Abstract
This study was conducted in order to investigate the function of IL-21 in intervertebral disc degeneration. The serum concentration of IL-21 in patients with lumbar disc herniation (LDH) was examined by ELISA. Immunohistochemistry and western blot analysis were performed to detect the expression of IL-21, a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS-7), and tumor necrosis factor alpha (TNF-α) in degenerated intervertebral disc (IVD) tissues of human and rat. Moreover, nucleus pulposus (NP) cells were treated with 0, 10, 100, and 1000 ng/mL of IL-21 cytokine with and without AG490. TNF-α, ADAMTS-7, and matrix metalloproteinases-13 (MMP-13) mRNA expression was determined by RT-PCR. The expression of signal transducers and activators of transcription, STAT-1, STAT-3, and STAT-5b, was detected by western blot. IL-21 concentration level is higher in the degenerated group and positively correlates with the visual analog score (VAS). IL-21, ADAMTS-7, and TNF-α can be detected in the degenerative NP tissues in both human and rat degenerated NP tissues. The mRNA expression of ADAMTS-7, TNF-α, and MMP-13 was enhanced after stimulation with IL-21. Compared to control, STAT-1, STAT-3, and STAT-5b expression was also enhanced after IL-21 treatment, with STAT-3 being the most significantly enhanced; furthermore, expression was significantly reduced after treatment with AG490. The mRNA expression of TNF-α was markedly reduced after treatment with AG490 compared to treatment with IL-21 only. IL-21 is involved in the pathological development of IVD degeneration and IL-21 could aggravate IVD degeneration by stimulating TNF-α through the STAT signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Lumbar disc herniation (LDH) is a common clinical disease that causes inconveniences in life and work for many patients due to degeneration of the intervertebral disc (IVD) [1]. The IVD is a kind of soft tissue located between vertebral bodies that can bear weight. Less than 50% of lower back pain (LBP) cases involve IVD degeneration and 90% of sciatica cases involve LDH [2–4]. Bobechko and Hirsch [5] demonstrated that IVD degeneration may also be a kind of autoimmune disease, as herniated nucleus pulposus (NP) tissue was considered to be an antigen that could immediately induce inflammation.
The process of IVD degeneration is based on many factors, and a large number of causes have been implicated in the pathogenesis of lumbar disc degeneration, such as end plate degeneration, excessive mechanical loading, congenital factors, unhealthy habits, aging, and spine infection. In addition to these causes, inflammation plays an important role in IVD degeneration [6]. The ability of the IVD tissue to secrete or attract proinflammatory cytokines supports the hypothesis that IVD-originated cells are capable of inducing and enhancing inflammation [7].
Interleukin-21 (IL-21) is produced by activated CD4+ T cells [8]. Previous studies have shown that IL-21 is overexpressed in many chronic inflammatory diseases [9–12]. Furthermore, IL-21 plays a central role in the survival and differentiation of both T cells and B cells, thus making it an attractive target for therapeutic intervention in a wide range of immune-inflammatory diseases [13]. The IL-21 signaling network is involved mainly in the JAK-STAT pathway. However, there are no published studies regarding the role of IL-21 in IVD degeneration and the mechanism underlying the effects of IL-21 in degeneration. Based on the discussion above, we decided to investigate the expression of IL-21 in degenerated IVD tissues to elucidate the effect of IL-21 in IVD degeneration.
METHODS
Ethics
Our experiments were approved by the Shandong University Human Subjects Institutional Review Board and followed the guidelines for ethical principles in medical research involving human subjects. Patients agreed to participate in this study and signed approval documentation. Patient privacy was protected.
Our animal experiment was approved by the Animal Ethical Committee of Shandong University and followed the requests of the Animal Management Rules of the Chinese Ministry of Health (Document no. 55, 2001). The animals were placed in a warm room after surgery, supplied with food and water for nutrition, and were monitored every 12 h.
Patients
Forty patients with single-level IVD herniation (25–61 years; mean 43.68 ± 12.72 years) were selected according to their MRI results. Before surgery, all of the patients had taken a conservative form of treatment, such as taking painkillers and resting for at least 3 months. All of the patients decided to undergo surgery under the condition that the conservative treatment was ineffective or their conditions were getting worse (Table 1).
