Abstract
Crohn’s disease (CD) and ulcerative colitis (UC) are the most widely known types of inflammatory bowel diseases (IBD) and have been paid more attention due to their increasing incidence and a substantial increase in the risk of colorectal cancer (CRC). However, the phenotype and, more importantly, the function in the regulation of mucosal inflammation by different macrophages are poorly understood, even though macrophages constitute a major subset of intestinal myeloid cells. The results firstly showed that the subset of peritoneal CD11b+CD169+ macrophages increased and CCL22 expression level decreased significantly during the DSS-induced colitis. DSS-induced colitis was alleviated in CD169-DTR mice at least partially due to the deletion CD169+ macrophages. Moreover, the CCL22 expression level in peritoneal macrophages from CD169-DTR mice was much higher than that from WT mice with DSS-induced colitis. And, the cell-sorting result revealed that CD11b+CD169+ macrophage cells did not express CCL22 dominantly. Further experiment in vivo demonstrated that treatment with recombinant murine CCL22 (rmCCL22) ameliorated the clinical symptoms of DSS-induced colitis. All these data indicated that macrophage subset of CD11b+CD169+ from peritoneal cavity played critical role probably together with low levels of CCL22 in DSS-induced colitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Crohn’s disease (CD) and ulcerative colitis (UC) are the most widely known types of inflammatory bowel diseases (IBD) which are characterized by chronic inflammation of the gastrointestinal tract and have been the focus of attention due to their increasing incidence [1, 2]. The correlation between inflammation and the development of cancer has been confirmed by many studies, and IBD is also related to a substantial increase in the risk of colorectal cancer (CRC). Patients with UC and CD are at increased risk for developing CRC [3]. At present, the factors that contribute to the development of IBD and the pathogenesis remain elusive. Thus, it is necessary to elucidate the critical mechanism of IBD for prevention of inflammation progression further.
Although the etiology of IBD has not been fully elucidated, an interaction between genetic, immune, and environmental risk factors is thought to play a role in the pathogenesis of IBD [4]. Macrophages, known for their role as immune sentinels, play a critical role in inflammation diseases. These cells are strategically located throughout the body tissues and play important roles in development, homeostasis, tissue repair, and immunity. Under steady state conditions, many tissues and serous cavities contain multiple types of macrophages with distinct phenotypes and functions [5].
Macrophages in gastrointestinal tract and peritoneal cavity are both related to IBD [6]. Abundant macrophages are found spread throughout the gut lamina propria (LP) and it has been reported a LP-resident macrophage subset expressing CD169 links mucosal damage and inflammatory monocyte recruitment [7]. Peritoneal macrophages also play important roles in the control of inflammatory pathologies, as well as in the defense against infection of the abdominal cavity [8]. Large populations of resident macrophages in gastrointestinal tract are blood monocyte derived in the steady state; however, F4/80Hi peritoneal cavity macrophages were confirmed to have a prenatal origin which derived from cells in the yolk sac [9]. However, the phenotype and, more importantly, the function in the regulation of mucosal inflammation by different macrophages are poorly understood, even though macrophages constitute a major subset of intestinal myeloid cells. Thus, it is necessary to investigate the role of peritoneal macrophage further due to the different origins of these cells.
The highly heterogeneous macrophages can rapidly change their function to adapt distinct tissue environments. CD169 (also known as sialoadhesin, Siglec-1) is a cell surface sialic-acid-binding receptor, which is expressed by specific macrophage subsets [10]. In lymph node, CD169+ macrophages dominate antitumor immunity by phagocytizing dead tumor cells transported via lymphatic flow and cross presenting tumor antigens to CD8+ T cells subsequently [11]. In spleen, CD169+ macrophages that located in the marginal zone (MZ) have a role in inducing immune tolerance [12]. However, whether peritoneal macrophages expressing CD169 play an important role in mucosal inflammation has not been reported yet.
During the initial inflammatory response of IBD, innate immune cells such as dendritic cells (DC) and macrophages activated by foreign antigens and secrete several cytokines to regulate the inflammatory response in UC and CD. Then these cytokines trigger T cells and activate the adaptive immune response [13]. The production and release of chemokines are considered to be important factors in the pathogenesis of IBD. Chemokines bind to G-protein-coupled trans-membrane receptors and participate in the recruitment and migration of both effector and memory T cells to specific intestine tissue sites. The expression of several chemokines and their receptors such as CCL2, CCL3, CCL4, CCL5, CXCL8, CXCL10, CCR2, and CCR5 has been demonstrated to be increased in IBD tissue [14]. Some studies indicated chemokines involved in the IBD disease initiation and progression, such as CCR9/CCL25 [15, 16]. Therefore, chemokines are believed to be key regulators in the progression of intestinal injury and inflammation.
