ABSTRACT
We isolated JEUD-38, a new sesquiterpene lactone from Inula japonica Thunb. JEUD-38 dramatically attenuated lipopolysaccharide (LPS)-induced nitric oxide (NO) production. Consistent with this finding, the protein expression of inducible nitric oxide synthase (iNOS) was blocked by JEUD-38 in a concentration-dependent manner. To elucidate the mechanism, we examined the effect of JEUD-38 on LPS-stimulated nuclear factor-κB (NF-κB) nuclear translocation, inhibitory factor-κB (IκB) phosphorylation, and degradation. JEUD-38 reduced the translocation of p65, via abrogating IκB-α phosphorylation and degradation. In addition, JEUD-38 inhibited LPS-stimulated phosphorylation of mitogen-activated protein kinases (MAPKs) including extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), and p38. Since iNOS as well as the upstream NF-κB and MAPKs are known to be closely involved in inflammation, these results suggest that JEUD-38 is a promising candidate for prevention and therapy of inflammatory diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Chronic or acute inflammation is a multiple process mediated by activated inflammatory or immune cells [1, 2]. Macrophages play a key role in mediating many different immunopathological phenomena including the overproduction of pro-inflammatory cytokines and inflammatory mediators such as nitric oxide (NO), prostaglandin (PG) E2, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 [3, 4].
NO is a reactive radical molecule generated via the oxidative deamination of l-arginine by a family of NO synthases (NOS) [5]. Among them, inducible NOS (iNOS) is expressed in response to lipopolysaccharide (LPS) and various pro-inflammatory cytokines [6]. NO production has beneficial effects for host innate immune response to pathogens such as viruses, bacteria, fungi, helminthes, and protozoa [7, 8]. However, excessive NO production can be harmful to the host, resulting in various inflammatory diseases [9].
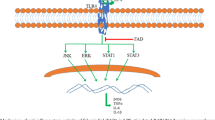
NF-κB is a ubiquitous transcription factor which plays a crucial role in immune and inflammatory responses through regulation of genes encoding pro-inflammatory cytokines, adhesion molecules, chemokines, and inducible enzymes such as cyclooxygenase-2 (COX-2) and iNOS [10–12]. In resting cells, NF-κB is associated with the inhibitor IκB in the cytoplasm. Many stimuli activate NF-κB, mostly through IκB kinase (IKK)-dependent phosphorylation and subsequent degradation of IκB proteins. Following activation, NF-κB dimmers dissociate from the inhibitor and enter the nucleus [13].
The mitogen-activated protein kinases (MAPK) are known to play a crucial role in mediating inflammatory responses [14]. The MAPK family consists of three well-characterized subfamilies including extracellular regulated kinases (ERK1/2), c-Jun N-terminal kinases (JNK), and p38 MAP kinases. MAPK pathway is involved in the up-regulation of iNOS and pro-inflammatory cytokines in murine macrophages [15, 16]. Therefore, NF-κB and MAPKs are known as important targets for anti-inflammatory therapy.
The flowers of Inula japonica Thunb (I.japonica) have been used in traditional Chinese medicine, owing to their activities of relieving phlegm, detumescence, peptic, vermifuge, and anti-inflammation [17]. In our previous studies, we demonstrated that the flower extract of I. japonica showed anti-inflammatory activities, such as alleviation of ovalbumin (OVA)-induced airway inflammation in murine model of asthma [18], suppression of mast cell-mediated allergic reaction and mast cell activation [19], and reduction of pro-inflammatory cytokines release and NO production in RAW264.7 cells [20].
JEUD-38, 1-oxo-4aH-eudesma-5 (6),11 (13)-dien-12,8β-olide, is a new sesquiterpene lactone isolated from I. japonica. In a preliminary experiment, we found that JEUD-38 inhibited NO production in RAW264.7 cells. To investigate the mechanism, we evaluated the effect on iNOS expression, NF-κB activation, and MAPK phosphorylation in LPS-stimulated RAW264.7 cells.
