipore, Billerica, MA, USA), with or without M-CSF (50 ng/ml, Peprotech, Rocky Hill, NJ, USA). For longer incubations, in some experiments, cells were cultured in serum-containing medium, with human AB serum 5–10% (Mediatech, Manassas, VA, USA), heat-inactivated fetal calf serum (FCS) 5% (to avoid formation of clumps induced by autologous serum), and X-Vivo™15 medium for 7 days, with or without growth factors and/or cytokines orientating differentiation towards M1 (GM-CSF 100 ng/ml, Peprotech; and/or IFNγ 2.5 ng/ml, R&D Systems, Minneapolis, MN, USA), M2a (IL-4 20 ng/ml, Novus Biologicals, Littleton, CO, USA), or M2c (M-CSF 50 ng/ml plus serum, or M-CSF plus IL-10 37.5 ng/ml, Peprotech), as specified in the text. Prior to participation, all subjects gave informed consent to donate their blood. The study was approved by the Institutional Review Board of Temple University.
Cell Stimulation with Antibody Cross-Linking and MerTK Ligands
Ab cross-linking of membrane receptors was performed by resuspending the cultured cells in fresh medium in the presence of one of the following primary mouse anti-human monoclonal Abs (mAbs, 5 μg/ml): anti-CD14 (LEAF™ purified clone M5E2 or clone HCD14, both from Biolegend, San Diego, CA, USA), anti-CD16 (LEAF™ purified clone 3G8, Biolegend), anti-CD32 (clone FUN-2, Biolegend; or clone 3D3 mIgG1, BD Biosciences, San Jose, CA, USA), anti-CD163 (clone EDHu-1, Novus Biologicals), anti-MerTK (clone 125518, R&D Systems), anti-SR-A/CD204 (clone 351620, R&D Systems), and murine IgG1 or IgG controls (Southern Biotech). After 20 min, cells were incubated with a goat anti-mouse (GAM) IgG (H + L) (15 μg/ml, Southern Biotech) for an additional 20 min. In other experiments, cells were stimulated with (protein S containing) AB serum 2–10%, or with recombinant human Gas6 (rhGas6 1 μg/ml, R&D Systems), in the presence or absence of a neutralizing goat polyclonal anti-human Gas6 Ab (10 μg/ml, R&D Systems), or with a goat polyclonal anti-MerTK Ab (5 μg/ml, R&D System) or appropriate isotype control (5 μg/ml, Southern Biotech) for 40 min. Phosphorylation of MerTK was then investigated by western blot.
Western Blot
Cell lysates were obtained in a buffer containing 50 mM Hepes, 150 mM NaCl, 10% glycerol, 1% Triton X-100, and freshly added cocktails of protease and phosphatase inhibitors (Sigma-Aldrich, EMD Millipore). Lysates were resolved on a SDS-PAGE 8% polyacrylamide gel. Proteins, transferred to PVDF membranes (EMD Millipore), were probed with: biotinylated goat polyclonal anti-human MerTK (R&D Systems), recognizing amino acid sequence Arg26-Ala499 (corresponding to N-terminal extracellular region and possibly part of a transmembrane segment [11]), followed by horseradish peroxidase (HRP)-conjugated streptavidin (Biolegend); rabbit polyclonal anti-phospho-MerTK (Fabgennix, Frisco, TX, USA), raised against a synthetic peptide mapping amino acids 746–757 (corresponding to C-terminal intracellular region [11]); rabbit polyclonal anti-Syk (Cell Signaling Technology, Beverly, MA, USA); rabbit polyclonal anti-phospho-Syk (Cell Signaling Technology); rabbit anti-β-actin (Santa Cruz Biotechnology, Dallas, TX, USA); all of them followed by secondary HRP-conjugated goat anti-rabbit Ab (Santa Cruz Biotechnology). Immunoblots were developed and visualized by enhanced chemiluminescence using Amersham ECL™ reagents (GE Healthcare). Densitometry of phosphorylated MerTK bands was calculated using ImageJ software (imagej.nih.gov), and normalized to β-actin expression.
Phagocytosis of Apoptotic Neutrophils
Human neutrophils were isolated from Ficoll-Hypaque pellets through dextran (20%) erythrocyte sedimentation followed by lysis of contaminating erythrocytes, by incubation with ice-cold ammonium chloride (0.15 M) and potassium bicarbonate (0.01 M) solution. They were then resuspended at 1 × 106 cell/ml in 10% FCS-RPMI in the presence of 1% penicillin and streptomycin, labeled with 2.5 μM carboxyfluorescein succinimidyl ester (CFSE, Sigma-Aldrich, EMD Millipore), and incubated for 20 h at 37 °C in 5% CO2. ANs were co-incubated for 60 min with 7-day differentiated macrophages, at a 5:1 ratio. In some experiments, macrophages were pre-treated for 40 min with an anti-CD14 Ab (clone M5E2), or an IgG control (5 μg/ml), and secondarily with a GAM Ab (H + L) (15 μg/ml) to obtain cross-linking of CD14. Flow cytometry was used to quantify the percentages of CD14-labeled macrophages that phagocytosed CFSE-labeled ANs.
Flow Cytometry
Flow cytometry was carried out after washing the cells in 2% bovine serum albumin-containing buffer. Surface expression of CD14 and MerTK was detected using a PE-Cy7-labeled anti-CD14 (Biolegend) and a PE-labeled anti-MerTK ab (R&D Systems, clone 125518), respectively, along with appropriate isotype controls (Biolegend). Viable monocytes in cell cultures were quantified as double negative for APC-conjugated annexin-V (BD Biosciences) and propidium iodide (Sigma-Aldrich). Efferocytic macrophages were measured as percentages of CD14 (PeCy7) and CFSE (FITC) double positive macrophages. Cells were analyzed using FACSCalibur™ (BD Biosciences) and FlowJo software (Tree Star, Ashland, OR, USA).
Isolation of Macrophage-Derived Microvesicles
Cells were cultured for 7 days in serum-containing medium in the presence of M-CSF. In some experiments, cells were subsequently co-incubated with LPS (10 μg/ml, Sigma-Aldrich) or unesterified cholesterol (100 μg/ml, a gift from Drs. Ming-Lin Liu and Kevin J. Williams) for an additional 24 h. Culture supernatants, including two washes with PBS to remove residual MVs still adhering to the cell surfaces and to the wells, were ultracentrifuged at 100,000×g for 1 h to precipitate MVs.
Statistical Analysis
Data are expressed as mean ± standard deviation (SD). Statistical differences among different cell treatments were calculated by one-way repeated measures ANOVA with Newman-Keuls multiple comparisons test or by paired t test. Statistical significance was defined as P < 0.05. Analysis and graphing of the data were performed using GraphPad Prism™ 7 software (La Jolla, CA, USA).
RESULTS
Ab cross-linking of CD14 and CD32 induces MerTK phosphorylation
Among human circulating monocytes, the CD16+ subsets are considered more mature cell types which can recognize and phagocytose ANs [12]. While CD14dimCD16+ (“non-classical”) monocytes mostly utilize CD36 to uptake ANs [12], the minor population of M2c-like CD14brightCD16+ (“intermediate”) monocytes expresses and utilizes MerTK to phagocytose ANs [2]. We asked whether MerTK activation in monocytes was itself favored by the engagement of CD14 or CD16. To address this question, we coincubated monocytes in the presence or absence of purified anti-CD14 mAb (clone M5E2) or anti-CD16 mAb (clone 3G8), and measured MerTK phosphorylation by western blot. We found that Ab engagement of CD14, but not CD16, provoked MerTK phosphorylation. This phenomenon was more obvious when cell cultures were secondarily co-incubated with a GAM (H + L) Ab to further cross-link the anti-CD14 Abs (Fig. 1a).