We herein investigated the role of the macrophage subset expressing CD169 from peritoneal cavity in IBD. The CD169-human diphtheria toxin receptor (DTR) transgenic mouse in which CD169+ macrophages could be deleted by diphtheria toxin (DT) injection was used to study the role of CD169+ peritoneal macrophages in dextran sulfate sodium (DSS)-induced colitis [12, 17]. The present study demonstrated CD169+ macrophages transiently deleted by DT injection caused higher expression of CCL22 in DSS-induced colitis. The in vivo treatment with rmCCL22 ameliorated the clinical symptoms of DSS-induced colitis. These result revealed the macrophage subset expressing CD169 from peritoneal cavity played essential roles partially together with lower levels of CCL22 in DSS-induced colitis.
MATERIALS AND METHODS
Mice
C57BL/6 mice were obtained from the Vital River Laboratories, Beijing. CD169-DTR mice were provided by the RIKEN BioResource Center (RBRC NO 04395), Japan with the approval of depositors [18, 19]. All mice were maintained under specific-pathogen-free conditions. All experiments using mice described herein were approved by the Animal Care and Utilization Committee of Shandong University and performed in accordance with applicable guidelines and regulations.
Induction of Colitis by DSS
Female mice of 8 to 12 weeks of age were administrated orally with 3.5% DSS (MW 5000, Wako, Japan) in drinking water for 7 days. The body weight of each mouse was recorded daily. Ten microgram per kilogram body weight of DT (Sigma, MO) was intraperitoneally injected 1 day (day -1) before and 3 days (day 3) after the administration of DSS.
Histopathology and Immunofluorescence
The distal portion of the colon was fixed in neutralized 10% formalin, embedded in paraffin. The samples were sectioned and stained with hematoxylin and eosin. Peritoneal macrophages were obtained from WT or CD169-DTR mice. Then, the peritoneal macrophages were cultured in a 24-well plate with 1640 medium for 24 h to make the cells adhere to the wall. Peritoneal macrophages were fixed in 4% paraformaldehyde and blocked with 10% normal goat serums for 45 min. Cells were incubated with FITC-conjugated anti-mouse CD11b (Biolegend, CA) and PE-conjugated anti-mouse CD169 (Biolegend, CA) at 4 °C for overnight and observed by fluorescence microscopy (Nikon, Japan) and analyzed using NIS-Elements BR 3.2.
RNA Extraction and Quantitative Real-Time RT-PCR
Total RNA was extracted from peritoneal macrophages and colon tissues using Trizol Reagent (Invitrogen, CA). Complementary DNAs were synthesized using the ReverTra Ace qPCR RT kit (Toyobo, Japan) according to the manufacturer’s instructions. qPCR reactions were performed in a CFX96 real-time PCR detection system (Bio-Rad, CA, USA) using SYBR green PCR master mix (Toyobo, Japan) according to the manufacturer’s instructions and specific primer pairs. Primers for mouse IL1β, IL-6, IL-12, IL-17, IL-23, CCL3, CCL8, CCL22, TNFα, Foxp3, Siglec 1, and β-actin were as follows: IL-1β, GGATGAGGACATGAGCACCT (sense) and AGCTCATATGGGTCCGACAG (antisense); IL-6, CTGGAGTACCATAGCTACC (sense) and CTGTTAGGAGAGCATTGGA (antisense); IL-12, AGCAGTAGCAGTTCC CCTGA (sense) and AGTCCCTTTGGTCCAGTGTG (antisense); IL-17, CTCCAG AAGGCCCTCAGACTAC (sense) and AGCTTTCCCTCCGCATTGACACAG (antisense); IL-23, CAGGGAACAAGATGCTGGAT (sense) and GGCTAGCATGC AGAGATTCC (antisense); CCL3, GGCATTCAGTTCCAGGTCAG (sense) and TCCC AGCCAGGTGTCATTT (antisense); CCL8, GCTGTGGTTTTCCAGACCAA (sense) and GAAGGTTCAAGGCTGCAGAA (antisense); CCL22, CAGGCAGGTCTGGGT GAA (sense) and TAAAGGTGGCGTCGTTGG (antisense); TNFα, ACCCTCACA CTCAGATCATC (sense) and GAGTAGACAAGGTACAACCC (antisense); Foxp3, GGCAGTTCAGGACGAGGG (sense) and GGTTCTTGTCAGAGGCA (antisense); Siglec1, CAATTTCCGGTGCTTACGGTG (sense) and CATAGTCTAGGC TTCTGT GC (antisense); and β-actin, TGCGTGACATCAAAGAGAAG (sense) and TCCATACCCA AGAAGGAAGG (antisense). The messenger ribonucleic acid (mRNA) expression was calculated by the △△Ct method and depicted as relative expression of target genes to an endogenous reference gene actin in the control group.
Flow Cytometry and Cell Sorting
Peritoneal macrophages were incubated with Fc blocker (clone 93; Biolegend, CA) for 10 min at 4 °C and then stained with antibodies for indicated surface molecular. Anti-CD11b (M1/70) and anti-CD169 (3D6.112) antibodies were purchased from Biolegend Biosciences. Cells were acquired by fluorescence-activated cell sorter (FACS) Aria 3 (BD Biosciences, NJ) and analyzed by FlowJo software version 8.8.7 (Tree Star). CD11b+CD169− and CD11b+CD169+ cells were sorted and used for quantitative PCR analysis of CD169, CCL22, and IL17 as described above.
