Abstract
To determine whether IL-21 receptor signaling plays a significant role in promoting Tfh cell-mediated cardiac injury in viral myocarditis (VMC), we compared IL-21R-deficient mice for some parameters of VMC. Balb/c and IL-21R−/− mice were infected with CVB3. Frequencies of splenic Tfh cells were determined by flow cytometric analysis, and productions of anti-adenine nucleotide translocator (ANT) autoantibodies were detected by enzyme-linked immunosorbent assay. To determine the effects of IL-21R signal on the proliferation of B cells, lymphocytes from spleens of the IL-21R−/− and Balb/c mice infected by CVB3 were tagged with carboxyfluorescein succinimidyl ester (CFSE) and then were stimulated with lipopolysaccharides plus IL-21 or anti-IL-21 neutralizing antibody for 3 days. The proliferation of B cells was analyzed by flow cytometry. Anti-ANT antibodies in the supernatants were detected by ELISA. Results showed that IL-21R−/− mice developed significantly less inflammation of the myocardium than Balb/c mice. Numbers of the Tfh cells and levels of anti-ANT antibody were decreased in IL-21R−/− mice, indicating IL-21 signaling plays a role on the Tfh cell response. The percentage of CD19+CFSE+ B cells decreased in IL-21R−/− mice compared to VMC mice. And anti-ANT antibodies were detected at lower levels in cultured supernatant from IL-21R−/− mice than in those from VMC mice. These data suggest that IL-21R signal may contribute to anti-ANT antibody production and expansion of B cells in VMC mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Myocarditis is inflammation of the myocardium associated with microbial infections, which may explain 10% of patients with acute heart failure for unknown causes and account for 12% of sudden deaths in young adults less than 40 years old [1]. Among viral causes, coxsackievirus B3 (CVB3) is the leading cause of viral myocarditis (VMC), which can develop into dilated cardiomyopathy (DCM) [2]. Excessive immune response and activated CD4+ T lymphocytes, such as Th1, Th2, Th17, and Th22 cells, have been reported participating in the procedure of myocardial cell damage [3,4,5,6,7]. Upon activation, CD4+ T cells can activate autoreactive B cells via cytokines and chemokines. The autoantibodies thus produced can precipitate inflammation by triggering complement protein cascade. Assisting autoantibody, produced by activated CD4+ T cells, is a fundamental respect of immune responses. Anti-β1-adrenergic receptor autoantibodies, anti-adenine nucleotide translocator (ANT) autoantibodies, and anti-cardiac myosin autoantibodies were commonly seen in VMC and DCM patients, suggesting the existence of abnormal humoral autoimmune responses in VMC.
Interleukin-21 (IL-21) is produced by activated CD4+ T cells and regulates the T cell, B cell, and natural killer (NK) cell responses. Follicular helper T cells (Tfh) are one of the potential sources [8, 9]. IL-21 receptor (IL-21R), which specifically binds IL-21, together with the common cytokine receptor γ-chain, is expressed on multiple immune cell types, such as T cells, B cells, NK cells, and dendritic cells. In mouse models of systemic lupus erythematosus (SLE), type 1 diabetes mellitus, and arthritis, IL-21R deficiency completely stops the progress of disease [10,11,12]. Our initial studies have suggested a role for IL-21 acting through Tfh in VMC [13]. In the present study, we investigated whether IL-21R signaling is essential to induction of Tfh cells and anti-ANT antibodies in the CVB3 model of myocarditis. Our results indicate that mice lacking the IL-21R develop less serious myocarditis. And IL-21R defects correlate with decrease of the proportion of Tfh cells and the level of anti-ANT antibody. These findings suggest a novel mechanism that IL-21R signaling by which Tfh cells can contribute to the pathogenesis of mice models of VMC.
MATERIAL AND METHODS
Mice
Male IL-21R−/− mice were purchased from Jackson Laboratories. Four-week-old SPF male Balb/c mice were obtained from Hunan Silaike Jingda Animal Center, Chinese Academy of Sciences. All mice were 4 weeks of age when infected. All of the studies have been reviewed and approved by the Guangxi Medical University Animal Care and Use Committee. Animals were housed according to standard procedures and maintained under a standard regimen of food and water ad libitum.