The selection criteria were as follows: (1) sciatica, (2) positive for Lasegue’s sign (+) or Bragard test, (3) positive for femoral nerve traction test (+), (4) loss of disc height on lumbar MRI and X-ray or reduced signal intensity of the nucleus pulposus as determined by T2-weighted MRI, and (5) single-level IVD herniation. Determination of the disc degeneration grade was made according to the Pfirrmann grading system based on MRI. The exclusion criteria were as follows: (1) IVD combined with osteoarthritis, rheumatoid osteoarthritis, or tuberculosis; (2) IVD combined with a malignant tumor; (3) recent upper respiratory tract infection; (4) recent history of glucocorticoid use; and (5) previous surgery for IVD disease.
Patients were assigned to three groups. Group P had 20 patients (average age is 51.3 ± 24.4) and the annulus fibrosus was intact; group E had 20 patients (average age is 61.9 ± 27.8) and the annulus fibrosus was not intact. NP tissues were obtained from the annulus fibrosus. All patients were given a lumbar MRI to determine whether the annulus fibrosus was intact. NP tissues of the normal control group were obtained from patients admitted to our department because of lumbar vertebral fracture or lumbar scoliosis without any signal intensity change in the NP tissue according to MRI.
Four milliliters of blood was drawn using a heparin sodium anticoagulant tube from patients early in the morning after fasting. Blood samples were sent to the laboratory immediately and centrifuged at 4 °C at 3000 rpm for 10 min. Supernatant was extracted and stored in EP tubes. VAS scoring was used to evaluate pain sensation.
Animals
Rats (weighing 230–250 g) were randomly assigned to two groups: sham operation group (n = 10) and needle puncture group (n = 10). Needle puncture surgery was conducted in a rat needle puncture model [14] under anesthesia induced by pentobarbital sodium (20 mg/kg). A 20-gauge needle was inserted into the Co6-7 disc at a depth of 5 mm, rotated 360°, and held for 5 s. For the sham operation group, the disc was exposed without needle puncture. Disc tissues were extracted from the rats after 4 weeks.
Human NP Cell Culture
Disc tissues were extracted during operation, washed in normal saline, and clipped into 2–3 mm2 fragments. After the end plate and tendons were removed, tissues were then digested with 0.25% trypsin for 1 h, followed by digestion with type II collagen enzyme for 4 h. Cells were cultured in DMEM/F12 media (Gibco, Invitrogen Corporation, USA) with 10% FCS, 1% PS, 0.05% fungizone, and 50 mg/mL of ascorbate at a density of 25,000 cells per cm2 under standard incubation conditions (37 °C, 5% CO2). Cells were used at the second passage for all experiments. First, NP cells were cultured in six-well plates with IL-21 added at 0, 10, 100, and 1000 ng/mL concentrations. After 24 h, NP cells were harvested for western blot analysis of tumor necrosis factor alpha (TNF-α), a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS-7), and matrix metalloproteinases-13 (MMP-13) expression level. In another six-well plate, cells were cultured in normal media (as described above), media with IL-21 (100 ng/mL), or media with both IL-21 (100 ng/mL) and 10 μM AG490. After a 2-h incubation, NP cells were harvested for western blot analysis of STAT-1 expression. The same steps were repeated to analyze STAT-3 and STAT-5b expression.
Serum Concentrations of IL-21
The serum concentration of IL-21 in patients with LDH was measured by ELISA, following the manufacturer’s instructions (BlueGene, Shanghai, China). All the operations were performed at room temperature, and all samples were measured in duplicate. The color reaction was assayed at 450 nm using a Varioskan flash multifunction plate reader (Thermo Fisher Scientific, Waltham, MA).
Immunohistochemical Staining
Tissues embedded in paraffin were cut into 4-μm sections and stained by IHC. Tissues were incubated with primary antibody (goat anti-IL-21 1:300, Santa, USA; rabbit anti-ADAMTS-7 1:300, Abcam, USA; goat anti-TNF-α 1:300, Santa, USA). Tissues were then washed in PBS and incubated with secondary antibodies (goat anti-rabbit immunoglobulin-HRP secondary antibody 1:200; Beijing Golden Bridge Biotechnology Co., Ltd, Beijing, China). Slides were visualized on an IX71-SIF type microscope (Olympus, Japan), and the Image-Pro Plus 5.0 software (Media Cybernetics, MD, USA) was used for digital photograph analysis.
