Abstract
Spilanthol a phytochemical derived from the Spilanthes acmella plant has antimicrobial, antioxidant, and anti-inflammatory properties. This study evaluated its effects on the expression of intercellular adhesion molecule 1 (ICAM-1) and inflammation-related mediators in IL-1β-stimulated human lung epithelial A549 cells. Human lung epithelial A549 cells were pretreated with various concentrations of spilanthol (3–100 μM) followed by treatment with IL-1β to induce inflammation. The protein levels of pro-inflammatory cytokines, chemokines, and prostaglandin E2 (PGE2) were measured using ELISA. Cyclooxygenase-2 (COX-2), heme oxygenase (HO-1), nuclear transcription factor kappa-B (NF-κB), and mitogen-activated protein kinase (MAPK) were measured by immunoblotting. The mRNA expression levels of ICAM-1 and MUC5AC were determined by real-time polymerase chain reaction. Spilanthol decreased the expression of PGE2, COX-2, TNF-α, and MCP-1. It also decreased ICAM-1 expression and suppressed monocyte adhesion to IL-1β-stimulated A549 cells. Spilanthol also significantly inhibited the phosphorylation of MAPK and I-κB. These results suggest that spilanthol exerts anti-inflammatory effects by inhibiting the expression of the pro-inflammatory cytokines, COX-2, and ICAM-1 by inhibiting the NF-κB and MAPK signaling pathways.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The airway inflammation associated with asthma is regulated by mutually interacting cytokines [1]. Asthma, a heterogeneous chronic inflammatory airway disease, is associated with interleukin-1β (IL-1β), a pro-inflammatory cytokine that drives inflammation and induces airway smooth muscle (ASM) responsiveness in asthma [2]. Studies have shown that IL-1β levels are elevated in the early phases of the inflammatory response and in the airways of patients with asthma, which show altered responsiveness [3]. IL-1β acts by upregulating adhesion molecules on endothelial cells and by inducing the synthesis of tumor necrosis factor-α (TNF-α) and MCP-1, both of which are cytokines. Notably, TNF-α causes airway hyper-responsiveness and stimulates ASM cells, thereby playing an important role in the pathogenesis of asthma [4].
In inflamed airway epithelium, ICAM-1 upregulation mediates increased eosinophil adhesion to the endothelium [5]. Specifically, when endothelial cells are exposed to the pro-inflammatory mediators TNF-α and IL-1β, ICAM-1 is upregulated on the endothelial cell surface [6]. Studies indicate that the inflammatory response is mediated by phosphatidylinositol 3-kinase (PI3K), protein kinase (PKC), and reactive oxygen species (ROS), leading to NF-κB activation [7]. Lung endothelial cell ICAM-1 mRNA expression is induced by NF-κB activation, suggesting that the induction of ICAM-1 is mediated by NF-κB in the A549 cell by IL-1β [8]. Notably, NF-κB acts as an activator of multiple cytokines, chemokines, and adhesion molecules that play important roles in inflammatory diseases such as asthma. Consequently, NF-κB is considered as an attractive therapeutic target in asthma [9]. Indeed, airway epithelial NF-κB activation is observed in asthma patients, implying that NF-κB is a critical modulator of inflammation in the pathogenesis of this lung disease [10, 11].
Airway epithelial NF-κB is a central mediator in the inflammatory and immune responses to infections and environmental insults that modulate allergic sensitization and the severity of subsequent allergic airway disease [12]. Furthermore, MCP-1 is involved in mast cell, eosinophil, and macrophage recruitment and promotes PGE2 generation. PGE2 is biosynthesized from arachidonic acid, showing that COX-2 is primarily responsible [13]. COX-2 can be induced by cytokines IL-β and TNF-α, which are involved in pathological processes such as cancer and inflammatory diseases and play important roles in the pathogenesis of asthma [14]. The MAPK pathways regulate the production of chemokines in primary epithelial cells [15]. Studies show that asthmatic patients have increased phosphorylated- (p)-ERK1/2, p-p38 (p-p38) and pJNK1/2/3 (pJNK) in their airway epithelium and smooth muscle cells [12]. Indeed, MAPK activity is significantly higher in the lungs of asthmatic mice than in normal controls [16].
A previous study reported that spilanthol has an anti-inflammatory effect in lipopolysaccharide (LPS)-stimulated macrophages [17]. We assumed that spilanthol could inhibit IL-1β-induced inflammatory responses and suppress ICAM-1 expression to reduce leukocyte adherence to lung epithelial cells. Hence, in this study, we evaluated the anti-inflammatory effect of spilanthol and the signaling mechanism of the NF-κB and MAPK pathways in IL-1β-induced A549 human lung epithelial cells.
