Abstract
Antibody repertoires of healthy humans and animals contain a fraction of antibodies able to acquire additional polyspecificity following exposure to several biologically relevant redox molecules (free heme, reactive oxygen species, ferrous ions, HOCl, etc.). The physiological role of these “hidden” polyspecific antibodies is poorly understood. Similar to inherently polyspecific antibodies, those with induced polyspecificicty may also have immunoregulatory properties. We have previously shown that a pooled human IgG preparation, modified by the exposure to ferrous ions, acquires the ability to significantly improve survival of animals with polymicrobial sepsis or aseptic systemic inflammation induced by bacterial lipopolysaccharide or zymosan administration. In the present study, we have analyzed the effects of administration of heme-exposed pooled human IgG in the same models of sepsis and aseptic systemic inflammation. The administration of a single dose of heme-exposed pooled IgG has resulted in a significant increase in the survival of mice with endotoxinemia, but not in those with polymicrobial sepsis and zymosan-induced severe generalized inflammation. Finally, we have provided evidence that the anti-inflammatory effect of heme-exposed IgG can be explained by scavenging of pro-inflammatory mediators.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
An antibody molecule that is able to recognize multiple structurally unrelated antigens is defined as polyspecific [1]. The polyspecific antibodies represent approximately 20 % of normal immunoglobulin repertoires and their levels may further increase in certain pathological conditions [2]. Various functions of polyspecific antibodies have been proposed; particularly, they were suggested to participate in the immune defense against pathogens and exert to anti-inflammatory properties [2–4].
In addition to inherently polyspecific antibodies, normal immune repertoires also contain antibodies that can acquire polyspecificity following interaction with various redox-active substances: reactive oxygen species, ferrous ions, hypochloric acid, heme, etc. [5–9]. Importantly, these redox-active substances when released in vivo in sites of inflammation have also been shown to enhance the antigen-binding specificities of circulating antibodies [10]. The functions of induced polyspecific antibodies are not well understood. One could speculate that once their polyspecificity is unmasked, they would have similar functional properties as their natural counterparts [11]. Indeed, we observed that the post-translational induction of polyspecificity of antibodies is accompanied by a potentiation of their in vitro and in vivo anti-inflammatory activity [5, 12, 13].
The treatment of sepsis and aseptic severe inflammatory response syndromes (SIRS) is a major unsolved medical problem. A dramatic change in the expression of more than 80 % of all human genes has been reported recently in patients, referred to as “genomic storm” [14]. These chaotic events in severe systemic inflammation could explain the failure of all clinical trials so far that have used blocking of individual pro-inflammatory molecules to improve the patients’ survival. We have hypothesized before that a multifunctional multispecific therapeutic agent might be successful in calming, at least partially, the effects of this storm. Indeed, a recent study by our group has shown that a single dose of pooled therapeutic human IgG (intravenous immunoglobulin, IVIg) with additional ferrous ions-induced polyspecificity significantly improved animal survival in all models of severe generalized inflammation used [12].
Free heme influences the functions of different plasma proteins [15]. It can induce polyspecificity of antibodies [7, 16]. Importantly, free heme is also known to play a detrimental role in the pathogenesis of sepsis [17]. However, the heme-induced additional polyspecificity of sensitive circulating immunoglobulins could also enhance anti-inflammatory activity thus contributing to the regulation of ongoing systemic inflammation. To test this hypothesis and to evaluate the anti-inflammatory potential of heme-exposed polyspecific antibodies, we used three mouse models of systemic inflammation. The administration of heme-exposed pooled human IgG had a protective effect in the LPS-induced endotoxemia but not in other models of severe generalized inflammation.
