Abstract
Inflammation following hemorrhagic shock/resuscitation (HS/RES) induces acute lung injury (ALI). Dimethyl sulfoxide (DMSO) possesses anti-inflammatory and antioxidative capacities. We sought to clarify whether DMSO could attenuate ALI induced by HS/RES. Male Sprague-Dawley rats were allocated to receive either a sham operation, sham plus DMSO, HS/RES, or HS/RES plus DMSO, and these were denoted as the Sham, Sham + DMSO, HS/RES, or HS/RES + DMSO group, respectively (n = 12 in each group). HS/RES was achieved by drawing blood to lower mean arterial pressure (40–45 mmHg for 60 min) followed by reinfusion with shed blood/saline mixtures. All rats received an intravenous injection of normal saline or DMSO immediately before resuscitation or at matching points relative to the sham groups. Arterial blood gas and histological assays (including histopathology, neutrophil infiltration, and lung water content) confirmed that HS/RES induced ALI. Significant increases in pulmonary expression of tumor necrosis factor-α (TNF-α), malondialdehyde, nuclear factor-kappa B (NF-κB), inducible nitric oxide synthase (iNOS), and cyclooxygenase 2 (COX-2) confirmed that HS/RES induced pulmonary inflammation and oxidative stress. DMSO significantly attenuated the pulmonary inflammation and ALI induced by HS/RES. The mechanisms for this may involve reducing inflammation and oxidative stress through inhibition of pulmonary NF-κB, TNF-α, iNOS, and COX-2 expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Reduced blood flow to organs during hemorrhagic shock initiates cellular hypoxia and oxidative stress in susceptible tissues [1, 2]. Further release of toxic metabolites during resuscitation induces the systemic inflammatory response, which may cause upregulation of cytokine expression and neutrophil infiltration in a variety of organs, especially the lungs [3, 4]. Acute lung injury (ALI) following hemorrhagic shock and resuscitation (HS/RES) is a major clinical problem leading to mortality and morbidity [2, 5]. Strategies aimed at modulating pulmonary inflammation may be beneficial to patients through the reduction of ALI induced by HS/RES [4, 5].
Dimethyl sulfoxide (DMSO) is an amphipathic molecule with one highly polar and two nonpolar domains that dissolve both aqueous and organic compounds [6, 7]. It is frequently used as a solvent in biological research and as a vehicle for drug therapy. Moreover, DMSO possesses a variety of biological activities and has been used for diverse laboratory and clinical purposes [8]. DMSO has been used clinically as an anti-inflammatory agent to treat several dermatological, rheumatological, and gastrointestinal diseases [9–11], as well as interstitial cystitis and amyloidosis [8, 12]. Recent animal experiments further confirmed the anti-inflammatory and antioxidative effects of DMSO against endotoxin sepsis and inflammatory bowel disease [13], hepatic necrosis [14], as well as intestinal inflammation and barrier dysfunction [15].
To date, a determination of whether DMSO has protective effects on ALI after HS/RES remains unclear. Based on the anti-inflammatory property of DMSO, we hypothesized that DMSO could attenuate ALI induced by HS/RES in rats. Assays of pulmonary function, histology, and inflammation were preformed to elucidate the therapeutic effects of DMSO and the possible mechanisms involved.
MATERIALS AND METHODS
Animal Preparation
Adult male Sprague-Dawley rats (BioLASCO Taiwan Co., Ltd, Taipei, Taiwan) weighing 250 to 300 g were used in this study. All animal experiments were approved by the Institutional Animal Use and Care Committee, Taipei Tzu Chi Hospital (102-IACUC-012). All experiments were completed according to the guidelines of the National Institutes of Health. The rats used were anesthetized with an intramuscular injection of ketamine (110 mg/kg) and xylazine (10 mg/kg). Additional doses of ketamine (30 mg/kg) and xylazine (3 mg/kg) were also given hourly throughout the experiments. Polyethylene catheters (PE-50, Becton Dickinson, Sparks, MD, USA) were cannulated into the right femoral artery of the rats for blood pressure monitoring and into the femoral vein for blood withdrawal and intravenous injections, respectively. In addition, tracheostomies were performed on the rats.
HS/RES Protocols
HS/RES was achieved as described previously [4]. In brief, hemorrhagic shock was induced by drawing arterial blood over 10 min to lower mean arterial pressure (MAP; BIOPAC System, Santa Barbara, CA, USA) from the baseline level to 40–45 mmHg. The drained blood was kept in a syringe containing heparin. The low MAP was maintained for 60 min by drawing or reinfusing blood as needed. Resuscitation was achieved by reinfusing the drained blood plus twice the maximum blood volume drawn of normal saline over a 10-min period. The rats were then monitored for another 300 min before euthanasia.
