Abstract
The goal of this study was to trace the course of multiple sclerosis (MS) by evaluating the lymphocyte subpopulation counts and the levels of CD4+ and CD8+ T cell activation using flow cytometry. Samples obtained from healthy subjects (N = 40) and patients with MS (N = 290) were analyzed. Lymphocytes were labeled for the surface markers CD4+, CD8+, CD3+, CD16+, CD19+, CD45+, and CD53+ and the activation marker HLA-DR+. Cell counts were then determined using flow cytometry. A high degree of inter-individual variability was observed in the counts of all lymphocyte subtypes in the MS group. A significantly lower proportion of CD3+ T cells (69 ± 14 % in healthy subjects and 60 ± 17 % as a percent of total lymphocytes in MS patients), CD4+ T cells (41 ± 11 and 28 ± 18 %, respectively), and a significantly higher proportion of NK T cells (12 ± 5 and 25 ± 21 %, respectively) were observed in patients with MS than in healthy subjects. These differences led to a lowered CD4+/CD8+ T cell ratio. Furthermore, a significantly lower proportion of activated CD4+ T cells (HLA-DR+ CD4+; from 48 ± 10 to 38 ± 15 % as a percent of CD4+ cells) was observed in patients with MS than in healthy subjects. The high level of inter-individual variability in lymphocyte cell counts and the counts of activated T cells suggest that MS is a complex and heterogeneous disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Laboratory analyses are useful for monitoring the status of a disease and other clinically relevant patient characteristics. Therefore, whole blood samples were obtained from patients as part of routine diagnostic procedures, and the proportions of lymphocyte subtypes were characterized in these whole blood samples using flow cytometry. The markers evaluated in this study included CD3+, CD4+, CD8+, CD16+, CD19+, CD45+, and CD53+. In addition to these markers, the activation marker human leukocyte antigen D related (HLA-DR) was examined in this study. In this report, the distributions of activated CD4+ and CD8+ T cells are described in healthy subjects and patients with multiple sclerosis (MS). Furthermore, the distributions of cells associated with these markers were analyzed. The results showed that specific autonomous active T cell clones remained in the blood of MS patients and that these cells may explain the differences that were observed in activated T cell populations between these two groups.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board (IRB) of Dresden University and conformed to the directives of the Helsinki Declaration. All patients who were involved in this study provided written informed consent. Patient data and whole blood were collected at the Multiple Sclerosis Center at Dresden University.
Whole blood from MS patients (N = 290) and from healthy subjects (N = 40) was collected in ethylenediaminetetraacetic acid (EDTA)-containing monovettes and immediately transported to the laboratory.
Flow cytometry analyses were then performed using a FACSCanto II flow cytometer (Becton Dickinson (BD) Biosciences, Heidelberg, Germany). In a parallel experiment, the same whole blood samples were used to determine complete cell counts in a Sysmex XE5000 analyzer (Sysmex, Kobe, Japan).
For the flow cytometry analyses, 50 μl of whole blood was mixed with an antibody solution that contained 2 μl of each of the following antibodies (all BD Biosciences, Heidelberg, Germany): CD45-APC, CD3-APC-Cy7, CD4-PE-Cy7, CD8-PerCP, CD16-FITC, CD56-FITC, and HLA-DR-PE.
As a part of routine laboratory procedures, the immune status of each patient was analyzed using flow cytometry to determine the lymphocyte region, which was gated with the sideward scatter versus CD45+ (labeled with APC) cells using a sideward/APC scatter plot. Within the lymphocyte region, the lymphocyte populations were evaluated by plotting the CD3+ marker versus the CD4+ and CD8+ markers. To determine the proportion of activated CD4+ and CD8+ lymphocytes, cells expressing the markers CD3+, CD4+, and CD8+ were plotted versus cells expressing HLA-DR+. The number of CD4+ HLA-DR+ double-positive T cells was considered the activated CD4+ population and was expressed as N act CD4 in absolute numbers or qCD4 in relative terms (the number of double-positive CD4+ HLA-DR+ T cells divided by the number of total CD4+ T cells). The number of CD8+ HLA-DR+ double-positive T cells was considered equivalent as the population of activated CD8+ T cells and is represented in absolute numbers as N act CD8 or in relative terms as qCD8 (the number of double-positive HLA-DR+ CD8+ T cells divided by the number of total CD8+ T cells).