Antibody cross-linking of CD14, but not CD16, on human monocytes elicits MerTK activation. a Fresh human monocytes were plated and soon co-incubated in the presence of mouse anti-human CD14 (clone M5E2) or anti-CD16 (clone 3G8) monoclonal antibodies (mAbs). When indicated, after 20 min, cells were co-incubated with a goat anti-mouse (GAM) Ab (H + L) (15 μg/ml), for an additional 20 min. Phosphorylated MerTK (P-Mer) was then studied by western blot, and densitometric areas of P-Mer bands were calculated. b Viability of monocytes in 48-h cell cultures, in the presence or absence of dexamethasone (100 nM), was quantified by flow cytometry. Viable cells showed higher forward scatter (FSC), and were double negative for annexin-V (early and late apoptosis marker) and propidium iodide (late apoptosis and necrosis marker). For better clarity, the experiment shown also includes a 96-h assessment of living and dying cells. Data are representative of three independent experiments.
We then expanded our study to include a panel of surface receptors known to be induced by M-CSF and IL-10 or glucocorticoids, and to be specifically upregulated on MerTK-expressing M2c monocytes/macrophages, thereby including CD163, CD204, and CD32, in addition to CD14 and CD16 [2, 13]. In these experiments, we cultured cells in the presence of dexamethasone in order to upregulate M2c receptors on their membranes and obtain more signal in case Ab-mediated stimulation had induced MerTK phosphorylation. To keep good viability in serum-free conditions, cells were cultured for 2 days only (Fig. 1b). Also, in this set of experiments, we confirmed that Ab cross-linking of CD14 led to MerTK phosphorylation (Fig. 2a). A lesser degree of phosphorylation of MerTK was also observed in the presence of the mouse IgG control. In this regard, phosphorylation of human MerTK was also previously reported in the presence of rat IgG [6]. In these cases, MerTK activation was likely mediated by Fc-gamma receptors (FcγRs); specifically, we observed that it was the cross-linking of IgG receptor CD32 or FcγRII (by means of anti-CD32 mAb clone FUN-2) to account for a certain amount of MerTK phosphorylation (Fig. 2a). On the other hand, because control IgG purified from mouse serum is aggregated and polyclonal, it might have a more potent effect than the IgG monoclonals for which it served as control. In contrast, MerTK activation was not seen upon CD16 (FcγRIII) cross-linking, while we did not test the effects of CD64 (FcγRI) cross-linking, since this receptor is not upregulated under M2c polarizing conditions [13]. Contrary to what was previously reported in mice [6], no synergism between CD204 and MerTK was observed, nor we detect any MerTK phosphorylation by cross-linking CD163. In our hands, Ab cross-linking of MerTK (by means of anti-MerTK Ab clone 125518) provoked the appearance of multiple bands at lower molecular weights (MWs) corresponding to the MWs of poorly or non-glycosylated MerTK isoforms [14,15,16], thus suggesting that membrane receptor internalization and deglycosylation may have occurred upon engagement (Fig. 2a).
Antibody cross-linking of either CD14 or CD32 on M2c cells induces a significant increase in MerTK phosphorylation, irrespective of the antibody clones and isotypes used. a Monocytes were cultured in the presence of M-CSF (50 ng/ml) and dexamethasone (100 nM) for 48 h; subsequently, cells were co-incubated with anti-MerTK (clone 1255128), anti-CD204 (clone 351620), anti-CD163 (clone EDHu-1), anti-CD14 (clone M5E2), anti-CD16 (clone 3G8), or anti-CD32 (clone FUN-2) mAbs or mouse (m)IgG control (5 μg/ml). b, c Monocytes were cultured in the presence of dexamethasone (100 nM) for 48 h; cells were subsequently co-incubated in (b) with different anti-CD14 mAbs (clone M5E2 mIgG2a and clone HCD14 mIgG1) and in (c) with different anti-CD32 mAbs (clone FUN-2 mIgG2b and clone 3D3 mIgG1) (5 μg/ml) for 20 min; cells were finally co-incubated with GAM (H + L) (15 μg/ml), for an additional 20 min. Phosphorylated MerTK (P-Mer) was then studied by western blot, and densitometry of P-Mer bands was calculated. d, e Densitometric values of P-Mer bands were assessed in unstimulated conditions and upon stimulation with anti-CD14 (d) or anti-CD32 (e) and IgG controls, and corrected for beta-actin densitometry. The MerTK phosphorylating effect of Ab cross-linking was calculated as fold increase in P-Mer densitometry compared to unstimulated conditions (mean ± standard deviation). Note: as one western blot sample of the three experiments represented in (e) had particularly low background and the P-Mer densitometry of unstimulated cells was very low (i.e., around 1/200 compared to the area percent values of the other two experiments), we arbitrarily multiplied the corresponding densitometric absolute value by 200, in order to attenuate data variability and detect potential statistical significances among the pooled data. Each set of data is representative of three independent experiments. Double asterisks indicate P < 0.01. Asterisk indicates P < 0.05.
MerTK phosphorylation occurs irrespective of the clone and isotype used for Ab cross-linking, and is statistically significant
We wanted to rule out the possibility that CD14 and CD32 cross-linking effects on MerTK phosphorylation were dependent on the clone or the isotype of the antibodies used. For this purpose, we repeated the experiments using other anti-CD14 and anti-CD32 mAbs targeting different epitopes (clones HCD14 and 3D3, respectively) and possessing the poorest Fc receptor binding affinity isotype (i.e., IgG1 class). Even in these cases, either CD14 or CD32 stimulation was confirmed to provoke intense MerTK phosphorylation (Fig. 2b, c). Whereas MerTK phosphorylation induced by anti-CD32 Abs was similar to that obtained by control IgG (Fig. 2c, e), probably due to control IgG-induced MerTK phosphorylation via CD32, MerTK phosphorylation induced by anti-CD14 Abs was visibly and statistically stronger compared to unstimulated and control IgG-stimulated conditions (Fig. 2b, d), while MerTK phosphorylation induced by control IgG was not statistically different compared to unstimulated conditions (Fig. 2d, e).
MerTK can be phosphorylated in both its glycoforms, localized at 175–205 KDa and 135–155 kDa
The MW of MerTK varies depending on glycosylation rates, and at least two differentially glycosylated isoforms of MerTK are well recognized [14, 15]. The standard, fully glycosylated, membrane MerTK isoform (or full MerTK glycoform) is clearly expressed on the surface of macrophages [16] and is upregulated by M2c polarizing conditions (e.g., M-CSF + IL-10 or serum, IL-10 + GM-CSF or IL-17, glucocorticoids, PPARγ-antagonist GW9662), as previously assessed by flow cytometry in non-permeabilized cells [1, 2, 17]. The partially glycosylated MerTK isoform (or partial MerTK glycoform) is instead poorly expressed on the cell surface, and is upregulated by prolonged cell exposure (> 2–4 h) to exogenous Gas6 [16] or by discrete cytokine microenvironments (e.g., IL-4 + M-CSF or GM-CSF, IL-10 + M-CSF or GM-CSF, glucocorticoids + IL-4 or IL-17) previously reported to stimulate endogenous Gas6 release by monocytes/macrophages [1, 2]. Also, it might be possible that certain amounts of the partial glycoform are generated upon internalization and deglycosylation of the membrane MerTK (Fig. 2a). During our present experiments, MerTK phosphorylation was observed at the 175–205 kDa glycoform level (Figs. 1a and 2c) or in both 175–205 and 135–155 kDa glycoforms (Fig. 2a, b). Presumably, slight differences in the timing and/or duration of Ab stimulation among the several experiments could account for variable MerTK internalization and/or glycosylation rates. To further demonstrate that the 135–155 kDa bands we observed were truly corresponding to the phosphorylated partial MerTK glycoform, we stimulated MerTK by co-incubating (for 40 min only) cell cultures with either MerTK ligands rhGas6 and protein S-containing serum, or with a polyclonal MerTK Ab, and then looked at MerTK glycoform phosphorylation. Detection of phosphorylated 135–155 kDa MerTK bands was observed in the presence of either bioactive concentrations of protein S (contained in 10%, not 2%, human AB serum-supplemented medium) or rhGas6. Accordingly, addition of an anti-Gas6 neutralizing antibody attenuated the 135–155 kDa band intensity (Fig. 3a). On the other hand, the polyclonal anti-MerTK Ab induced the 175–205 kDa MerTK glycoform phosphorylation.