Determination of DMSO Dosage
The rats were randomly assigned to five groups (n = 4 in each group). The Sham group received 0.6 mL/kg of normal saline. The HS/RES groups received either 0, 0.1, 0.3, or 0.6 mL/kg of DMSO (purity > 99.9%) diluted in normal saline to an equal volume for injection (i.e., 0.6 mL/kg of normal saline or 0.6 mL/kg of 16.7, 50.0, and 99.9% DMSO, respectively). All rats received an intravenous injection of normal saline or DMSO immediately before resuscitation or at matching points for the Sham group where resuscitation is not applicable. The dosage of 0.3 mL/kg DMSO was chosen for subsequent experiments according to our preliminary bronchoalveolar lavage fluid (BALF) data (Fig. 1).
Dose-dependent responses to dimethyl sulfoxide (DMSO) of the total cell number in bronchoalveolar lavage fluid (BALF). Animals were treated with DMSO at doses of 0, 0.1, 0.3, or 0.6 mL/kg (n = 4 in each group). NS normal saline, Sham the Sham group, Sham + DMSO the Sham plus DMSO group, HS/RES the hemorrhagic shock/resuscitation group, HS/RES + DMSO the HS/RES plus DMSO group. Data were expressed as mean ± standard deviation. *P < 0.05 vs. the Sham group. #P < 0.05 the HS/RES + DMSO group vs. the HS/RES group.
Experimental Protocols
The rats were randomly assigned to four groups (n = 12 in each group). The Sham group received the sham operation which consisted of vascular cannulations and a tracheostomy plus 0.3 mL/kg of normal saline. The Sham + DMSO group received the sham operation plus 0.3 mL/kg of DMSO. The HS/RES group received HS/RES plus 0.3 mL/kg normal saline. The HS/RES + DMSO group received HS/RES plus 0.3 mL/kg of DMSO. All rats received an intravenous injection of normal saline or DMSO immediately before resuscitation or at matching points in the sham groups.
Arterial Blood Gas (ABG) and Plasma Laboratory Parameters
At the end of each experiment, an arterial blood sample (0.5 mL) was drawn for ABG analysis, using a blood gas analyzer (Gem Premier 3000; Instrumentation Laboratory, Bedford, MA, USA). Additional blood sample (3 mL) was obtained and centrifuged to separate plasma. Because the metabolism of DMSO takes place primarily in the liver and kidneys [16], the plasma levels of alanine aminotransferase (ALT, liver function test) and creatinine (kidney function test) were measured using the DXC 800 general chemistry systems (Beckman Coulter, Brea, CA, USA). All rats were subsequently euthanized in a carbon dioxide chamber.
Lung Sample Collection and Bronchoalveolar Lavage (BAL)
For all of the rats, the left main bronchus was tied and the left lung was removed. The superior and inferior lobes of the left lung were then separated. The inferior lobe was snap frozen in liquid nitrogen and stored at −80 °C for subsequent analysis, while the left superior lobe was used for wet/dry weight ratio measurement. For the six rats of each group, the right lungs were perfused with 4% formaldehyde and then excised for histological analysis. For the remaining six rats of each group, the right lung was lavaged 5 times with 3 mL normal saline and then the BALF was collected [17, 18].
Total Cell Number in BALF and Wet/Dry Weight Ratio
The total cell number in BALF and the wet/dry weight ratio (i.e., lung water content) were measured using the protocols we have previously reported [4, 19]. In brief, an aliquot of BALF (50 μL) was diluted to 1:1 with trypan blue dye and the total cell number was counted using a standard hemocytometer. For the wet/dry weight ratio, the freshly harvested left superior lobe was weighed and then dried in the oven at 80 °C for 24 h. After that, the lobe in dry condition was weighed again to calculate the wet/dry weight ratio.
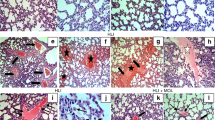
Histological Analysis
The formalin-fixed and paraffin-embedded lung tissues were serial sectioned and stained with hematoxylin and eosin. Histological features including alveolar wall edema, vascular congestion, hemorrhage, and polymorphonuclear (PMN) leukocyte infiltration were examined under a light microscope using our previously published protocol [1]. Each histological feature was scored on a 5-grade scale: 0 (normal) to 5 (severe). The overall lung injury in each rat was classified according to the sum of the scores (0–5: normal to minimal injury; 6–10: mild injury; 11–15: moderate injury; 16–20: severe injury).
Assays of Tumor Necrosis Factor-α (TNF-α) and Malondialdehyde (MDA) Levels
The harvested lung tissues were processed as we have previously reported [17, 18]. The concentration of pulmonary TNF-α was measured using an enzyme-linked immunosorbent assay (R&D Systems, Inc, Minneapolis, MN, USA) according to the manufacturer’s instructions. The concentrations of pulmonary MDA, a lipid peroxidation marker, were measured using a commercial MDA assay kit (TBARS assay kit, Cayman Chemical Co., Ann Arbor, MI, USA) according to the manufacturer’s instruction.