Gating Strategy
In the first diagram, the sideward scatter was drawn versus cells expressing CD45+ (labeled with APC), and all lymphocytes were gated in the CD45+ positive region in the lower right corner. In a second diagram, the sideward scatter was drawn versus the forward scatter, and the lymphocyte region was further adjusted and defined as a single population. In a third diagram, the sideward scatter was drawn versus CD3+ cells that were labeled with APC-Cy7. In this diagram, the CD3+ positive and CD3- negative lymphocytes were separated and then counted separately. Next, CD4+ PE-Cy7 versus CD3+ APC-Cy7 and CD8+ PerCP versus CD3+ APC-Cy7 diagrams were generated and used to separate and count the CD3 + CD4+ and CD3 + CD8+ double-positive T cell populations. Then, a CD16+ CD56+ (both FITC-labeled) versus CD3+ APC diagram was used to count CD3+ CD16+ CD56+ NK T cells (equivalent to NK T cells). A HLA-DR+ PE versus CD3+ APC-Cy7 diagram was then used to define the count of activated T cells (defined as CD3+ HLA-DR+ T cells). Two separate diagrams were derived from this single diagram. In one, HLA-DR+ PE cells were drawn versus CD4+ PE-Cy7 cells to identify activated CD4+ T cells, and in the other, HLA-DR+ PE cells were drawn versus CD8+ PerCP cells to define and count the activated CD8+ T cells.
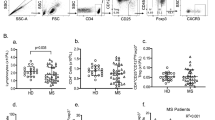
The percentage of cells in each cell subpopulation was evaluated for by each patient and healthy subject. The differences in these percentages were compared using a constructing diagram (Fig. 1).
Overview of the results of the FACS analysis to determine cell count measurements. The percentages of cell types were compared between MS patients and healthy subjects (normal controls). There were significant fewer CD4+ T and total CD3+ T cells and more CD3+ CD16+ CD56+ (NK) T cells in MS patients than in the healthy controls. In addition, the number of activated CD4+ T cells (CD4 + HLA-DR+ T cells) was significantly lower in MS patients than in healthy subjects. All percentages are shown as a percentage of all counted lymphocytes, except for activated CD4+ T cells, which are shown as a percentage of all CD4+ T cells, and activated CD8+ T cells, which are shown as a percentage of all CD8+ T cells.
The flow cytometric analysis was performed using the BD FacsDiva 8.0 software (BD Biosciences, Heidelberg, Germany) and FlowJo software (Treestar, USA). The data obtained from these analyses were statistically analyzed using SPSS 20.0 statistical software (SPSS Inc., Chicago, IL, USA). Variables were tested for normality using the Kolmogorov-Smirnov test. Values are presented as the mean ± standard deviation. Percentages are provided, and the chi-squared test was used. Independent sample t tests, paired t tests, one-way analysis of variance, parametric tests, and Wilcoxon t tests were used to calculate statistical significance. A p value less than 0.02 was considered to indicate statistical significance.
RESULTS
A high degree of inter-individual variability was observed in the cell counts of all lymphocyte subpopulations in the MS patients group. In this study, significantly lower counts of CD3+ T cells (60 ± 17 % in MS patients and 69 ± 14 % in healthy subjects) and CD4+ T cells (28 ± 18 % in MS patients and 41 ± 11 % in healthy subjects) were observed in the MS group than in the healthy group (data are described as a percent of total lymphocytes, see Fig. 1). Furthermore, there was a significantly higher proportion of NK T cells (25 ± 21 % in MS patients and 12 ± 5 % in healthy subjects, expressed as percent of total lymphocytes) in the MS group than in the healthy group.
While the results showed that CD8+ T cell counts were non-significantly higher in the MS group (27 ± 12 % as a percent of all lymphocytes) than in the healthy group (23 ± 9 % as a percent of all lymphocytes), the CD4+/CD8+ ratio was significantly lower in MS patients (1.05 in MS patients versus 2 in normal controls).