MATERIALS AND METHODS
Plant Material
JEUD-38 was isolated from the ethanol extract of I. japonica. The plants of I. japonica were collected from Henan Province, China, and identified by Professor Y. Zhou (Department of Pharmacognosy, School of Pharmacy, Tianjin Medical University). A voucher specimen (IJ201105) was deposited at the School of Pharmacy, Tianjin Medical University, China. Prior to use, JEUD-38 was dissolved in dimethyl sulfoxide (DMSO).
Extraction and Isolation
The powdered and dried flowers of I. japonica (8.0 kg) were extracted with 75 % ethanol (15 L × 3, 2 h each time) under reflux. The extracts were concentrated to give a residue (600 g), which was suspended in water and partitioned with petroleum ether (P.E.), ethyl acetate (EtOAc), and n-butyl alcohol (n-BuOH) successively.
The P.E.-partitioned extract (36 g) was chromatographed on a silica gel column (500 g, 300–400 mesh, 80 × 10 cm) and eluted with a gradient solvent system (P.E.-EtOAc, v/v, 6:1, 5:1, 4:1, 3:1, 2:1, 1:1, 1:2, with EtOAc of 2000 mL) to produce 39 fractions (fractions 1–39). The fraction 15 (2.1 g) was re-chromatographed on a silica gel column (50 g, 300–400 mesh, 60 × 3 cm), eluted with a gradient solvent system (CH2Cl2-MeOH, v/v, 99:1, 97:3, 95:5, 1:1, with MeOH of 200 mL) to produce 22 fractions (fractions 15.1–15.22). The fraction 15.4 (725.5 mg) was then separated by gel permeation chromatography (GPC) (2 × 50 cm × 2 cm, MeOH, flow rate 3 mL/min) to afford 12 fractions (fractions 15.4.1–15.4.12). Finally, the fraction 15.4.3 was separated by HPLC (ODS, 5 μm, 2 × 25 cm, MeOH-H2O, v/v, 9:1, flow rate 3 mL/min, Rt =22 min) to afford compound JEUD-38 (12.0 mg).
Reagents
The antibodies specific for iNOS, phospho-IκB-α, IκB-α, phospho-ERK1/2, ERK1/2, phospho-p38, p38, phospho-JNK, JNK, β-actin, and the horseradish peroxidase-conjugated goat anti-rabbit secondary antibody were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). The antibodies for p65 and lamin B were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). The enhanced chemiluminescence (ECL) Western blot detection reagent was from Thermo Fisher Scientific (Rockford, IL, USA). The bacterial LPS was purchased from Sigma-Aldrich (Louis, MO, USA).
Cell Culture
The RAW264.7 macrophage cells were obtained from the Korea Cell Line Bank (Seoul, Korea) and cultured in DMEM supplemented with 10 % FBS, 100 U/mL of penicillin, and 100 μg/mL of streptomycin.
Determination of Cell Viability
MTT assay was used to measure cell viability. RAW 264.7 cells were treated with different concentrations of JEUD-38 for 24 h. Then, MTT (5 mg/mL) was added and further incubated for 4 h. The culture medium was discarded and the formazan blue formed in the cells was resolved with DMSO. The absorbance at 490 nm was measured with a microplate absorbance reader (BIO-RAD iMark, Hercules, CA, USA).
Measurement of NO
RAW264.7 cells (2 × 105 cells/mL) were pre-treated with or without JEUD-38 for 1 h and stimulated with LPS (200 ng/mL) for 18 h. NO production was evaluated by measuring nitrite level in the culture media using Griess reagent (1 % sulfanilamide, 0.1 % N-1-naphthylenediamine dihydrochloride, and 2.5 % phosphoric acid). The signal was determined by measuring the absorbance at 570 nm with multi-mode microplate reader (Molecular Devices FilterMax F5, Sunnyvale, CA, USA). l-N6-(1-iminoethyl) lysine (l-NIL, a selective inhibitor of iNOS) was used as a positive control.
Extraction of Nuclear Protein
RAW264.7 cells pretreated with or without JEUD-38 or ammonium pyrrolidinedithiocarbonate (PDTC) for 1 h were incubated with LPS for 30 min. Then, the nuclear extracts were prepared as described in the manufacturer’s protocol (Panomics Nuclear Extraction Kit, Fremont, CA, USA).