Virus and Infection of Mice
Heart-passaged CVB3 (Nancy strain) was propagated in Hep-2 cells, the 50% tissue culture infectious dose (TCID50) titer was determined by the cytopathic effects visible after 72 h. The TCID50 assay result for Hep-2 cells was 1 × 10−7. IL-21R−/− mice (IL-21R−/− group, n = 8) and Balb/c mice were infected by intraperitoneal (i.p.) injection of 0.1 ml of PBS containing approximately 100TCID50 of the virus (VMC group, n = 8). The day of injection was defined as week 0. Surviving mice were separately sacrificed by cervical dislocation on 2 weeks after i.p. injection.
Histology
Ventricular tissue was fixed in 10% buffered formalin for 48 h and then paraffin embedded, sectioned, and stained by hematoxylin and eosin. Histopathological change was observed by using light microscopy (Nikon Eclipse E800 Microscope, Japan). Image analysis of cardiac inflammation was blindly graded by two independent pathologists separately based on the following semi-quantitative scale: 0, no inflammatory infiltrates; 1, small foci of inflammatory cells between myocytes or inflammatory cells surrounding individual myocytes; 2, larger foci of 100 inflammatory cells or involving at least 30 myocytes; 3, 10% of a myocardial cross-section involved; and 4, 30% of a myocardial cross-section involved [14].
Lymphocyte Isolations and Flow Cytometry
The spleens were removed and pressed through fine-mesh screens aseptically. Lymphoid cells were isolated by Ficoll Plaque (Solarbio Science & Technology, China) gradient centrifugation and then maintained in a 24-well flat-bottom tissue culture plate with RPMI 1640 supplemented with 10% fetal calf serum (Gibco, USA) at 37 °C in a humidified atmosphere with 5% CO2. Lymphocytes (106/tube) were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD4, phycoerythrin-Cy5 (PE-Cy5)-conjugated anti-ICOS, and phycoerythrin (PE)-conjugated anti-CXCR5 (BD PharMingen San Diego, CA), or isotype-matched control IgG, according to the manufacturer’s protocol and fixed in 4% paraformaldehyde. Flow cytometric analysis was performed by using conventional multiparameter procedures. Data were analyzed on a FACS Calibur flow cytometer (BD Biosciences). Cell Quest software (BD Biosciences) was used for data acquisition. Viable cells were determined by trypan blue exclusion. At least 50,000 cells were evaluated. Cells were gated on the forward scatter of living cells and then centered on CD4+ T cells. After setting the threshold using isotype control staining for CXCR5 and ICOS, the proportion of Tfh cells was analyzed as the percentage of CXCR5+ ICOS+ cells within total CD4+ T cells for each sample, and percentages of CXCR5+ ICOS+/CD4+ cell proportion were defined as Tfh cells.
Enzyme-Linked Immunosorbent Assay
Blood was collected via retro-orbital bleeding, and serum was separated. The amounts of ANT autoantibody in the blood serum were detected using the ANT Immunoassay (CUSABIO, China). No cross-reactivity was observed in detection. All samples were measured in triplicate.
Lymphocyte Preparation, Culture Conditions, and Differentiation Stimulation
To determine the effects of IL-21R signal on the proliferation of B cells, the spleens from the IL-21R−/− and Balb/c mice which were infected by CVB3 were harvested aseptically. The lymphocyte fractions of these samples were obtained by Ficoll Paque (Solarbio Science & Technology) gradient centrifugation. Lymphocytes were tagged with carboxyfluorescein succinimidyl ester (CFSE, Invitrogen) and then were stimulated with 10 μg/ml lipopolysaccharides (LPS) (Sigma-Aldrich) plus 20 ng/ml IL-21 (eBioscience) or stimulated with 10 μg/ml LPS (Sigma-Aldrich) plus 0.5 ng/ml anti-IL-21 neutralizing antibody (eBioscience) for 3 days. The proliferation of B cells was analyzed by flow cytometry using phycoerythrin PE-conjugated anti-CD19 antibody. Anti-ANT antibodies in the supernatants were detected by ELISA.