In addition to these observations, significantly fewer CD4+ HLA-DR+ T cells (activated CD4+ T cells) were found in the MS patient group (38 ± 15 % as a percent of all CD4+ T cells) than in the healthy group (48 ± 10 %). Additionally, there were only slightly (non-significantly) more activated CD8+ T cells (CD8+ HLA-DR+ T cells) in the MS group (56 ± 14 % percent of all CD8+ T cells) than in the healthy group (53 ± 9 % as a percent of CD8+ T cells). This was leading to a ratio of activated T cells, or a CD4+ HLA-DR+/CD8+ HLA-DR+ ratio, which was significantly lower in MS patients (0.68 in MS patients versus 0.95 in healthy controls). These results indicate that activated T cells shift toward CD8+ T cell activation in MS patients.
A comparison of the proportions of CD3 + HLA-DR+, CD8 + HLA-DR+, CD8 + CD3+, and CD19+ (B) cell counts as a percentage of the total number of lymphocytes showed that there were no significant differences between MS patients and normal controls.
DISCUSSION
Figure 1 provides an overview of the results, which were used to compare the proportions of cell types in the evaluated cell populations between MS patients and healthy subjects. There were significantly fewer CD4+ T cells and CD3+ T cells in MS patients than in healthy subjects. In contrast, there were significantly more CD3+ CD16+ CD56+ T cells (NK T cells) in the blood obtained from MS patients. Furthermore, the counts of activated CD4+ T cells (CD4+ HLA-DR+) were significantly lower in MS patients than in healthy subjects. There were slight (non-significant) differences in other T cell counts, such as CD8 + HLA-DR+ counts, CD3 + HLA-DR+ counts, the total lymphocyte count, CD8 + CD3+ counts, and B cell counts (CD19+). A high degree of inter-individual variability was observed for all lymphocyte subpopulation cell counts between MS patients and healthy subjects, as is evident given the large standard deviation values that were obtained.
The results of the current study are in agreement with those of previous studies that have been explored on the compositions of T lymphocyte subsets in MS patients. Several of these studies have found that there is high inter-individual variability of the CD4+/CD8+ T cell ratio in MS patients who receive therapy [1, 2]. One study observed a CD4+/CD8+ T cell ratio of less than 2 in most MS patients who received natalizumab therapy [1]. This ratio indicates that the CD4+ T cell count was reduced in the peripheral blood, a finding, which is in-line to the results of the present study.
Another study on this subject also found that the CD4+/CD8+ T cell ratio was lower in patients who were treated with fingolimod [2]. This reduction resulted from a decrease of the CD4+ T cell count from 58 to 11 % after 1 month of treatment. However, the authors of that second study also observed an increase in the proportion of NK T cells from 11 to 51 % after 1 month of fingolimod therapy [2]. Here, a similar increase was observed in the proportion of NK T cells. Similarly, another study also found that patients who were treated with natalizumab had lower CD4+/CD8+ T cell ratios [3]. Thus, the results presented in this study are consistent with the results described in the recent literature.
Another recent study demonstrated that the clones of at least some activated T cells are specifically directed against myelin basic protein in patients with MS [4]. Thus, different antigens may sequentially activate different T cell clones, thereby adding to the number of activated T cells in affected patients.
So several studies have previously discussed MS-related alterations in the CD4+/CD8+ T cell ratio, and shifts in the CD4+/CD8+ T cell ratio have also been observed in several other diseases and conditions [5–10]. However, none of these studies have explored differences in CD4+ and CD8+ T cell activation states similar to those observed in MS patients in the present study.
T cell activation, at least in HIV patients, is thought to result from the antigenic stimulation of T cells [11]. This might also be true in MS patients given the changes in activated T cell populations that were observed in this study. Antigenic stimulation could be induced by myelin, at least in some MS patients, as was demonstrated in a recent paper [4].
One study reported that it was primarily newly produced T and B lymphocytes that were reduced in fingolimod-treated patients [12]. Furthermore, the authors of that last study found that fingolimod-treated patients had smaller repertoires of T cell receptors.
Another study reported that fingolimod-treated patients had higher levels of regulatory T cells (Treg cells) and memory T cells, whereas the proportion of naive T cells was lower in these patients [13]. The authors of that last study believed that these naive T cells were the “main drivers” of pathogenesis in MS.
