ABSTRACT
Circulating lymphocyte number was significantly decreased in patients with sepsis. However, it remains unknown which severity phase (sepsis, severe sepsis, and septic shock) does it develop and what happen on each subpopulation. Eight patients with differing severities of sepsis (31 sepses, 33 severe sepses, and 16 septic shocks) were enrolled. Quantitative real-time polymerase chain reaction (RT-PCR) of Th1, Th2, and Th17; regulatory T (Treg) cell-specific transcription factor T-bet; GATA-3; RORgammat (RORγt); forkhead box P3 (FOXP3); and IL-17 mRNA were performed, and the enzyme-linked immunosorbent assay (ELISA) was used to detect serum interferon (IFN)-γ, IL-4, and IL-10. In this study, the Th1, Th2, Treg transcription factors, and related cytokines IFN-γ, IL-4, and IL-10 levels of sepsis and severe sepsis patients in peripheral blood were significantly higher than those of the normal controls. Except for IL-17, the T-bet, GATA-3, and IFN-γ levels of septic shock patients were lower than those of sepsis patients. We also observed that the proportions of Th17/Treg in the sepsis and septic shock groups were inversed. From the above, the inflammatory response especially the adaptive immune response is still activated in sepsis and severe sepsis, but significant immunosuppression was developed in septic shock. In addition, the proportion of Th17/Treg inversed may be associated with the illness aggravation of patients with sepsis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Sepsis, the systemic inflammatory response syndrome (SIRS) induced by pathogenic microorganism infection, is a common cause of death in ICUs. Although the treatment of sepsis appears significantly improved over the past 20 years (e.g., effective antibiotics, hemofiltration, immune therapy), the mortality of sepsis still remained high. More than 200,000 patients die of sepsis each year in the USA [1].
It is known that immune system dysfunction is the main pathophysiological procedure in the development of sepsis. The immune homeostasis of septic patients deeply perturbs which performance as pro-inflammation accompanied by an anti-inflammation process [2]. The alternation of pro-inflammation and anti-inflammation leads to immunosuppressive state [3]. Septic patients with immunosuppression could have reactivation of dormant viruses (cytomegalovirus or herpes simplex virus) or opportunistic germ infections [2–4]. Several researches have confirmed immunosuppression in the innate side of the immune system. However, T lymphocytes, major effector cells of the adaptive immune system, are also involved. The pathological mechanism of immunosuppression on lymphocytes is the disorder of cell differentiation and function and an increase of apoptosis in sepsis [5–8].
T helper lymphocytes play an important role in anti-infection response. According to differentiation and different phenotypes, T helper cells can be divided into four subpopulations: Th1, Th2, Th17, and regulatory T (Treg) lymphocytes [9]. Researches were initially focused on the Th1 and Th2 subpopulations to find out the mechanism of immune dysfunction [10]. In recent years, with the discovery of Th17 cells and deep understanding of Treg cells, studies on these four subpopulations shed new light on the pathogenesis of sepsis. Venet et al. found that four T helper lymphocyte subpopulations in the peripheral blood decreased at the same time in septic shock patients [11]. Wu et al. observed that the number and differentiation of four subpopulations decreased and were associated with the mortality of severe sepsis patients [12]. At present, it still remains unclear in which severity phase do those pathological phenomenons appear and what happen on each subpopulation.
In this study, we detected the transcription factors and the related cytokines of the Th1, Th2, Th17, and Treg subpopulations in the peripheral blood under different severities of sepsis to investigate the pathological changes of T helper cells.