SDS-PAGE/Immunoblot Analysis
SDS-PAGE/immunoblot analysis was performed as described by us previously [21]. Equal amounts of protein were electrophoresed on 10 % SDS-polyacrylamide gels and blotted onto a PVDF membrane. Membranes were blocked with 5 % non-fat dry milk in TTBS (20 mM Tris–HCl, 150 mM NaCl, and 0.05 % Tween-20) and probed with various primary antibodies. After overnight incubation with primary antibody followed by three times of washes, the membrane was hybridized with HRP-conjugated secondary antibody for 1 h and washed three times with TTBS. The protein bands were then visualized with an ECL system.
Statistical Analysis
Results are expressed as mean ± S.D. One-way analysis of variance (ANOVA) was utilized to determine the statistical significance.
RESULTS
Structure Elucidation of JEUD-38
JEUD-38 was isolated as a pale yellow solid, with the formula of C15H18O3 as determined by HR ESI-MS at m/z 269.1150 [M + Na]+, 1H-, and 13C-NMR spectral data. 13C NMR and DEPT spectra exhibited 15 carbons in the molecule, involving two methyls, four methylenes, four methines, and five quaternary carbons. The low field region of the 13C NMR spectrum revealed the presence of one carbonyl carbon [δ C 213.2 (C-1)], one carboxyl carbon [δ C 169.9 (C-12)], and four olefinic carbons [δ C 145.7 (C-5), 139.1 (C-11), 122.3 (C-13), 121.7 (C-6)]. Its 1H NMR spectral data (Table 1) exhibited two methyl proton signals [δ H 1.26 (3H, d, J =7.2 Hz, H-15), 1.39 (3H, s, H-14)], three olefinic proton signals [δ H 5.37 (1H, d, J =4.0 Hz, H-6), 5.68 (1H, d, J =1.6 Hz, H-13a), 6.25 (1H, d, J =1.6 Hz, H-13b)], and one oxygenated methines [δ H 4.86 (1H, m, H-8)]. Two double bonds and two carbonyl groups derived from 13C NMR analysis accounted for four of the seven degrees of unsaturation, thus implying a polycyclic nature for JEUD-38.
The 13C NMR and DEPT spectra of JEUD-38 showed that this compound had 15 carbons for a sesquiterpene-type compound (Table 1), with a structure very similar to the known compound 1β-hydroxyalantolactone [22], except for the ketone group at C-1 instead of a hydroxyl group in reported compound. The protons at δ1.39 (CH3-14), 2.25 (H-2a), 1.97 (H-3a) revealed HMBC correlations with the ketone group at δ213.2, indicating that the ketone group is positioned at C-1. Finally, the structure of JEUD-38 was elucidated as 1-oxo-4αH-eudesma-5 (6),11 (13)-dien-12,8β-olide (Fig. 1).
The Cytotoxicity of JEUD-38 in RAW264.7 Cells
Firstly, the cytotoxic effect of JEUD-38 (Fig. 2) was measured on RAW264.7 cells by MTT assay. Treatment of RAW264.7 cells with JEUD-38 for 24 h showed that cell viability was affected by 25 μM but was a little changed by 10 μM (Fig. 2). Therefore, concentrations of equal to or lower than 10 μM were selected for subsequent experiments.
Effect of JEUD-38 on LPS-induced NO Production and iNOS Protein Expression in RAW264.7 Cells
To investigate the effects of JEUD-38 on LPS-induced NO production in RAW264.7 cells, cells were pretreated with various concentrations of JEUD-38 for 1 h and then stimulated with LPS (200 ng/mL) for 18 h. Upon stimulation with LPS, NO production increased approximately 11-fold, but these increases were inhibited in a dose-dependent manner by JEUD-38 with IC50 as 2.56 μM which was calculated by the use of GraphPad Prism 4 (GraphPad software, San Diego, CA, USA) (Fig. 3a). l-NIL (10 μM), an NO production inhibitor [23], was used as a positive control. In addition, to investigate if the inhibition of NO production by JEUD-38 is associated with iNOS protein expression, Western blotting was performed. The expression level of iNOS was significantly up-regulated in RAW264.7 cells when exposed to LPS, and JEUD-38 markedly suppressed the increased iNOS protein level (Fig. 3b).