RESULTS
Effect of Pooled Human IgG with Heme-Exposure Induced Additional Polyspecificity on Survival of Mice with Systemic Inflammation
The revealing of the cryptic polyspecificity of heme-sensitive IgG molecules in the therapeutic IVIg preparation was achieved by adding of hemin (ferriprotoporphyrin IX, oxidized form of heme) to the native immunoglobulin preparation. Single doses of the native- and heme-exposed pooled IgG were administrated to mice with three types of systemic inflammation: induced by the administration of bacterial LPS, of zymosan as well as with polymicrobial sepsis caused by cecal ligation and puncture (CLP) (Fig. 1). The treatment with the native, commercially available IVIg preparation did not affect the survival in any of the studied models (Fig. 1). This result is in agreement with the data from the literature showing lack of efficacy of IVIg infusions in sepsis [18]. In contrast, the treatment of mice with heme-exposed IVIg at dose of 50 mg/kg significantly improved survival in LPS-induced endotoxemia (Fig. 1a, right panel). The administration of lower doses (2 and 10 mg/kg) of the heme-exposed preparation were, however, inefficient (Fig. 1a, left and middle panels). The heme-exposed IVIg did not demonstrate significant improvement of survival of mice with zymozan-induced severe inflammation and with CLP sepsis, even when used at doses of 250 mg/kg (Fig. 1b, c). However, in these cases there was a tendency for a better survival of animals. To rule out a potential effect on the animal survival of hemin itself administered as a complex with IVIg, we injected free hemin to mice with endotoxemia. The administration of hemin, at doses corresponding to those introduced by injection of the heme-exposed IVIg, did not influence survival (Fig. 2).
Survival of animals after treatment with a heme-exposed IVIg preparation in three different experimental models of systemic inflammation. a Bacterial lipopolysacharide (LPS)-induced endotoxemia. The survival curve of the control group, treated i.v. with PBS pH 7.4 (open squares, n = 12) was compared with that of the group treated with 2 mg/kg (left panel), 10 mg/kg (middle panel) or 50 mg/kg (right panel) native IVIg (black triangles, n = 12), or heme-exposed IVIg (black circles, n = 12); *p < 0.05, Mantel-Haenszel logrank test. b Zymosan-induced systemic inflammation. A control group, treated i.v. with PBS (open squares, n = 12), was compared to a group treated with 10 mg/kg (left panel), 50 mg/kg (middle panel) or 250 mg/kg (right panel) native IVIg (black triangles, n = 12), or heme-exposed IVIg (black circles, n = 12). c Cecal ligation and puncture (CLP)-induced polymicrobial sepsis. Survival was compared between a control group, injected i.v. with PBS only (open squares, n = 12), a group treated with 10 mg/kg (left panel), 50 mg/kg (middle panel) or 250 mg/kg (right panel) of native IVIg (black triangles, n = 12), and a group treated with 10 mg/kg (left panel), 50 mg/kg (middle panel), or 250 mg/kg (right panel) of heme-exposed IVIg (black circles, n = 12).
Administration of hemin alone does not prevent death in endotoxemia. Groups of ICR mice (n = 12/group) were injected intraperitoneally with 10 mg/kg LPS and then injected i.v. with hemin: 0.8 μg/kg (open circles), 4 μg/kg (black circles) or 20 μg/kg (black squares) or with PBS pH 7.4 alone (open squares).
Mechanism of Anti-inflammatory Effect of Heme-IVIg
The anti-inflammatory activity of therapeutic immunoglobulin preparations was proposed to be partly mediated by natural polyspecific antibodies that interact and scavenge various mediators of inflammation such as complement proteins, cytokines, or modulate the inflammatory cells by interaction with surface receptors [11]. Hence, the acquisition of polyspecificity by IgG, induced by heme exposure, may result in better recognition of pro-inflammatory molecules released during severe inflammation, thus explaining their enhanced anti-inflammatory potential. To provide evidence for the validity of this hypothesis, we studied the binding of heme-exposed IVIg to complement proteins C3 and C5 and their degradation products. We selected these complement proteins as they are known to play key role in the pathogenesis of systemic inflammation. The induction of additional polyspecificity of IVIg was accompanied by the acquisition of binding to C3 and C5 as observed by surface plasmon resonance-based technology (Fig. 3a). Moreover, heme-exposed IVIg acquired binding potential to anaphylatoxins C3a and C5a, as well as to C3b. The analyses of the binding kinetics indicated that the heme-exposed pooled IgG interacted with different complement proteins with varying values of the apparent affinity (KD in the range 2.9–12.6 nM) (Fig. 3b). However, in all cases, the KD values were in nanomolar range, suggesting that these interactions are characterized with high affinity and might have physiological relevance (Fig. 3b).