MATERIALS AND METHODS
Materials
Figure 1a shows the chemical structure of spilanthol (ChromaDex, Irvine, CA, USA) which was prepared as a 100 mM stock solution in dimethyl sulfoxide (DMSO) and stored at − 20 °C. The final DMSO concentration was ≤ 0.1% in culture medium, as described previously. All chemicals and reagents were purchased from Sigma (St. Louis, MO, USA). Antibodies against β-actin, COX-2, and HO-1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against JNK, ERK, p38, phosphorylated (phospho)-JNK, phospho-ERK, and phospho-p38 were purchased from Millipore (Billerica, MA, USA). Enzyme-linked immunosorbent assay (ELISA) kits were obtained from R&D Systems (Minneapolis, MN, USA).
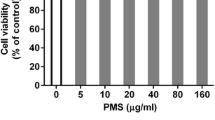
The chemical structure (a) and cytotoxicity of spilanthol in A549 cells (b). The effects of spilanthol on IL-1β-induced production of TNF-α (c) and MCP-1 (d). Cells (106 cells/well) were pretreated with the indicated concentrations of spilanthol (SP) for 1 h and then stimulated with IL-1β (1 ng/mL) for 24 h. The data are presented as the mean ± SD; *p < 0.05, **p < 0.01 compared with the IL-1β-treated group.
Cell Line and Treatment
The A549 human lung epithelial cell line was purchased from the Bioresource Collection and Research Center (BCRC, Taiwan) and cultured in F12 medium (Invitrogen-Gibco™, Paisley, Scotland) supplemented with 10% heat-inactivated fetal bovine serum (Biological Industries, Haemek, Israel), penicillin (100 units/mL), streptomycin (100 μg/mL), and 2 mM L-glutamine. All cells were incubated under a humidified atmosphere of 5% CO2 at 37 °C, and the cells were sub-cultured twice each week.
Cell Viability Assay
The inhibitory effect of spilanthol on cell viability was assessed using 3-(4,5-dimethylthiazol -2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma) as described previously [18]. In brief, cells (104 cells/well) were seeded in 96-well plates and treated with various concentrations of spilanthol for 24 h. After treatment, the supernatant was removed and incubated with 5 mg/mL MTT solution for 4 h. Then, the medium was removed, and isopropanol was added to dissolve the formazan crystals. Absorbance was measured at 570 nm with a microplate reader (Multiskan FC, Thermo Fisher Scientific, Waltham, MA, USA).
Measurement of Pro-inflammatory Cytokines, Chemokines, and PGE2
A549 cells (106 cells/mL) were cultured and pretreated with different concentrations of spilanthol (50–150 μ5) in 24-well plates for 1 h, then IL-1β (1 ng/mL) was added and the cells were cultured for an additional 24 h. The supernatants were tested using ELISA kits specific for MCP-1, TNF-α, PGE2, and ICAM-1 (R&D Systems, Minneapolis, MN, USA). The OD was determined spectrophotometrically at 450 nm in a microplate reader (Multiskan FC).
Preparation of Total and Nuclear Proteins
To assay the total protein and phosphorylated protein content, A549 (106 cells/mL) cells were pretreated with or without spilanthol for 1 h in 6-well plates. Then, the cells were stimulated with or without IL-1β (1 ng/mL) for 30 min (before assaying for protein phosphorylation) or for 24 h (to evaluate total protein. The cells were harvested in 300 μL protein lysis buffer (50 mM Tris-HCl, pH 8, 150 mM NaCl, 1 mM EDTA, 0.5% NP40, 0.1% SDS) containing protein inhibitor cocktail and phosphatase inhibitors (Sigma). Cells were also cultured for 1 h to detect NF-κB. To investigate the expression of nuclear proteins, cells were treated using the NE-PER® nuclear and cytoplasmic extraction reagent kits (Pierce, Rockford, IL, USA). All protein concentrations were measured with the BCA protein assay kit (Pierce).