Statistical Analysis
All data were expressed as mean ± SD. One-way ANOVA and q test were used for comparison among groups. All data were analyzed with SPSS 17.0 for Windows. A significant difference was defined as P < 0.05.
RESULTS
IL-21R Deficiency Partially Alleviated the Development of Myocarditis and the Severity of Myocardium Inflammation
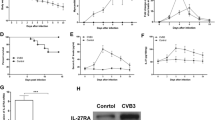
Mice of Balb/c and IL-21R−/− were infected with CVB3, and surviving animals were sacrificed 2 weeks post infection for determination of myocarditis. Results showed that IL-21R deficiency alleviates the severity of myocarditis. The survival rate of IL-21R−/− group mice was significantly improved comparing with the VMC groups (Fig. 1). The number of mice survived to 14 days was eight and five for IL-21R−/− group and VMC groups separately. Figure 2 gives representative histology of CVB3-infected Balb/c and IL-21R−/− mice. Less cardiac inflammation and T, B cell infiltration were observed in IL-21R−/− mice (Supplement Material). As our preliminary results showed that anti-IL-21mAb can partially protect from CVB3-induced myocarditis, these results indicate that IL-21/IL-21R signaling has a significant role in myocarditis.
Effects of IL-21R deficiency on survival rate. The survival rate of IL-21R−/− group mice was significantly improved comparing with the VMC group (P < 0.05). Eight mice in the IL-21R−/− group, and five mice in VMC group survival to 14 days. Kaplan-Meier life span analysis indicated a significant difference for survival differences.
IL-21R deficiency prevents myocarditis in IL-21R−/− mice. Representative histopathological images of heart tissue. Heart sections from VMC and IL-21R−/− mice were stained with H&E, original magnification ×400. a BABL/C mice after 2 weeks CVB3 infection. b IL-21R−/− mice after 2 weeks CVB3 infection.
Analysis of Involvement of Tfh
Total numbers of spleen CD4+ Tfh cells were determined in Balb/c mice and IL-21R−/− mice 14 days after CVB3 infection. Tfh cell numbers were significantly increased in infected Balb/c mice compared to the infected IL-21R−/− mice. Compared with those in the VMC group, the percentages of Tfh cells in the IL-21R−/− group trended lower, and there was significant difference between them (P < 0.05, Fig. 3). The frequencies of the Tfh cells in VMC and IL-21R−/− groups on day 0 and day 14 were (0.15 ± 0.03%) vs (0.15 ± 0.01%) and (12.17 ± 2.76%) vs (3.26 ± 0.69%), respectively.
Decreased Tfh cells in IL-21R−/− mice 14 days after CVB3 infection. Numbers in the upper right quadrants indicate the mean percentages of Tfh cells. a Representative picture of CXCR5+ICOS+CD4+ Tfh cells in VMC mice on week 0. b Representative pictures of Tfh cells in IL-21R−/− mice on week 0. c Tfh cells in VMC mice on week 2 after CVB3 infection. d Tfh cells in IL-21R−/− mice on week 2 after CVB3 infection.
Reduction of Anti-ANT Antibody from IL-21R−/− Mice
Our preliminary study showed that the humoral manifestations in VMC mice include marked elevation in serum levels of anti-ANT antibody, and there is a positive relationship between IL-21 and anti-ANT antibody. Inhibiting IL-21 in vivo can decrease the level of anti-ANT antibody and reduce the frequency of Tfh cells [13]. To examine whether IL-21R signal contributes to the Tfh cells and production of anti-ANT antibody, we studied the change of Tfh cells’ anti-ANT antibody in IL-21R−/− mice. We compared serum levels of anti-ANT antibody in normal and IL-21R−/− which received CVB3 i.p. The levels of anti-ANT antibody were markedly lower in serum of IL-21R−/− mice than those in the VMC groups (Fig. 4, P < 0.05). These data showed that ANT antibody was greatly decreased in mice unable to signal through the IL-21R.
The levels of serum anti-ANT antibody in different groups, measured by ELISA. The levels of anti-ANT antibody were (1.41 ± 0.11) vs (1.57 ± 0.16) pg/ml (0 week) and (2.16 ± 0.67) vs (9.26 ± 0.67) pg/ml in IL-21R−/− group and VMC group, respectively. ★ P < 0.05, vs week 0 VMC mice. ▲ P < 0.05, vs week 2 IL-21R−/− mice.