Elisa
The peritoneal macrophages and colon tissues (0.5 cm) were cultured in RPMI with 10% fetal calf serum. The supernatants were harvested after 24 h. Concentrations of CCL22 and IL-17 in the culture medium were measured by ELISA (PeproTech) according to the manufacturer’s protocols.
Treatment with RmCCL22
Recombinant mouse CCL22 was purchased from PeproTech. Mice were received a total of six daily subcutaneous injections of rmCCL22 (150 μg/kg/day). Meanwhile, the control groups received saline, and treatment started at 2 days after the administration of DSS. Non-colitic and DSS-induced colitis mice were sacrificed by cervical dislocation for tissue collection at day 8.
Statistical Analysis
All experiments were performed at least three times using three or five mice in each group. Unpaired, two-tailed Student t test was performed by using Prism version 5.0 in all experiments. Data are presented as the mean ± SEM. A value of p < 0.05 was considered significant.
RESULTS
Increase of Macrophage Subset Expressing CD169 from Peritoneal Cavity in DSS-Induced Colitis
It is necessary to clarify the role of peritoneal macrophages in IBD because of the heterogeneity of macrophages in the peritoneal cavity. The present study firstly investigated the changes of the peritoneal macrophage in the process of IBD by using the DSS-induced colitis, one of the most widely used to resemble human UC. WT mice were administrated with 3.5% DSS in drinking water for 7 days. The results in Fig. S1 indicated that DSS-induced colitis model worked well in the present study.
Firstly, the number of peritoneal macrophages expressing CD169 was compared between wild-type control mice and DSS-induced colitis mice. Flow cytometry analysis showed the percentage of CD11b+CD169+ population cells in peritoneal macrophages increased from 4.33 to 15.8% after DSS treatment for 3 days. At day 7, the percentage of CD11b+CD169+ macrophages also increased to 12.2% compared with control mice (Fig. 1a). In addition, results of immunofluorescence histochemistry showed the percentages of CD11b+CD169+ macrophages increased significantly during the DSS-induced colitis (Fig. 1b). These results indicated that peritoneal macrophages expressing CD169 was an important subset during mucosal inflammation development.
Increase of macrophage subset expressing CD169 from peritoneal cavity in DSS-induced colitis. Mice were administrated with 3.5% DSS in drinking water for 3 days or 7 days to induce colitis (n = 5 per group). a Peritoneal cells from control mice and DSS-treated mice for the indicated number of days were stained with antibodies for CD11b and CD169 and the percentage of CD11b+CD169+ was analyzed by flow cytometry. *P < 0.05 compared to naïve. b Peritoneal cells from control mice and colitis mice were stained for CD11b and CD169 (left). Original magnification, ×20. The percentage of CD11b+CD169+ macrophages in each random field from the control mice and DSS-treated mice was calculated and the average of five fields was shown (right). **P < 0.01 compared to control.
Decreased CCL22 Level in Peritoneal Macrophage During Development of DSS-Induced Colitis
The present study next examined expression levels of inflammatory cytokines and chemokines during the pathogenesis of IBD. Peritoneal macrophages were collected from the control mice and DSS-treated colitis mice for detection of the expression of inflammatory cytokines and the expression levels of IL-1β, IL-6, and TNFα increased significantly in the mice at day 3 and day 7 of DSS treatment (Fig. 2a). Meanwhile, the expression levels of chemokines such as CCL22, CCL8, and CCL3 were also detected to elucidate the role of chemokines during the inflammation. The results showed that the expression levels of CCL3 and CCL8 increased at day 7 of DSS treatment. However, CCL22 decreased at both day 3 and day 7 of DSS treatment (Fig. 2b). To confirm the role of CCL22 in the colitis further, the production of CCL22 in peritoneal macrophages was also detected using an enzyme-linked immunosorbent assay (ELISA) and the results showed the level of CCL22 secretion in the culture medium of peritoneal macrophages from the colitic mice was significantly decreased (Fig. 2c). These results indicated that peritoneal macrophages played important roles in DSS-induced colitis probably together with specific cytokines or chemokines.
Decreased CCL22 level in peritoneal macrophage during development of DSS-induced colitis. a, b Pro-inflammatory cytokine and chemokine mRNA level in peritoneal cells from control mice and DSS-treated mice for the indicated number of days were determined by qRT-PCR (n = 5 per group). Data are expressed as the ratios of the target mRNA levels to the β-actin mRNA level. **P < 0.01, ***P < 0.001 compared to naive. c CCL22 production in vitro by peritoneal cells from control mice and colitic mice at the indicated number of days were detected using ELISA. ***P < 0.001compared to naive.