Assay of Inhibitor Kappa B (IκB) Phosphorylation
Nuclear factor-kappa B (NF-κB) activation was measured by immunoblotting assay of phosphorylated IκB (p-IκB) according to our previous report [20]. In brief, equal amounts of protein samples from lung tissues were separated by polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. The membranes were blocked, followed by overnight incubation at 4 °C with the primary antibody solution of p-IκB (1:1000 dilution, monoclonal anti-p-IκB antibody; Cell Signaling Technology, Inc, Danvers, MA). Horseradish peroxidase-conjugated IgG antibody (Amersham Pharmacia Biotech, Inc, Piscataway, NJ) was used as a secondary antibody. Reacting bands were detected by chemiluminescence (ECL plus kit; Amersham). Band intensities were quantified by densitometric techniques (ImageJ software, http://imagej.nih.gov/ij/download).
Transcriptional Expression of Inducible Nitric Oxide Synthase (iNOS) and Cyclooxygenase 2 (COX-2)
Transcriptional expression of iNOS and COX-2 of the lung tissues was determined using reverse transcription and polymerase chain reaction (RT-PCR). The protocols of primer sequences and amplification for iNOS, COX-2, and β-actin were performed according to our previous report [19]. After separation, band intensities of PCR-amplified cDNA were quantified by densitometric techniques (ImageJ software).
Survival Analysis After Resuscitation
Twenty-four rats were randomly divided into HS/RES and HS/RES + DMSO groups. All rats underwent hemorrhagic shock as above, followed by receiving an intravenous injection of normal saline or DMSO immediately before resuscitation. At the end of resuscitation, the cannulation catheters were removed and the vessels were ligated. The rats were returned to their cages and supplied with standard rat chow and water ad libitum. All rats were observed for 48 h. The 48-h survival rates were then calculated.
Statistical Analysis
One-way analysis of variance (ANOVA) was performed to examine differences among groups. If one-way ANOVA revealed significant differences, Tukey tests were performed for pairwise multiple comparisons. Data were expressed as mean ± standard deviation. The 48-h survival data were analyzed using Kaplan-Meier survival analysis. Log rank test was used to compare survival distributions. The significance level was set at 0.05. A commercial software package (SigmaStat for Windows, SPSS Science, Chicago, IL) was used for data analysis.
RESULTS
Dose-Response Effects of DMSO on Total Cell Number in BALF
The BALF total cell number for the Sham group was low (Fig. 1). The BALF total cell numbers for the HS/RES, HS/RES + DMSO (0.1 mL/kg), HS/RES + DMSO (0.3 mL/kg), and HS/RES + DMSO (0.6 mL/kg) groups were significantly higher than those of the Sham group (P < 0.001, <0.001, =0.045, and 0.021, Fig. 1). In contrast, the BALF total cell numbers for the HS/RES + DMSO (0.3 mL/kg) and HS/RES + DMSO (0.6 mL/kg) groups were significantly lower than those of the HS/RES group (P = 0.003 and 0.006, Fig. 1). However, the BALF total cell numbers for the HS/RES + DMSO (0.3 mL/kg) and HS/RES + DMSO (0.6 mL/kg) groups were not significantly different from one another (P = 0.99, Fig. 1).
Hemodynamic Data
The baseline MAP and heart rate (HR) were not significantly different among the groups (data not shown). The MAP and HR of the Sham and the Sham + DMSO groups were stable throughout the experiments. The end state MAP and HR of the HS/RES group were significantly lower than those of the Sham group (both P < 0.001, Table 1). Moreover, the end state MAP and HR of the HS/RES + DMSO group were significantly higher than those of the HS/RES group (P = 0.008 and 0.001, Table 1).
ABG Data
The ABG data, including pH, PaO2, PaCO2, and base excess (BE) values, of the Sham and the Sham + DMSO groups were not significantly different from one another (Table 1). The pH, PaO2, and BE of the HS/RES group were significantly lower than those of the Sham group (all P < 0.001), whereas the PaCO2 values between the two groups were not significantly different (Table 1). The pH, PaO2, and BE values of the HS/RES + DMSO group were significantly higher than those of the HS/RES group (P = 0.006, 0.002, and <0.001), whereas the PaCO2 values between the two groups were not significantly different (Table 1).
Plasma Laboratory Data
The plasma levels of ALT for the Sham, Sham + DMSO, HS/RES, and HS/RES + DMSO groups were 56.3 ± 8.9, 57.8 ± 10.4, 70.2 ± 17.6, and 65.0 ± 9.4 IU/L, respectively. There were no significant differences among the groups (P = 0.355). The plasma levels of creatinine for the Sham, Sham + DMSO, HS/RES, and HS/RES + DMSO groups were 0.24 ± 0.02, 0.21 ± 0.04, 0.30 ± 0.09, and 0.24 ± 0.05 mg/dL, respectively. Similarly, there were no significant differences among the groups (P = 0.181).