MATERIALS AND METHODS
Patients
The patients in this study were from the surgery ICU, emergency ICU, and respiratory ICU of the Chinese PLA General Hospital; patient data were collected between July 2011 and March 2013. The diagnosis of sepsis and the criteria used to define different severities of sepsis were based on the 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference [13]. Disease severity was assessed according to the Sequential Organ Failure Assessment (SOFA) scores. Sepsis was defined as SIRS associated with infection. Severe sepsis was defined as sepsis complicated by organ dysfunction. Patients with severe sepsis exhibited at least one of the following: (1) hypoxemia (oxygenation index partial pressure of oxygen in the arteries/fraction of inspired oxygen <300); (2) acute renal injury as evidenced by either oliguria (urine output <0.5 mL/kg/h for ≥2 h) or a serum creatinine increase of >0.5 mg/dL (44.2 μmol/L); (3) coagulation abnormalities, in which the activated partial thromboplastin time was >60 s, the international normalized ratio was >1.5 s, or the platelet (PLT) count was <100 × 109/L; (4) hyperbilirubinemia, in which the total bilirubin was either >4 mg/dL or 70 mmol/L; (5) metabolic acidosis, in which either the pH was <7.3 or the lactic acid levels were >2 times higher than normal (lactic acidosis); or (6) infection-associated hypotension, in which the systolic blood pressure was <80 mmHg, mean arterial pressure (MAP) was <70 mmHg, or systolic blood pressure had a decrease of >40 mmHg.
Septic shock was defined by persistent arterial hypotension (systolic arterial pressure below 90 mmHg, mean arterial pressure lower than 60, or a reduction in systolic blood pressure of more than 40 mmHg from baseline, despite adequate volume resuscitation) that could not be explained by other causes. The control group consisted of age-matched healthy individuals selected from the Health Center of the Chinese PLA General Hospital. The exclusion criteria were as follows: (1) patients under 18 years of age; (2) patients with other conditions that could affect immunity, including cancer, chronic viral infections (human immunodeficiency virus and hepatitis B or C), or autoimmune diseases; and (3) refusal to participate. Patients who survived longer than 28 days after ICU admission were defined as survivors. The protocol was approved by the Hospital Committee on Ethics of the Chinese PLA hospital (20111013-008). An informed consent form was signed by patients or their legal representatives prior to enrollment in the study.
Study Protocol and Methods
Peripheral arterial blood from patients with sepsis was collected within 24 h after their diagnosis. All samples were anticoagulated with ethylenediaminetetraacetic acid. Each 400 μL sample of blood was isolated and stored at −80 °C. The remaining plasma was stored at −80 °C until determination of IFN-γ, IL-4, and IL-10 was made.
Quantitative Real-Time (RT)-PCR
Gene expression was analyzed using two-step quantitative RT-PCR. Total RNA was extracted from 200 μL of the blood sample, which had been stored at −80 °C. A QuantiTect Reverse Transcription Kit (QIAGEN, Beijing, China) was used to reverse transcribe RNA (0.2–1 μg) in a 20-μl reaction volume (42 °C, 30 min; 95 °C, 5 min); then, complementary DNA (cDNA; 2 μL) was amplified using a SYBR Green I Master Mix (Roche, Basel, Switzerland) and a LightCycler 480 PCR System (Roche). All tests were performed in duplicate 20 μL reaction mixtures in 96-well plates, and a negative control without cDNA template was included in each run. Visual inspection of the melting curves was used to confirm the specificity of the products during PCR. The expression level of a gene was calculated using the 2−ΔΔCt method with GAPDH as the internal control.
Enzyme-Linked Immunosorbent Assay (ELISA)
Plasmas IFN-γ, IL-4, and IL-10 were determined using a commercial ELISA kit (Dakewe, Beijing, China) according to manufacturer’s instructions. The lower detection limit of the IFN-γ, IL-4, and IL-10 assays was 5, 2.5, and 5 pg/mL, respectively.
Statistical Analysis
All data were analyzed using the SPSS 20.0 software (IBM, Chicago, USA) for Windows. Abnormal distributed data were expressed as the medians ± interquartile range and compared using the Kruskal-Wallis test. Normally distributed data were expressed as the means ± standard error and compared using Student’s t test. Categorical variables were analyzed using the χ 2 test. The p values <0.05 were considered statistically significant.