Alleviated Symptoms of DSS-Induced Colitis in CD169-DTR Mice by DT Injection
The increase of CD169 expressing macrophages and decrease of CCL22 levels in peritoneal macrophages during the inflammation promote us to hypothesize that peritoneal CD169+ macrophages and CCL22 are both essential in the inflammation of IBD. Thus, the CD169-DTR mice were used to investigate the role of CD169+ peritoneal macrophages in colitis. Firstly, the deletion of CD169 expressing cells in peritoneal macrophage from CD169-DTR mice was confirmed. Figure 3a showed that the percentage of CD11b+CD169+ macrophages in the CD169-DTR mice was dramatically decreased by DT injection. And immunofluorescence histochemistry results also showed that CD169+ macrophages from peritoneal cavity of CD169-DTR mice significantly decreased by DT injection compared to that of WT mice (Fig. 3b). mRNA expression level of CD169 in peritoneal macrophages was significantly decreased in DT-injected CD169-DTR mice (Fig. 3c). All these data clearly demonstrated that the peritoneal CD169 expressing macrophages could be specially deleted in the CD169-DTR mice by DT injection.
Selective deletion of CD169-expressing cells in peritoneal macrophage from CD169-DTR mice. a Peritoneal cells from WT mice and CD169-DTR mice with DT injection for 3 days were stained with antibodies for CD11b and CD169. The percentage of CD11b+CD169+ was analyzed by flow cytometry. *P < 0.05 compared to WT. b CD169 mRNA level in peritoneal cells from WT mice and CD169-DTR mice with DT injection for 3 days was detected by qRT-PCR. ***P < 0.001 compared to WT group. c Immunohistochemistry of peritoneal macrophages from WT mice and CD169-DTR mice with DT injection for 3 days (n = 3 per group). Original magnification, ×10.
Next, CD169-DTR mice were used to study the role of CD169 expressing subset in peritoneal cells in colitis induced by DSS. 3.5% DSS in drinking water was administrated to both CD169-DTR and WT mice for 7 days, and DT was injected on day −1 and 3. The results showed the colitis symptoms of CD169-DTR mice were more moderate than those of WT mice. The body weight of DT-injected CD169-DTR mice did not change obviously during the DSS-induced colitis, although 7-day administration caused more than 20% of their body weight loss in WT mice (Fig. 4a). Besides, CD169-DTR mice did not show the symptom of rectal bleeding and the length of colons from CD169-DTR mice was longer than that of WT mice (Fig. 4b). Histological examination of the colons from CD169-DTR mice showed a lower degree of mucosal injury and greatly reduced numbers of infiltrating inflammatory cells (Fig. 4c). Taken together, these results indicated that typical colitis symptoms could not be observed in CD169-DTR mice with DT injection, which is due to, at least partially the deletion of CD169+ cells in peritoneal macrophage.
Alleviated symptoms of DSS-induced colitis in CD169-DTR mice by DT injection. WT mice and CD169-DTR mice were administrated with 3.5% DSS in drinking water for 7 days and DT was injected on day - 1 and 3(n = 5 per group).a The body weight (%) of WT mice and CD169-DTR mice (n = 5 per group) was examined. *P < 0.05, **P < 0.01compared to WT colitis. b The colon length of DSS-exposed WT and CD169-DTR mice and control mice was measured. *P < 0.05, compared to WT naïve; **P < 0.01 compared to WT colitis. c Hematoxylin and eosin staining of colon sections from WT naïve, WT colitic, and CD169-DTR colitic mice were shown. Original magnification, ×10.
Higher CCL22 Expression Level in Peritoneal Cells from DSS-Treated CD169-DTR Mice
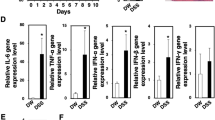
To understand the mechanism of peritoneal CD169+ macrophages in the DSS-induced colitis, the expression level of cytokines in peritoneal cells from DSS administrated WT mice and 169-DTR mice were detected. Compared to the expression level in WT colitic mice, the expression level of inflammatory cytokines including IL-1β, IL-6, IL-12, IL-23, and TNFα in the peritoneal cells from DSS-treated CD169-DTR mice decreased significantly (Fig. 5a). Contrast to these results, CCL22 expression level in DSS-treated CD169-DTR mice increased significantly although the expression of CCL3 and CCL8 decreased (Fig. 5b). The secretion of CCL22 in peritoneal macrophages was also detected by ELISA and it showed that CCL22 production in DSS-treated CD169-DTR mice was higher than that in WT colitis mice (Fig. 5c). These results suggested that peritoneal macrophage subset expressing CD169 regulates the colonic inflammation probably together with lower CCL22 levels.
Higher CCL22 expression level in peritoneal cells from CD169-DTR mice. a IL-1β, IL-6, IL-12, IL-23, and TNFα mRNA levels in peritoneal cells from DSS-treated WT and CD169-DTR mice were detected by qRT-PCR (n = 5 per group). DT was injected on day - 1 and 3. *P < 0.05, **P < 0.01, ***P < 0.001 compared to WT colitis. b CCL3, CCL8, and CCL22 mRNA levels in peritoneal cells from DSS-treated WT and CD169-DTR mice were detected by qRT-PCR. DT was injected on day - 1 and 3.*P < 0.05, ***P < 0.001 compared to WT colitis. c Peritoneal cells from DSS-treated WT and CD169-DTR mice were cultured in vitro for CCL22 production detection using ELISA. DT was injected on day 1. *P < 0.05, compared to WT colitis.