Additionally, Salou et al. reported that for decades, CD4+ T cells were thought to play the most important role in the pathogenesis of MS; whereas in recent years, new evidence has increased the interest in the possibility that CD8+ T cells may also play an important role [14]. Another previous study showed that fingolimod-treated MS patients showed strong fluctuations in total lymphocyte cell counts [15].
The major differences between the present study and previous reports are our observation of the widespread occurrence of inter-individual variability also in other, further lymphocyte subpopulations, especially CD3+ T cells, CD4 + HLA-DR+ cells, CD8 + HLA-DR+ cells, CD3 + CD16 + CD56+ (NK T) cells and CD19+ cells (B cells).
Furthermore, in this study, the results demonstrate that in MS patients, there are changes in the activation states of CD4+ and CD8+ T cells. The results also demonstrate that there is a shift in the relationship between the number of activated CD4+ T cells and the number of activated CD8+ T cells. In MS patients, the CD4 + HLA-DR+/CD8 + HLA-DR+ ratio is shifted to a similar extent and in the same direction as has previously been described for the CD4+/CD8+ T cell ratio. This finding regarding the CD4+/CD8+ T cell ratio is widely accepted and also supported by the results of the present study.
The results here showed that a shift occurs in the CD4 + HLA-DR+/CD8 + HLA-DR+ ratio in patients with MS. These data are in-line with the hypotheses that CD8+ T cells might play a role in the pathogenesis underlying MS. Sinha et al. and Salon et al. summarized the growing amount of clinical and animal study-based evidence that shows that CD8+ T cells might play an important role in the pathogenesis of MS [14, 16]. However, the specific mechanisms underlying the role of CD8+ T cells in the pathogenesis of MS remain undefined. One study evaluated the role of T cells in MS and showed that “myelin-specific” CD8+ T cells are involved in its onset [17]. This process seems to involve the clonal expansion of CD8+ T cells, which are also involved in the differentiation of “encephalogenic” CD4+ T cells. These “encephalogenic” CD4+ T cells are, in turn, capable of invading the cerebrospinal fluid (CSF) and consequently the central nervous system (CNS) [17].
CONCLUSIONS
The results of this study show that MS patients display significantly fewer CD3+, CD4+, and CD4+ HLA-DR+ T cells and significantly more CD3 + CD16 + CD56+ (NK) T cells. Recent results in the literature indicate that CD8+ T cells may play an important role in MS. The results of our study, which show that the numbers of activated CD8+ T cells are slightly higher in MS patients, may support these previous findings. Furthermore, the results here show that in patients with MS, there was a high degree of inter-individual variability in all lymphocyte subpopulations which have been investigated.
References
Harrer, A., G. Pilz, P. Wipfler, K. Oppermann, J. Sellner, W. Hitzel, E. Haschke-Becher, S. Afazel, T. Rispens, D. van der Kleij, E. Trinka, and J. Kraus. 2015. High interindividual variability in the CD4/CD8 T cell ratio and natalizumab concentration levels in the cerebrospinal fluid of patients with multiple sclerosis. Clinical Experimental Immunology 180: 383–392.
Rudnicka, J., M. Czerwiec, E. Grywalska, D. Siwicka-Gieroba, M. Walankiewicz, A. Grafka, M. Zgurski, A. Surdacka, H. Bartosik-Psujek, and J. Rolinski. 2015. Influence of fingolimod on basic lymphocyte subsets frequencies in the peripheral blood of multiple sclerosis patients—preliminary study. Central European Journal of Immunology 40: 354–359.
Stüve, O., C.M. Marra, A. Bar-Or, M. Niino, P.D. Cravens, S. Cepok, E.M. Frohman, J.T. Phillips, G. Arendt, K.R. Jerome, L. Cook, F. Grand’Maison, B. Hemmer, N.L. Monson, and M.K. Racke. 2006. Altered CD4+/CD8+ T-cell ratios in cerebrospinal fluid of natalizumab-treated patients with multiple sclerosis. Archives of Neurology 63: 1383–1387.