IL-21R Signal Deficiency Is Related to B Cell Expansion and Anti-ANT Antibody Production
By flow cytometry, we found that the percentage of CD19+CFSE+ B cells increased in VMC mice (5.53 ± 0.61%) compared to IL-21R−/− mice (1.88 ± 0.50%, P < 0.05, Fig. 5a). By use of ELISA, anti-ANT were detected at higher levels in cultured supernatant from VMC mice than in those from IL-21R−/− mice (6.03 ± 0.70 pg/ml) compared to IL-21R−/− mice (2.05 ± 0.21 pg/ml), Fig. 5b. These data indicate that the expansion of B cells and the increase of anti-ANT antibody in VMC mice may be related to IL-21. To test this possibility, a neutralizing antibody to IL-21 was added in the culture medium. Results showed that anti-ANT antibody decreased the proportion of B cells in VMC mice (2.61 ± 0.46%), but a litter bit higher than those of IL-21R−/− mice (1.90 ± 0.52%), Fig. 5b. These data suggest that IL-21R signal may contribute to anti-ANT antibody production and expansion of B cells in VMC mice.
IL-21R signal deficiency is related to B cell expansion and anti-ANT antibody production. a Splenocytes were isolated from VMC and IL-21R−/− mice and stimulated with LPS, or LPS + IL-21, or LPS + anti-IL-21 for 72 h. CFSE+ cells among CD19+ B cells were detected by flow cytometry analysis. a Numbers in the upper right quadrants indicate the mean percentages of CD19+ B cells. b The levels of serum anti-ANT antibody in different groups, measured by ELISA. The levels of anti-ANT antibody were (3.33 ± 0.17) vs (2.07 ± 0.27) pg/ml (LPS) and (6.03 ± 0.70) vs (2.05 ± 0.21) pg/ml (LPS + IL-21) and (1.87 ± 0.19) vs 1.99 ± 0.19 pg/ml (LPS + anti-IL-21) in VMC group and IL-21R−/− group, respectively. ▲ P < 0.05.
DISCUSSION
In 2000, IL-21 and its receptor IL-21R were first identified [15]. IL-21 is primarily produced by a cluster of CD4+ cells and natural killer cells, while IL-21R is broadly expressed on B cells, activated T cells, and other myeloid cells. A large number of studies confirm that IL-21 can stimulate B cell proliferation and differentiation in the context of a co-stimulatory T cell signal. Due to its ability to regulate immune responses, numerous studies have shown that enhancing or inhibiting the action of IL-21 as antitumor treatment. What more, blocking IL-21R in autoimmune disease has therapeutic effects in animal models of a wide range of diseases, and various clinical trials are ongoing [16].
Interaction of IL-21 with its receptor leads to regulation of activation, proliferation, and survival of both Tfh and B cells. Moreover, IL-21 plays an important role in the development of Tfh [17]. Recent studies have shown that IL-21 is not required for the generation of Tfh cells, but Tfh cell-derived IL-21 is essential for formation of GC by acting directly on B cells [8]. It has been suggested that the expression of IL-21/IL-21R has high relationship with different autoimmune diseases, such as multiple sclerosis [18] and EAE [19, 20]. Several studies have reported that IL-21 contributes to the pathogenesis of myocarditis [13, 21]. As IL-21 is a pleiotropic cytokine, which effects on multiple lymphocyte subpopulations, a major question in this study was whether IL-21/IL-21R signals impact Tfh cell responses. In the previous study, we sought to block IL-21 signaling by treating VMC mice with anti-IL-21mAb. Results showed that neutralization of IL-21 with anti-IL-21 can ameliorate the myocardium inflammation and decrease Tfh cells and ANT autoantibody, indicating IL-21 signaling plays a role on the Tfh cell response. Here, we show that IL-21R signaling is essential for the VMC mice and also documented changes in myocarditis. The results described here demonstrate that development of the myocarditis is critically dependent on IL-21/IL-21R signaling. To further determine how IL-21R signal could impact the Tfh and anti-ANT antibodies, the spleens of the IL-21R−/− and BALB/c mice infected by CVB3 were tagged with CFSE and then were stimulated with LPS plus IL-21 or anti-IL-21 neutralizing antibody. Results showed that the percentage of CD19+CFSE+ B cells decreased in IL-21R−/− mice compared to VMC mice. And anti-ANT antibodies were detected at lower levels in cultured supernatant from IL-21R−/− mice than in those from VMC mice. These data suggest that IL-21R signal may contribute to anti-ANT antibody production and expansion of B cells in VMC mice.