Interaction of human pooled therapeutic IgG with complement proteins. a Real time interaction profiles of binding of native (gray line) and heme-exposed pooled IgG (black line) to immobilized human C3, C3a, C3b, C5, and C5a. The binding profiles of 25 nM of IgG are presented. b Apparent kinetic parameters of binding of heme-exposed pooled human IgG to complement proteins. The kinetic values were evaluated by using Proteone manager software. Presented values are mean ± SD obtained from four different measurements.
DISCUSSION
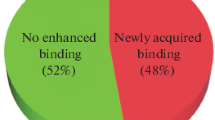
In the present study, we demonstrated that the anti-inflammatory potential of pooled human IgG was increased as a result of revealing of the cryptic antibody polyspecificity after heme exposure. We have compared the binding intensity of native and heme-exposed IVIg preparation to antigens from human liver extract. This assay demonstrated considerable increase in the number of recognized antigens following heme exposure (shown in Supplemental Figure 1).
Heme-exposed IVIg significantly improved the survival of mice with endotoxemia. However, the same preparation was not protective in CLP- and zymozan-induced sytemic inflammation.
Our previous studies of post-translationally acquired IgG polyspecificity implied that different redox-active agents use different mechanisms of polyspecificity induction [6]. Comparative analyses of various redox-active substances have revealed that the most potent inducer of polyspecificity is heme [6]. These observations allowed us to speculate that the degree of increase of polyspecificity may correlate with the augmentation of anti-inflammatory potential of IgG. In an independent study, we performed comprehensive analyses of the anti-inflammatory potential of IVIg modified by exposure to ferrous ions [12]. Although, ferrous ions are a less potent agent than heme in inducing antibody polyspecificity [6], the anti-inflammatory activity of Fe(II)-modified IVIg has been shown to be more robust. Its administration resulted in an improved survival in all studied models of sepsis and aseptic systemic inflammation [12].
A possible explanation in the absence of correlation between the level of polyspecificity and the enhanced anti-inflammatory potential of pooled IgG could be due be the difference of the mechanisms of polyspecificity induction after exposure to Fe(II) ions or to heme. We have demonstrated before that in the case of heme this effect is due to a direct binding of this cofactor to immunoglobulin molecules [7, 19]. Thus, sensitive antibodies use heme as a promiscuous cofactor for recognition of multiple unrelated antigens. Therefore, the heme presence on the IgG scaffold is of critical importance for polyspecific antigen binding, and its dissociation would result in loss of the newly acquired polyspecificity. The lower anti-inflammatory potential of heme-modified IVIg may be due to a dissociation of heme from IgG and its chelation by high affinity heme-binding proteins in plasma (e.g., hemopexin). As free heme is demonstrated to exacerbate systemic inflammation in sepsis [17], other explanation of lower anti-inflammatory potential of the IgG-heme complex is the possibility that heme which is introduced with immunoglobulins has a negative impact on the survival of animals.
The observed anti-inflammatory efficacy of heme-exposed IVIg in LPS-induced endotoxemia may be due to the particular characteristics of the systemic inflammation in this model, where the attainment of a therapeutic effect might be less demanding than in the other two variants of this syndrome. The severe inflammatory syndrome after LPS administration is due to the binding of the latter and signaling through TLR4, while that after zymosan injection it is due to signaling through TLR2. The induction of polymicrobial peritonitis and sepsis is the result of the access of large numbers of hundreds of species of intestinal microorganisms into the previously sterile peritoneum. It is obvious that in this case multiple severe inflammation-inducing mechanisms are activated.