Effect of JEUD-38 on NO production and iNOS protein expression in LPS-stimulated RAW264.7 cells. RAW264.7 cells were pretreated with various concentrations of JEUD-38 for 1 h and then stimulated with 20 ng/mL of LPS for 18 h. a The amount of NO in the medium was measured using the Griess reagent. b iNOS protein expression levels were determined by Western blotting analysis. Data are shown as the mean ± S.D. of three different samples. ***P < 0.001, compared with LPS-induced cells in the absence of JEUD-38.
Effect of JEUD-38 on LPS-induced Nuclear Translocation of NF-κB p65, Degradation and Phosphorylation of IκBα in RAW264.7 Cells
NF-κB regulates numerous genes, particularly those involved in immune and inflammatory responses. NF-κB is retained in the cytoplasm in an inactive form through binding to IκB proteins. Diverse cell stimuli induce phosphorylation and subsequent proteasomal degradation of IκB, leading to NF-κB release from the cytoplasmic IκBα/NF-κB complex and translocation to the nucleus [24].
The p65 is the major subunit of NF-κB. The p65 levels in nuclear extract and in cytoplasm were evaluated respectively by Western blotting analysis. As shown in Fig. 4a, b, after treatment by JEUD-38, the p65 level in the nucleus was decreased while that in the cytoplasm was increased, suggesting that LPS-induced nuclear translocation of p65 was strongly inhibited in a dose-dependent manner. Lamin B and β-actin were used as internal controls. We also explored whether JEUD-38 inhibits the LPS-stimulated degradation of IκBα. As shown in Fig. 5, LPS-induced IκBα was suppressed by JEUD-38 pretreatment. To confirm that this IκBα degradation was associated with IκBα phosphorylation, we also examined the effect of JEUD-38 on LPS-induced p-IκBα. As shown in Fig. 5, IκBα phosphorylation was also significantly blocked in the presence of JEUD-38, consistent to the result for the IκBα degradation. As a positive control, we used PDTC which is a specific NF-κB inhibitor.
Effect of JEUD-38 on LPS-induced p65 nuclear translocation in RAW264.7 cells. RAW264.7 cells were pre-incubated with indicated concentrations of JEUD-38 for 1 h, followed by stimulation with LPS (200 ng/mL) for 30 min. The nuclear (a) and cytosolic (b) extracts were prepared in accordance with the manufacturer’s instructions and subjected to Western blotting analysis. As a positive control, a specific NF-κB inhibitor named PDTC was also applied. Data are expressed as the mean ± S.D. of three different samples. *P < 0.05, **P < 0.01, ***P < 0.001, compared with LPS-induced cells in the absence of JEUD-38.
Effect of JEUD-38 on LPS-induced degradation and phosphorylation of IκBα in RAW264.7 cells. RAW264.7 cells were stimulated with LPS in the presence or absence of JEUD-38 for 15 min (for detection of p-IκB) or 30 min (for detection of IκB). The phosphorylation and degradation of IκBα were determined by Western blotting analysis. Data are shown as the mean ± S.D. of three different samples. *P < 0.05, ***P < 0.001, compared with LPS-induced cells in the absence of JEUD-38.
Effect of JEUD-38 on LPS-Induced Activation of MAPK in RAW264.7 Cells
MAPK pathway activates a variety of transcription factors and coordinates induction of many genes encoding inflammatory mediators [25]. Signal proteins of this pathway such as ERK, JNK, and p38 are known to be closely involved in the expression of iNOS and NO production [12, 13, 15, 16, 26]. To investigate whether JEUD-38 inhibits the MAPK pathway, we tested the effects of JEUD-38 on LPS-induced phosphorylation of ERK, JNK, and p38 in RAW264.7 cells by Western blotting. As a result, the phosphorylation of ERK, JNK, and p38 were increased in cells treated with LPS alone. However, JEUD-38 inhibited phosphorylated ERK, JNK, and p38 levels in a dose-dependent manner (Fig. 6). PD98059, SP600125, and SB203580 were used as positive controls because they are known as ERK, JNK, and p38 pathway inhibitors, respectively [21, 27]. These findings suggest that phosphorylation of MAPKs might be involved in the JEUD-38-mediated suppression of LPS-induced iNOS expression.
