Abstract
Shock is associated with inflammation-induced endothelial dysfunction. The aim of this study was to determine time-dependent alteration of blood biomarkers related to endothelial function in hemorrhagic and septic shocks. Hemorrhagic shock was induced by bleeding the animals. A cecal ligation and incision model was used to induce septicemia. Resuscitation was carried out by infusion of lactated Ringer’s solution. Resuscitation extended survival time in both shock groups. Blood pressure increased by resuscitation in the hemorrhagic shock but not in the septic shock. While hemorrhage caused a decrease in plasma levels of nitric oxide (NO) and hydrogen sulfide (H2S), asymmetric dimethylarginine (ADMA) and total antioxidant capacity (TAC) levels were increased. Only NO and TAC levels at the late phase were reversed by resuscitation. On the other hand, plasma levels of NO, ADMA, and TAC were increased by septicemia and resuscitation did not alter the septicemia-induced increase. These results indicate that blood biomarkers related to endothelial function were differentially affected by hemorrhage and septicemia. The time scale of biomarker production should be taken into consideration for the diagnostic and therapeutic approaches to these life-threatening diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Circulatory shock is an acute, life-threatening disease characterized by reduced effective blood flow of the body tissues. In addition, a systemic inflammatory response is also present during shock. Hemorrhage and sepsis are the most common causes of circulatory shock in humans [1, 2].
Functions of the endothelium in circulatory shock are critically important for vascular response and host survival. Although the exact mechanism of shock remains unknown, it has been suggested that endothelial dysfunction is probably the cause and/or result of the general inflammatory response [3, 4]. Systemic activation and dysfunction of the endothelium always end in inadequate tissue perfusion and blood-tissue barrier disruption. With increasing dysfunction, uncontrolled clotting activation, capillary microthrombi formation, tissue edema, local hypoxia, and ischemia are initiated. This in turn enhances a vicious cycle leading to multiple organ failure and death [5,6,7].
Reduced production or availability of nitric oxide (NO) derived from endothelial cells implies endothelial dysfunction. NO is synthesized by many cell types in various tissues and is involved in multiple physiological and pathological responses, including vasorelaxation, platelet function, host defense, and neurotransmission. It is synthesized from L-arginine by nitric oxide synthases (NOS). The neuronal and endothelial NOS (nNOS and eNOS) produce low levels of NO for physiological functions, whereas the inducible form of NOS (iNOS) is activated by several immunological stimuli and generates higher concentrations of NO [8]. While eNOS-derived NO production is reduced, iNOS-derived NO is increased by the general inflammatory response seen in hemorrhagic and septic shocks and resuscitation [9, 10].
During shock and resuscitation, reactive oxygen species (ROS) are also produced in large amounts. Immune cells are the main source of ROS, but endothelial cells also synthesize ROS in response to oxidative agents or cytokines. ROS decrease NO bioavailability through formation of reactive nitrogen species and eNOS inhibition, and ultimately affect vascular tone, platelet adhesion, and permeability. These modifications lead to vascular occlusion and exacerbate organ hypoperfusion [11, 12]. However, the time course of production and the role of NO and ROS in the critical periods of circulatory shock are not well understood or distinguished between different shock types.
Impaired endothelial function due to decreased NO bioavailability is a potential mechanism linking increased plasma asymmetric dimethylarginine (ADMA) levels with organ failure and death in shock [13]. ADMA, an endogenous NOS inhibitor, is associated with endothelial dysfunction, but its role in the setting of hemorrhagic and septic shocks has been less well characterized.
Hydrogen sulfide (H2S) is a naturally occurring gaseous transmitter, which may play important roles in normal physiology and diseases [14]. Although enhanced formation of H2S has been reported in shock, the timing of H2S production in hemorrhagic and septic shocks remains unclear.
During shock, increased or decreased release of some endogenous active substances may contribute to pathology. Therefore, biomarkers reflecting this pathological situation may help in the detection of systemic inflammatory and circulatory conditions. The present study was designed to compare the time-dependent production of blood biomarkers related to endothelial function (NO, ADMA, TAC, H2S) in hemorrhagic and septic shock models in rats.
MATERIALS AND METHODS
Animals
Male adult Wistar albino rats (250–350 g; Experimental Animals Breeding and Research Center at Uludag University, Bursa, Turkey) were used in the experiments. Ten animals in each group were housed at a constant temperature of 22 ± 2 °C with 12-h light/dark cycles and fed a standard laboratory rat diet with water ad libitum. Surgical and experimental protocols were approved by the Local Ethical Committee of Uludag University and are in accordance with the guidelines of national (Turkish Animal Welfare Act) and international (2010/63/EU) authorities.