Western Blot Analysis
Protein samples (10–30 μg) were loaded onto 10% SDS polyacrylamide gels, and the gels were transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The membranes were blocked and incubated overnight at 4 °C with the following primary antibodies: antibodies to HO-1, COX-2, IκB-α, phosphorylated-IκB-α, and p65 (Santa Cruz, CA, USA); to ERK1/2, p38, JNK, phospho-ERK 1/2, phospho-p38, and phospho-JNK (Millipore); and to ICAM-1 and β-actin (Sigma). After the membranes were washed three times in Tris-buffered saline with Tween 20 (TBST) buffer (150 mM NaCl, 10 mM Tris-HCl pH 8.0, 0.1% Tween 20), they were incubated with secondary antibodies for 1 h at room temperature. Next, the membranes were incubated with HRP-conjugated secondary antibodies for 1 h at room temperature. Finally, the membranes were washed with TBST and incubated with Luminol/Enhancer Solution (Millipore), exposed to film, and bands were quantitated using the BioSpectrum 600 system (UVP, Upland, CA, USA).
Real-Time PCR to Quantitate Gene Expression
The mRNA expression levels of ICAM-1 and MUC5AC were determined by real-time polymerase chain reaction (PCR) using the β-actin-encoding gene as an internal reference. Total RNA was prepared using TRIzol solution (Invitrogen, Carlsbad, CA, USA). RNA was isolated according to the manufacturer’s instructions. TaqMan real-time quantitative PCR was performed and analyzed according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA, USA). The primers used for amplification were as follows: for ICAM-1, forward 5′-AGA CGC AGA GGA CCT TAA-3′ and reverse 5’-CAC ACT TCA CAG TTA CTT GG-3′; for MUC5AC, forward 5’-CTG TTA CTA TGC GAT GTG TAG-3′ and reverse 5′-GTG GCG TGG TAG ATG TAG-3′; and for β-actin, forward 5′-AAG ACC TCT ATG CCA ACA CAG T -3′ and reverse 5′- AGC CAG AGC AGT AAT CTC CTT C -3′. The results are expressed as arbitrary units and normalized against β-actin mRNA expression.
Cell Adhesion Assay
A549 cells were treated with spilanthol for 1 h and incubated with 1 ng/mL IL-1β for 24 h. Then, human monocytic THP-1 cells in calcein AM solution (Sigma) were co-cultured with A549 cells for 1 h. Cells were washed and the adhesion of THP-1 cells to A549 cells was observed under fluorescence microscopy (Olympus, Tokyo, Japan).
Statistical Analysis
Data are reported as the mean ± standard deviation (SD). The significance of differences was assessed using one-way analysis of variance (ANOVA) and Tukey’s test. Differences were considered statistically significant at p < 0.05.
RESULTS
The Effects of Spilanthol on the Viability of Human Lung Epithelial A549 Cells
To determine the cytotoxicity of spilanthol in A549 cells, the MTT assay was used to measure cell viability. Spilanthol did not significantly affect cell viability at concentrations ≤ 150 μM (Fig. 1b). Therefore, all subsequent experiments used concentrations of spilanthol that ranged from 50 to 150 μM.
Pretreatment with Spilanthol Efficiently Inhibits Pro-inflammatory Cytokines and Chemokines in IL-1β-Induced A549 Cells
Spilanthol is reported to have anti-inflammatory effects on LPS-induced macrophages [18]. Therefore, we investigated whether spilanthol could suppress the inflammatory response in terms of affecting IL-1β-induced pro-inflammatory cytokine and chemokine production. A549 cells were pretreated with 50, 75, 100, and 150 μM spilanthol and stimulated with IL-1β (1 ng/mL) for 24 h. Analysis showed that IL-1β significantly induced the release of the inflammatory cytokine TNF-α and the chemokine MCP-1, and addition of spilanthol significantly suppressed the secretion of these inflammatory mediators at all concentrations of spilanthol compared with IL-1β alone. We found that 50–150 μM spilanthol significantly decreased TNF-α and MCP-1 levels compared with IL-1β alone (TNF-α; SP50 10.18 ± 0.45 ng/mL, p < 0.05; SP75 8.19 ± 0.16 ng/mL, p < 0.01; SP100 7.43 ± 0.15 ng/mL, p < 0.01; SP150 5.14 ± 0.11 ng/mL, p < 0.01 versus IL-1β alone 13.53 ± 0.25 ng/mL, respectively. MCP-1; SP50 10.12 ± 0.13 ng/mL, p < 0.05; SP75 10.11 ± 0.14 ng/mL, p < 0.05; SP100 9.21 ± 0.19 ng/mL, p < 0.01; SP150 8.65 ± 0.12 ng/mL, p < 0.01 versus IL-1β alone 13.41 ± 0.88 ng/mL, respectively). Further, the inhibitory effects of spilanthol on TNF-α and MCP-1 levels were dose-dependent (Fig. 1c, d).