The C-terminal phospho-MerTK antibody is more sensitive than the N-terminal MerTK antibody in detecting the (poorly N-glycosylated) partial MerTK glycoform. a Monocytes were cultured in the presence of M-CSF (50 ng/ml) and dexamethasone (100 nM) for 48 h. Cells were then stimulated with increasing amounts of (protein S containing) AB serum (2–10%), or with recombinant human Gas6 (rhGas6, 1 μg/ml), in the presence or absence of a neutralizing Gas6 Ab (10 μg/ml), or with a polyclonal anti-MerTK Ab or an appropriate isotype control (5 μg/ml), for 40 min. b Monocytes were cultured in M1 conditions in the presence of GM-CSF (100 ng/ml); in some experiments, IL-4 (20 ng/ml) or IL-10 (37.5 ng/ml) was added to switch cells into M2a or M2c conditions. MerTK expression and phosphorylated MerTK (P-Mer) were then studied by western blot. Shorter or longer exposure refers to duration of film exposure during immunoblot development. The figure represents two independent experiments.
In contrast to what was observed using the C-terminal phospho-MerTK Ab, the partial MerTK glycoform was not easily detectable by the N-terminal MerTK Ab in these 2-day serum-free cultured monocytes/macrophages. On the other hand, in our previous experiments conducted on cells cultured for 4–8 days or in the presence of serum-supplemented medium, such anti-MerTK antibody was instead able to recognize the smaller MerTK isoform [1]. Therefore, we presume that optimal detection of the partial MerTK glycoform by this antibody needs some post-translational modifications which may occur later during differentiation, and/or in the presence of certain nutrients contained in serum. Nevertheless, a longer film exposure still allowed the detection, by western blot, of the whole range of MerTK MWs encompassing both glycoforms (Fig. 3a).
As a further proof that MerTK glycoform binding affinity differed between the two antibodies, we noted that, by means of the N-terminal MerTK Ab, we could detect the full MerTK glycoform being upregulated by IL-10 (consistent with the previously reported IL-10-induced MerTK upregulation on cell surfaces by flow cytometry [2]), whereas, by means of the C-terminal phospho-MerTK Ab, we could detect the partial MerTK glycoform being strongly phosphorylated by IL-4 (consistent with the previously reported strong IL-4 induction of Gas6 release in cell culture supernatants [1, 2]) (Fig. 3b).
Altogether, we verified that the bands detected by the C-terminal phospho-MerTK Ab at both 175-205 and 135–155 kDa glycoform levels were actually corresponding to the phosphorylated MerTK glycoforms; however, the two detection antibodies we used preferentially recognized different MerTK glycoforms, and in 2-day serum-free cell cultures, the poorly N-glycosylated 135–155 kDa isoform (i.e., the partial MerTK glycoform) was probably not sufficiently modified (e.g., N-glycosylated) to be clearly recognized by the N-terminal MerTK detection Ab.
Ab cross-linking of CD14 improves macrophage clearance of apoptotic neutrophils
MerTK is crucial for anti-inflammatory clearance of ANs by M2c monocytes/macrophages [2]. A role for CD14 in tethering ACs has been clearly demonstrated for apoptotic lymphoid cells, but not for ANs [12, 18,19,20,21,22,23]. Since CD14 is upregulated in M2c conditions [2, 24], is co-expressed with MerTK, and is here found to promote MerTK activation, we wanted to further investigate whether CD14 stimulation favored the clearance of ANs as well. For this purpose, monocytes were differentiated into M1, M2a, and M2c macrophages, and then co-incubated with anti-CD14 mAb and GAM (H + L) Ab, prior to co-incubation with CFSE-labeled ANs. In fact, we observed that Ab cross-linking of CD14 increased the amounts of efferocytic (CFSE+) macrophages compared to non-crosslinked cells (Fig. 4a, b).
Antibody cross-linking of CD14 enhances macrophage clearance of apoptotic neutrophils. Macrophages were differentiated for 7 days in the presence of cytokines polarizing M1 (IFNγ 2.5 ng/ml), M2a (IL-4 20 ng/ml), or M2c (M-CSF 50 ng/ml and IL-10 37.5 ng/ml) conditions. In some experiments, macrophages were pre-treated with anti-CD14 Ab (clone M5E2) or IgG control (5 μg/ml) for 20 min, and secondly with GAM (H + L) (15 μg/ml) for 20 min, prior to co-incubation with apoptotic neutrophils (ANs). Macrophages were then co-incubated with ANs, at a 1:5 ratio, for 60 min. Efferocytic cells were measured by flow cytometry as percentages of CD14-labeled macrophages that phagocytosed CFSE-labeled ANs. a Representative dot plots are shown. Dashed lines and small red arrows indicate CD14bright efferocytic cell subsets in M1 and M2a conditions. b Representative histograms referring to CD14+ cell gates (depicted in a) are shown, whereby peaks on the right correspond to percentages of CSFE+ efferocytic macrophages. c Data representative of two independent experiments are shown as mean ± standard deviation. Changes in percentages of efferocytic cells among CD14-stimulated and unstimulated cells are also calculated.
We had previously shown that, even in M1 and M2a polarizing conditions, minority populations of CD14brightMerTK+ M2c-like cells still emerged [2]. Accordingly, here we found that Ab cross-linking of CD14 enabled macrophages to clear ANs efficiently even under M1 and M2a differentiating conditions. In these settings, in fact, the efferocytic (CSFE+) macrophages powered by cross-linking CD14 were characterized by a brighter expression of CD14 (Fig. 4a), and the gain of efferocytosis in M1 and M2a conditions was indeed superior to that acquired under M2c or unpolarized conditions (≅ + 60% versus + 20%) (Fig. 4c).
Taken together, CD14 acts to phosphorylate MerTK and to foster the clearance of ANs, thus facilitating human macrophages to reach the optimal efferocytic capacity that is characteristic of specialized M2c cells.