Total Cell Number in BALF and Wet/Dry Weight Ratio
The BALF total cell numbers for the Sham and the Sham + DMSO groups were low (Fig. 2a). The BALF total cell number for the HS/RES group was significantly higher than that of the Sham group (P < 0.001, Fig. 2a). In contrast, the BALF total cell number for the HS/RES + DMSO group was significantly lower than that of the HS/RES group (P < 0.001, Fig. 2a). Also, data for the wet/dry weight ratio (Fig. 2b) essentially matched the data for BALF total cell numbers (Fig. 2a).
a The total cell number in bronchoalveolar lavage fluid (BALF; n = 6 in each group) and b the wet/dry weight ratio of the lungs (n = 12). NS normal saline, DMSO dimethyl sulfoxide, Sham the Sham group, Sham + DMSO the Sham plus DMSO group, HS/RES the hemorrhagic shock/resuscitation group. HS/RES + DMSO the HS/RES plus DMSO group. Data were expressed as mean ± standard deviation. *P < 0.05 vs. the Sham group. #P < 0.05 the HS/RES + DMSO group vs. the HS/RES group.
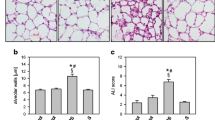
Histological Lung Injury Score
Histological analysis revealed normal to minimal lung injury in rats of the Sham and the Sham + DMSO groups (Fig. 3a). Histological analysis also revealed moderate lung injury in rats of the HS/RES group (Fig. 3a) and mild injury in rats of the HS/RES + DMSO group (Fig. 3a). Lung injury scores (Fig. 3b) quantify the histological analysis.
a Representative microscopic findings of the lung tissues stained with hematoxylin-eosin (×200) and b the lung injury scores (n = 6 in each group). NS normal saline, DMSO dimethyl sulfoxide, Sham the Sham group, Sham + DMSO the Sham plus DMSO group, HS/RES the hemorrhagic shock/resuscitation group, HS/RES + DMSO the HS/RES plus DMSO group. Data were expressed as mean ± standard deviation. *P < 0.05 vs. the Sham group. #P < 0.05 the HS/RES + DMSO group vs. the HS/RES group.
Pulmonary TNF-α Level
The pulmonary TNF-α levels for the Sham and the Sham + DMSO groups were low (Fig. 4). The pulmonary TNF-α level for the HS/RES group was significantly higher than that of the Sham group (P < 0.001, Fig. 4). Moreover, the pulmonary TNF-α level for the HS/RES + DMSO group was significantly lower than that of the HS/RES group (P < 0.001, Fig. 4).
The concentration of tumor necrosis factor-α (TNF-α) in rat lungs (n = 12 in each group). NS normal saline, DMSO dimethyl sulfoxide, Sham the Sham group, Sham + DMSO the Sham plus DMSO group, HS/RES the hemorrhagic shock/resuscitation group, HS/RES + DMSO the HS/RES plus DMSO group. Data were expressed as mean ± standard deviation. *P < 0.05 vs. the Sham group. #P < 0.05 the HS/RES + DMSO group vs. the HS/RES group.
Pulmonary MDA level
The pulmonary MDA levels for the Sham and the Sham + DMSO groups were low (Fig. 5). The pulmonary MDA level for the HS/RES group was significantly higher than that of the Sham group (P < 0.001, Fig. 5). Moreover, the pulmonary MDA level for the HS/RES + DMSO group was significantly lower than that of the HS/RES group (P < 0.001, Fig. 5).
The concentration of malondialdehyde (MDA) in rat lungs (n = 12 in each group). NS normal saline, DMSO dimethyl sulfoxide, Sham the Sham group, Sham + DMSO the Sham plus DMSO group, HS/RES the hemorrhagic shock/resuscitation group, HS/RES + DMSO the HS/RES plus DMSO group. Data were expressed as mean ± standard deviation. *P < 0.05 vs. the Sham group. #P < 0.05 the HS/RES + DMSO group vs. the HS/RES group.
IκB Phosphorylation
The pulmonary levels of p-IκB for the Sham and Sham + DMSO groups were low (Fig. 6). The pulmonary level of p-IκB for the HS/RES group was significantly higher than that of the Sham group (P < 0.001, Fig. 6). Moreover, the pulmonary level of p-IκB for the HS/RES + DMSO group was significantly lower than that of the HS/RES group (P < 0.001, Fig. 6).
Representative gel photography of the proteins of p-IκB and actin in rat lungs (n = 6 in each group). NS normal saline, DMSO dimethyl sulfoxide, Sham the Sham group, Sham + DMSO the Sham plus DMSO group, HS/RES the hemorrhagic shock/resuscitation group, HS/RES + DMSO the HS/RES plus DMSO group. Data were expressed as mean ± standard deviation. *P < 0.05 vs. the Sham group. #P < 0.05 the HS/RES + DMSO group vs. the HS/RES group.