RESULTS
Clinical Characteristics of Sepsis Patients and Normal Controls
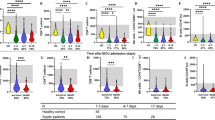
The clinical data of sepsis patients and the normal control group is shown in Table 1. The average ages of the sepsis, severe sepsis, septic shock patients, and the normal controls were 45(28, 72), 54(18, 87), 63.67(18, 84), and 51.5(35, 70) years, respectively. There were no significant differences among these groups (p = 0.341). The mortality on day 28 in the sepsis, severe sepsis, and septic shock groups were 9.7, 27.3, and 95 %, respectively. The WBC counts of each experimental group were significantly higher than that of the normal controls, but the lymphocyte percentages were significantly lower than those of the normal controls. The highest proportion of infection sources was pneumonia, followed by multiple trauma and peritonitis. The SOFA scores of the severe sepsis and septic shock groups were significantly higher than those of the sepsis group, and the SOFA scores of the septic shock patients were significantly higher than those of the severe sepsis patients. The APACHE II scores of the severe sepsis and the septic shock groups were higher than those of the sepsis group.
The Th1 Subpopulation and IFN-γ Levels
The specific transcription factor T-bet of the Th1 subpopulation of the sepsis and severe sepsis groups was significantly higher than that of the normal controls (p < 0.05), and the mRNA level of T-bet of the sepsis group was significantly higher than that of the septic shock group (p < 0.05, Fig. 1a). Compared with the normal controls, the IFN-γ levels of each experimental group were significantly higher (sepsis, severe sepsis vs. normal control (NC), p < 0.001; septic shock vs. NC, p < 0.05); the IFN-γ level of the sepsis group was higher than that of the septic shock group (p < 0.05, Fig. 1b).
a The mRNA level of T-bet of the sepsis and severe sepsis groups was significantly higher than that of the normal controls. The mRNA level of T-bet of sepsis group was significantly higher than that of the septic shock group. b Compared with the normal controls, the IFN-γ levels of the sepsis, severe sepsis, and septic shock patients were significantly higher. The IFN-γ level of the sepsis group was higher than that of the septic shock group (*p< 0.05, **p < 0.001). NC normal control.
The Th2 Subpopulation and IL-4 Levels
The GATA-3 mRNA levels of the sepsis and severe sepsis groups were significantly higher than those of the normal controls (p < 0.01) and the septic shock group (p < 0.05, Fig. 2a). The serum IL-4 levels of the sepsis and severe sepsis groups were significantly higher than those of the normal control group (p < 0.05, Fig. 2b).
a The GATA-3 mRNA levels of the sepsis and severe sepsis groups were significantly higher than those of the normal controls and the septic shock group. b The serum IL-4 levels of the sepsis and severe sepsis groups were significantly higher than those of the normal controls (*p < 0.01, **p < 0.05) NC normal control.
The Th17 Subpopulation and IL-17 Levels
There was no significant difference between the experimental groups and the normal control group on the specific transcription factor RORgammat (RORγt) of the Th17 subpopulation (Fig. 3a). Compared with the normal controls, the IL-17 mRNA level of the sepsis and septic shock groups was significantly higher (p < 0.01). The IL-17 mRNA level of the septic shock group was higher than that of the severe sepsis groups (p < 0.05, Fig. 3b).
a There was no significant difference between the experimental groups and the normal controls on RORγt. b Compared with normal controls, the IL-17 mRNA level of the sepsis and septic shock groups was significantly higher. The IL-17 mRNA level of the septic shock group was higher than that of the severe sepsis group (*p < 0.01, **p < 0.05) NC normal controls.
The Treg Subpopulation and IL-10 Levels
Compared with the normal controls, the specific transcription factor forkhead box P3 (FOXP3) of the Treg subpopulation (sepsis, severe sepsis vs. NC, p < 0.01; septic shock vs. NC, p < 0.05; Fig. 4a) and the serum IL-10 level (p < 0.01; Fig. 4b) of the experimental groups were significantly higher.