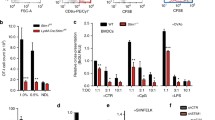
LPS reshapes the CD14/Syk endocytosis pathway by suppressing MerTK in CD14bright M1 cells, whereas it poorly affects MerTK expression in M2c cells and M2c-derived microvesicles. a Syk expression and phosphorylation (P-Syk) was assessed in monocytes, cultured for 48 h in the presence of M-CSF (50 ng/ml) and dexamethasone (100 nM), upon Ab cross-linking of M2c receptors. b CD14 and MerTK expression was studied by flow cytometry in M1 (GM-CSF 100 ng/ml) and M2 (M-CSF 50 ng/ml) conditions, in the presence or absence of LPS (10 μg/ml). Arrows indicate disrupted MerTK expression following LPS exposure in M1 conditions, occurring specifically in the CD14bright cell subset. c M2c macrophages were differentiated for 7 days in the presence of M-CSF (50 ng/ml) and 10% human AB serum, with or without LPS (10 μg/ml) or unesterified cholesterol (UC) (100 μg/ml) for an additional 24 h. Microvesicles (MVs) were isolated by ultracentrifugation of cell culture supernatants at 100,000×g for 1 h. MerTK expression was then studied in M2c cells and in constitutively released M2c MVs by western blot. The figure represents three independent experiments.
LPS redirects the CD14/Syk endocytosis pathway by suppressing MerTK in CD14bright M1 cell subsets, yet it poorly impairs MerTK in M2c cells and M2c-derived MVs
CD14 is an anchored—not a transmembrane—surface molecule [25]; therefore, it needs to recruit downstream docking molecules for signaling transduction. Among these, in the presence of LPS, CD14 was shown to recruit spleen tyrosine kinase (Syk), which is needed for toll-like receptor (TLR) 4 endocytosis and TIR-domain-containing adapter-inducing interferon-β (TRIF) activation [26]. CD32 is also known to signal via Syk, which is required for FcγR-induced phagocytosis [27, 28]. Here, we observed that, even in the absence of LPS, Ab cross-linking of CD14 could still activate Syk, as could cross-linking of CD32 (Fig. 5a).
On the other hand, in the presence of LPS, a disintegrin and metallopeptidase domain 17 (ADAM17) has been reported to cleave surface MerTK into soluble Mer ectodomain (sMer) [29]. Here, we pointed out that co-incubation of cells with LPS under inflammatory M1 differentiating conditions (i.e., in the presence of GM-CSF and IFNγ) abolished surface expression of MerTK specifically in the minor CD14bright cell population, whereas the expression of its co-receptor CD14 was preserved (Fig. 5b). Altogether, by disrupting the anti-inflammatory CD14/MerTK axis in CD14bright cells, LPS may thus redirect the CD14/Syk endocytosis pathway towards proinflammatory TLR4 internalization rather than anti-inflammatory AC phagocytosis.
In M2c conditions (e.g., in the presence of M-CSF and IL-10, or M-CSF and serum), MerTK was instead brightly expressed along with CD14 on the membranes of the great majority of cells (Fig. 5b), and its expression was only partially downregulated by LPS (Fig. 5c). Remarkably, MerTK was also detected in M2c macrophage-derived MVs. Of note, LPS partially reduced the amounts of the full MerTK glycoform expressed on cell surfaces, but did not impair the partial MerTK glycoform discovered in M2c MVs (Fig. 5c). LPS is known to induce macrophage release of proinflammatory MVs [30,31,32,33]. Here, LPS downregulation of MerTK on cell surfaces proved not to be related with increased release of MerTK into MVs (Fig. 5c). Also, unesterified cholesterol (UC) was previously shown to induce proinflammatory MVs, at least in part, by eliciting macrophage apoptosis [34]. By light microscopy, we did observe a clear secretion of MVs or apoptotic debris following UC treatment (not shown), yet these particles did not express MerTK, as their presence did not enhance global MerTK expression in MVs. In fact, by treating macrophages with either LPS or UC, the amounts of MerTK+ MVs constitutively released by M2c cells were not significantly altered (Fig. 5c). Taken together, here, we revealed the existence of MerTK+ M2c MVs, which seem to differ from LPS and UC-induced proinflammatory MVs.
DISCUSSION
Apart from recognizing pathogen-associated molecular patterns (PAMPs) and serving as LPS co-receptor [35], CD14 is believed to recognize putative AC-associated molecular patterns (ACAMPs), likely certain phosphatidylinositides exposed on the AC surface [23, 36, 37], and act in a phosphatidylserine (PS)-independent manner to tether ACs, a first step in the efferocytic process that precedes AC engulfment [38,39,40]. By using discrete blocking anti-CD14 mAb clones, CD14 was previously shown to tether apoptotic lymphoid cells of either human or murine T and B cell lines [18,19,20], yet not human ANs [12, 21,22,23]. MerTK is crucially involved in both tethering and engulfment of ACs, and is key in regulation of innate immunity [2, 41, 42]. It binds to ACs in a PS-dependent manner, by means of protein S or Gas6 [3, 10, 43], and is required for phagocytosis of both apoptotic lymphoid cells [41, 42] and ANs [1,2,3, 10]. Although the Mer kinase activity is necessary only for AC engulfment, MerTK probably already undergoes phosphorylation during the tethering stage [10]. CD14 KO mice show impaired AC recognition and binding, and accumulate large amounts of ACs in multiple tissues [44]. However, unlike MerTK Kd mice, whose tyrosine kinase activity, AC engulfment, and anti-inflammatory transduction signal are impaired [41, 42], these mice do not develop autoimmunity [44], thus confirming that CD14’s role in anti-inflammatory clearance of ACs is complementary to the central role of MerTK.
Here, we discovered that co-expression of CD14 and MerTK, a condition that occurs in human M2c macrophages, as well as in minor M2c-like subsets such as CD14bright M1 and M2a cells and circulating CD14brightCD16+ monocytes [1, 2], predisposes the cooperation between these two receptors, apt to ensure an efficient clearance of ANs. Specifically, we show that CD14 engagement, by means of Ab cross-linking, provokes Syk activation and MerTK phosphorylation. This event is associated with increased macrophage phagocytosis of ANs, to the extent that even cells obtained in M1 and M2a conditions reach the optimal efferocytic capacity of specialized M2c cells. As is seen for conventional M2c cells, in fact, the efferocytic M2c-like macrophage subsets observed in M1 and M2a conditions show a brighter expression of CD14 compared to the other cells. In our experimental conditions, Ab cross-linking would then mimic certain PS-independent interactions between CD14 and ACAMPs that might concur to stabilize PS and MerTK-dependent binding to early ACs and, in any case, act to promote MerTK phosphorylation and efficient phagocytosis. Therefore, although CD14 seems not to be directly involved in tethering ANs [12, 21,22,23], it still has a role in the clearance of ANs by human M2c or M2c-like cells through its cooperation with MerTK. Because our lab is now no longer active, we could not assess the direct role of MerTK in CD14-stimulated clearance of ANs, e.g., by using MerTK inhibitors. Nevertheless, since we showed that CD14 cross-linking stimulates MerTK activation, that CD14 cross-linking elicits AC clearance, and that MerTK is key for AC clearance, we conclude that CD14 cross-linking is likely to elicit AC clearance via MerTK. Such interaction might also concern other myeloid phagocytes that express CD14 and utilize MerTK for AC clearance, like dendritic cells (DCs) [45] and microglia [46].
The downstream molecule Syk, so far known to mediate CD14-dependent endocytosis in the presence of LPS [26], is here shown to be activated by CD14 even in the absence of LPS. In M1 conditions, however, the presence of LPS abolishes membrane expression of MerTK in the efferocytic CD14bright cells, while preserving CD14 and Syk expression. Therefore, in this context, LPS disrupts the anti-inflammatory CD14/MerTK axis by redirecting the CD14/Syk endocytosis pathway towards TLR4 internalization and activation of the endosomal TLR pathway [26], rather than AC phagocytosis.