CD11b+CD169+ Macrophage Cells Did Not Express CCL22 Dominantly
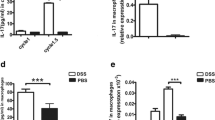
The higher CCL22 level of peritoneal cells from DSS-treated CD169-DTR mice with moderate colitis revealed that CD169-expressing subset was a key subset regulating mucosal inflammation probably together with CCL22. Thus, the cell sorting of total peritoneal cells was performed to examine the relationship between CD169 expressing subset and CCL22 production. CD11b+CD169+ and CD11b+CD169− subpopulations of total peritoneal cells from WT mice were sorted by flow cytometry (Fig. 6a). The result of real-time PCR showed that the CCL22 expression level was much lower in CD169+ peritoneal cells compared to that in CD169− peritoneal cells. Contrary to the result, CD169+ peritoneal cells produced much higher pro-inflammatory IL-17 than CD169− peritoneal cells (Fig. 6b). These results suggested that CD169+ macrophages could not express anti-inflammatory CCL22 dominantly.
CD11b+CD169+ macrophage cells did not express CCL22 dominantly. Peritoneal macrophages from wild-type C57BL/6 mice were stained with antibodies for CD11b and CD169 (n = 3 per group).CD11b+CD169+ (R1) and CD11b+CD169− (R2) were sorted (a) and used for examining the mRNA expression levels of CCL22 and IL17 (b).*P < 0.05, **P < 0.01 compared with levels of CD11b+CD169+, respectively.
The In Vivo Treatment with RmCCL22 Could Alleviate the Symptoms of DSS-Induced Colitis
The higher expression level of CCL22 and moderate colitis symptoms in CD169-DTR mice with DT injection suggested that CCL22 could be considered as a potential pathway for colitis therapy. To investigate the direct role of CCL22 on colitis, the wild-type mice were received a total of six daily subcutaneous injections of rmCCL22 (150 μg/kg/day) at 2 days after the administration of DSS and the control mice were received saline. The results showed that treatment with rmCCL22 inhibited the clinical symptoms of colitis induced by DSS. The body weight loss in rmCCL22-treated mice was less than that in the control mice (Fig. 7a). Histological examination of colons showed the inflammatory infiltrate and tissue injury were moderate in rmCCL22-treated mice compared with the control mice (Fig. 7b). The expression levels of IL-1β, IL-6, IL-12, IL-23, and TNFα were lower in the peritoneal cells of rmCCL22-treated mice than those in the control mice (Fig. 7c). These results showed treatment with rmCCL22 at least partially could alleviate the symptoms of DSS-induced colitis.
The in vivo treatment with rmCCL22 alleviated the symptoms of DSS-induced colitis. C57BL/6 mice were exposed to drinking water containing 3.5% DSS for 7 days and received daily subcutaneous injections of rmCCL22 (150 μg/kg/day) or saline from day 2 to day 7 (n = 5 per group). a The body weight (%) was compared between rmCCL22-treated mice and saline-treated mice. *P < 0.05 compared to saline group. b Hematoxylin and eosin staining of colon sections from rmCCL22-treated mice and saline-treated mice. Original magnification, ×10. c The mRNA expression levels of IL-1β, IL-6, IL-12, IL-23, and TNFα were examined in the peritoneal cells from saline-treated and rmCCL22-treated mice. *P < 0.05, **P < 0.01, ***P < 0.001 compared to saline group.
Discussion
Macrophages throughout the body tissues play important roles during the maintenance of tissue homeostasis, pathogenic infection, and tissue injury [20, 21]. Many studies have revealed that macrophages are highly heterogeneous to be consistent with their distinct tissue environments and specific functions [5]. Mouse peritoneal macrophages are among the best-studied macrophage populations in the control of mucosal immunity and inflammatory responses [22,23,24,25,26]. It has been confirmed that peritoneal cavity macrophages have a prenatal origin which derived from cells in the yolk sac; however, the resident macrophages of gastrointestinal tract are blood monocyte derived in the steady state. The different origins of these cells support that it is necessary to investigate the role of peritoneal macrophage further. The present study revealed new findings that mouse peritoneal macrophage expressing CD169 was a key subset in DSS-induced colitis by using CD169-DTR mice study. The percentage of peritoneal CD169+ macrophages increased and CCL22 expression level of peritoneal macrophage decreased in DSS-induced colitic mice than those in non-colitic mice. Meanwhile, the typical colitis was not induced by DSS in CD169-DTR mice and the CCL22 level in colitic CD169-DTR mice significantly increased than that in colitic WT mice, which might be caused by transiently deletion by CD169+ macrophages by DT injection. Moreover, the in vivo treatment with rmCCL22 ameliorated the clinical symptoms of DSS-induced colitis.
CD169+ macrophages are mainly reported in sinus macrophage [27, 28]. Animal studies have suggested that CD169+ macrophages have a pro-inflammatory property. In addition, recent studies revealed the role of CD169+ macrophage in human studies [29,30,31]. Therefore, the roles of the CD169+ macrophages have diversities and heterogeneities in both humans and animals. The present study mainly reported the increase of CD169-expressing cells in peritoneal cavity and the moderate colitis symptoms in CD169-DTR mice, which strongly revealed that peritoneal macrophage expressing CD169 was an important regulator in the pathogenesis of IBD using DSS-induced colitis model. Moreover, cell-sorting result revealed the specific expression characteristic of cytokine and chemokine of the peritoneal subset expressing CD169 (Fig. 6b). Further studies need to be performed to understand its specific roles clearly in mucosal immunity.