Expression of iNOS and COX-2
The pulmonary levels of iNOS and COX-2 for the Sham and Sham + DMSO groups were low (Fig. 7a, b). The pulmonary levels of iNOS and COX-2 for the HS/RES group were significantly higher than those of the Sham group (both P < 0.001, Fig. 7a, b). Moreover, the pulmonary levels of iNOS and COX-2 for the HS/RES + DMSO group were significantly lower than those of the HS/RES group (both P < 0.001, Fig. 7a, b).
a Representative gel photography and densitometric analysis data illustrate the transcriptional expression of inducible nitric oxide synthase (iNOS) and b cyclooxygenase 2 (COX-2) in rat lungs (n = 6 in each group). NS normal saline, DMSO dimethyl sulfoxide, Sham the Sham group, Sham + DMSO the Sham plus DMSO group, HS/RES the hemorrhagic shock/resuscitation group, HS/RES + DMSO the HS/RES plus DMSO group. Data were expressed as mean ± standard deviation. *P < 0.05 vs. the Sham group. #P < 0.05 the HS/RES + DMSO group vs. the HS/RES group.
Survival Rates
The survival rates at 48 h after resuscitation for the HS/RES and HS/RES + DMSO groups were 58 and 92%, respectively (Fig. 8). However, there was no significant difference between the two groups (P = 0.071, Fig. 8).
DISCUSSION
In the present study, we used a rodent model of HS/RES to assess the protective effects of DMSO against HS/RES-induced ALI. Consistent with previous findings [1, 4, 17, 21, 22], our results confirmed that HS/RES induces ALI, manifested as increases in BALF total cell number, lung wet/dry weight ratio, histological lung injury score, pulmonary proinflammatory cytokine production, pulmonary lipid peroxidation, pulmonary NF-κB activation, expression of pulmonary iNOS and COX-2 mRNA, as well as functionally impaired gas exchange. Furthermore, our results revealed that an intravenous injection of DMSO immediately before resuscitation alleviated all experimental indices of ALI induced by HS/RES. Collectively, our findings confirmed that DMSO can mitigate HS/RES-induced ALI. Our data further confirmed that DMSO can also mitigate pulmonary inflammation and oxidative stress induced by HS/RES.
ALI remains a common contributor to the complications following HS/RES [23, 24]. The shock state causes tissue ischemia and the subsequent resuscitation acts as an inducing mechanism of reperfusion. The resuscitation can induce dispersion of toxic metabolites from ischemic tissues that prompt local and distal inflammation cascades [4, 25, 26]. Oxidants, infiltrated neutrophils, and inflammatory mediators work synergistically to damage pulmonary microvascular endothelial cells that lead to microvascular integrity loss and lung dysfunction [27, 28]. DMSO has been reported to decrease neutrophil infiltration and endothelial injury in several experimental animal models of lung injury and thus has been reported to alleviate lung injury in these models [29–31]. In a similar way, our study revealed that DMSO also significantly decreased the BALF total cell count and lung water content in HS/RES-induced lung injury, which indicates that DMSO may attenuate inflammatory cell infiltration and pulmonary edema related to HS/RES-induced lung injury. Moreover, histological findings also supported the protective role of DMSO, expressed as reduced alveolar wall edema, vascular congestion, hemorrhage, and PMN leukocyte infiltration. As a result, further lung injury was prevented and the blood gas exchange was significantly improved by administration of DMSO before resuscitation.
HS/RES induces an inflammatory response characterized by upregulation of proinflammatory cytokine expression in a variety of tissues [1, 25]. TNF-α is known to be a major initiator of a proinflammatory mediator cascade in the pathophysiology of HS/RES [32]. TNF-α has been reported to trigger the release of other proinflammatory cytokines, such as interleukin-1β and interleukin-6, and itself has more potency than that of other cytokines in inducing neutrophil activation [15, 33, 34]. Previous data revealed that TNF-α is directly related to the development of lung dysfunction during acute hemorrhage and may ultimately induce multiple organ failure [32, 35]. Moreover, the release of TNF-α into circulation during HS/RES may initiate a lethal cardiovascular collapse [35, 36]. DMSO has been reported to inhibit synthesis and release of TNF-α and thus reduce the intestinal TNF-α level in a rodent model of gut inflammation [15]. Likewise, the present data clearly revealed that DMSO significantly suppressed the pulmonary TNF-α level, thus contributing to a reduced level of ALI and a better hemodynamic profile following HS/RES.