Surgical Procedures
The surgical procedures were carried out under ketamine/xylazine (100/10 mg/kg) anesthesia. The animals were kept on a heating pad and rectal temperature was monitored and maintained at 37 °C during surgery. The left common carotid artery and left jugular vein of rats were cannulated with PE-50 tubing filled with heparinized saline (250 U/ml). During the arterial cannulation procedure, the vagus nerve and the cervical sympathetic trunk were carefully separated. The catheters were exteriorized at the nape of the neck and sealed until use.
Blood Pressure Recording
At the end of the surgical procedures, the arterial cannula was connected to a volumetric pressure transducer (BPT 300) attached to a DA100 B general-purpose transducer amplifier (Commat Ltd., Turkey). Mean arterial pressure (mmHg) and heart rate (beats/min) were recorded and analyzed using the MP100 system and AcqKnowledge software (BIOPAC Systems, USA).
Experimental Procedure for Hemorrhagic Shock
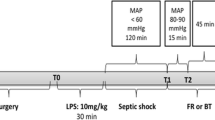
The animals were bled through the arterial catheter to a mean arterial blood pressure of 30 mmHg within the first 15 min. Hypotension (mean arterial pressure 30 mmHg) was maintained for 60 min. At the end of the shock period, lactated Ringer’s solution (3× the shed blood volume) was infused within 10 min to provide adequate fluid resuscitation. Control rats in the hemorrhagic shock group received no resuscitation. Cardiovascular parameters were recorded continuously throughout the experiments. The body temperature of hemorrhaged rats were kept around 37–37.6 °C by laying the animals on a heating pad throughout the experiments. Blood samples were obtained before hemorrhage (0 min) and at 15 (end of first hemorrhage), 75 (end of hypotensive period), and 135 min (end of experiment), in order to determine the plasma NO, ADMA, TAC, and H2S levels.
Experimental Procedure for Septic Shock
The cecal ligation and incision model was used to induce septic shock [15]. Briefly, the cecum was tightly ligated with a 3.0 silk suture just distal to the ileocecal valve to avoid any intestinal obstruction and a 1.5-cm incision was made. The cecum was then returned to the peritoneal cavity and the abdominal incision was closed with atraumatic 4.0 silk sutures. Sham-operated animals underwent the same surgical procedure except that the cecum was neither ligated nor incised. Immediately after the surgery, intraperitoneal saline (2 ml/kg) was administered. This is essential for producing the hyperdynamic earlier phase of sepsis in this experimental model.
After cecal ligation and incision, animals were placed in a separate cage and cardiovascular parameters were continuously monitored. Since body temperature (above 38.3 °C or below 36 °C) is one of the main symptoms of sepsis, we continuously monitored the rats’ body temperature as well to check the stage of shock. Body temperature gradually increased from 37.1 ± 0.3 °C to 38.4 ± 0.2 °C in both control and resuscitated cecal ligation and incision (CLI) groups. There were no significant differences in the body temperatures of rats in both CLI groups at the end of the experiments. At the end of 180 min, rats in the resuscitation group were resuscitated by the infusion of lactated Ringer’s solution (3 ml/100 g) within 10 min [2, 16] and monitored for an additional 180 min. Control CLI group did not receive fluid replacement, as in the hemorrhagic shock model. Surviving animals were observed for 24 h without cardiovascular monitoring. Blood samples were obtained before cecal ligation and incision (0 min) and at 180 (before resusCitation) and 360 min (end of cardiovascular monitoring) in order to determine the plasma NO, ADMA, TAC, and H2S levels.
Biochemical Examination
For the measurement of NO, ADMA, TAC, and H2S concentrations, blood samples (1.5 ml) were withdrawn from the arterial catheter in ice-cold tubes containing EDTA (50 μg/ml blood) at the time points described above. Samples were centrifuged (10.000 rpm; 4 °C, 10 min) and the plasma was separated and stored at − 80 °C until analysis.
The plasma nitrite/nitrate level was measured as a representation of NO production. It was measured by using the spectrophotometric method based on the Griess reaction [17]. This method was modified in our laboratories for 96-well plates.
The TAC of plasma was measured using a previously described method [18] based on the reduction of Cu+2 to Cu+ by antioxidants in the plasma. Neocuproine was used as a chromogenic agent and the colored complex was detected spectrophotometrically at 455 nm.