Although LPS downregulates surface MerTK and promotes TNFα secretion, in both cases by activating ADAM17 [29, 47,48,49], on the other hand, MerTK continues to signal even in the presence of LPS. When Gas6 is co-added to LPS, in fact, MerTK acts to mitigate LPS-stimulated TNFα production [50, 51], while enhancing LPS-stimulated IL-10 production [2]. It was shown that Gas6 is able to induce de novo synthesis of a poorly N-glycosylated MerTK isoform, which is barely expressed on cell surfaces [1, 16], and is thereby scarcely susceptible to LPS-triggered cleavage. Here, we show that Gas6 specifically phosphorylates the partial MerTK glycoform, while Ab cross-linking of CD14 induces phosphorylation of both glycoforms. Thus, the Gas6/MerTK axis counterbalances the proinflammatory effects of LPS stimulation and might interfere with LPS through a CD14-mediated phosphorylation of the Gas6-inducible partial MerTK glycoform. In fact, both surface and intracellular MerTK glycoforms were shown to localize into nuclei and modify gene expression [16]. Schematic representations of the interactions herein suggested to occur between CD14 and MerTK, in the presence of ACs, LPS, and/or Gas6, are illustrated in Fig. 6.
MerTK cooperation with CD14 in anti-inflammatory conditions. a In M2c polarizing conditions, such as in the presence of M-CSF, IL-10, and/or glucocorticoids (GCs) [2], CD14 engagement by certain apoptotic cell (AC)-associated molecular patterns (ACAMPs), possibly some phosphoinositides exposed on the AC surface [23, 36], leads to Syk activation and MerTK phosphorylation, thereby favoring phosphatidylserine-dependent, Gas6 or protein S mediated, clearance of ACs by MerTK. Collaterally, by stimulating autocrine Gas6 release [1, 2], M2c polarizing agents, as well as M2a cytokine IL-4, also stimulate macrophage production of the partial MerTK glycoform, which is distributed intracellularly and less expressed on the cell membrane [16]. Both full and partial MerTK glycoforms can be phosphorylated upon CD14 engagement. In anti-inflammatory conditions, the CD14/Syk pathway would therefore serve to foster MerTK-driven AC endocytosis by M2c (or M2c-like) cells. b In M1 polarizing conditions, such as in the presence of LPS, CD14 engagement by the pathogen-associated molecular pattern (PAMP) LPS itself leads to Syk activation as well, but in this case, the CD14/Syk pathway will serve to foster LPS-driven TLR4 endocytosis [26]. This step is required for TLR4 to induce the TRIF-dependent signal leading to IFNα production [26], while membrane TLR4 activates the MyD88-dependent cascade that accounts for IL-6 and proTNFα production. In proinflammatory conditions, LPS also stimulates membrane sheddase ADAM17 to cleave both proTNFα, so allowing TNFα secretion [49], and full MerTK glycoform ectodomain, so generating soluble Mer (sMer) [29] and disrupting the CD14-MerTK axis driving AC clearance. CD14 interactions with either ACAMPs (e.g., phosphoinositides) or PAMPs (i.e., LPS) would therefore provoke opposite and mutually exclusive effects in monocytes/macrophages, with CD14 being placed between M2c-related MerTK-dependent response and M1-related TLR4-dependent response, respectively. c In M2c cells, Gas6 enhances LPS-stimulated IL-10 production [2], while it inhibits LPS-stimulated TNFα production [2, 50, 51]. These effects might be due to LPS binding to CD14, which can in turn promote, possibly via Syk, the phosphorylation of the Gas6-induced partial MerTK glycoform (i.e., mostly intracellular and non-susceptible to LPS-triggered ectodomain cleavage), so that the latter [16] can translocate to the nucleus and modulate gene expression. Furthermore, the partial MerTK glycoform may exert additional anti-inflammatory effects from inside the microvesicles (MVs) that are constitutively released by M2c macrophages.
Remarkably, for the first time, we also report that MerTK, specifically its partial glycoform, is present in M2c macrophage-derived MVs. In M2c conditions, LPS partially downregulates the full MerTK glycoform expression on cell surfaces, but does not affect the MV-associated isoform. By stimulating macrophage activation or apoptosis [32, 34], LPS and unesterified cholesterol (UC) are known to induce macrophage release of proinflammatory, proapoptotic, and oxidative MVs [30,31,32,33,34, 52]. Here, we observed that neither LPS nor UC increase the amounts of MerTK+ MVs, which are constitutively released by M2c cells. In accordance with the anti-inflammatory, anti-apoptotic, and anti-oxidant roles exerted by MerTK [2, 53], MerTK+ M2c MVs would thus be antithetic to LPS or UC-induced MVs. Nonetheless, the mechanisms by which MerTK is released into M2c MVs and therein acts remain obscure. Further investigation will be required to assess whether such MerTK+ MVs may transfer certain immune regulatory capacities to other cells or have a role, for instance, in determining free protein S levels in the circulation of lupus and HIV-positive patients [54,55,56], antagonizing complement and endothelial activation induced by other MVs [33, 52], modulating lipoprotein macrophage metabolism in metabolic syndrome and atherosclerosis [17], or competing for Gas6 with MerTK-expressing platelets and tumor-associated macrophages in regulation of platelet aggregation [57] and cancer immune surveillance [58], respectively.
Finally, in this study, we show that antibody cross-linking of CD32/FcγRII is able to activate MerTK. As for CD14, in fact, CD32 cross-linking phosphorylates Syk, which is known to be critical for FcγR-mediated phagocytosis [27, 28]. Whether Syk, previously shown to physically associate with CD32 as well as with the CD14/TLR4 complex [27, 59], may serve as an adaptor molecule for these receptors to recruit and activate MerTK is a fascinating hypothesis for which additional studies will be required. In favor of this hypothesis, CD14 interaction with ACs and CD14 activation of Syk can occur independently of TLR4 [26, 48]; moreover, Syk is required for LPS-stimulated macrophage production of IL-10 [59], a process that in M2c conditions is specifically enhanced by Gas6 via MerTK [2]. CD32 (FcγRII) is involved in pathological clearance of ACs in lupus [60, 61], probably mediated by M2b and/or altered M2c macrophages [55, 62]. In the presence of immune complexes, in fact, TLR agonists (e.g., LPS or DNA-containing immune complexes) and cytokines (e.g., TNFα) elicit M2b differentiation [62] and impair M2c activity through ADAM17-mediated cleavage of the M-CSF receptor [63], MerTK [29], and CD163 [64]. In the absence of MerTK, CD32 drives a proinflammatory clearance of antinuclear antibody-opsonized late ACs and remnants, leading to further release of TNFα and IL-6 from macrophages [60] and IFNα from DCs [65]. By contrast, in healthy conditions and in the absence of inflammation, CD32 may synergize with undisrupted MerTK, so fostering anti-inflammatory clearance of early ACs by intact M2c macrophages. Interestingly, ACs release sphingosine-1-phosphate (S1P), which in turn elicits macrophage production of IL-10 [66] and upregulates CD32 [67]. As for CD14, in fact, IL-10 is required for upregulation and co-expression of CD32 with MerTK in M-CSF-conditioned macrophages [2, 13], and may thus allow their cooperation in M2c cells. The presence or lack of interaction with MerTK may therefore account for two counterposed CD32 pathways, depicted in Fig. 7.