IBD, including Crohn’s disease and ulcerative colitis, is characterized as a chronic inflammation in the gastrointestinal tract. The IBD patients especially with extended severe disease phenotypes have an increased risk of colitis-associated cancer [3]. Although the precise cause of IBD is unknown, mice with the absence of lymphocytes developed severer inflammation [32, 33], which suggests innate immune cells are sufficient for disease onset and development. The present study revealed that peritoneal macrophage expressing CD169 subset was a key regulator of IBD. CD169 is expressed in a specific sialic acid on the surface of the macrophages adhesion receptor. In the lymph nodes, CD169+ macrophage could mediate antitumor immunity by engulfing the dead tumor cells and presenting subsequent tumor-related antigen to CD8+ T cell. In the spleen, CD169+ macrophages are located in the marginal zone of the spleen and play a critical role in the induction of immune tolerance. In the colon, CD169+ macrophages are mainly located in the lamina propria layer and are associated with mucosal injury and inflammatory monocyte recruiting [7, 34]. Human CD169 was expressed strongly by tissue macrophages in the spleen, lymph node, bone marrow, liver, lungs, and colon [35]. CD169+macrophages are found in the lamina propria of the human colon tissues and the expression is similar to the mouse [31]. All these reports indicated that CD169-expressing cells are essential subset in both innate and adoptive immunity. The present study reported moderate colitic symptoms in DSS-treated CD169-DTR mice and much higher CCL22 level in peritoneal cells from DSS-treated CD169-DTR mice than that from DSS-treated WT mice. It suggested that CD169-expressing cells in peritoneal play its role in mucosal inflammation probably together with CCL22. The precise mechanism of the process and the crosstalk between the peritoneal cells and the resident macrophages in colon are necessary to be clarified.
The present data showed that chemokines played different roles in colitis induced by DSS. The expression level of CCL22 was decreased in DSS-induced colitis; however, expression levels of both CCL3 and CCL8 increased in colitis. The therapy role of CCL22 on DSS-induced colitis in the present study (Fig. 7) suggested its potential use in anti-inflammatory therapy in clinical symptoms. CCL22 is a member of the CC-chemokine family that attracts regulatory T cells [36]. And our recent studies have shown that CCL22 could induce tolerance probably by attracting CCR4+ Tregs migration in the microenvironment during apoptotic cell clearance [37]. These reported data supported that CCL22 played its anti-inflammatory role probably by attracting regulatory cells to maintain hemostats. The further experiments would be necessary to understand the molecular mechanism.
Numerous studies had demonstrated that CD4+ T helper (Th) 1 and Th2 cells are essential in the pathogenesis of IBD. Recently, Th17 cells, a subset of Th cells capable of producing IL-17, was reported to have a relationship with the IBD pathogenesis [38, 39]. Expression level of IL-17 increased in inflamed mucosa obtained from IBD patients [40]. In addition, the symptoms of colitis were improved in IL-17R knockout mouse by using trinitrobenzenesulfonic acid (TNBS) to induce colitis [41]. Th1-related cytokines (IFN gamma) and Th17-related cytokines (IL-17, IL-21, 22, 23, and 26) are associated with CD [42]. Th2-related cytokines (IL-13 and IL-5) and Th17-related cytokines are selectively increased in CD [43]. Some cytokines also implicated in the pathogenesis of UC, such as TNF alpha, IL-6, IL-1β, IL-33, TNF-like cytokine1A (TL1A), and lymph toxin-like inducible protein (LIGHT). Moreover, decreased expression of IL-17 in colon (Fig. S2) from CD169-DTR mice also suggested peritoneal CD169+ macrophage- CCL22 mediated process of colitis by regulating T cells subset balances. CD169-DTR mice study revealed that the CCL22 level in local sites could be as a biomarker of autoimmune diseases.
In conclusion, the present study verified peritoneal macrophage subset expressing CD169 is a critical regulator in DSS-induced colitis, probably combined with CCL22 production by CD169− cells. The moderate colitis symptom and higher CCL22 levels in peritoneal macrophages from DSS-treated CD169-DTR mice are at least partially due to the deletion of the CD169 expressing cells in peritoneal cavity. Further studies revealed CCL22 are mainly produced by CD169− subset and treatment of CCL22 moderated the colitis symptoms. These results verified the possibility that CCL22 could be potentially used as a preventive agent or as an adjunct in anti-inflammatory therapy in clinical symptoms. Thus, the precise role of CCL22 in the regulation of mucosal inflammation diseases is needed to be clarified.
Abbreviations
- CD169:
-
Cluster of differentiation 169
- CCL22:
-
C-C motif chemokine 22
- DSS:
-
Dextran sulfate sodium
- DT:
-
Diphtheria toxin
- DTR:
-
Diphtheria toxin receptor
- Tregs:
-
Regulatory T cells
- UC:
-
Ulcerative colitis
- IBD:
-
Inflammatory bowel diseases.