Protein Extraction and Western Blot for IL-21, ADAMTS-7, and TNF-α
Tissue samples were preserved in liquid nitrogen. Total protein was extracted and determined using the BCA Protein Assay Kit according to the manufacturer’s instructions. Equivalent amounts of protein for each sample were resolved using 12% acrylamide-SDS-PAGE. The samples were subsequently transferred to polyvinylidene difluoride membranes (PVDF; Millipore, Billerica, MA, USA). Primary antibodies (IL-21, 1:500, Santa; ADAMTS-7, 1:1000, Abcam; TNF-α, 1:500, Santa) and a rabbit anti-goat immunoglobulin (IgG)-horseradish peroxidase (HRP) secondary antibody (1:5000; Beijing Golden Bridge Biotechnology) were applied. Equal protein loading was confirmed by reprobing the membranes with the mouse anti-GAPDH-HRP antibody (1:10,000, Abcam). Protein bands were detected using a FluorChem E Chemiluminescent Western Blot Imaging System (Amersham Imager 600, GE Amersham, USA) and quantified by densitometry analysis using ImageJ software (National Institutes of Health, USA).
Real-Time PCR
Total RNA was extracted using the TRIzol (Takara Biotechnology Co., Ltd, Dalian, China) method according to the manufacturer’s recommendations. Total RNA (1 μg) was reverse transcribed in a total volume of 10 μL, including 2 μL of RT buffer, 0.5 μL of RT enzyme mix, and 0.5 μL of primer mix (Toyobo Co, Ltd, Japan). The reaction was performed at 37 °C for 15 min followed by 95 °C for 5 min. The complementary DNA (cDNA) was stored at −20 °C until further use. RT-PCR reactions were carried out on a Roche LightCycler (Roche, USA). The cDNA was detected with SYBR Green Dye I Master Mix. To normalize TNF-α gene expression, GADPH was used as an internal control. Cycle threshold (Ct) data were obtained and the relative expression level of TNF-α was analyzed using the 2−△△Ct method. The messenger RNA (mRNA) expression of TNF-α was also detected after IL-21 treatment (100 ng/mL) with and without AG490 and normal controls.
Statistical Analysis
The software used for the analyses was SPSS version 19.0. Mean values were calculated and presented with an error bar representing ±SD. Statistical analysis of data was performed by one-way ANOVA using Dunnett’s test and Pearson correlation. Differences were regarded to be statistically significant if p < 0.05.
Funding Source
The study was supported by the General Program of the National Natural Science Foundation of China. All experimental reagents and tools were provided by this program.
RESULTS
Serum Secretion of IL-21 in LDH Patients and Correlation with VAS Score
VAS score was significantly higher in degenerated groups compared to the normal control (0.258 ± 0.238) as shown in Fig. 1a. Furthermore, preoperative VAS score was significantly higher in group E than in group P (8.334 ± 1.343 vs. 5.852 ± 1.089, respectively, p < 0.001). IL-21 level showed a similar trend to VAS score. Compared with the normal control (127.728 ± 25.355 pg/mL), IL-21 expression level was markedly higher in the degenerated groups preoperatively, especially in group E. The statistical analysis of the IL-21 ELISA data acquired from the protruded group and extruded group is shown in Fig. 1b (689.139 ± 121.688 vs. 508.455 ± 98.220 pg/mL, respectively, p < 0.001). As shown in Fig. 1c, d, the postoperative VAS score dramatically decreased in both the protruded group and extruded group (2.894 ± 1.398 vs. 2.452 ± 0.991, respectively, p < 0.001). The Pearson correlation coefficients were used to examine the correlation of VAS score with IL-21 preoperatively. A positive correlation between IL-21 and VAS score was found (r = 0.834, p < 0.001). This result implied that IL-21 may positively correlate with the extent of pain that the patients experienced.
Preoperative IL-21 serum concentration and VAS score in LDH patients before and after surgery. Group P represents patients whose annulus fibrosus was intact; group E represents patients whose annulus fibrosus was not intact. The VAS score of group E was higher than that of group P and control (p < 0.001, a). IL-21 concentration was higher in degenerated groups, especially group E, compared to the normal control group (p < 0.001, b). The VAS score was markedly reduced postoperation compared to preoperation in group P and group E (p < 0.001, c, d).