Spilanthol Inhibits PGE2 Production and Affects COX-2 and HO-1 Protein Expression in IL-1β-Induced A549 Cells
To investigate the effect of spilanthol on COX-2 protein expression, A549 cells were pretreated with spilanthol, followed by stimulation with IL-1β for 24 h. Pretreatment with 75–150 μM spilanthol significantly inhibited COX-2 expression compared with IL-1β-stimulated cells that were not treated with spilanthol (Fig. 2a, b). In addition, spilanthol modulated the expression of HO-1, which has anti-inflammatory and anti-oxidant activity [19]. Specifically, ≥ 100 μM spilanthol concentrations increased HO-1 expression compared with IL-1β treatment alone (Fig. 2a, c). Spilanthol also significantly decreased the level of PGE2 compared to IL-1β alone (Fig. 2d).
The effects of spilanthol on IL-1β-induced production of COX-2 and HO-1 (a), COX-2 and HO-1relative protein expressions were measured relative to the expression of β-actin were detected by Western blots (n = 3 per group) in (b) (c), respectively. The effects of spilanthol on IL-1β-induced production of PGE2 (d). Data are presented as the mean ± SD; *p < 0.05, **p < 0.01 compared with the IL-1β-treated group.
The Effects of Spilanthol on IκBα Phosphorylation, and Suppression of IκB Degradation in IL-1β-Activated Human Lung Epithelial Cells
The NF-κB pathway plays a critical role in the inflammatory response by regulating the expression of inflammatory cytokines, including IL-1β, IL-6, and TNF-α. The rapid protease-mediated degradation of IκBα leads to NF-κB release from the cytoplasm into the nucleus [20]. Therefore, we investigated whether spilanthol could regulate NF-κB activity in A549 cells treated with IL-1β (Fig. 3a). Interestingly, spilanthol significantly decreased IκBα phosphorylation, and suppression IκB degradation which is an indication that spilanthol suppresses NF-κB transcriptional activation and translocation to the nucleus induced by IL-1β-stimulated in A549 cells (Fig. 3b, c, d).
The effects of spilanthol on IL-1β-induced the phosphorylation of IκBα, total IκB-α and NF-κB protein expressions (a). Phosphorylation of IκBα, total IκB-α and NF-κB relative protein expressions were measured relative to the expression of β-actin in (b) (c) (d), respectively. Phosphorylation of IκBα, total IκBα and NF-κB proteins were detected by western blots (n = 3 per group). Data are presented as the mean ± SD; *p < 0.05, **p < 0.01 compared with the IL-1β-treated group.
Spilanthol Inhibits MAPK Phosphorylation in IL-1β-Activated Human Lung Epithelial Cells
The MAPK signaling pathways regulate cellular activities and the release of pro-inflammatory cytokines. We investigated whether spilanthol could modulate MAPK signaling pathways, including the ERK1/2, p38, and JNK proteins, in A549 cells treated with IL-1β (Fig. 4a). We found that spilanthol significantly decreased the phosphorylation of ERK1/2, p38, and JNK in a concentration-dependent manner compared with IL-1β treatment alone (Fig. 4b, c, d). These results indicated that spilanthol modulated the activation of the MAPK signaling pathways in A549 cells that were treated with IL-1β.
The effects of spilanthol on the IL-1β-induced phosphorylation of MAPK. A549 cells were pretreated with varying concentrations of spilanthol (SP) for 1 h and then incubated with or without IL-1β (1 ng/mL) for 30 min. Protein samples were analyzed by Western blotting with phospho-specific antibodies (a). The phospho-specific JNK, ERK, and p38 relative protein expressions were measured relative to the expression of total MAPK levels were used as the internal control in (b) (c) (d), respectively. Data are presented as the mean ± SD; *p < 0.05, **p < 0.01 compared with the IL-1β-treated group.
Spilanthol Inhibited ICAM-1 and MUC5AC Expression in IL-1β-Stimulated Human Lung Epithelial Cells
Previous studies have demonstrated that the expression of ICAM-1 by inflammatory endothelial cells results in eosinophil adhesion [11]. Here, we found that spilanthol significantly reduced ICAM-1 production compared to IL-1β treatment alone (Fig. 5a). In addition, ≥ 50 μM spilanthol concentrations significantly suppressed ICAM-1 protein expression (Fig. 5b, c). Together, these results showed that spilanthol significantly decreased ICAM-1 production and suppressed its release into the cell culture medium. We used real-time PCR to assess gene expression and found that spilanthol significantly decreased the mRNA levels of ICAM-1 and MUC5AC in A549 cells (Fig. 5d, e).