Reference
Kilcoyne, A., J.L. Kaplan, and M.S. Gee. 2016. Inflammatory bowel disease imaging: Current practice and future directions. World Journal of Gastroenterology 22: 917–932.
Kaistha, A., and J. Levine. 2014. Inflammatory bowel disease: The classic gastrointestinal autoimmune disease. Current Problems in Pediatric and Adolescent Health Care 44: 328–334.
Itzkowitz, S.H., and X. Yio. 2004. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: The role of inflammation. American Journal of Physiology: Gastrointestinal and Liver Physiology 287: G7–17.
de Souza, H.S., and C. Fiocchi. 2016. Immunopathogenesis of IBD: Current state of the art. Nature Reviews. Gastroenterology & Hepatology 13: 13–27.
Davies, L.C., S.J. Jenkins, J.E. Allen, and P.R. Taylor. 2013. Tissue-resident macrophages. Nature Immunology 14: 986–995.
Gross, M., T.M. Salame, and S. Jung. 2015. Guardians of the Gut-Murine Intestinal Macrophages and Dendritic Cells. Frontiers in Immunology 6: 254.
Yona, S., K.W. Kim, Y. Wolf, A. Mildner, D. Varol, M. Breker, et al. 2013. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38: 79–91.
Asano, K., N. Takahashi, M. Ushiki, M. Monya, F. Aihara, E. Kuboki, et al. 2015. Intestinal CD169(+) macrophages initiate mucosal inflammation by secreting CCL8 that recruits inflammatory monocytes. Nature Communications 6: 7802.
Martinez-Pomares, L., and S. Gordon. 2012. CD169+ macrophages at the crossroads of antigen presentation. Trends in Immunology 33: 66–70.
Asano, K., A. Nabeyama, Y. Miyake, C.H. Qiu, A. Kurita, M. Tomura, et al. 2011. CD169-positive macrophages dominate antitumor immunity by cross presenting dead cell-associated antigens. Immunity 34: 85–95.
Ravishankar, B., R. Shinde, H. Liu, K. Chaudhary, J. Bradley, H.P. Lemos, et al. 2014. Marginal zone CD169+ macrophages coordinate apoptotic cell-driven cellular recruitment and tolerance. Proceedings of the National Academy of Sciences of the United States of America 111: 4215–4220.
Hiemstra, I.H., M.R. Beijer, H. Veninga, K. Vrijland, E.G. Borg, B.J. Olivier, et al. 2014. The identification and development requirements of colonic CD169+ macrophages. Immunology: 142, 269–278.
Cassado Ados, A., M.R. D'Império Lima, and K.R. Bortoluci. 2015. Revisiting mouse peritoneal macrophages: heterogeneity, development, and function. Frontiers in Immunology 6: 225.
Geremia, A., P. Biancheri, P. Allan, G.R. Corazza, and A. Di Sabatino. 2014. Innate and adaptive immunity in inflammatory bowel disease. Autoimmunity Reviews 13: 3–10.
Wang, D., R.N. Dubois, and A. Richmond. 2009. The role of chemokines in intestinal inflammation and cancer. Current Opinion in Pharmacology 9: 688–696.
Zhang, J., J. Romero, A. Chan, J. Goss, S. Stucka, J. Cross, et al. 2015. Biarylsulfonamide CCR9 inhibitors for inflammatory bowel disease. Bioorganic & Medicinal Chemistry Letters 25: 3361–3364.
Evans-Marin, H.L., A.T. Cao, S. Yao, F. Chen, C. He, H. Liu, et al. 2015. Unexpected Regulatory Role of CCR9 in Regulatory T Cell Development. PloS One 10: e0134100.
Saito, Michiko, l Takao Iwawaki, Choji Taya, Hiromichi Yonekawa, Munehiro Noda, et al. Diphtheria toxin receptor–mediated conditional and targeted cell ablation in transgenic mice. Nature Biotechnology 19: 746–750.
Miyake, Y. l, K. Asano, H. Kaise, M. Uemura, M. Nakayama, and M. Tanaka. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. The Journal of Clinical Investigation 117: 2268–2278.
Saito, M., T. Iwawaki, C. Taya, H. Yonekawa, M. Noda, Y. Inui, et al. 2001. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nature Biotechnology 19: 746–750.
Herwald, H., and A. Egesten. 2013. Macrophages: past, present and future. Journal of Innate Immunity 5: 657–658.
Wynn, T.A., A. Chawla, and J.W. Pollard. 2013. Macrophage biology in development, homeostasis and disease. Nature 496: 445–455.
Ghosn, E.E., A.A. Cassado, G.R. Govoni, T. Fukuhara, Y. Yang, D.M. Monack, et al. 2010. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc Natl Acad Sci U S A107: 2568–2573.
Davies, L.C., M. Rosas, P.J. Smith, D.J. Fraser, S.A. Jones, and P.R. Taylor. 2011. A quantifiable proliferative burst of tissue macrophages restores homeostatic macrophage populations after acute inflammation. European Journal of Immunology 41: 2155–2164.