Expression Pattern of IL-21, ADAMTS-7, and TNF-α in Human and Rat Disc Tissues
As shown in Fig. 2a–c, IL-21, ADAMTS-7, and TNF-α immunoreactivity was detected in the NP cells. Different levels of IL-21, ADAMTS-7, and TNF-α immunoreactivity were detected in the normal control group, group P, and group E. For IL-21, ADAMTS-7, and TNF-α, the degree of degeneration in group P was clearly lower than in group E. Image-Pro Plus software was used to analyze the mean density of positive cells. Mean density is the average optical density of the objects and represents the extent of expression of the cytokines. As shown in Fig. 2g–i, compared to the normal control group, the expression of IL-21, TNF-α, and ADAMTS-7 in groups P and E was significantly increased (IL-21: group E, 0.193 vs. group P, 0.127 vs. control, 0.003, p < 0.001; ADAMTS-7: group E, 0.158 vs. group P, 0.143 vs. control, 0.113, p < 0.001; TNF-α: group E, 0.162 vs. group P, 0.123 vs. control, 0.052, p < 0.001). Immunohistochemistry results of the rat NP tissues showed elevated expression levels of IL-21, ADAMTS-7, and TNF-α in degenerated rat NP tissues (Fig. 2d–f). Image-Pro Plus software was also used to analyze the results (Fig. 2j–l). Compared to the normal control group, the expression of IL-21, ADAMTS-7, and TNF-α was higher in group P (IL-21: group P, 0.167 vs. control, 0.078, p < 0.001; ADAMTS-7: group P, 0.153 vs. control, 0.143, p < 0.001; TNF-α: group P, 0.173 vs. control, 0.139, p < 0.001).
Immunohistochemical localization of IL-21, ADAMTS-7, and TNF-α in human and rat NP tissues. Group E represents heavier degree of NP cell degeneration than group P. However, group P in rats just means degenerated NP cells induced by the needle puncture model. IL-21, ADAMTS-7, and TNF-α expression levels, respectively, showed an increasing trend in the control group, group P, and group E in human (a–c). IL-21, ADAMTS-7, and TNF-α expression levels, respectively, showed an increasing trend in the control group and group P in rats (p < 0.001, d–f). Mean density of IL-21, ADAMTS-7, and TNF-α in human and rat NP tissues (p < 0.001, g–l).
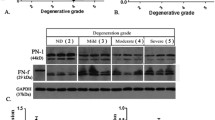
IL-21, ADAMTS-7, and TNF-α Protein Level in NP Tissues
The protein level of IL-21, ADAMTS-7, and TNF-α was examined in human degenerated NP tissue by western blot (Fig. 3a). Western blot verified a gradual increase in IL-21, ADAMTS-7, and TNF-α in group P and group E compared to the normal control group. The protein level of IL-21, ADAMTS-7, and TNF-α was also examined in rat degenerated NP tissue by western blot (Fig. 3c). ImageJ software was used to detect the relative density of IL-21, ADAMTS-7, and TNF-α in both human and rat tissues. The relative density was chosen to represent the average protein level, which is regarded as the extent of expression of IL-21, ADAMTS-7, and TNF-α. In the human tissues (Fig. 3b), the expression level of IL-21, ADAMTS-7, and TNF-α was higher in group E than in group P or in the normal control group (IL-21: group E, 1.228 vs. group P, 0.697 vs. control, 0.245, p < 0.001; ADAMTS-7: group E, 0.896 vs. group P, 0.749 vs. control, 0.228, p < 0.001; TNF-α: group E, 0.958 vs. group P, 0.683 vs. control, 0.287, p < 0.001). In the rat tissues (Fig. 3d), the protein level of IL-21, ADAMTS-7, and TNF-α was higher in group P than in the control group (IL-21: group P, 1.297 vs. control, 0.387, p < 0.001; ADAMTS-7: group P, 0.819 vs. control, 0.418, p < 0.001; TNF-α: group P, 1.253 vs. control, 0.607, p < 0.001).
IL-21, ADAMTS-7, and TNF-α protein level in human and rat NP tissues. In terms of human, group P represents patients whose annulus fibrosus was intact; group E represents patients whose annulus fibrosus was not intact and more serious degeneration degree than group P. However, group P in rats means the rats’ nucleus pulposus cells were induced into degeneration by the needle puncture model and fibrosus was not intact. Western blot analysis showed that IL-21, ADAMTS-7, and TNF-α expression levels were higher in degenerate NP tissues compared to control tissues in human (a). Western blot showed that IL-21, ADAMTS-7, and TNF-α expression levels are higher in degenerate NP tissue in rat compared to control tissue (c). The relative density of IL-21, ADAMTS-7, and TNF-α is shown above (b, d).