The effects of spilanthol on the IL-1β-induced production of ICAM-1 and MUC5AC. A549 cells (106 cells/mL) were stimulated with IL-1β for 24 h, then the level of ICAM-1 in the supernatant was assayed by ELISA (a), the level of ICAM-1 protein in the cells was assayed by Western blot (n = 3 per group) (b), and ICAM-1 protein relative expressions were measured relative to β-actin (c). The cells were pretreated with the indicated concentrations of spilanthol (SP) for 1 h and then stimulated with IL-1β (1 ng/mL) for 4 h before assaying the ICAM-1 (d) and MUC5AC (e) gene expression levels, which were determined using real-time RT-PCR. Data are presented as the mean ± SD; *p < 0.05, **p < 0.01 compared with the IL-1β-treated group.
Spilanthol Suppresses Monocyte Adhesion to Human Lung Epithelial Cells
The human cell line THP-1 with monocytic properties can be active to macrophage-like cells in the inflammatory response. Activated THP-1 release more inflammatory mediators and adhesion molecules and caused cells adherence [27]. We found that spilanthol significantly reduced ICAM-1 gene expression in IL-1β-stimulated A549 cells (Fig. 5d). Therefore, we further investigated whether spilanthol inhibited monocyte adhesion to IL-1β-stimulated A549 cells (Fig. 6a–f). THP-1 cells were stained with calcein AM and co-cultured with IL-1β-induced A549 cells. We found that THP-1 cells adhered to IL-1β-activated A549 cells and that pretreatment with spilanthol significantly reduced this adhesion (Fig. 6g).
Spilanthol inhibits the adherence of THP-1 cells to activated A549 cells. THP-1 cells were labeled with calcein AM, mixed with A549 cells, and observed using fluorescence microscopy. The adherence of THP-1 cells to normal (a) and IL-1β-activated A549 cells (b) is shown. THP-1 cells were treated with 50 μM spilanthol (c), 75 μM spilanthol (d), 100 μM spilanthol (e), or 150 μM spilanthol (f). Fluorescence intensity of THP-1 cell adhesion to A549 cells (g). THP-1 cell adhesion in spilanthol treated cells were detected by fluorescence microscope (n = 3 per group). Data are presented as the mean ± SD; *p < 0.05, **p < 0.01 compared with the IL-1β-treated group.
DISCUSSION
When respiratory epithelial cells and macrophages are activated, they release pro-inflammatory cytokines increasing the secretion of chemokines and evoking an inflammatory response [20]. One study indicated that bacterial stimuli induce respiratory epithelial cells to secrete pro-inflammatory mediators such as IL-1β and TNF-α, which leads to upregulation of adhesion molecules [21]. There is also evidence that LPS-stimulated airway bronchiolar and alveolar epithelial cells induce NF-κB activation and increase ICAM-1 expression [22]. In adult respiratory distress syndrome (ARDS) the levels of pro-inflammatory cytokines TNF-α and IL-1β are increased. Notably, IL-1β is the major cytokine that maintains lung inflammation in ARDS [23]. Studies show that IL-1β is an important factor in inflammatory responses as well as in fibroproliferative processes in the lungs of adult patients with ARDS [24].
Spilanthol is the compound (2E,6Z,8E)-N-isobutylamide-2,6,8-decatrienamide and a physiologically active substances from Spilanthes acmella, which is known as the anti-toothache plant and which has anti-bacterial effects [25]. An aqueous extract of S. acmella has important anti-inflammatory and analgesic properties in animal models [26]. One study suggested that spilanthol attenuates the LPS-induced inflammatory responses in murine RAW 264.7 macrophages, in part due to the inactivation of NF-κB, which negatively regulates the production of proinflammatory mediators [27]. However, the anti-inflammatory effects of spilanthol have not been shown previously in human lung epithelial cells. In this study, we found that spilanthol can downregulate COX-2 production and decrease TNF-α and MCP-1 production in IL-1β-stimulated lung epithelial cells. In particular, spilanthol significantly decreased the phosphorylation of IκBα and MAPK pathways compared with IL-1β alone. These results indicated that spilanthol can inhibit the activation of NF-κB and MAPK pathways in IL-1β-activated A549 cells. We demonstrated hypothesis that spilanthol dampen the inflammatory effects of IL-1β in stimulated lung epithelial cells.