In addition to TNF-α, our data also revealed that HS/RES induced the upregulation of iNOS and COX-2 in rat lungs and this upregulation could be inhibited by DMSO. During the inflammatory processes, iNOS is capable of producing a large amount of nitric oxide, a highly reactive free radical. Furthermore, COX-2 involves the synthesis of prostaglandin E2 that can exacerbate inflammation. Of note, expression of TNF-α, iNOS, and COX-2 is regulated by the upstream transcription factor NF-κB [37–39]. Consistent with previous reports [40, 41], data from this study confirmed that DMSO can effectively suppress the activation of NF-κB. Judging from these data, we thus speculated that DMSO may very likely act through suppressing NF-κB activation to exhibit its effects on inhibiting the upregulation of TNF-α, iNOS, and COX-2 induced by HS/RES. However, the definitive mechanism underlying the effects of DMSO on suppressing NF-κB activation in this study remains to be elucidated. NF-κB is an oxidative stress reactor that free radicals can induce significant NF-κB activation [42]. Of note, several animal studies have confirmed that DMSO is a potent antioxidant, as DMSO can directly scavenge free radicals that gather at the site of injury [43, 44]. In line with this notion, we thus speculated that DMSO may very likely act through its antioxidative potentials to suppress NF-κB activation in this study. This concept is supported by our data that DMSO could inhibit the HS/RES-induced lipid peroxidation in rat lungs. More studies are needed before further conclusions can be reached.
Certain concerns relating to DMSO need to be addressed in future studies. Firstly, the plasma ALT and creatinine levels were not significantly increased after HS/RES in our experiments. Moreover, the plasma ALT and creatinine levels were not significantly different among the four groups. Those measurements were limited to the early phase of HS/RES. Whether HS/RES induces liver and kidney injuries in the delayed phase of HS/RES and whether DMSO has protective effects on those organs have yet to be determined. Secondly, our data did not demonstrate significantly superior survival rate for the HS/RES + DMSO group. However, there was a trend toward the beneficial effects of DMSO since the P value of 0.071 was just over the arbitrary threshold for significance. More studies with larger sample sizes are required before further conclusions can be reached. Thirdly, DMSO has been reported to have a dose-dependent inhibitory effect on cellular model of inflammation [41]. The concentration of neutrophils in BALF is known to correlate with the severity and outcome in the animal model of ALI [45]. Viewing data from our dosage determination in the present study, DMSO revealed a narrow therapeutic range and a ceiling effect. Optimal dosage and potential side effects of DMSO for future clinical application remain to be determined. Finally, the anti-inflammatory and antioxidative properties of DMSO may raise doubts about its use as a drug vehicle for in vivo and in vitro experiments. In majority of the cases, DMSO is used as a control vehicle to properly evaluate the drug effects [27]. DMSO effects largely depend on the models and doses used, and we suggest minimizing the volume as much as possible to abate its interference during drug evaluation.
In conclusion, DMSO attenuates ALI induced by HS/RES in rats. The mechanisms may involve reducing inflammation and oxidative stress through inhibition of pulmonary NF-κB, TNF-α, iNOS, and COX-2 expression.
References
Shenkar, R., W.F. Coulson, and E. Abraham. 1994. Hemorrhage and resuscitation induce alterations in cytokine expression and the development of acute lung injury. American Journal of Respiratory Cell and Molecular Biology 10: 290–297.
Niesler, U., A. Palmer, P. Radermacher, and M.S. Huber-Lang. 2014. Role of alveolar macrophages in the inflammatory response after trauma. Shock 42: 3–10.
Liu, H., X. Xiao, C. Sun, D. Sun, Y. Li, and M. Yang. 2015. Systemic inflammation and multiple organ injury in traumatic hemorrhagic shock. Frontiers in Bioscience (Landmark Edition) 20: 927–933.
Kao, M.C., C.H. Yang, J.R. Sheu, and C.J. Huang. 2015. Cepharanthine mitigates pro-inflammatory cytokine response in lung injury induced by hemorrhagic shock/resuscitation in rats. Cytokine 76: 442–448.
Godinho, M., P. Padim, P.R. Evora, and S. Scarpelini. 2015. Curbing Inflammation in hemorrhagic trauma: a review. Revista do Colégio Brasileiro de Cirurgiões 42: 273–278.
Murdoch, L. 1982. Dimethyl sulfoxide (DMSO)—an overview. The Canadian Journal of Hospital Pharmacy 35: 79–85.
Brayton, C.F. 1986. Dimethyl sulfoxide (DMSO): a review. The Cornell Veterinarian 76: 61–90.
Santos, N.C., J. Figueira-Coelho, J. Martins-Silva, and C. Saldanha. 2003. Multidisciplinary utilization of dimethyl sulfoxide: pharmacological, cellular, and molecular aspects. Biochemical Pharmacology 65: 1035–1041.
Capriotti, K., and J.A. Capriotti. 2012. Dimethyl sulfoxide: history, chemistry, and clinical utility in dermatology. The Journal of Clinical and Aesthetic Dermatology 5: 24–26.
Abdullaeva, G.K., and B.S.H. Shakimova. 1989. An evaluation of the efficacy of treating rheumatoid arthritis with preparations for local use. Revmatologiia (Moscow, Russia) 4: 35–39.