Effects of IL-21 on ADAMTS-7, TNF-α, and MMP-13 Secretion in NP Cells from Degenerate IVD Tissue
As shown in Fig. 4a, the IL-21 cytokine upregulated ADAMTS-7, TNF-α, and MMP-13 secretion in a dose-dependent manner (0, 10, 100, 1000) in cells exposed to IL-21 alone. ImageJ software was used to calculate relative density to determine the expression of ADAMTS-7, TNF-α, and MMP-13. As shown in Fig. 4b, the peak amount of ADAMTS-7, TNF-α, and MMP-13 expression appeared in cells treated with IL-21 alone at a concentration of 100 ng/mL.
Effect of IL-21 on ADAMTS-7, TNF-α, and MMP-13 mRNA Expression in Degenerate NP Cells
As shown in Fig. 5, NP cells were treated with IL-21, and mRNA expression of ADAMTS-7, TNF-α, and MMP-13 was determined by RT-PCR. TNF-α expression peaked in cells treated with IL-21 at a concentration of 100 ng/mL. A similar trend also occurred for the mRNA expression of ADAMTS-7 and MMP-13. The mRNA expression level of ADAMTS-7, TNF-α, and MMP-13 was not dose dependent.
Effects of IL-21 on STAT-1, STAT-3 and STAT-5b (With and Without AG490) in Degenerate NP Cells
As shown in Fig. 6, the IL-21 cytokine upregulated STAT-1, STAT-3, and STAT-5b secretion when cells were treated with IL-21 stimulation alone, with STAT-3 being especially upregulated. The expression of STAT-1, STAT-3, and STAT-5b dramatically decreased when cells were treated with both IL-21 and AG490. The mRNA expression of TNF-α increased when IL-21 treatment and decreased after treatment with IL-21 combined with AG490.
STAT-1, STAT-3, and STAT-5b protein level in NP cells treated with IL-21 with or without AG490 and mRNA expression of TNF-α in NP cells after treatment with IL-21 with or without AG490. (a) The western blot showed that STAT-1, STAT-3, and STAT-5b expression levels were higher with IL-21 treatment alone. (b) The protein level of STAT-1, STAT-3, and STAT-5b was significantly reduced in cells treated with IL-21 combined with AG490. The mRNA expression of TNF-α was consistent with the changes of STAT-1, STAT-3, and STAT-5b.
DISCUSSION
Autoimmunity is known to play a critical role in the inflammation processes associated with IVD degeneration [15]. IL-21 has been reported to be involved in several autoimmune diseases [16]. The effect of IL-21 in degenerated IVD tissues is still unclear. In the current study, we investigated whether IL-21 plays a role in IVD degeneration. Our study evaluated VAS score, a commonly accepted parameter for LBP in patients with degenerative disc diseases (DDD), and examined the expression pattern of IL-21. Our results indicated that IL-21 expression is positively associated with VAS score (r = 0.834, p < 0.001), which prompted us to investigate whether IL-21 increased along with the different stages of IVD degeneration. It is widely accepted that overexpressed cytokines are responsible for IVD degeneration [2]. Together, these data suggested that IVD degeneration is related to IL-21.
IL-21, a cytokine produced by activated CD4+ cells, is well accepted as a critical cytokine in autoimmune disorders, and IL-21 has emerged as the most potent inducer of plasma cell differentiation [17, 18]. IL-21 has been shown to play a vital role in differentiation of Th cells [24], which play a critical role in the process of IVD degeneration in humans. In the present study, expression of IL-21 was significantly increased in the extruded group compared to the protruded group. In rat, IL-21 expression was also higher in the degenerated NP tissues compared to the normal control. Based on the results of studies in both human and rat NP tissues, it is suggested that IL-21 has a high expression in NP tissues. The increased expression of IL-21 was related to the degree of IVD degeneration, suggesting that the more severe the inflammation, the worse the IVD degeneration might become.
ADAMTS-7, a recently identified metalloproteinase family member, was present and upregulated in NP tissues and induces a series of metabolic changes such as MMP-13 expression [19, 20]. It has been shown that ADAMTS-7 was upregulated by TNF-α and plays an important role in the pathogenesis of IVD degeneration and other autoimmune diseases [21]. Immunohistochemistry staining and western blot analysis of human and rat NP tissues showed a concurrent increase in ADAMTS-7 expression and expression of IL-21 and TNF-α in NP tissues. Based on our results and the literature, we suggest that IL-21 has a positive relationship with ADAMTS-7. In other words, it is reasonable to assert that IL-21 has a cause and effect relationship with ADAMTS-7 and that IL-21 may act as an initiator to enhance ADAMTS-7 expression through TNF-α to aggravate IVD degeneration.