MerTK cooperation with CD32 in anti-inflammatory conditions. a In anti-inflammatory conditions, apoptotic cells (ACs) release sphingosine-1-phosphate (S1P), which induces CD32 on the macrophage surface [67]. At the same time, macrophage exposure to ACs activates liver X receptors (LXRs), thereby inducing MerTK [2, 17]. M2c polarizing agents, such as M-CSF and IL-10 or glucocorticoids, may further enable anti-inflammatory macrophages to co-express CD32 and MerTK [2, 13]. CD32 engagement by immunoglobulin (Ig)-opsonized ACs may then result, possibly through Syk activation, into MerTK phosphorylation and anti-inflammatory clearance of early ACs by M2c cells, which is associated with IL-10 production. b By contrast, in proinflammatory conditions and autoimmune diseases, toll-like receptor (TLR) agonists (e.g., LPS, ssDNA, dsDNA, or DNA-containing immune complexes) would enable ADAM17 sheddase to generate the soluble MerTK ectodomain (sMer) [29], thus inactivating MerTK and disrupting the CD32-MerTK axis. Accumulating ACs therefore stimulates the formation of autoantibodies (e.g., anti-DNA and anti-phospholipoproteins), so that autoantibody and immune complex-opsonized late ACs will be subject to a CD32-mediated proinflammatory clearance, exerted by M1/M2b macrophages or dendritic cells (DCs), and associated with TNFα, IL-6, and IFNα production [60, 62, 65].
In conclusion, the herein recognized interactions of MerTK with CD14 and CD32 further support the complex and central role of MerTK in efficient efferocytosis. Although CD14 serves as LPS co-receptor during acute bacterial inflammation, once bacteria have been neutralized and LPS cleared, cooperation between CD14 and MerTK may subsequently promote the prompt removal of dying and ANs, needed for complete resolution of inflammation and prevention of post-infectious autoimmune disorders. In addition, the CD14/MerTK interaction may represent the molecular basis through which the Gas6/MerTK axis modulates LPS-induced cytokine release, so preventing excessive inflammation and endotoxic shock mortality. CD14 is therefore implicated in both pathogen surveillance and tissue homeostasis, and stands at the crossroads between LPS-induced proinflammatory M1 activation and MerTK-dependent anti-inflammatory M2c response. Moreover, cooperation between CD32 and MerTK in glucocorticoid-treated M2c cells may explain the beneficial effects of glucorticoid therapy in autoimmune and immune-complex diseases, by promoting an early and anti-inflammatory, instead of a late and proinflammatory, clearance of Ig-opsonized ACs. Finally, detection of MerTK in M2c-derived MVs opens new scenarios for a deeper comprehension in the near future of the mechanisms by which MerTK and M2c cells regulate autoimmunity, infections, atherosclerosis, and cancer.
References
Zizzo, G., and P.L. Cohen. 2013. IL-17 stimulates differentiation of human anti-inflammatory macrophages and phagocytosis of apoptotic neutrophils in response to IL-10 and glucocorticoids. Journal of Immunology 190: 5237–5246.
Zizzo, G., B.A. Hilliard, M. Monestier, and P.L. Cohen. 2012. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. Journal of Immunology 189: 3508–3520.
McColl, A., S. Bournazos, S. Franz, M. Perretti, B.P. Morgan, C. Haslett, and I. Dransfield. 2009. Glucocorticoids induce protein S-dependent phagocytosis of apoptotic neutrophils by human macrophages. Journal of Immunology 183: 2167–2175.
Wu, Y., S. Singh, M.M. Georgescu, and R.B. Birge. 2005. A role for Mer tyrosine kinase in αvbeta5 integrin-mediated phagocytosis of apoptotic cells. Journal of Cell Science 118: 539–553.
Nishi, C., S. Toda, K. Segawa, and S. Nagata. 2014. Tim4- and MerTK-mediated engulfment of apoptotic cells by mouse resident peritoneal macrophages. Molecular and Cellular Biology 34: 1512–1520.
Todt, J.C., B. Hu, and J.L. Curtis. 2008. The scavenger receptor SR-A I/II (CD204) signals via the receptor tyrosine kinase Mertk during apoptotic cell uptake by murine macrophages. Journal of Leukocyte Biology 84: 510–518.
Galvan, M.D., D.B. Foreman, E. Zeng, J.C. Tan, and S.S. Bohlson. 2012. Complement component C1q regulates macrophage expression of Mer tyrosine kinase to promote clearance of apoptotic cells. Journal of Immunology 188: 3716–3723.
Liao, D., X. Wang, M. Li, P.H. Lin, Q. Yao, and C. Chen. 2009. Human protein S inhibits the uptake of AcLDL and expression of SR-A through Mer receptor tyrosine kinase in human macrophages. Blood 113: 165–174.
Hulsebus, H.J., S.D. O'Conner, E.M. Smith, C. Jie, and S.S. Bohlson. 2016. Complement component C1q programs a pro-efferocytic phenotype while limiting TNFα production in primary mouse and human macrophages. Frontiers in Immunology 7: 230.
Dransfield, I., A. Zagórska, E.D. Lew, K. Michail, and G. Lemke. 2015. Mer receptor tyrosine kinase mediates both tethering and phagocytosis of apoptotic cells. Cell Death & Disease 6: e1646.
Graham, D.K., T.L. Dawson, D.L. Mullaney, H.R. Snodgrass, and H.S. Earp. 1994. Cloning and mRNA expression analysis of a novel human protooncogene, c-mer. Cell Growth & Differentiation 5: 647–657.
Mikołajczyk, T.P., J.E. Skrzeczyńska-Moncznik, M.A. Zarebski, E.A. Marewicz, A.M. Wiśniewska, M. Dzieba, J.W. Dobrucki, and J.R. Pryjma. 2009. Interaction of human peripheral blood monocytes with apoptotic polymorphonuclear cells. Immunology 128: 103–113.
Hashimoto, S., M. Yamada, K. Motoyoshi, and K.S. Akagawa. 1997. Enhancement of macrophage colony-stimulating factor-induced growth and differentiation of human monocytes by interleukin-10. Blood 89: 315–321.
Feng, W., D. Yasumura, M.T. Matthes, M.M. LaVail, and D. Vollrath. 2002. Mertk triggers uptake of photoreceptor outer segments during phagocytosis by cultured retinal pigment epithelial cells. The Journal of Biological Chemistry 277: 17016–17022.
Graham, D.K., D.B. Salzberg, J. Kurtzberg, S. Sather, G.K. Matsushima, A.K. Keating, X. Liang, M.A. Lovell, S.A. Williams, T.L. Dawson, M.J. Schell, A.A. Anwar, H.R. Snodgrass, and H.S. Earp. 2006. Ectopic expression of the proto-oncogene Mer in pediatric T-cell acute lymphoblastic leukemia. Clinical Cancer Research 12: 2662–2669.
Migdall-Wilson, J., C. Bates, J. Schlegel, L. Brandão, R.M. Linger, D. DeRyckere, and D.K. Graham. 2012. Prolonged exposure to a Mer ligand in leukemia: Gas6 favors expression of a partial Mer glycoform and reveals a novel role for Mer in the nucleus. PLoS One 7: e31635.
Zizzo, G., and P.L. Cohen. 2015. The PPAR-γ antagonist GW9662 elicits differentiation of M2c-like cells and upregulation of the MerTK/Gas6 axis: a key role for PPAR-γ in human macrophage polarization. Journal of Inflammation (Lond) 12: 36.
Schlegel, R.A., S. Krahling, M.K. Callahan, and P. Williamson. 1999. CD14 is a component of multiple recognition systems used by macrophages to phagocytose apoptotic lymphocytes. Cell Death and Differentiation 6: 583–592.