Salim, A.S. 1992. Role of oxygen-derived free radical scavengers in the management of recurrent attacks of ulcerative colitis: a new approach. The Journal of Laboratory and Clinical Medicine 119: 710–717.
Parkin, J., C. Shea, and G.R. Sant. 1997. Intravesical dimethyl sulfoxide (DMSO) for interstitial cystitis—a practical approach. Urology 49: 105–107.
Marchetti, C., E. Mezzaroma, and S. Toldo. 2015. Effects of dimethyl sulfoxide on the NLRP3 inflammasome. Immunobiology 220: 1030.
Iida, C., K. Fujii, E. Koga, Y. Washino, I. Ichi, and S. Kojo. 2007. Inhibitory effect of dimethyl sulfoxide (DMSO) on necrosis and oxidative stress caused by D-galactosamine in the rat liver. Journal of Nutritional Science and Vitaminology 53: 160–165.
Li, Y.M., H.B. Wang, J.G. Zheng, X.D. Bai, Z.K. Zhao, J.Y. Li, and S. Hu. 2015. Dimethyl sulfoxide inhibits zymosan-induced intestinal inflammation and barrier dysfunction. World Journal of Gastroenterology 21: 10853–10865.
He, X., and C.M. Slupsky. 2014. Metabolic fingerprint of dimethyl sulfone (DMSO2) in microbial-mammalian co-metabolism. Journal of Proteome Research 13: 5281–5292.
Yang, C.H., P.S. Tsai, T.Y. Wang, and C.J. Huang. 2009. Dexmedetomidine-ketamine combination mitigates acute lung injury in haemorrhagic shock rats. Resuscitation 80: 1204–1210.
Chou, W.C., M.C. Kao, C.T. Yue, P.S. Tsai, and C.J. Huang. 2015. Caffeine mitigates lung inflammation induced by ischemia-reperfusion of lower limbs in rats. Mediators of Inflammation 2015: 361638.
Chang, K.Y., P.S. Tsai, T.Y. Huang, T.Y. Wang, S. Yang, and C.J. Huang. 2006. HO-1 mediates the effects of HBO pretreatment against sepsis. The Journal of Surgical Research 136: 143–153.
Kao, M.C., C.H. Yang, W.C. Chou, J.R. Sheu, and C.J. Huang. 2015. Cepharanthine mitigates lung injury in lower limb ischemia-reperfusion. The Journal of Surgical Research 199: 647–656.
Kosaka, J., H. Morimatsu, T. Takahashi, H. Shimizu, S. Kawanishi, E. Omori, Y. Endo, et al. 2013. Effects of biliverdin administration on acute lung injury induced by hemorrhagic shock and resuscitation in rats. PloS One 8: e63606.
Wang, L., B. Zhao, Y. Chen, L. Ma, E.Z. Chen, and E.Q. Mao. 2015. Inflammation and edema in the lung and kidney of hemorrhagic shock rats are alleviated by biliary tract external drainage via the heme oxygenase-1 pathway. Inflammation 38: 2242–2251.
Fowler, A.A., R.F. Hamman, J.T. Good, K.N. Benson, M. Baird, D.J. Eberle, T.L. Petty, and T.M. Hyers. 1983. Adult respiratory distress syndrome: risk with common predispositions. Annals of Internal Medicine 98: 593–597.
Potter, D.R., G. Baimukanova, S.M. Keating, X. Deng, J.A. Chu, S.L. Gibb, Z. Peng, et al. 2015. Fresh frozen plasma and spray-dried plasma mitigate pulmonary vascular permeability and inflammation in hemorrhagic shock. The Journal of Trauma and Acute Care Surgery 78: S7–s17.
Dutton, R.P. 2007. Current concepts in hemorrhagic shock. Anesthesiology Clinics 25: 23–34.
Pfeifer, R., P. Lichte, H. Schreiber, R.M. Sellei, T. Dienstknecht, C. Sadeghi, H.C. Pape, and P. Kobbe. 2013. Models of hemorrhagic shock: differences in the physiological and inflammatory response. Cytokine 6: 585–590.
le Wang, F., M. Patel, H.M. Razavi, S. Weicker, M.G. Joseph, D.G. McCormack, and S. Mehta. 2002. Role of inducible nitric oxide synthase in pulmonary microvascular protein leak in murine sepsis. American Journal of Respiratory and Critical Care Medicine 165: 1634–1639.
Su, C.F., S.J. Kao, and H.I. Chen. 2012. Acute respiratory distress syndrome and lung injury: pathogenetic mechanism and therapeutic implication. World Journal of Critical Care Medicine 1: 50–60.
Boybeyi, O., B. Bakar, M.K. Aslan, P. Atasoy, U. Kisa, and T. Soyer. 2014. Evaluation of dimethyl sulfoxide and dexamethasone on pulmonary contusion in experimental blunt thoracic trauma. The Thoracic and Cardiovascular Surgeon 62: 710–715.