DISCUSSION
T helper lymphocytes are the main effector cells in the adaptive immune response and play an important role against pathogenic microorganisms. The naive T lymphocyte subgroup can be induced to differentiate toward Th1, Th2, Th17, and Treg subpopulations according to the local cytokine milieu [9]. The presence of IL-12 induces the differentiation of Th1 subpopulation which was controlled by Th1-specific T box transcription factor (T-bet) [14]. IL-4 and transcription factor GATA-3 induce Th2 subpopulation [15]. IL-6 and the transforming growth factor (TGF)-β induce the differentiation of Th17 cells, and only TGF-β induces differentiation of Treg cells. Those two progressions are controlled by the transcription factors RORγt and FOXP3 [16, 17]. All four transcription factors exist in the above four types of cells, so the level of these four transcription factors could indirectly reflect the number of those four subpopulations in the peripheral blood.
Several researchers have reported the pathologic changes of the T helper lymphocyte subpopulations in patients with sepsis, such as an inverse proportion of Th1/Th2 cells [10, 18, 19] and an increase of Th17 and Treg cells [20–22]. However, Venet et al. reported that the counts of peripheral blood Th1, Th2, Th17, and Treg cells decreased in patients with septic shock [11]. The different results between these studies may be accounted for the different illness severities of patients and their different immune status among these researches. In our study, we compared four T helper lymphocyte subpopulations by using their special transcription factors and related cytokines among three different illness severity groups (sepsis, severe sepsis, and septic shock).
We observed that T-bet and GATA-3, the specific transcription factors of Th1 and Th2 cells, and related cytokines IFN-γ and IL-4 of the sepsis and severe sepsis groups were significantly higher compared with those of the normal controls. However, except for IL-4, these transcription factors and cytokines were significantly reduced in the septic shock groups. This result was similar to the study reported by Pachot et al. [23]. As pathogenic microorganisms invade, the activation of the innate immune system promotes the secretion of IL-12 which then induces naive T cells differentiated to Th1 cells and triggers the adaptive immune response. At the same time, some naive T cell differentiates to the Th2 cells, which participate in the humoral immune system and the anti-inflammation response [10]. In our study, the dramatic increasing of the transcription factor and cytokines of Th1 and Th2 suggested that pro-inflammation is simultaneous with anti-inflammation in the early phase of sepsis. In addition, by dividing sepsis patients into different levels of illness severity, we found that the number and cytokines of both Th1 and Th2 cells decreased in the septic shock phase. This result could give us a suggestion that immunosuppression is significantly developed in septic shock patients.
Th17 cells are one of the important participants in inflammation and the autoimmune response. In our study, there was no significant difference in RORγt among the experimental groups, but the IL-17 mRNA of the septic shock group was higher than that of the sepsis group. Several researches have indicated that IL-17 levels were increased in mice with sepsis induced by cecal ligation and puncture (CLP) [24, 25]. In addition, Shimura et al. found that macrophages, DCs, and eosinophils, but not Th17 cells or γδT cells, may be sources of IL-17 (also called IL-17A) during LPS-induced endotoxin shock. That is to say, although the number of Th17 cells did not significantly increase, a large amount of IL-17 may come from other activated cells. Flierl et al. found that IL-17 antibodies could improve the survival rate of sepsis model mice through the plasma IL-6 and TNF-α [24]. IL-17 could promote an inflammation response together with IL-23 in extracellular bacterial and fungal infections [26]. The high level of IL-17 in septic shock shows that this highly activated pro-inflammation response may not be a good protection but an aggravation.
The additional finding of our study is that the proportion of Th17/Treg cells between sepsis and septic shock patients appears inverted. The differentiation of Th17 and Treg cells is a mutual restriction relation. When the immune system inactivated, TGF-β induces native T cells to differentiate into Treg cells. As immune system turns activated, especially during the inflammatory response, a large number of IL-6 and TGF-β together induce naive T cells to differentiate into Th17 cells. In some cases, the differentiation direction of Th17 and Treg cells may determine the prognosis [27]. Treg cells downregulate the immune response by targeting the APC and effector T cell in the inflammation to maintain the balance of the immune response [28]. The early stage of sepsis is considered excessive inflammation, while the differentiation of Treg cells may be an adjustment between pro- and anti-inflammatory responses, which was indicated by our results. However, the fact that the Th17 cells increased as the Treg cells and IL-10 decreased in our septic shock group indicates that the immune modulating function may significantly disorder. Under this situation, inflammation cannot be effectively controlled, leading to the excessive release of inflammatory cytokines and an increased risk of nosocomial infection and multiple organ dysfunction syndromes (MODS).