During sepsis, numerous endogenous danger-associated molecules, such as HMGB-1 [20], histones [21], and CIRP [22], as well as pro-inflammatory cytokines and anaphylatoxines, are released into the circulation [23, 24]. These molecules have been demonstrated to play a detrimental role and to contribute to organ failure. The protective effect of pooled IgG with additionally induced polyspecificity in endotoxemia could be explained by scavenging of some endogenous pro-inflammatory mediators. As a proof of concept, we demonstrated here, that heme-exposed IVIg gained high-binding affinity to complement proteins C3 and C5 as well as to their degradation fragments C3a, C3b, and C5a. As C5a is known to play a detrimental role in sepsis [23, 25], its neutralization by polyspecific IgG would have a beneficial effect. Owing to their marked antigen-binding breadth, induced polyspecific IgG may also scavenge many other mediators of inflammation and tissue damage in systemic inflammation, thus contributing to the improved outcome.
In conclusion, here we demonstrated that the induction of cryptic polyspecificity of antibodies in IVIg by exposure to heme results in a moderate increase in the anti-inflammatory activity of the immunoglobulin preparation. The existence of heme-sensitive antibodies in the circulation may represent a defense strategy for dealing with the strong pro-inflammatory effects of free heme liberated in result of tissue damage or other pathologies.
MATERIALS AND METHODS
Immunoglobulin Preparations and Their Modification by Exposure to Heme
The intravenous immunoglobulin preparation (IVIg) Endobulin S/D (Baxter) was used in the experiments. The preparation was exposed to heme (hemin) as described before [7]. Briefly, a hemin (ferriprotoporphyrine IX chloride, from Fluka) stock solution was prepared in DMSO (cell culture grade, Sigma-Aldrich, USA) at a concentration of 25 mmol/L. This solution was kept in dark at 4 °C until use. The IVIg preparation (at 50 mg/ml) was incubated for 1 h at 4 °C in the dark in the presence of 50 μM of heme (added from the stock solution) then filtered through a 0.22 μm filter and kept at 4 °C.
Mice and Experimental Models of Severe Generalized Inflammation
Outbred female ICR mice (8–12 weeks old, 18–22 g) were purchased from the Breeding Farm of the Bulgarian Academy of Sciences and kept in a conventional animal facility. The experimental protocols were approved by the Animal Care Commission of the Institute of Microbiology in accordance with National and European Regulations (BABH protocol #105/10 July 2014).
Endotoxemia was induced by the intraperitoneal injection of 10 mg/kg E. coli LPS (B 055:B5, #L2880, Sigma-Aldrich). Multiple organ dysfunction was induced by the i.p. injection of 500 mg/kg zymosan (Sigma-Aldrich) [26]. Colon ligation and puncture (CLP) was performed as previously described [27]. All the three models were adjusted to approximately 90 % mortality in the control groups, treated with PBS only. The single doses of the native or of the heme-exposed IVIg preparations were administered i.v. minutes before the LPS i.p. injection. The control groups were infused i.v. with PBS. The animals’ survival was observed for 7 days. The statistical significance of the differences in survival curves was evaluated by the Mantel-Haenszel logrank test.
Surface Plasmon Resonance
The interaction of native and heme-exposed IVIg was analyzed in real time using a ProteOn XPR36 SPR equipment (BioRad). Complement proteins—C3, C3a, C3b, C5, and C5a (all from Complement technologies, Tylor, TX) were immobilized to GLC biosensor chip using standard amino-coupling procedure. Six different concentrations (starting from 100 nM) of native IVIg (Endobulin, Baxter) or IVIg exposed at 67 μM (10 mg/ml) to 50 μM hematin were injected through the six channels of the microfluidic system at a 30 μl/min flow rate and allowed to interact with each of the five immobilized proteins for 300 s. The dissociation was followed also for 300 s. The running buffer was PBS. The surface was regenerated with a pulse of 25 mM NaOH. Binding curves were double referenced by subtracting the signal from the interspots and the signal after a buffer injection as recommended by the manufacturer. The experiment was repeated four times on two different chips. The 1:1 Langmuir model was used for calculating the kinetic parameters.