Effects of JEUD-38 on LPS-induced MAPKs phosphorylation in RAW264.7 cells. RAW264.7 cells were pretreated with various concentrations of JEUD-38, 25 μM of SP600125 (JNK inhibitor), 30 μM of SB203580 (p38 inhibitor), or 50 μM of PD98059 (ERK inhibitor) for 1 h and then incubated for an additional 30 min with LPS (200 ng/mL). Cells were harvested, and the cell lysates were prepared. The phosphorylation of the MAPKs was analyzed by immunoblotting. All experiments were performed three times, and representative results are shown. Data are expressed as the mean ± S.D. of three different samples. **P < 0.01, ***P < 0.001, compared with LPS-induced cells in the absence of JEUD-38.
DISCUSSION
The pro-inflammatory mediator NO plays critical roles in inflammatory diseases [28]. NO is synthesized from l-arginine after the activation of NOS. There are three isoforms of the NOS, including neuronal NOS (nNOS), endothelial NOS (eNOS) which is constitutive, and iNOS which is produced by activated macrophages [29]. In this study, we demonstrated that JEUD-38 suppresses LPS-induced NO production and iNOS protein expression in mouse macrophage RAW264.7 cells with an IC50 of 2.56 μM (Fig. 3).
LPS stimulation induces inflammation through the activation of NF-κB and MAPK pathways [30, 31]. NF-κB plays a key role in controlling the expression of multiple inflammatory and immune genes and therefore leads to the development of inflammatory diseases including asthma, rheumatoid arthritis, type 1 diabetes, and Parkinson’s disease [10, 32]. NF-κB is located in the cytoplasm in a quiescent form bound to IκB [33]. In the majority of cells, NF-κB is composed of a p50 and p65 subunit. NF-κB translocates into the nuclei after the degradation of IκB. Therefore, the amount of NF-κB protein in nuclear extracts could authentically reflect the activation status of NF-κB [13]. In the present study, we found that the translocation of activated NF-κB p65 to the nucleus was reduced by JEUD-38 (Fig. 4), and the degradation and phosphorylation of IκBα were also inhibited in a concentration-dependent manner by JEUD-38 (Fig. 5). Activated ERK, JNK, and p38 have been postulated to play important roles in controlling iNOS gene expression [34, 35]. In this study, active ERK, JNK, and p38 were significantly inhibited by JEUD-38 (Fig. 6). These results suggest that JEUD-38 reduction of LPS-induced iNOS expression could occur through blocking NF-κB signaling and MAPK activation.
The flower of I. japonica is well known in traditional Chinese herbal medicine. It is often used to treat emesis, chronic bronchitis, and acute pleurisy in China (Pharmacopoeia Commission of People’s Rebublic of China, 2010). The extract of I. japonica has beneficial pharmacologic effects, including anti-diabetic and hypolipidemic effects [36, 37]. In the previous study, our group reported that I. japonica extract has anti-asthmatic and anti-inflammatory activities [18–20]. In this paper, we isolated and identified a new sesquiterpene lactone named JEUD-38 from I. japonica, which dramatically inhibited NO production in LPS-induced RAW264.7 cells.
In summary, the present study revealed the anti-inflammatory activities and the related mechanism of a new compound JEUD-38. We demonstrated that JEUD-38 inhibits LPS-induced NO production accompanied with down-regulation of iNOS protein expression in murine RAW264.7 macrophage cells. Moreover, JEUD-38 interferes the activation of NF-κB transcription factor by inhibiting IκBα phosphorylation and degradation. In addition, JEUD-38 also reduces MAPK activation. Based on these results, JEUD-38 might become a promising drug candidate for prevention and treatment of inflammatory diseases.
REFERENCES
Su, X., and H.J. Federoff. 2014. Immune responses in Parkinson's disease: Interplay between central and peripheral immune systems. BioMed Research International 275178.
Virdis, A., U. Dell'Agnello, and S. Taddei. 2014. Impact of inflammation on vascular disease in hypertension. Maturitas 78: 179–183.
Gordon, S. 2003. Alternative activation of macrophages. Nature Reviews. Immunology 3: 23–35.