Plasma H2S levels were measured spectrophotometrically, according to a previously described method [5]. The assay is based on measurement of the absorbance of methylene blue, which produces a chemical reaction between N,N-dimethyl-p-phenylenediamine and FeCl3, at 670 nm.
ADMA levels were measured by using ELISA kits (Immunodiagnostic A.G., Germany) according to the manufacturer’s instructions.
Plasma levels of NO, ADMA, TAC, and H2S were plotted as a percentage of the pre-shock levels (0 min).
Statistical Analysis
Values are expressed as mean ± standard error of the mean (SEM). Repeated-measures of one-way or two-way ANOVA were used to test the time-dependent effects of hemorrhagic and septic shocks on blood biomarkers or on blood pressure and heart rate, respectively. When the p value was statistically significant, comparisons were performed by using the Tukey or Holm-Sidak tests. Survival rate was analyzed by using the Kaplan-Meier test. Student’s t test was used to analyze the specific time points in the same group or between the groups, respectively. Values were considered significantly different when p < 0.05.
RESULTS
The basal, pre-bleeding mean arterial pressure and heart rate values did not reveal significant differences between the hemorrhagic shock and resuscitation groups. The total bleeding volume necessary to induce hemorrhagic shock within 15 min was approximately 2.0–2.3 ml per 100 g of body weight. During the first 15 min of the hemorrhage procedure, blood pressure and heart rate decreased in the hemorrhagic shock group (Fig. 1). Fluid resuscitation increased blood pressure significantly in the resuscitation group. Heart rate increased gradually in both groups with no differences between the values (Fig. 1). Fluid resuscitation extended survival time (Fig. 2).
Blood pressure and heart rate changes in hemorrhagic shock and resuscitation. Bleeding caused a decrease in blood pressure and heart rate of rats. While resuscitation increased blood pressure, heart rate of rats increased gradually in both groups. *p < 0.05, significantly different from the hemorrhagic shock group. Values are expressed as mean ± SEM.
While hemorrhage significantly decreased plasma NO levels in a time-dependent manner, there was no time-dependent difference in the resuscitation group (Fig. 3). However, resuscitation inhibited hemorrhage-induced decrease at the 135-min time point. The level of ADMA was significantly increased by hemorrhage (Fig. 3) and resuscitation did not reverse this increase. Hemorrhage significantly increased plasma TAC levels, but resuscitation prevented this increase at the 135-min time point (Fig. 3). Plasma H2S levels were significantly decreased by hemorrhage at 75 and 135 min and resuscitation did not affect H2S levels (Fig. 3).
Plasma NO, ADMA, TAC, and H2S levels of rats in hemorrhage and resuscitation. While plasma nitrite and H2S levels were significantly decreased by hemorrhage, ADMA and TAC levels were significantly increased (p < 0.05). Resuscitation inhibited only hemorrhage-induced alteration of NO and TAC levels after 135 min. Differences from 0-min time point within each group (asterisk) and differences from hemorrhage group at each time point (plus sign). Values are expressed as mean ± SEM.
Basal blood pressure and heart rate values were similar in septic shock and resuscitation groups. The cecal ligation and incision procedure caused a progressive decrease in blood pressure without affecting heart rate (Fig. 4). Fluid resuscitation did not influence cardiovascular parameters; however, survival time and survival rate in resuscitated animals increased significantly (Fig. 5).
Septicemia significantly increased plasma NO levels in a time-dependent manner; resuscitation did not alter this increase (Fig. 6). The levels of ADMA were also significantly increased by septicemia (Fig. 6) and resuscitation did not reverse the increased ADMA level. Septicemia significantly increased plasma TAC levels only in the late phase of shock (Fig. 6) and resuscitation did not prevent this increase. Plasma H2S levels were not significantly changed by septicemia. However, resuscitation decreased plasma H2S level in 180 min (Fig. 6).
Plasma NO, ADMA, TAC, and H2S levels of rats in septic shock and resuscitation. Plasma nitrite, ADMA, and TAC levels were significantly increased by septicemia (p < 0.05). While plasma H2S level was not significantly changed by septicemia, resuscitation significantly decreased H2S level after 180 min (p < 0.05). Differences from 0-min time point within each group (asterisk) and differences from septic shock group at each time point (plus sign). Values are expressed as mean ± SEM.