Earlier studies showed that a hydroethanolic extract of S. acmella has anti-inflammatory properties that could decrease the levels of the lipid peroxidation product malondialdehyde (MDA) and increase antioxidant enzyme activity (catalase and superoxide dismutase) [26, 28]. We found that spilanthol enhanced heme oxygenase-1 (HO-1) protein expression. HO-1 can regulate the balance of anti-inflammatory mediators and has antioxidant properties [29], prompting us to evaluate the anti-inflammatory molecular mechanisms underlying the effects of spilanthol. Our results showed that spilanthol significantly reduced COX-2 and PGE2 production in LPS-stimulated macrophages and IL-1β-activated human lung epithelial cells. We suggest that spilanthol may be useful as a COX-2 inhibitor to attenuate the inflammatory response. In addition, bronchoalveolar lavage fluid from patients with interstitial lung disease contains more MCP-1, TNF-α, and IL-8 than normal tissue [4]. We found that spilanthol significantly decreased TNF-α and MCP-1 in IL-1β-stimulated lung epithelial cells, suggesting that spilanthol might suppress the inflammatory response of lung epithelial cells and improve lung disease symptoms.
As part of their response to inflammation of lung epithelial cells express cell adhesion molecules including ICAM-1 and VCAM-1, which induce monocyte adherence and lead to neutrophil migration and infiltration into lung tissue [5]. Inflammatory mediators are released from activated monocytes and neutrophils, thereby increasing lung inflammation. In this study, real-time PCR and Western blot analysis showed that spilanthol suppressed ICAM-1 gene and protein expression. In addition, inflammatory epithelial cells release “soluble” intercellular adhesion molecule-1 (sICAM-1), which is a marker of inflammation and promote the inflammatory responses. In inflammation-relative diseases, as in autoimmune diseases, viral infections and bronchoalveolar lavage fluid of asthma patients serum sICAM-1 levels increase more than healthy individual. Inflammatory mediators (IL-1β, TNF-α, IL-6 and angiotensin II) could stimulate epithelial cells and endothelial cells to secrete sICAM-1 in circulating [30]. Inflammatory lung epithelial cells express ICAM-1 were cleaved to sICAM-1 and released into the supernatant. In this study, ELISA analysis showed that spilanthol decreased ICAM-1 levels. Although the roles of sICAM-1 have not been completely clear, the evidence suggests its implication in lung epithelial cells inflammatory progression at this works.
Further, to understand whether the spilanthol decrease in ICAM-1 expression could decrease lymphocyte adhesion to inflammatory lung epithelial cells, we co-cultured monocyte THP-1 cells with IL-1β-stimulated A549 cells. We found that spilanthol decreased THP-1 adherence to A549 cells compared with IL-1β treatment alone. Lung epithelial cells secrete excessive mucus as part of the inflammatory response, which obstructs the airway and leads to difficulty breathing and even to suffocation [6]. By preventing ICAM-1 expression, spilanthol decreases inflammatory leukocyte infiltration into lung tissue, possibly reducing mucus production. Comprehensive detections of sICAM-1, ICAM-1 gene and protein expression and THP-1 adherence to A549 cells indicated that spilanthol decreased ICAM-1 expression in adherent monocytes as well. We confirmed hypothesis that spilanthol could suppress ICAM-1 expression to reduce leukocyte adherence to lung epithelial cells.
To summarize, we found that spilanthol not only inhibited the levels of TNF-α and MCP-1, but it also suppressed COX-2 protein expression and promoted HO-1 protein expression by suppressing NF-κB activation and MAPK pathways in IL-1β-activated human lung epithelial cells. In addition, we found evidence that spilanthol decreased ICAM-1 expression in these cells. Based on these results, we propose a model that explains the anti-inflammatory effects of spilanthol (Fig. 7).
Model explaining the mechanism underlying the anti-inflammatory effects of spilanthol (SP). Spilanthol decreased the levels of pro-inflammatory cytokine (TNF-α) and chemokine (MCP-1), and inhibited COX-2 and ICAM-1 expression via suppression of the NF-κB and MAPK signaling in IL-1β-stimulated human lung epithelial cells. SP also inhibited leukocyte adherence to lung epithelial cells by reducing ICAM-1 expression via inhibition of the IL-1β pathway. Spilanthol is a potential anti-inflammatory compound that could ameliorate inflammatory in human lung epithelial cells.
CONCLUSION
We conclude that spilanthol, which is a natural anti-inflammatory agent, acts as a regulatory factor in MAPK pathways and in NF-κB activation of COX-2 and ICAM-1 expression. Further studies are needed to investigate its effects in vivo.