Rim, H.K., W. Cho, S.H. Sung, and K.T. Lee. 2012. Nodakenin suppresses lipopolysaccharide-induced inflammatory responses in macrophage cells by inhibiting tumor necrosis factor receptor-associated factor 6 and nuclear factor-kappaB pathways and protects mice from lethal endotoxin shock. Journal of Pharmacology and Experimental Therapeutics 342: 654–664.
Wimalawansa, S.J. 2008. Nitric oxide: New evidence for novel therapeutic indications. Expert Opinion on Pharmacotherapy 9: 1935–1954.
Chesrown, S.E., J. Monnier, G. Visner, and H.S. Nick. 1994. Regulation of inducible nitric oxide synthase mRNA levels by LPS, INF-gamma, TGF-beta, and IL-10 in murine macrophage cell lines and rat peritoneal macrophages. Biochemical and Biophysical Research Communications 200: 126–134.
Bogdan, C., M. Rollinghoff, and A. Diefenbach. 2000. The role of nitric oxide in innate immunity. Immunological Reviews 173: 17–26.
MacMicking, J., Q.W. Xie, and C. Nathan. 1997. Nitric oxide and macrophage function. Annual Review of Immunology 15: 323–350.
Sharma, J.N., A. Al-Omran, and S.S. Parvathy. 2007. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 15: 252–259.
Barnes, P.J., and M. Karin. 1997. Nuclear factor-kappaB: A pivotal transcription factor in chronic inflammatory diseases. New England Journal of Medicine 336: 1066–1071.
Chen, F., V. Castranova, X. Shi, and L.M. Demers. 1999. New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clinical Chemistry 45: 7–17.
Baldwin Jr., A.S. 2001. Series introduction: the transcription factor NF-kappaB and human disease. Journal of Clinical Investigation 107: 3–6.
Luo, J.L., H. Kamata, and M. Karin. 2005. IKK/NF-kappaB signaling: Balancing life and death—a new approach to cancer therapy. Journal of Clinical Investigation 115: 2625–2632.
Brown, M.D., and D.B. Sacks. 2008. Compartmentalised MAPK pathways. Handbook of Experimental Pharmacology 205–235.
Chan, E.D., and D.W. Riches. 1998. Potential role of the JNK/SAPK signal transduction pathway in the induction of iNOS by TNF-alpha. Biochemical and Biophysical Research Communications 253: 790–796.
Ajizian, S.J., B.K. English, and E.A. Meals. 1999. Specific inhibitors of p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways block inducible nitric oxide synthase and tumor necrosis factor accumulation in murine macrophages stimulated with lipopolysaccharide and interferon-gamma. Journal of Infectious Diseases 179: 939–944.
Yang, C., C.M. Wang, and Z.J. Jia. 2003. Sesquiterpenes and other constituents from the aerial parts of Inula japonica. Planta Medica 69: 662–666.
Park, Y.N., Y.J. Lee, J.H. Choi, M. Jin, J.H. Yang, Y. Li, J. Lee, X. Li, K.J. Kim, J.K. Son, H.W. Chang, J.Y. Kim, and E. Lee. 2011. Alleviation of OVA-induced airway inflammation by flowers of Inula japonica in a murine model of asthma. Bioscience, Biotechnology, and Biochemistry 75: 871–876.
Lu, Y., Y. Li, M. Jin, J.H. Yang, X. Li, G.H. Chao, H.H. Park, Y.N. Park, J.K. Son, E. Lee, and H.W. Chang. 2012. Inula japonica extract inhibits mast cell-mediated allergic reaction and mast cell activation. Journal of Ethnopharmacology 143: 151–157.
Choi, J.H., Y.N. Park, Y. Li, M.H. Jin, J. Lee, Y. Lee, J.K. Son, H.W. Chang, and E. Lee. 2010. Flowers of Inula japonica attenuate inflammatory responses. Immune Network 10: 145–152.
Jin, M., Q. Zhou, E. Lee, S. Dan, H.Q. Duan, and D. Kong. 2014. AS252424, a PI3Kgamma inhibitor, downregulates inflammatory responsiveness in mouse bone marrow-derived mast cells. Inflammation 37: 1254–1260.
Bohlmann, F., P.K. Mahanta, J. Jakupovic, R.C. Rastogi, and A.A. Natu. 1978. New sesquiterpene lactones from Inula species. Phytochemistry 17: 1165–1172.