The four T helper lymphocyte subpopulations can be divided into two groups: (1) pro-inflammatory cells—Th1 and Th17 lymphocytes and (2) anti-inflammatory cells—Th2 and Treg lymphocytes. In patients with sepsis, we found that the immune response was almost balanced between pro- and anti-inflammation. However, this kind of balance was broken in septic shock patients, as the lymphocyte subpopulations are all decreased, except Th17. These four T cells’ specific transcription factors reflect the number of these four T cells’ subpopulations in peripheral blood, and this situation has been confirmed by apoptosis of CD4+ T cells in sepsis shock [3, 29]. That is to say, immune function of sepsis shock patients appears significantly suppressed.
Our study has some limitations. First, a small sample size in this study was not adequate, especially the patients with septic shock, which may bias the results. Second, although we tried to collect the blood samples of sepsis patients within 24 h after their diagnosis, we could not ensure that all the patients were really in the definite stage of the disease. Finally, within only one sample window, we just compare the alteration of T helper lymphocytes at the early stage of sepsis, but keeping track of the immune function in later period and comprising the septic patients with other critically ill individuals are also important, which we will view as important areas of future studies.
In conclusion, by detecting the alteration of four transcription factors and related cytokines in patients with different levels of illness severity, we found that the inflammatory response especially the adaptive immune response is still activated in sepsis and severe sepsis. However, the decrease of number and cytokine of Th1, Th2, and Treg cells suggested that significant immunosuppression was developed in septic shock. In addition, the inverse proportion of Th17/Treg cells may be associated with aggravated sepsis. Thus, we hope our study provides complementary information to increase understanding of lymphocytic alterations in sepsis. Further studies are needed to confirm these findings in a large cohort of sepsis patients and to investigate the mechanism of immunosuppression in septic shock.
REFERENCES
Angus, D.C., W.T. Linde-Zwirble, J. Lidicker, et al. 2001. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Critical Care Medicine 29: 1303–1310.
Monneret, G., F. Venet, A. Pachot, et al. 2008. Monitoring immune dysfunctions in the septic patient: A new skin for the old ceremony. Molecular Medicine 14: 64–78.
Hotchkiss, R.S., and I.E. Karl. 2003. The pathophysiology and treatment of sepsis. New England Journal of Medicine 348: 138–150.
Limaye, A.P., K.A. Kirby, G.D. Rubenfeld, et al. 2008. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA 300: 413–422.
Nishijima, M.K., J. Takezawa, K.K. Hosotsubo, et al. 1986. Serial changes in cellular immunity of septic patients with multiple organ-system failure. Critical Care Medicine 14: 87–91.
Roth, G., B. Moser, C. Krenn, et al. 2003. Susceptibility to programmed cell death in T-lymphocytes from septic patients: A mechanism for lymphopenia and Th2 predominance. Biochemical and Biophysical Research Communications 308: 840–846.
Hotchkiss, R.S., P.E. Swanson, B.D. Freeman, et al. 1999. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Critical Care Medicine 27: 1230–1251.
Hotchkiss, R.S., K.W. Tinsley, P.E. Swanson, et al. 2001. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. Journal of Immunology 166: 6952–6963.
Afzali, B., G. Lombardi, R.I. Lechler, et al. 2007. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clinical and Experimental Immunology 148: 32–46.
Ferguson, N.R., H.F. Galley, and N.R. Webster. 1999. T helper cell subset ratios in patients with severe sepsis. Intensive Care Medicine 25: 106–109.