TNF-α is a widely studied mediator of the inflammatory response. Previous studies revealed a noticeable elevation in TNF-α in patients with LDH [22]. According to the results from our immunohistochemistry staining and western blot analysis, TNF-α is elevated in degenerate NP tissues compared to normal tissues in both human and rats, which is consistent with the expression of IL-21. Therefore, our results suggest that IL-21 could induce TNF-α, indicating that TNF-α might act as an important mediator of IL-21.
Spolski and Leonard [23] believed that IL-21 acts through the JAK-STAT signaling pathway. When IL-21 was bound to IL-21R, then STAT-1, STAT-3, and STAT-5 become phosphorylated, especially STAT-3 [24]. Therefore, it is speculated that IL-21 might enhance TNF-α through the STAT pathway. The results of our western blot analysis showed that in NP cells stimulated by IL-21 alone, an increase in mRNA expression and protein level of ADAMTS-7, TNF-α, and MMP-13 was observed. The expression of STAT-1, STAT-3, and STAT-5b was also upregulated after stimulation with IL-21 alone, especially STAT-3, which is consistent with previous studies. There was a slight difference between the normal control cells and cells treated with IL-21 combined with AG490. AG490, also known as Tyrphostin AG or Tyrphostin B42, is a permeable membrane protein tyrosine kinase inhibitor of JAK-2 protein and has no significant effect on other kinases such as Lck, Lyn, Btk, Syk, or Src. AG490 is a common reagent for studying the function of JAK in the process of cell signal transduction [25]. The mRNA expression of TNF-α in cells treated with IL-21 and AG490 was lower than cells treated with IL-21 alone. Our results showed that IL-21 could induce expression of TNF-α through the JAK-STAT signaling pathway, which indicated that STAT proteins play a role in TNF-α gene expression. It is interesting to summarize that IL-21 could induce TNF-α to trigger a series of inflammatory changes to aggravate IVD degeneration.
In conclusion, IL-21 might act as an initiator of inflammation by inducing a large number of cytokines and metabolic substances to start inflammation pathways, which in turn aggravates IVD degeneration. Moreover, TNF-α might be a crucial mediator of IL-21 in this process.
References
Le, M.C.L., A.J. Freemont, and J.A. Hoyland. 2004. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. Journal of Pathology 204(1): 47–54.
Burke, J.G., R.W. Watson, D. Mccormack, F.E. Dowling, M.G. Walsh, and J.M. Fitzpatrick. 2002. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. Journal of Bone and Joint Surgery (British) 84: 196–201.
Schwarzer, A.C., C.N. Aprill, R. Derby, J. Fortin, G. Kine, and N. Bogduk. 1995. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine (Phila Pa 1976) 20: 1878–1883.
Koes, B.W., M.W. Van Tulder, and W.C. Peul. 2007. Diagnosis and treatment of sciatica. British Medical Journal 334: 1313–1317.
Bobechko, W.P., and C. Hirsch. 1965. Auto-immune response to nucleus pulposus in the rabbit. Journal of Bone and Joint Surgery (British) 47: 574–580.
De Souza Grava, A.L., L.F. Ferrari, and H.L. Defino. 2012. Cytokine inhibition and time-related influence of inflammatory stimuli on the hyperalgesia induced by the nucleus pulposus. European Spine Journal 21(3): 537–545.
Le Maitre, C.L., J.A. Hoyland, and A.J. Freemont. 2007. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1β and TNFα expression profile. Arthritis Research and Therapy 9: R77.
Liu, R., Q. Wu, D. Su, N. Che, H. Chen, L. Geng, et al. 2012. A regulatory effect of IL-21 on T follicular helper-like cell and B cell in rheumatoid arthritis. Arthritis Research and Therapy 14: R255.
Nakou, M., E. Papadimitraki, A. Fanouriakis, G. Bertsias, C. Choulaki, N. Goulidaki, et al. 2012. Interleukin-21 is increased in active systemic lupus erythematosus patients and contributes to generation of plasma B cells. Clinical and Experimental Rheumatology 31: 172–179.