DISCUSSION
The results of this study show that some blood biomarkers related to endothelial function, such as NO, ADMA, TAC, and H2S, are affected differently in hemorrhagic and septic shocks and resuscitation. In addition, blood biomarkers are altered in a time-dependent manner and the time scales of these changes are also different between hemorrhagic and septic shocks.
Circulatory shock is the inability of the body to maintain adequate end-organ perfusion and is responsible for significant morbidity, mortality, and health care resource consumption worldwide. Shock is associated with cardiovascular abnormalities characterized by hypotension and altered vascular reactivity [1, 2]. Two main causes of circulatory shock are hemorrhage and sepsis. In our study, rat models of hemorrhagic and septic shock were used and vital parameters were recorded to verify manifestations of each type of shock. Both procedures decreased blood pressure significantly but at different ranges. In hemorrhaged rats, blood pressure of animals decreased to 30 mmHg at the end of the shock procedure and fluid replacement increased these levels to around 60–70 mmHg (Fig. 1). However, in septic shock, fluid replacement did not significantly affect blood pressure values (Fig. 4). The replacement volume (3 ml/100 g of bw) of crystalloids which was used in this study is the recommended initial fluid challenge in sepsis in order to maintain mean arterial pressure ≥ 65 mmHg [2]. The observation of no changes in blood pressure after fluid challenge is somehow expected because (i) the lowest levels of blood pressure of rats in the CLI group before resuscitation are around 60–65 mmHg which are levels aimed to maintain during sepsis conditions [2]; (ii) hemorrhagic shock is a result of blood loss and hypovolemia while septic shock is initiated by the entry of an invasive microorganism into the circulation through the gut, lung, skin, or genitourinary tract and represents the normovolemic shock situation in the beginning. The increase in survival rates in the resuscitated group may be due to an increase in tissue perfusion through the increase in blood volume that is not reflected in blood pressure.
The hemorrhagic and septic shock-induced systemic responses share many biological features. The pathogenesis of circulatory shock is still not fully understood and the lack of effective treatment is due partly to an incomplete understanding of shock pathophysiology [1, 2]. Circulatory shock causes a systematic inflammatory response that leads to multiple organ failure. Endothelial cells play an important role in the immunoinflammatory response and dysfunction of the endothelium contributes to organ damage in shock [3, 4]. It has been shown that endothelial dysfunction develops early following circulatory shock and plays a major role in the development of reduced perfusion of tissues and activation of clotting pathways [5,6,7]. The vascular endothelium releases substances that play a pivotal role in maintenance of vascular homeostasis. One of these substances is NO, which is known to function as a modulator of blood flow, resulting in vascular relaxation. Other important effects of NO include the regulation of microvascular permeability, leukocyte-endothelium interactions, and platelet functions. Decreased endothelium-derived NO production is a prominent feature of circulatory shock-induced endothelial dysfunction [9, 10].
NO is synthesized from L-arginine by NOS enzymes. Three types of NOS have been identified. eNOS and nNOS are expressed constitutively under physiological conditions. The iNOS isoform can be stimulated in inflammatory conditions [8]. In circulatory shock, while endothelial dysfunction causes decreased eNOS-derived NO production, systemic inflammatory response triggers iNOS expression and a huge amount of NO is released. In hemorrhagic and septic shocks, decreased, increased, and unchanged blood NO levels have been reported [5, 19,20,21,22,23,24,25,26]. But, the time scale of NO production has not been documented. In the present study, while the plasma NO level was increased by septicemia, hemorrhage caused a decrease in the blood NO level. These opposite changes of NO levels were time-dependent. Thus, it could be posited that different NO levels in hemorrhagic and septic shocks are related to the manifestation of inflammatory responses on different time scales in the different shock models.
On the other hand, it has been suggested that mechanisms involved in endothelial dysfunction are dependent on oxidative stress in shocks [11, 12]. NO bioavailability is reduced by oxidative stress, due to the removal of NO by a superoxide radical and decreasing NO synthesis and release from the endothelium. In the current study, plasma TAC levels were increased in the late phases of septic and hemorrhagic shocks. Similar results have been reported in septic shock and attributed to the activation of antioxidant defense mechanisms [27]. These results are the first to show the time scale of the redox state of systemic circulation in these shock models.