Venet, F., F. Davin, C. Guignant, et al. 2010. Early assessment of leukocyte alterations at diagnosis of septic shock. Shock 34: 358–363.
Wu, H.P., K. Chung, C.Y. Lin, et al. 2013. Associations of T helper 1, 2, 17 and regulatory T lymphocytes with mortality in severe sepsis. Inflammation Research 62: 751–763.
Levy, M.M., M.P. Fink, J.C. Marshall, et al. 2003. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Critical Care Medicine 31: 1250–1256.
Szabo, S.J., S.T. Kim, G.L. Costa, et al. 2000. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100: 655–669.
Zheng, W., and R.A. Flavell. 1997. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 89: 587–596.
Ivanov II, B.S., L. Zhou McKenzie, et al. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126: 1121–1133.
Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science 299: 1057–1061.
Zedler, S., R.C. Bone, A.E. Baue, et al. 1999. T-cell reactivity and its predictive role in immunosuppression after burns. Critical Care Medicine 27: 66–72.
Heidecke, C.D., T. Hensler, H. Weighardt, et al. 1999. Selective defects of T lymphocyte function in patients with lethal intraabdominal infection. American Journal of Surgery 178: 288–292.
Brunialti, M.K., M.C. Santos, O. Rigato, et al. 2012. Increased percentages of T helper cells producing IL-17 and monocytes expressing markers of alternative activation in patients with sepsis. PLoS One 7: e37393.
Ono, S., A. Kimura, S. Hiraki, et al. 2013. Removal of increased circulating CD4 + CD25 + Foxp3+ regulatory T cells in patients with septic shock using hemoperfusion with polymyxin B-immobilized fibers. Surgery 153: 262–271.
Leng, F.Y., J.L. Liu, Z.J. Liu, et al. 2013. Increased proportion of CD4(+)CD25(+)Foxp3(+) regulatory T cells during early-stage sepsis in ICU patients. Journal of Microbiology, Immunology and Infection 46: 338–344.
Pachot, A., G. Monneret, N. Voirin, et al. 2005. Longitudinal study of cytokine and immune transcription factor mRNA expression in septic shock. Clinical Immunology 114: 61–69.
Flierl, M.A., D. Rittirsch, H. Gao, et al. 2008. Adverse functions of IL-17A in experimental sepsis. FASEB Journal 22: 2198–2205.
Bosmann, M., F. Meta, R. Ruemmler, et al. 2013. Regulation of IL-17 family members by adrenal hormones during experimental sepsis in mice. American Journal of Pathology 182: 1124–1130.
Chen, Z., and J.J. O’Shea. 2008. Th17 cells: A new fate for differentiating helper T cells. Immunologic Research 41: 87–102.
Vernal, R., and J.A. Garcia-Sanz. 2008. Th17 and Treg cells, two new lymphocyte subpopulations with a key role in the immune response against infection. Infectious Disorders Drug Targets 8: 207–220.
Tang, Q., and J.A. Bluestone. 2008. The Foxp3+ regulatory T cell: A jack of all trades, master of regulation. Nature Immunology 9: 239–244.
Le Tulzo, Y., C. Pangault, A. Gacouin, et al. 2002. Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock 18: 487–494.
ACKNOWLEDGMENTS
This study was supported by the Beijing Municipal Science & Technology Commission Program (program number Z11110706730000, project number Z111107067311029) and the National Natural Sciences Foundation of China (Grant No.81172800). The authors thank the nurses of RICU/SICU/EICU at the Chinese PLA General Hospital for their work in this study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jia Li and Ming Li contributed equally to this work and should be considered co-first authors.
Rights and permissions
About this article
Cite this article
Li, J., Li, M., Su, L. et al. Alterations of T Helper Lymphocyte Subpopulations in Sepsis, Severe Sepsis, and Septic Shock: A Prospective Observational Study. Inflammation 38, 995–1002 (2015). https://doi.org/10.1007/s10753-014-0063-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-0063-3