References
Notkins, A. 2004. Polyreactivity of antibody molecules. Trends in Immunology 25(4): 174–9.
Dimitrov, J.D., C. Planchais, L.T. Roumenina, T.L. Vassilev, S.V. Kaveri, and S. Lacroix-Desmazes. 2013. Antibody polyreactivity in health and disease: statu variabilis. Journal of Immunology 191(3): 993–9.
Zhou, Z.H., A.G. Tzioufas, and A.L. Notkins. 2007. Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. Journal of Autoimmunity 29(4): 219–228.
Zhou, Z.H., Y. Zhang, Y.F. Hu, L.M. Wahl, J.O. Cisar, and A.L. Notkins. 2007. The broad antibacterial activity of the natural antibody repertoire is due to polyreactive antibodies. Cell Host & Microbe 1(1): 51–61.
Dimitrov, J.D., N.D. Ivanovska, S. Lacroix-Desmazes, V.R. Doltchinkova, S.V. Kaveri, and T.L. Vassilev. 2006. Ferrous ions and reactive oxygen species increase antigen-binding and anti-inflammatory activities of immunoglobulin G. Journal of Biological Chemistry 281(1): 439–446.
Dimitrov, J.D., C. Planchais, J. Kang, A. Pashov, T.L. Vassilev, S.V. Kaveri, and S. Lacroix-Desmazes. 2010. Heterogeneous antigen recognition behavior of induced polyspecific antibodies. Biochemical and Biophysical Research Communications 398(2): 266–271.
Dimitrov, J.D., L.T. Roumenina, V.R. Doltchinkova, N.M. Mihaylova, S. Lacroix-Desmazes, S.V. Kaveri, and T.L. Vassilev. 2007. Antibodies use heme as a cofactor to extend their pathogen elimination activity and to acquire new effector functions. Journal of Biological Chemistry 282(37): 26696–26706.
Dimitrov, J.D., T.L. Vassilev, S. Andre, S.V. Kaveri, and S. Lacroix-Desmazes. 2008. Functional variability of antibodies upon oxidative processes. Autoimmunity Reviews 7(7): 574–578.
McIntyre, J.A., D.R. Wagenknecht, and W.P. Faulk. 2005. Autoantibodies unmasked by redox reactions. Journal of Autoimmunity 24(4): 311–317.
Mihaylova, N.M., J.D. Dimitrov, I.K. Djoumerska-Alexieva, and T.L. Vassilev. 2008. Inflammation-induced enhancement of IgG immunoreactivity. Inflammation Research 57(1): 1–3.
Kazatchkine, M.D., and S.V. Kaveri. 2001. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. New England Journal of Medicine 345(10): 747–755.
Djoumerska-Alexieva, I., L. Roumenina, A. Pashov, J. Dimitrov, M. Hadzhieva, S. Lindig, et al. 2015. Intravenous immunoglobulin with enhanced polyspecificity improves survival in experimental sepsis and aseptic systemic inflammatory response syndromes. Molecular Medicine 21: 1002–1010.
Pavlovic, S., N. Zdravkovic, J.D. Dimitrov, A. Djukic, N. Arsenijevic, T.L. Vassilev, and M.L. Lukic. 2011. Intravenous immunoglobulins exposed to heme (heme IVIG) are more efficient than IVIG in attenuating autoimmune diabetes. Clinical Immunology 138(2): 162–171.
Xiao, W., M.N. Mindrinos, J. Seok, J. Cuschieri, A.G. Cuenca, H. Gao, et al. 2011. A genomic storm in critically injured humans. Journal of Experimental Medicine 208(13): 2581–2590.