Davies, L.C., M. Rosas, S.J. Jenkins, C.T. Liao, M.J. Scurr, F. Brombacher, et al. 2013. Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation. Nature Communications 4: 1886.
Okabe, Y., and R. Medzhitov. 2014. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell 157: 832–844.
Dahdah, A., G. Gautier, T. Attout, F. Fiore, E. Lebourdais, R. Msallam, et al. 2014. Mastcells aggravate sepsis by inhibiting peritoneal macrophage phagocytosis. The Journal of Clinical Investigation 124: 4577–4589.
Saunderson, S.C., A.C. Dunn, P.R. Crocker, and A.D. McLellan. 2014. CD169 mediates the capture of exosomes in spleen and lymph node. Blood 123: 208–216.
Chávez-Galán, L., M.L. Olleros, D. Vesin, and I. Garcia. 2015. Much More than M1 and M2 Macrophages, There are also CD169+ and TCR+ Macrophages. Frontiers in Immunology 6: 263.
Ohnishi, K., M. Yamaguchi, C. Erdenebaatar, F. Saito, H. Tashiro, H. Katabuchi, et al. 2016. Prognostic significance of CD169-positive lymph node sinus macrophages in patients with endometrial carcinoma. Cancer Science 107: 846–852.
Li, C., X. Luo, Y. Lin, X. Tang, L. Ling, L. Wang, et al. 2015. A Higher Frequency of CD14+ CD169+ Monocytes/Macrophages in Patients with Colorectal Cancer. PloS One 10: e0141817.
Saito, Y., K. Ohnishi, A. Miyashita, S. Nakahara, Y. Fujiwara, H. Horlad, et al. 2015. Prognostic Significance of CD169+ Lymph Node Sinus Macrophages in Patients with Malignant Melanoma. Cancer Immunology Research 3: 1356–1363.
Kim, T.W., J.N. Seo, Y.H. Suh, H.J. Park, J.H. Kim, J.Y. Kim, et al. 2006. Involvement of lymphocytes in dextran sulfate sodium-induced experimental colitis. World Journal of Gastroenterology 12: 302–305.
Tlaskalová-Hogenová, H., L. Tucková, R. Stepánková, T. Hudcovic, L. Palová-Jelínková, H. Kozáková, et al. 2005. Involvement of innate immunity in the development of inflammatory and autoimmune diseases. Annals of the New York Academy of Sciences 1051: 787–798.
Hartnell, A., J. Steel, H. Turley, M. Jones, D.G. Jackson, and P.R. Crocker. 2001. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood 97 (1): 288–296.
Yoshie, O., and K. Matsushima. 2015. CCR4 and its ligands: from bench to bedside. International Immunology 27: 11–20.
Hao, S., X. Han, D. Wang, Y. Yang, Q. Li, X. Li, et al. 2016. Critical role of CCL22/CCR4 axis in the maintenance of immune homeostasis during apoptotic cell clearance by splenic CD8a+CD103+ DCs. Immunology 148: 174–186.
Owaga, E., R.H. Hsieh, B. Mugendi, S. Masuku, C.K. Shih, and J.S. Chang. 2015. Th17 Cells as Potential Probiotic Therapeutic Targets in Inflammatory Bowel Diseases. International Journal of Molecular Sciences 16: 20841–20858.
Gálvez, J. 2014. Role of Th17 Cells in the Pathogenesis of Human IBD. ISRN Inflamm 2014: 928461.
Sartor, R.B. 2006. Mechanisms of disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepato 3: 390–407.
Zhang, Z., M. Zheng, J. Bindas, P. Schwarzenberger, and J.K. Kolls. 2006. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflammatory Bowel Diseases 12: 382–388.
Monteleone, Ivan, Francesco Pallone, and Giovanni Monteleone. 2011. Th17-related cytokines: new players in the control of chronic intestinal inflammation. BMC Medicine 9: 122.
Sanchez-Munoz, F., A. Dominguez-Lopez, and J.K. Yamamoto-Furusho. 2008. Role of cytokines in inflammatory bowel disease. World Journal of Gastroenterology 14: 4280–4288.
Acknowledgments
The present work was supported by grants from the (NSFC), (No. 81202306), China Postdoctoral Science Foundation (201252M1343, 2013T60674). We would like to thank Dr. Yunxue Zhao and Ms. Limei Wang at Shandong University for FACS analysis. Qiu C. and Wang D. designed the study. Wang D., Li Q., Yang Y., Hao S., Han X., Song J., and Yin Y. performed experiments. Wang D., Li X, and Qiu C. analyzed the data. Qiu C. and Wang D. wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest
Electronic supplementary material
ESM 1
(PPTX 266 kb)
Rights and permissions
About this article
Cite this article
Wang, D., Li, Q., Yang, Y. et al. Macrophage Subset Expressing CD169 in Peritoneal Cavity-Regulated Mucosal Inflammation Together with Lower Levels of CCL22. Inflammation 40, 1191–1203 (2017). https://doi.org/10.1007/s10753-017-0562-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0562-0