Kawada, T., K. Kambara, M. Arakawa, T. Segawa, F. Ando, S. Hirakawa, S. Emura, et al. 1992. Pretreatment with catalase or dimethyl sulfoxide protects alloxan-induced acute lung edema in dogs. Journal of Applied Physiology 73: 1326–1333.
Leff, J.A., M.A. Oppegard, E.C. McCarty, C.P. Wilke, P.F. Shanley, C.E. Day, N.K. Ahmed, et al. 1992. Dimethyl sulfoxide decreases lung neutrophil sequestration and lung leak. Journal of Laboratory and Clinical Medicine 120: 282–289.
Colucci, M., F. Maione, M.C. Bonito, A. Piscopo, A. Di Giannuario, and S. Pieretti. 2008. New insights of dimethyl sulphoxide effects (DMSO) on experimental in vivo models of nociception and inflammation. Pharmacological Research 57: 419–425.
Suzuki, K., M. Hino, H. Kutsuna, F. Hato, C. Sakamoto, T. Takahashi, N. Tatsumi, and S. Kitagawa. 2001. Selective activation of p38 mitogen-activated protein kinase cascade in human neutrophils stimulated by IL-1beta. The Journal of Immunology 167: 5940–5947.
Suitters, A.J., R. Foulkes, S.M. Opal, J.E. Palardy, J.S. Emtage, M. Rolfe, S. Stephens, et al. 1994. Differential effect of isotype on efficacy of anti-tumor necrosis factor alpha chimeric antibodies in experimental septic shock. The Journal of Experimental Medicine 179: 849–856.
Sato, H., K. Kasai, T. Tanaka, T. Kita, and N. Tanaka. 2008. Role of tumor necrosis factor-alpha and interleukin-1beta on lung dysfunction following hemorrhagic shock in rats. Medical Science Monitor 14: BR79–87.
Rhee, P., K. Waxman, L. Clark, C.J. Kaupke, N.D. Vaziri, G. Tominaga, and G. Scannell. 1993. Tumor necrosis factor and monocytes are released during hemorrhagic shock. Resuscitation 25: 249–255.
Lee, S.J., S.K. Bai, K.S. Lee, S. Namkoong, H.J. Na, K.S. Ha, J.A. Han, et al. 2003. Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing I(kappa)B kinase-dependent NF-kappaB activation. Molecules and Cells 16: 97–105.
Ma, Z., Y. Wang, T. Piao, and J. Liu. 2016. Echinocystic acid inhibits IL-1beta-Induced COX-2 and iNOS expression in human osteoarthritis chondrocytes. Inflammation 39: 543–549.
Vane, J.R., J.A. Mitchell, I. Appleton, A. Tomlinson, D. Bishop-Bailey, J. Croxtall, and D.A. Willoughby. 1994. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proceedings of the National Academy of Sciences of the United States of America 91: 2046–2050.
Essani, N.A., M.A. Fisher, and H. Jaeschke. 1997. Inhibition of NF-kappa B activation by dimethyl sulfoxide correlates with suppression of TNF-alpha formation, reduced ICAM-1 gene transcription, and protection against endotoxin-induced liver injury. Shock 7: 90–96.
Kelly, K.A., M.R. Hill, K. Youkhana, F. Wanker, and J.M. Gimble. 1994. Dimethyl sulfoxide modulates NF-kappa B and cytokine activation in lipopolysaccharide-treated murine macrophages. Infection and Immunity 62: 3122–3128.
Li, N., and M. Karin. 1999. Is NF-kappaB the sensor of oxidative stress? FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology 13: 1137–1143.
Phillis, J.W., A.Y. Estevez, and M.H. O’Regan. 1998. Protective effects of the free radical scavengers, dimethyl sulfoxide and ethanol, in cerebral ischemia in gerbils. Neuroscience Letters 244: 109–111.
Chiappa, A., M. Makuuchi, A.P. Zbar, F. Biella, M. Bellomi, R. Biffi, E. Bertani, et al. 2003. Effects of the free radical scavenger dimethyl sulphoxide on experimental normothermic ischaemia of the liver. Digestive Surgery 20: 238–245.
Hu, Z., Z. Gu, M. Sun, K. Zhang, P. Gao, Q. Yang, and Y. Yuan. 2015. Ursolic acid improves survival and attenuates lung injury in septic rats induced by cecal ligation and puncture. The Journal of Surgical Research 194: 528–536.
Acknowledgements
This work was supported by a grant from Taipei Tzu Chi Hospital (TCRD-TPE-104-48) awarded to M.C. Kao.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Ming-Chang Kao and Chun-Jen Huang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Tsung, YC., Chung, CY., Wan, HC. et al. Dimethyl Sulfoxide Attenuates Acute Lung Injury Induced by Hemorrhagic Shock/Resuscitation in Rats. Inflammation 40, 555–565 (2017). https://doi.org/10.1007/s10753-016-0502-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0502-4