Ogden, C.A., J.D. Pound, B.K. Batth, S. Owens, I. Johannessen, K. Wood, and C.D. Gregory. 2005. Enhanced apoptotic cell clearance capacity and B cell survival factor production by IL-10-activated macrophages: implications for Burkitt's lymphoma. Journal of Immunology 174: 3015–3023.
Xu, W., A. Roos, N. Schlagwein, A.M. Woltman, M.R. Daha, and C. van Kooten. 2006. IL-10-producing macrophages preferentially clear early apoptotic cells. Blood 107: 4930–4937.
Flora, P.K., and C.D. Gregory. 1994. Recognition of apoptotic cells by human macrophages: inhibition by a monocyte/macrophage-specific monoclonal antibody. European Journal of Immunology 24: 2625–2632.
Fadok, V.A., M.L. Warner, D.L. Bratton, and P.M. Henson. 1998. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (α v beta 3). Journal of Immunology 161: 6250–6257.
Devitt, A., S. Pierce, C. Oldreive, W.H. Shingler, and C.D. Gregory. 2003. CD14-dependent clearance of apoptotic cells by human macrophages: the role of phosphatidylserine. Cell Death and Differentiation 10: 371–382.
Hodrea, J., G. Majai, Z. Doró, G. Zahuczky, A. Pap, É. Rajnavölgyi, and L. Fésüs. 2012. The glucocorticoid dexamethasone programs human dendritic cells for enhanced phagocytosis of apoptotic neutrophils and inflammatory response. Journal of Leukocyte Biology 91: 127–136.
Haziot, A., X.Y. Lin, F. Zhang, and S.M. Goyert. 1998. The induction of acute phase proteins by lipopolysaccharide uses a novel pathway that is CD14-independent. Journal of Immunology 160: 2570–2572.
Zanoni, I., R. Ostuni, L.R. Marek, S. Barresi, R. Barbalat, G.M. Barton, F. Granucci, and J.C. Kagan. 2011. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell 147: 868–880.
Crowley, M.T., P.S. Costello, C.J. Fitzer-Attas, M. Turner, F. Meng, C. Lowell, V.L. Tybulewicz, and A.L. DeFranco. 1997. A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages. The Journal of Experimental Medicine 186: 1027–1039.
Pan, X.Q., C. Darby, Z.K. Indik, and A.D. Schreiber. 1999. Activation of three classes of nonreceptor tyrosine kinases following Fc gamma receptor crosslinking in human monocytes. Clinical Immunology 90: 55–64.
Thorp, E., T. Vaisar, M. Subramanian, L. Mautner, C. Blobel, and I. Tabas. 2011. Shedding of the Mer tyrosine kinase receptor is mediated by ADAM17 protein through a pathway involving reactive oxygen species, protein kinase Cδ, and p38 mitogen-activated protein kinase (MAPK). The Journal of Biological Chemistry 286: 33335–33344.
Satta, N., F. Toti, O. Feugeas, A. Bohbot, J. Dachary-Prigent, V. Eschwège, H. Hedman, and J.M. Freyssinet. 1994. Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. Journal of Immunology 153: 3245–3255.
Sarkar, A., S. Mitra, S. Mehta, R. Raices, and M.D. Wewers. 2009. Monocyte derived microvesicles deliver a cell death message via encapsulated caspase-1. PLoS One 4: e7140.
Distler, J.H., D.S. Pisetsky, L.C. Huber, J.R. Kalden, S. Gay, and O. Distler. 2005. Microparticles as regulators of inflammation: novel players of cellular crosstalk in the rheumatic diseases. Arthritis and Rheumatism 52: 3337–3348.
Braig, D., T.L. Nero, H.G. Koch, B. Kaiser, X. Wang, J.R. Thiele, C.J. Morton, J. Zeller, J. Kiefer, L.A. Potempa, N.A. Mellett, L.A. Miles, X.J. Du, P.J. Meikle, M. Huber-Lang, G.B. Stark, M.W. Parker, K. Peter, and S.U. Eisenhardt. 2017. Transitional changes in the CRP structure lead to the exposure of proinflammatory binding sites. Nature Communications 8: 14188.
Liu, M.L., M.P. Reilly, P. Casasanto, S.E. McKenzie, and K.J. Williams. 2007. Cholesterol enrichment of human monocyte/macrophages induces surface exposure of phosphatidylserine and the release of biologically-active tissue factor-positive microvesicles. Arteriosclerosis, Thrombosis, and Vascular Biology 27: 430–435.
Wright, S.D., R.A. Ramos, P.S. Tobias, R.J. Ulevitch, and J.C. Mathison. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249: 1431–1433.
Wang, P.Y., R.L. Kitchens, and R.S. Munford. 1998. Phosphatidylinositides bind to plasma membrane CD14 and can prevent monocyte activation by bacterial lipopolysaccharide. The Journal of Biological Chemistry 273: 24309–24313.
Akashi, S., H. Ogata, F. Kirikae, T. Kirikae, K. Kawasaki, M. Nishijima, R. Shimazu, Y. Nagai, K. Fukudome, M. Kimoto, and K. Miyake. 2000. Regulatory roles for CD14 and phosphatidylinositol in the signaling via toll-like receptor 4-MD-2. Biochemical and Biophysical Research Communications 268: 172–177.
Devitt, A., O.D. Moffatt, C. Raykundalia, J.D. Capra, D.L. Simmons, and C.D. Gregory. 1998. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature 392: 505–509.
Hoffmann, P.R., A.M. de Cathelineau, C.A. Ogden, Y. Leverrier, D.L. Bratton, D.L. Daleke, A.J. Ridley, V.A. Fadok, and P.M. Henson. 2001. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J Cell Biol. 155: 649–659.
Lingnau, M., C. Höflich, H.D. Volk, R. Sabat, and W.D. Döcke. 2007. Interleukin-10 enhances the CD14-dependent phagocytosis of bacteria and apoptotic cells by human monocytes. Human Immunology 68: 730–738.
Scott, R.S., E.J. McMahon, S.M. Pop, E.A. Reap, R. Caricchio, P.L. Cohen, H.S. Earp, and G.K. Matsushima. 2001. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411: 207–211.
Cohen, P.L., R. Caricchio, V. Abraham, T.D. Camenisch, J.C. Jennette, R.A. Roubey, H.S. Earp, G. Matsushima, and E.A. Reap. 2002. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. The Journal of Experimental Medicine 196: 135–140.
Shao, W.H., Y. Zhen, R.A. Eisenberg, and P.L. Cohen. 2009. The Mer receptor tyrosine kinase is expressed on discrete macrophage subpopulations and mainly uses Gas6 as its ligand for uptake of apoptotic cells. Clinical Immunology 133: 138–144.
Devitt, A., K.G. Parker, C.A. Ogden, C. Oldreive, M.F. Clay, L.A. Melville, C.O. Bellamy, A. Lacy-Hulbert, S.C. Gangloff, S.M. Goyert, and C.D. Gregory. 2004. Persistence of apoptotic cells without autoimmune disease or inflammation in CD14-/- mice. The Journal of Cell Biology 167: 1161–1170.
Sen, P., M.A. Wallet, Z. Yi, Y. Huang, M. Henderson, C.E. Mathews, H.S. Earp, G. Matsushima, A.S. Baldwin Jr., and R.M. Tisch. 2007. Apoptotic cells induce Mer tyrosine kinase-dependent blockade of NF-kappaB activation in dendritic cells. Blood 109: 653–660.
Healy, L.M., G. Perron, S.Y. Won, M.A. Michell-Robinson, A. Rezk, S.K. Ludwin, C.S. Moore, J.A. Hall, A. Bar-Or, and J.P. Antel. 2016. MerTK is a functional regulator of myelin phagocytosis by human myeloid cells. Journal of Immunology 196: 3375–3384.