References
Adcock, I.M., and P.J. Barnes. 2008. Molecular mechanisms of corticosteroid resistance. Chest 134 (2): 394–401.
Gao, P., P.G. Gibson, K.J. Baines, I.A. Yang, J.W. Upham, P.N. Reynolds, S. Hodge, A.L. James, C. Jenkins, M.J. Peters, J. Zhang, and J.L. Simpson. 2015. Anti-inflammatory deficiencies in neutrophilic asthma: Reduced galectin-3 and IL-1RA/IL-1β. Respiratory Research 16: 5. https://doi.org/10.1186/s12931-014-0163-5.
Whelan, R., C. Kim, M. Chen, J. Leiter, M.M. Grunstein, and H. Hakonarson. 2004. Role and regulation of interleukin-1 molecules in pro-asthmatic sensitised airway smooth muscle. The European Respiratory Journal 24 (4): 559–567.
Schwingshack, A., M. Duszyk, N. Brown, and R. Moqbe. 1999. Human eosinophils release matrix metalloproteinase-9 on stimulation with TNF-alpha. The Journal of Allergy and Clinical Immunology 104 (5): 983–989.
Wegner, C.D., R.H. Gunde, P. Reilly, N. Haynes, L.G. Letts, and R. Rothlein. 1990. Intercellular adhesion molecule-1 (ICAM-1) in the pathogenesis of asthma. Science 247 (26): 456–459.
Min, J.K., Y.M. Kim, S.W. Kim, M.C. Kwon, Y.Y. Kong, and I.K. Hwang. 2005. TNF-related activation-induced cytokine enhances leukocyte adhesiveness: Induction of ICAM-1 and VCAM-1 via TNF receptor-associated factor and protein kinase C-dependent NF-B activation in endothelial cells. Journal of Immunology 175 (1): 531–540.
Hoesel, B. Schmid, J. A. 2013. The complexity of NF-κB signaling in inflammation and cancer. https://doi.org/10.1186/1476-4598-12-86.
Fakler, C.R., B. Wu, H.W. McMicken, R.S. Geske, and S.E. Welty. 2000. Molecular mechanisms of lipopolysaccharide induced ICAM-1 expression in A549 cells. Inflammation Research 49 (2): 63–72.
Neil, S.H., C.C. Matthew, M.C. Lisa, J.B. Peter, and N. Robert. 2004. ICAM-1 expression is highly NF-•B-dependent in A549 cells No role for ERK and p38 MAPK. European Journal of Biochemistry 271 (4): 785–791.
Pantano, C., J.L. Ather, J.F. Alcorn, M.E. Poynter, A.L. Brown, and A.S. Guala. 2008. Nuclear factor-kB activation in airway epithelium induces inflammation and hyperresponsiveness. American Journal of Respiratory and Critical Care Medicine 177 (9): 959–969.
Kato, A., and R.P. Schleimer. 2007. Beyond inflammation: Airway epithelial cells are at the interface of innate and adaptive immunity. Current Opinion in Immunology 19 (6): 711–720.
Jennifer, L.A., R.H. Samantha, M.W. Yvonne, H. Janssen, and E.P. Matthew. 2011. Airway epithelial NF-κB activation promotes allergic sensitization to an innocuous inhaled antigen. American Journal of Respiratory Cell and Molecular Biology 44 (5): 631–638.
Conti, P., and M. DiGioacchino. 2001. MCP-1 and RANTES are mediators of acute and chronic inflammation. Allergy and Asthma Proceedings 22 (3): 133–137.
Min, T., D. Yunfei, and H.H. Tai. 2006. Reciprocal regulation of cyclooxygenase-2 and 15-hydroxyprostaglandin dehydrogenase expression in A549 human lung adenocarcinoma cells. Carcinogenesis 27 (11): 2170–2179.
Liu, W., Q. Liang, S. Balzar, S. Wenzel, M. Gorska, and R. Alam. 2008. Cell-specific activation profile of extracellular signal-regulated kinase 1/2, Jun N-terminal kinase, and p38 mitogen-activated protein kinases in asthmatic airways. The Journal of Allergy and Clinical Immunology 12 (4): 893–902.
Wei, D., H.P.C. Jasmine, H.W. Chui, P.L. Bernard, and W. Fred. 2004. Anti-inflammatory effects of mitogen-activated protein kinase inhibitor U0126 in an asthma mouse model. Journal of Immunology 172 (11): 7053–7059.