ADMA is an endogenous competitive inhibitor of NO synthase and an increased level of ADMA also causes NO reduction due to competitive inhibition of NOS. Elevated plasma levels of ADMA have been related to organ failure and can predict patient mortality in sepsis [13]. However, increased ADMA levels have been reported only in hemorrhagic shock in the pig and endotoxemia in the guinea pig [28, 29]. The current study shows, for the first time, that there is a time-dependent increase in the blood ADMA level in septic and hemorrhagic shocks in rats. In addition, resuscitation did not affect ADMA levels. This suggests that the time-dependency of increased blood ADMA could be a key point for the observation of disease status in circulatory shock.
H2S is a biologically active gas molecule with a regulatory role in various physiological functions [14]. While increased plasma and tissue levels of H2S have been reported in septic shock, the opposite has been observed in hemorrhagic shock [30,31,32]. In the present study, although there was a trend toward increased plasma H2S levels in septic shock, it was not significant. However, hemorrhage decreased plasma H2S levels in the late phase of our experimental protocol. It is hypothesized that plasma H2S levels in different phases of circulatory shock could be affected differently in different animal models of shock.
The results of this study indicate that although endothelial dysfunction is the main result of all types of circulatory shock, blood biomarkers related to endothelial function are affected differently in the hemorrhagic and septic shock models we investigated. In addition, plasma biomarker levels are altered by shock in a time-dependent manner. Various biomarkers have been evaluated to aid in the diagnosis of shock grade and therapeutic approach. Unfortunately, no biomarker to date has demonstrated sufficient predictive value for the assessment of disease status. These findings may have important implications for consideration of time scale of biomarker level in the circulatory shock.
References
Kobayashi, L., T.W. Costantini, and R. Coimbra. 2012. Hypovolemic shock resuscitation. The Surgical Clinics of North America 92 (6): 1403–1423.
Dellinger, R.P., M.M. Levy, A. Rhodes, Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup, et al. 2013. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Critical Care Medicine 41 (2): 580–637.
Paulus, P., C. Jennewein, and K. Zacharowski. 2011. Biomarkers of endothelial dysfunction: Can they help us deciphering systemic inflammation and sepsis? Biomarkers Suppl 1: S11–S21.
Tunctan, B., B. Korkmaz, A.N. Sari, et al. 2012. A novel treatment strategy for sepsis and septic shock based on the interactions between prostanoids, nitric oxide, and 20-hydroxyeicosatetraenoic acid. Antiinflamm Antiallergy Agents Med Chem 11 (2): 121–150.
Zhang, H., S.M. Moochhala, and M. Bhatia. 2008. Endogenous hydrogen sulfide regulates inflammatory response by activating the ERK pathway in polymicrobial sepsis. Journal of Immunology 181: 4320–4331.
Ait-Oufella, H., E. Maury, S. Lehoux, et al. 2010. The endothelium: Physiological functions and role in microcirculatory failure during severe sepsis. Intensive Care Medicine 36 (8): 1286–1298.
Lundy, D.J., and S. Trzeciak. 2011. Microcirculatory dysfunction in sepsis. Critical Care Nursing Clinics of North America 23 (1): 67–77.
Szabó, C., and C. Thiemermann. 1994. Invited opinion: Role of nitric oxide in hemorrhagic, traumatic, and anaphylactic shock and thermal injury. Shock 2 (2): 145–155.
Shah, N.S., and T.R. Billiar. 1998. Role of nitric oxide in inflammation and tissue injury during endotoxemia and hemorrhagic shock. Environmental Health Perspectives Supplements 5: 1139–1143.
Förstermann, U., and W.C. Sessa. 2012. Nitric oxide synthases: Regulation and function. European Heart Journal 33 (7): 829–837.
Szabó, C., and K. Módis. 2010. Pathophysiological roles of peroxynitrite in circulatory shock. Shock Suppl 1: 4–14.
Andrades, M.É., A. Morina, S. Spasić, et al. 2011. Spasojević I. Bench-to-bedside review: Sepsis—from the redox point of view. Critical Care 15 (5): 230.
Koch, A., R. Weiskirchen, J. Kunze, et al. 2013. Elevated asymmetric dimethylarginine levels predict short- and long-term mortality risk in critically ill patients. Journal of Critical Care 28 (6): 947–953.
Van de Louw, A., and P. Haouzi. 2012. Oxygen deficit and H2S in hemorrhagic shock in rats. Critical Care 16 (5): R178.
Scheiermann, P., S. Hoegl, M. Revermann, et al. 2009. Cecal ligation and incision: An acute onset model of severe sepsis in rats. The Journal of Surgical Research 151 (1): 132–137.