As IL-21 promotes development of Tfh cells involved in the inflammatory process associated with VMC, therefore, inhibition of IL-21 or IL-21R signal may be considered a potential target for VMC therapy. However, it should be noted that IL-21 affects the development and function of other immune cells, including Th17, Treg, NK, and B cells. Since these cells play an important role in the immune responses, Treg and Th17 cells are in reciprocal regulation and development of each cell is associated with expansion of another one. Although IL-21 has immunomodulatory effects on multiple lymphoid cell subsets, including CD4+, CD8+, B cells, and NK cells, in the CVB3 myocarditis model, IL-21 inhibition resulting in the Tfh cell population reduction provides the same level of myocarditis protection as observed in IL-21R−/− mice lacking IL-21 signal transduction in Tfh subpopulations.
Although it remains to be shown definitely that IL-21R signal contributes to mice VMC, the observation that defects in the IL-21R is a protect factor for mice VMC. Taken together, these results suggest that interruption of the IL-21/IL-21R signaling pathway may be a therapeutic option for treating VMC. Although our studies have shown that IL-21R signal plays an important role in the pathogenesis of VMC, it is still necessary to conduct studies to clarify the precise function of IL-21R signal in the pathogenesis of this disease. Further investigations regarding the immunobiology of IL-21 are required to enable the designing of novel therapeutics based on IL-21 targeting.
References
Blauwet, L.A., and L.T. Cooper. 2010. Myocarditis. Progress in Cardiovascular Diseases 52: 274–288.
Fairweather, D., and N.R. Rose. 2007. Coxsackievirus-induced myocarditis in mice: a model of autoimmune disease for studying immunotoxicity. Methods 41: 118–122.
Rangachari, M., N. Mauermann, R.R. Marty, S. Dirnhofer, M.O. Kurrer, V. Komnenovic, J.M. Penninger, and U. Eriksson. 2006. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. The Journal of Experimental Medicine 203: 2009–2019.
Valaperti, A., R.R. Marty, G. Kania, D. Germano, N. Mauermann, S. Dirnhofer, B. Leimenstoll, P. Blyszczuk, C. Dong, C. Mueller, L. Hunziker, and U. Eriksson. 2008. CD11b+ monocytes abrogate Th17 CD4+ T cell-mediated experimental autoimmune myocarditis. Journal of Immunology 180: 2686–2695.
Yang, F., W.F. Wu, Y.L. Yan, Y. Pang, Q. Kong, and Y.L. Huang. 2011. Expression of IL-23/Th17 pathway in a murine model of coxsackie virus B3-induced viral myocarditis. Virology Journal 8: 301.
Fan, Y., W. Weifeng, Y. Yuluan, K. Qing, P. Yu, and H. Yanlan. 2011. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of coxsackievirus b3-induced viral myocarditis reduces myocardium inflammation. Virology Journal 8: 17.
Kong, Q., W. Wu, F. Yang, Y. Liu, Y. Xue, M. Gao, W. Lai, X. Pan, Y. Yan, Y. Pang, and Y. Deng. 2012. Increased expressions of IL-22 and Th22 cells in the coxsackievirus B3-induced mice acute viral myocarditis. Virology Journal 9: 232.
Vogelzang, A., H.M. McGuire, D. Yu, J. Sprent, C.R. Mackay, and C. King. 2008. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity 29: 127–137.
King, C., S.G. Tangye, and C.R. Mackay. 2008. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annual Review of Immunology 26: 741–766.
Bubier, J.A., T.J. Sproule, O. Foreman, R. Spolski, D.J. Shaffer, H.C. Morse 3rd, W.J. Leonard, and D.C. Roopenian. 2009. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proceedings of the National Academy of Sciences of the United States of America 106: 1518–1523.