An, H.J., I.T. Kim, H.J. Park, H.M. Kim, J.H. Choi, and K.T. Lee. 2011. Tormentic acid, a triterpenoid saponin, isolated from Rosa rugosa, inhibited LPS-induced iNOS, COX-2, and TNF-alpha expression through inactivation of the nuclear factor-kappab pathway in RAW 264.7 macrophages. International Immunopharmacology 11: 504–510.
Lindstrom, T.M., and P.R. Bennett. 2005. The role of nuclear factor kappa B in human labour. Reproduction 130: 569–581.
Guha, M., and N. Mackman. 2001. LPS induction of gene expression in human monocytes. Cellular Signalling 13: 85–94.
Chan, E.D., and D.W. Riches. 2001. IFN-gamma + LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38 (mapk) in a mouse macrophage cell line. American Journal of Physiology. Cell Physiology 280: C441–C450.
Jin, M., S.J. Suh, J.H. Yang, Y. Lu, S.J. Kim, S. Kwon, T.H. Jo, J.W. Kim, Y.I. Park, G.W. Ahn, C.K. Lee, C.H. Kim, J.K. Son, K.H. Son, and H.W. Chang. 2010. Anti-inflammatory activity of bark of Dioscorea batatas DECNE through the inhibition of iNOS and COX-2 expressions in RAW264.7 cells via NF-kappaB and ERK1/2 inactivation. Food and Chemical Toxicology 48: 3073–3079.
Alderton, W.K., C.E. Cooper, and R.G. Knowles. 2001. Nitric oxide synthases: Structure, function and inhibition. Biochemical Journal 357: 593–615.
Andrew, P.J., and B. Mayer. 1999. Enzymatic function of nitric oxide synthases. Cardiovascular Research 43: 521–531.
Sanghera, J.S., S.L. Weinstein, M. Aluwalia, J. Girn, and S.L. Pelech. 1996. Activation of multiple proline-directed kinases by bacterial lipopolysaccharide in murine macrophages. Journal of Immunology 156: 4457–4465.
He, X., Z. Jing, and G. Cheng. 2014. MicroRNAs: New regulators of Toll-like receptor signalling pathways. BioMed Research International 945169.
Sun, X.F., and H. Zhang. 2007. NFKB and NFKBI polymorphisms in relation to susceptibility of tumour and other diseases. Histology and Histopathology 22: 1387–1398.
Baeuerle, P.A., and D. Baltimore. 1996. NF-kappa B: Ten years after. Cell 87: 13–20.
Murakami, A. 2009. Chemoprevention with phytochemicals targeting inducible nitric oxide synthase. Forum of Nutrition 61: 193–203.
Bhat, N.R., P. Zhang, J.C. Lee, and E.L. Hogan. 1998. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. Journal of Neuroscience 18: 1633–1641.
Shan, J.J., M. Yang, and J.W. Ren. 2006. Anti-diabetic and hypolipidemic effects of aqueous-extract from the flower of Inula japonica in alloxan-induced diabetic mice. Biological & Pharmaceutical Bulletin 29: 455–459.
Shan, J.J., Y. Zhang, Y.L. Diao, W.S. Qu, and X.N. Zhao. 2010. Effect of an antidiabetic polysaccharide from Inula japonica on constipation in normal and two models of experimental constipated mice. Phytotherapy Research 24: 1734–1738.
ACKNOWLEDGMENTS
This study was supported by a grant from the National Natural Science Foundation of China (81373441, 81202542, 81402802), the Natural Science Foundation of Tianjin (12JCZDJC25800, 13JCYBJC24800), the New Teachers’ Fund for Doctor Stations from Ministry of Education, China (20121202120009), the Project Sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, a grant from “211” project of Tianjin Medical University, and a grant from Japan Society for the Promotion of Science (FY2013, BR131302).
Conflict of Interest
There is no conflict of interest for all authors of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, X., Tang, SA., Wang, R. et al. Inhibitory Effects of JEUD-38, a New Sesquiterpene Lactone from Inula japonica Thunb, on LPS-Induced iNOS Expression in RAW264.7 Cells. Inflammation 38, 941–948 (2015). https://doi.org/10.1007/s10753-014-0056-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-0056-2