Roumenina, L.T., J. Rayes, S. Lacroix-Desmazes, and J.D. Dimitrov. 2016. Heme: modulator of plasma systems in hemolytic diseases. Trends in Molecular Medicine 22(3): 200–213.
Lecerf, M., T. Scheel, A.D. Pashov, A. Jarossay, D. Ohayon, C. Planchais, et al. 2015. Prevalence and gene characteristics of antibodies with cofactor-induced HIV-1 specificity. Journal of Biological Chemistry 290(8): 5203–5213.
Larsen, R., R. Gozzelino, V. Jeney, L. Tokaji, F.A. Bozza, A.M. Japiassu, et al. 2010. A central role for free heme in the pathogenesis of severe sepsis. Science Translational Medicine 2(51): 51ra71.
Brocklehurst, P., B. Farrell, A. King, E. Juszczak, B. Darlow, K. Haque, A. Salt, B. Stenson, and W. Tarnow-Mordi. 2011. Treatment of neonatal sepsis with intravenous immune globulin. New England Journal of Medicine 365(13): 1201–1211.
Dimitrov, J.D., C. Planchais, T. Scheel, D. Ohayon, S. Mesnage, C. Berek, S.V. Kaveri, and S. Lacroix-Desmazes. 2014. A cryptic polyreactive antibody recognizes distinct clades of HIV-1 glycoprotein 120 by an identical binding mechanism. Journal of Biological Chemistry 289(25): 17767–17779.
Wang, H., O. Bloom, M. Zhang, J.M. Vishnubhakat, M. Ombrellino, J. Che, et al. 1999. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285(5425): 248–251.
Xu, J., X. Zhang, R. Pelayo, M. Monestier, C.T. Ammollo, F. Semeraro, et al. 2009. Extracellular histones are major mediators of death in sepsis. Nature Medicine 15(11): 1318–1321.
Qiang, X., W.L. Yang, R. Wu, M. Zhou, A. Jacob, W. Dong, et al. 2013. Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nature Medicine 19(11): 1489–1495.
Czermak, B.J., V. Sarma, C.L. Pierson, R.L. Warner, M. Huber-Lang, N.M. Bless, H. Schmal, H.P. Friedl, and P.A. Ward. 1999. Protective effects of C5a blockade in sepsis. Nature Medicine 5(7): 788–792.
Riedemann, N.C., R.F. Guo, and P.A. Ward. 2003. Novel strategies for the treatment of sepsis. Nature Medicine 9(5): 517–524.
Rittirsch, D., M.A. Flierl, B.A. Nadeau, D.E. Day, M. Huber-Lang, C.R. Mackay, et al. 2008. Functional roles for C5a receptors in sepsis. Nature Medicine 14(5): 551–557.
Volman, T.J., T. Hendriks, and R.J. Goris. 2005. Zymosan-induced generalized inflammation: experimental studies into mechanisms leading to multiple organ dysfunction syndrome. Shock 23(4): 291–297.
Hubbard, W.J., M. Choudhry, M.G. Schwacha, J.D. Kerby, L.W. Rue 3rd, K.I. Bland, and I.H. Chaudry. 2005. Cecal ligation and puncture. Shock 24(Suppl 1): 52–57.
Acknowledgments
This work was supported by grants from the Bulgarian Science Fund (grant DFNI B02/29) and from the Agence Nationale de la Recherche (ANR-13-JCV1-006-01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experimental protocols were approved by the Animal Care Commission of the Institute of Microbiology in accordance with National and European Regulations (BABH protocol #105/10 July 2014).
ELECTRONIC SUPPLEMENTARY MATERIAL
Below is the link to the electronic supplementary material.
ESM 1
(JPG 32 kb)
Rights and permissions
About this article
Cite this article
Djoumerska-Alexieva, I., Roumenina, L.T., Stefanova, T. et al. Heme-Exposed Pooled Therapeutic IgG Improves Endotoxemia Survival. Inflammation 40, 117–122 (2017). https://doi.org/10.1007/s10753-016-0460-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0460-x