Sutherland, A.P., T. Van Belle, A.L. Wurster, A. Suto, M. Michaud, D. Zhang, M.J. Grusby, and M. von Herrath. 2009. Interleukin-21 is required for the development of type 1 diabetes in NOD mice. Diabetes 58: 1144–1155.
Young, D.A., M. Hegen, H.L. Ma, M.J. Whitters, L.M. Albert, L. Lowe, M. Senices, P.W. Wu, B. Sibley, Y. Leathurby, T.P. Brown, C. Nickerson-Nutter, J.C. Keith Jr., and M. Collins. 2007. Blockade of the interleukin-21/interleukin-21 receptor pathway ameliorates disease in animal models of rheumatoid arthritis. Arthritis and Rheumatism 56: 1152–1163.
Yang, F., W.H. Mo, B.P. Tan, X.M. Wei, and H. Wang. 2015. Increased frequency of follicular helper T cells in mice viral myocarditis is relevant with anti-ANT antoantibody. Virology Journal 12: 20.
Stritesky, G.L., N. Yeh, and M.H. Kaplan. 2008. IL-23 promotes maintenance but not commitment to the Th17 lineage. Journal of Immunology 181: 5948–5955.
Parrish-Novak, J., S.R. Dillon, A. Nelson, A. Hammond, C. Sprecher, J.A. Gross, J. Johnston, K. Madden, W. Xu, J. West, S. Schrader, S. Burkhead, M. Heipel, C. Brandt, J.L. Kuijper, J. Kramer, D. Conklin, S.R. Presnell, J. Berry, F. Shiota, S. Bort, K. Hambly, S. Mudri, C. Clegg, M. Moore, F.J. Grant, C. Lofton-Day, T. Gilbert, F. Rayond, A. Ching, L. Yao, D. Smith, P. Webster, T. Whitmore, M. Maurer, K. Kaushansky, R.D. Holly, and D. Foster. 2000. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 408: 57–63.
Steele, N., A. Anthony, M. Saunders, B. Esmarck, E. Ehrnrooth, P.E. Kristjansen, A. Nihlén, L.T. Hansen, and J. Cassidy. 2012. A phase 1 trial of recombinant human IL-21 in combination with cetuximab in patients with metastatic colorectal cancer. British Journal of Cancer 106: 793–798.
Shekhar, S., and X. Yang. 2012. The darker side of follicular helper T-cells: from autoimmunity to immunodeficiency. Cellular & Molecular Immunology 9: 380–385.
Romme Christensen, J., L. Bornsen, R. Ratzer, F. Piehl, M. Khademi, T. Olsson, P.S. Sorensen, and F. Sellebjerg. 2013. Systemic inflammation in progressive multiple sclerosis involves follicular TH, TH17, and activated B-cells and correlates with progression. PloS One 8: e57820.
Korn, T., E. Bettelli, W. Gao, A. Awasthi, A. Jäger, T.B. Strom, M. Oukka, and V.K. Kuchroo. 2007. IL-21 initiates an alternative pathway to induce pro-inflammatory TH17 cells. Nature 448: 484–487.
Spolski, R., and W.J. Leonard. 2008. IL-21: basic biology and implications for cancer and autoimmunity. Annual Review of Immunology 26: 57–79.
Wang, Y., G. Li, J.X. Tang, and H. Chang. 2012. Time course of interleukin-21 and related cytokines expression in rats with experimental autoimmune myocarditis. Zhonghua Xin Xue Guan Bing Za Zhi 40: 43–49.
Acknowledgments
This work was supported by the National Natural Science Foundation of China Grant 81260046.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving animals were in accordance with the ethical standards of Guangxi Medical University.
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
ESM 1
(DOC 609 kb)
Rights and permissions
About this article
Cite this article
Yang, F., Wei, Xm., Liang, Ww. et al. A Critical Role for IL-21 Receptor Signaling in the Coxsackievirus B3-Induced Myocarditis. Inflammation 40, 1428–1435 (2017). https://doi.org/10.1007/s10753-017-0586-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0586-5