Wang, P., and I.H. Chaudry. 1998. A single hit model of polymicrobial sepsis: Cecal ligation and puncture. Sepsis 2: 227–233.
Navarro-Gonzalvez, J., C. Garcia-Benayas, and J. Arenas. 1998. Semiautomated measurement of nitrate in biological fluids. Clinical Chemistry 44 (3): 679–681.
Usanmaz, S.E., and E. Demirel-Yilmaz. 2008. A microplate based spectrophotometric method for the determination of the total antioxidant capacity of human plasma: Modified cupric reducing ability assay. Fundamental & Clin Pharmacol 22 (Suppl .2): 67–67.
Hua, T.C., and S.M. Moochhala. 2000. Role of nitric oxide in hemorrhagic shock-induced bacterial translocation. The Journal of Surgical Research 93 (2): 247–256.
Ng, K.C., S.M. Moochhala, S. Md, E.L. Yap, S.Y. Low, and J. Lu. 2003. Preservation of neurological functions by nitric oxide synthase inhibitors following hemorrhagic shock. Neuropharmacology 44 (2): 244–252.
Savage, S.A., C.M. Fitzpatrick, V.S. Kashyap, et al. 2005. Endothelial dysfunction after lactated Ringer’s solution resuscitation for hemorrhagic shock. The Journal of Trauma 59 (2): 284–290.
Douzinas, E.E., O. Livaditi, A.G. Xiarchos, et al. 2006. The effect of hypoxemic resuscitation of hemorrhagic shock on hemodynamic stabilization and inflammatory response: A pilot study in a rat experimental model. The Journal of Trauma 61 (4): 918–923.
Shih, C.C., S.J. Chen, A. Chen, et al. 2008. Therapeutic effects of hypertonic saline on peritonitis-induced septic shock with multiple organ dysfunction syndrome in rats. Critical Care Medicine 36 (6): 1864–1872.
Chen, K., R.N. Pittman, and A.S. Popel. 2009. Hemorrhagic shock and nitric oxide release from erythrocytic nitric oxide synthase: A quantitative analysis. Microvascular Research 78 (1): 107–118.
Barmaki, B., A. Nasimi, and M. Khazaei. 2011. Effects of hypertension on hemodynamic response and serum nitrite concentration during graded hemorrhagic shock in rats. J Res Med Sci 16 (9): 1168–1175.
Subeq, Y.M., B.G. Hsu, N.T. Lin, et al. 2012. Hypothermia caused by slow and limited-volume fluid resuscitation decreases organ damage by hemorrhagic shock. Cytokine 60 (1): 68–75.
Victor, V.M., M. Rocha, and M. De la Fuente. 2004. Immune cells: Free radicals and antioxidants in sepsis. International Immunopharmacology 4 (3): 327–347.
Aneman, A., V. Backman, J. Snygg, et al. 1994. Accumulation of an endogenous inhibitor of nitric oxide synthase during graded hemorrhagic shock. Circulatory Shock 44 (3): 111–114.
Balabanli, B., H. Erdamar, N. Türközkan, et al. 2007. Effect of taurine on endotoxin-induced alterations in plasma asymmetric dimethylarginine, L-arginine and nitric oxide in guinea pigs. Journal of Thrombosis and Thrombolysis 24 (1): 53–57.
Mok, Y.Y., M.S. Atan, C. Yoke Ping, et al. 2004. Role of hydrogen sulphide in haemorrhagic shock in the rat: Protective effect of inhibitors of hydrogen sulphide biosynthesis. British Journal of Pharmacology 143 (7): 881–889.
Li, L., M. Bhatia, Y.Z. Zhu, et al. 2005. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. The FASEB Journal 19 (9): 1196–1198.
Zhang, H., L. Zhi, P.K. Moore, and M. Bhatia. 2006. Role of hydrogen sulfide in cecal ligation and puncture-induced sepsis in the mouse. American Journal of Physiology. Lung Cellular and Molecular Physiology 290 (6): L1193–L1201.
Acknowledgements
The present study was supported by a grant from The Commission of the Scientific Research Projects of Uludag University (2008/43). We are grateful to Soner Mamuk for the great technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no conflict of interest.
Rights and permissions
About this article
Cite this article
Coskun, C.N., Usanmaz, S.E., Savci, V. et al. Time-Dependent Production of Endothelium-Related Biomarkers is Affected Differently in Hemorrhagic and Septic Shocks. Inflammation 41, 33–41 (2018). https://doi.org/10.1007/s10753-017-0660-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0660-z