Salzer, E., A. Kansu, H. Sic, et al. 2014. Early-onset inflammatory bowel disease and common variable immunodeficiency-like disease caused by IL-21 deficiency. Journal of Allergy and Clinical Immunology 133(6): 1651–1659.
Kwok, S.K., M.L. Cho, M.K. Park, et al. 2012. Interleukin-21 promotes osteoclastogenesis in humans with rheumatoid arthritis and in mice with collagen-induced arthritis. Arthritis and Rheumatism 64: 740–751.
Parrish-Novak, J., and S.R. Dillon. 2000. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 408: 57–63.
Zhu, X., D. Ma, J. Zhang, J. Peng, X. Qu, C. Ji, et al. 2010. Elevated interleukin-21 correlated to Th17 and Th1 cells in patients with immune thrombocytopenia. Journal of Clinical Immunology 30: 253–259.
Zhang, H., F. La Marca, S.J. Hollister, S.A. Goldstein, and C.Y. Lin. 2009. Developing consistently reproducible intervertebral disc degeneration at rat caudal spine by using needle puncture. Journal of Neurosurgery: Spine 10(6): 522–530.
Kang, J.D., M. Stefanovic-Racic, L.A. McIntyre, H.I. Georgescu, and C.H. Evans. 1997. Toward a biochemical understanding of human intervertebral disc degeneration and herniation: contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine 22: 1065–1073.
Monteleone, G., M. Sarra, and F. Pallone. 2009. Interleukin-21 in T cell-mediated diseases. Discovery Medicine 8: 113–117.
Niu, X., D. He, X. Zhang, T. Yue, N. Li, J.Z. Zhang, C. Dong, and G. Chen. 2010. IL-21 regulates Th17 cells in rheumatoid arthritis. Human Immunology 71: 334–341.
Monteleone, G., I. Monteleone, D. Fina, et al. 2005. Interleukin-21 enhances T-helper cell type I signaling and interferon-gamma production in Crohn’s disease. Gastroenterology 128: 687–694.
Porter, S., I.M. Clark, L. Kevorkian, and D.R. Edwards. 2005. The ADAMTS metalloproteinases. Biochemical Journal 386: 15–27.
Lai, Y., X. Bai, Y. Zhao, Q. Tian, B. Liu, E.A. Lin, Y. Chen, B. Lee, C.T. Appleton, F. Beier, X.P. Yu, and C.J. Liu. 2014. ADAMTS-7 forms a positive feedback loop with TNF-alpha in the pathogenesis of osteoarthritis. Annals of the Rheumatic Diseases 73(8): 1575–1584.
Wang, S.-S., W. Zhang, Y.Q. Zhang, et al. 2015. IL-17A enhances ADAMTS-7 expression through regulation of TNF-a in human nucleus pulposus cells. Journal of Molecular Histology 46(6): 475–483.
Weiler, C., A.G. Nerlich, B.E. Bachmeier, and N. Boos. 2005. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine (Phila Pa 1976) 30(1): 44–54.
Spolski, R., and W.J. Leonard. 2014. Interleukin-21: a double-edged sword with therapeutic potential. Nature Reviews Drug Discovery 13(5): 379–395.
Rajbhandary, S., M.F. Zhao, N. Zhao, W.Y. Lu, H.B. Zhu, X. Xiao, et al. 2013. Multiple cytotoxic factors involved in IL-21 enhanced antitumor function of CIK cells signaled through STAT-3 and STAT5b pathways. Asian Pacific Journal of Cancer Prevention : APJCP 14(10): 5825–5831.
Kobayashi, A., Y. Tanizaki, A. Kimura, Y. Ishida, M. Nosaka, S. Toujima, et al. 2015. Ag490, a jak2 inhibitor, suppressed the progression of murine ovarian cancer. European Journal of Pharmacology 766: 63–75.
Acknowledgements
This study was supported by the General Program of National Natural Science Foundation of China (Grant No. 81572191).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interest.
Additional information
Bin Chen and Yi Liu contributed equally to this article and should be considered equal first authors.
Rights and permissions
About this article
Cite this article
Chen, B., Liu, Y., Zhang, Y. et al. IL-21 Is Positively Associated with Intervertebral Disc Degeneration by Interaction with TNF-α Through the JAK-STAT Signaling Pathway. Inflammation 40, 612–622 (2017). https://doi.org/10.1007/s10753-017-0508-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0508-6