Diasa, A.M.A., P. Santosa, I.J. Seabraa, R.N.C. Júniorc, M.E.M. Bragaa, and H.C. de Sousaa. 2012. Spilanthol from Spilanthes acmella flowers, leaves and stems obtained by selective supercritical carbon dioxide extraction. Journal of Supercritical Fluids 61: 62–70.
Wu, L.C., N.C. Fan, M.H. Lin, I.R. Chu, S.J. Huang, C.Y. Hu, and S.Y. Han. 2008. Anti-inflammatory effect of spilanthol from Spilanthes acmella on murine macrophage by down regulating LPS-induced inflammatory mediators. Journal of Agricultural and Food Chemistry 56 (7): 2341–2349.
Wu, S.J. 2015. Osthole attenuates inflammatory responses and regulates the expression of inflammatory mediators in HepG2 cells grown in differentiated medium from 3T3-L1 Preadipocytes. Journal of Medicinal Food 18 (9): 972–979.
Yumi, Y., and B.G. Richard. 2001. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. The Journal of Clinical Investigation 107 (2): 35–42.
Richter, E., K. Ventz, M. Harms, J. Mostertz, and F. Hochgräfe. 2016. Induction of macrophage function in human THP-1 cells is associated with rewiring of MAPK signaling and activation of MAP3K7 (TAK1) protein kinase. Frontiers in Cell and Development Biology. https://doi.org/10.3389/fcell.2016.00021.
Moldoveanu, B., P. Otmishi, P. Jani, J. Walker, X. Sarmiento, J. Guardiola, M. Saad, and Y. Jerry. 2009. Inflammatory mechanisms in the lung. Inflammation Research 2 (16): 1–11.
I-Ta, L. 2013. Chuen-Mao, Y. Inflammatory signalings involved in airway and pulmonary diseases. Mediat Inflamm. Article ID 791231,12 pages
Beck, S.B., C. Madjdpour, S. Kneller, U. Ziegler, T. Pasch, and R.P. Wuthrich. 2002. Role of alveolar epithelial ICAM-1 in lipopolysaccharide-induced lung inflammation. The European Respiratory Journal 19 (6): 1142–1150.
Suter, P.M., S. Suter, E. Girardin, P. Roux-Lombard, G.E. Grau, and J.M. Dayer. 1992. High bronchoalveolar levels of tumor necrosis factor and its inhibitors, interleukin-1, interferon, and elastase, in patients with adult respiratory distress syndrome after trauma, shock, or sepsis. The American Review of Respiratory Disease 145 (5): 1016–1022.
Park, W.Y., R.B. Goodman, and K.P. Steinberg. 2001. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 164 (15): 1896–1903.
Prachayasittikul, V., S. Prachayasittikul, S. Ruchirawat, and V. Prachayasittikul. 2013. High therapeutic potential of Spilanthes Acmella, A Review. Excli J 12: 291–212.
Kevin, S., D. Delphine, M.C. Megan, M. Elisabeth, and G. Philippe. 2011. The traditional medicine Spilanthes acmella, and the alkylamides spilanthol and undeca-2E -ene -8,10-diynoic acid isobutylamide, demonstrate in vitro and in vivo anti-malarial activity. Phytotherapy Research 25 (7): 1098–1101.
Ivones, H., M. Lucía, M. Ioanna, D. Rodrigo, D. Carla, P. Sylvia, M.T. Jorge, and G. Gabino. 2009. Anti-inflammatory effects of ethanolic extract and alkamides-derived from Heliopsis longipes roots. Journal of Ethnopharmacology 124 (3): 649–652.
Witkowska, A.M., and M.H. Borawska. 2004. Soluble intercellular adhesion molecule-1 (sICAM-1):an overview. European Cytokine Network 15 (2): 91–98.
Funding
This study was supported in part by grants from the Chang Gung Memorial Hospital (CMRPF1G0201), the Ministry of Science and Technology in Taiwan (MOST 105-2320-B-255-004), and Chang Gung University of Science and Technology (EZRPF3FG0071).
Author information
Authors and Affiliations
Contributions
Wen-Chung Huang and Ling-Yu Wu designed the study and performed the experiments. Sindy Hu searched the literature and performed the experiments. Shu-Ju analyzed interpretation of data and drafting the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Huang, WC., Wu, LY., Hu, S. et al. Spilanthol Inhibits COX-2 and ICAM-1 Expression via Suppression of NF-κB and MAPK Signaling in Interleukin-1β-Stimulated Human Lung Epithelial Cells. Inflammation 41, 1934–1944 (2018). https://doi.org/10.1007/s10753-018-0837-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0837-0