Shiratsuchi, A., I. Watanabe, O. Takeuchi, S. Akira, and Y. Nakanishi. 2004. Inhibitory effect of Toll-like receptor 4 on fusion between phagosomes and endosomes/lysosomes in macrophages. Journal of Immunology 172: 2039–2047.
Thomas, L., A. Bielemeier, P.A. Lambert, R.P. Darveau, L.J. Marshall, and A. Devitt. 2013. The N-terminus of CD14 acts to bind apoptotic cells and confers rapid-tethering capabilities on non-myeloid cells. PLoS One 8: e70691.
Black, R.A., C.T. Rauch, C.J. Kozlosky, J.J. Peschon, J.L. Slack, M.F. Wolfson, B.J. Castner, K.L. Stocking, P. Reddy, S. Srinivasan, N. Nelson, N. Boiani, K.A. Schooley, M. Gerhart, R. Davis, J.N. Fitzner, R.S. Johnson, R.J. Paxton, C.J. March, and D.P. Cerretti. 1997. A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature 385: 729–733.
Camenisch, T.D., B.H. Koller, H.S. Earp, and G.K. Matsushima. 1999. A novel receptor tyrosine kinase, Mer, inhibits TNF-α production and lipopolysaccharide-induced endotoxic shock. Journal of Immunology 162: 3498–3503.
Alciato, F., P.P. Sainaghi, D. Sola, L. Castello, and G.C. Avanzi. 2010. TNF-α, IL-6, and IL-1 expression is inhibited by GAS6 in monocytes/macrophages. Journal of Leukocyte Biology 87: 869–875.
Liu, M.L., R. Scalia, J.L. Mehta, and K.J. Williams. 2012. Cholesterol-induced membrane microvesicles as novel carriers of damage-associated molecular patterns: mechanisms of formation, action, and detoxification. Arteriosclerosis, Thrombosis, and Vascular Biology 32: 2113–2121.
Anwar, A., A.K. Keating, D. Joung, S. Sather, G.K. Kim, K.K. Sawczyn, L. Brandão, P.M. Henson, and D.K. Graham. 2009. Mer tyrosine kinase (MerTK) promotes macrophage survival following exposure to oxidative stress. Journal of Leukocyte Biology 86: 73–79.
Suh, C.H., B. Hilliard, S. Li, J.T. Merrill, and P.L. Cohen. 2010. TAM receptor ligands in lupus: protein S but not Gas6 levels reflect disease activity in systemic lupus erythematosus. Arthritis Research & Therapy 12: R146.
Zizzo, G., J. Guerrieri, L.M. Dittman, J.T. Merrill, and P.L. Cohen. 2013. Circulating levels of soluble MER in lupus reflect M2c activation of monocytes/macrophages, autoantibody specificities and disease activity. Arthritis Research & Therapy 15: R212.
Gris, J.C., P. Toulon, S. Brun, C. Maugard, C. Sarlat, J.F. Schved, and J. Berlan. 1996. The relationship between plasma microparticles, protein S and anticardiolipin antibodies in patients with human immunodeficiency virus infection. Thrombosis and Haemostasis 76: 38–45.
Cosemans, J.M., R. Van Kruchten, S. Olieslagers, L.J. Schurgers, F.K. Verheyen, I.C. Munnix, J. Waltenberger, A. Angelillo-Scherrer, M.F. Hoylaerts, P. Carmeliet, and J.W. Heemskerk. 2010. Potentiating role of Gas6 and Tyro3, Axl and Mer (TAM) receptors in human and murine platelet activation and thrombus stabilization. Journal of Thrombosis and Haemostasis 8: 1797–1808.
Loges, S., T. Schmidt, M. Tjwa, K. van Geyte, D. Lievens, E. Lutgens, D. Vanhoutte, D. Borgel, S. Plaisance, M. Hoylaerts, A. Luttun, M. Dewerchin, B. Jonckx, and P. Carmeliet. 2010. Malignant cells fuel tumor growth by educating infiltrating leukocytes to produce the mitogen Gas6. Blood 115: 2264–2273.
Chaudhary, A., T.M. Fresquez, and M.J. Naranjo. 2007. Tyrosine kinase Syk associates with toll-like receptor 4 and regulates signaling in human monocytic cells. Immunology and Cell Biology 85: 249–256.
Hart, S.P., K.M. Alexander, and I. Dransfield. 2004. Immune complexes bind preferentially to Fc gamma RIIA (CD32) on apoptotic neutrophils, leading to augmented phagocytosis by macrophages and release of proinflammatory cytokines. Journal of Immunology 172: 1882–1887.
Muñoz, L.E., C. Janko, G.E. Grossmayer, B. Frey, R.E. Voll, P. Kern, J.R. Kalden, G. Schett, R. Fietkau, M. Herrmann, and U.S. Gaipl. 2009. Remnants of secondarily necrotic cells fuel inflammation in systemic lupus erythematosus. Arthritis and Rheumatism 60: 1733–1742.
Zhang, W., W. Xu, and S. Xiong. 2010. Blockade of Notch1 signaling alleviates murine lupus via blunting macrophage activation and M2b polarization. Journal of Immunology 184: 6465–6478.
Hiasa, M., M. Abe, A. Nakano, A. Oda, H. Amou, S. Kido, K. Takeuchi, K. Kagawa, K. Yata, T. Hashimoto, S. Ozaki, K. Asaoka, E. Tanaka, K. Moriyama, and T. Matsumoto. 2009. GM-CSF and IL-4 induce dendritic cell differentiation and disrupt osteoclastogenesis through M-CSF receptor shedding by up-regulation of TNF-α converting enzyme (TACE). Blood 114: 4517–4526.
Etzerodt, A., M.B. Maniecki, K. Møller, H.J. Møller, and S.K. Moestrup. 2010. Tumor necrosis factor α-converting enzyme (TACE/ADAM17) mediates ectodomain shedding of the scavenger receptor CD163. Journal of Leukocyte Biology 88: 1201–1205.
Båve, U., M. Magnusson, M.L. Eloranta, A. Perers, G.V. Alm, and L. Rönnblom. 2003. Fc gamma RIIa is expressed on natural IFN-α-producing cells (plasmacytoid dendritic cells) and is required for the IFN-α production induced by apoptotic cells combined with lupus IgG. Journal of Immunology 171: 3296–3302.
Weis, N., A. Weigert, A. von Knethen, and B. Brüne. 2009. Heme oxygenase-1 contributes to an alternative macrophage activation profile induced by apoptotic cell supernatants. Molecular Biology of the Cell 20: 1280–1288.
Duong, C.Q., S.M. Bared, A. Abu-Khader, C. Buechler, A. Schmitz, and G. Schmitz. 2004. Expression of the lysophospholipid receptor family and investigation of lysophospholipid-mediated responses in human macrophages. Biochimica et Biophysica Acta 1682: 112–119.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID), grant 5U19AI082726 (Philadelphia Autoimmunity Center of Excellence), by a bequest from Ms. B. Wicks, and by the Judith Shockman Memorial Fund.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zizzo, G., Cohen, P.L. Antibody Cross-Linking of CD14 Activates MerTK and Promotes Human Macrophage Clearance of Apoptotic Neutrophils: the Dual Role of CD14 at the Crossroads Between M1 and M2c Polarization. Inflammation 41, 2206–2221 (2018). https://doi.org/10.1007/s10753-018-0864-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0864-x