Arneth, B. 2015. Early activation of CD4+ and CD8+ T lymphocytes by myelin basic protein in subjects with MS. Journal of Translational Medicine 13: 341.
Hernández-Hoyos, G., M. Anderson, C. Wang, E. Rothenberg, and J. Alberola-Ila. 2003. GATA-3 expression is controlled by TCR signals and regulates CD4/CD8 differentiation. Immunity 19: 83–94.
Egerton, M., R. Scollay, and K. Shortman. 1990. Kinetics of mature T-cell development in the thymus. Proceedings of the National academy of Sciences of the United States of America 87: 2579–2582.
Merkenschlager, M. 1997. How many thymocytes audition for selection? Journal Experimental Medicine 186: 1149–1158.
Merkenschlager, M., C. Benoist, and D. Mathis. 1994. Evidence for a single-niche model ofpositive selection. Proceedings of the National academy of Sciences of the United States of America 91: 11694–11698.
Sinclair, C., I. Bains, A. Yates, and B. Seddon. 2013. Asymmetric thymocyte death underlies the CD4:CD8 T-cell ratio in the adaptive immune system. Proceedings of the National academy of Sciences of the United States of America 110: E2905–E2914.
Schlub, T., J. Sun, S. Walton, S. Robbins, A. Pinto, M. Munks, et al. 2011. Comparing the kinetics of NK cells, CD4, and CD8 T cells in murine cytomegalovirus infection. Journal of Immunology 187: 1385–1392.
Cohen, S.J., M. Hazebergh, D. Hamann, S. Otto, J. Borleffs, F. Miedema, et al. 2000. The dominant source of CD4+ and CD8+ T-cell activation in HIV infection is antigenic stimulation. Journal of Acquired Immune Deficiency Syndromes 25: 203–211.
Chiarini, M., A. Sottini, D. Bertoli, F. Serana, L. Caimi, S. Rasia, R. Capra, and L. Imberti. 2015. Newly produced T and B lymphocytes and T-cell receptor repertoire diversity are reduced in peripheral blood of fingolimod-treated multiple sclerosis patients. Multiple Sclerosis 6: 726–734.
Claes, N., T. Dhaeze, J. Fraussen, B. Broux, B. Van Wijmeersch, P. Stinissen, R. Hupperts, N. Hellings, and V. Somers. 2014. Compositional changes of B and T cell subtypes during fingolimod treatment in multiple sclerosis patients: a 12-month follow-up study. PLoS One 9, e111115.
Salou, M., B. Nicol, A. Garcia, and D.A. Laplaud. 2015. Involvement of CD8(+) T cells in multiple sclerosis. Frontiers in Immunology 6: 604.
Henault, D., L. Galleguillos, C. Moore, T. Johnson, A. Bar-Or, and J. Antel. 2013. Basis for fluctuations in lymphocyte counts in fingolimod-treated patients with multiple sclerosis. Neurology 81: 1768–1772.
Sinha, S., A.W. Boyden, F.R. Itani, M. Crawford, and N. Karandikar. 2015. CD8+ T-cells as immune regulators of multiple sclerosis. Frontiers in Immunology 6: 619.
Fischer, H.J., J. Brandt, T. Lingner, F. Odoardi, A. Flügel, A. Weishaupt, and H. Reichardt. 2016. Modulation of CNS autoimmune responses by CD8+ T cells coincides with their oligoclonal expansion. Journal Neuroimmunology 290: 26–32.
Acknowledgements
The author thanks Prof. Ziemssen and the team at MS Center Dresden for providing clinical data and patient samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Institutional Review Board (IRB) of Dresden University and conformed to the directives of the Helsinki Declaration. All patients who were involved in this study provided written informed consent.
Competing Interests
The author declares that he has no competing interests.
ELECTRONIC SUPPLEMENTARY MATERIAL
Below is the link to the electronic supplementary material.
ESM 1
(XLS 77 kb)
Rights and permissions
About this article
Cite this article
Arneth, B. Activated CD4+ and CD8+ T Cell Proportions in Multiple Sclerosis Patients. Inflammation 39, 2040–2044 (2016). https://doi.org/10.1007/s10753-016-0441-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0441-0