Abstract
Diatoms have been extensively used as paleolimnological indicators because they acutely respond to changes in their environment. Diatom assemblages recovered from sediment cores are a mixture of benthic and planktic assemblages that may have been transported away from their source environment or deposited near their habitat. Thus, there is an inherent variability in the diatom deposition across the sediments of a lake. With the aim of characterizing this variability and identifying how it may affect palaeoecological reconstructions, we identified diatom communities and assemblages from a series of sediment cores, surface sediment samples, and samples from different lake microenvironments (submerged macrophytes, sediments, marsh, meadow and attached algae). Comparing the sediment cores, we found differences in the timing of diatom assemblage shifts, which we attribute to differences in the diatom distribution in the sediments. Additionally, we identified gradients of diatom deposition where benthic and tychoplanktic diatoms dominate assemblages near shorelines and planktic assemblages dominate toward the lake center. We attribute benthic and tychoplanktic distribution to distance to the source and recognize that diatoms associated with modern microenvironments are underrepresented in the sediments because of their attachment to a substrate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When recovering a sediment core from a lake, a common practice is to collect a single core from the deepest part of a lake basin. It is assumed that these sediments will be the least disturbed, since shallow water cores are liable to be influenced by processes of erosion or episodes of non-deposition (hiatuses), particularly if substantial lake-level change (lowering) influences the basin. It is also assumed that the collection of deep-water cores will reflect lake-wide processes (Smol, 2002). On the other hand, if other lake processes are being reconstructed, such as changes in littoral habitat availability and diversity, taking cores from sediments near shore might be a better option. The use of diatoms as indicators of ecological change in paleolimnology has been extensive (Smol & Stoermer, 2010). Typically, diatom records from deep-water environments are dominated by planktic assemblages, except for benthic taxa-dominated acidic lakes. This allows for ecological reconstructions of lake-wide dynamics such as nutrient cycling and thermal stratification, which are important in the context of climate changes and aquatic resource resilience (Winder et al., 2009; DeNicola, 2000). Yet when a record is dominated by a high biomass of planktic organisms, benthic assemblages are necessarily underrepresented. This has implications for data analysis, as rare occurrences and low abundances (below the selected threshold; ~ 3%) generally are not included in the statistical analysis because they do not produce robust interpretations. This bias often limits the interpretation of other ecological characteristics of lakes, such as fluctuations in shallow-water microenvironments (i.e., substrate availability, macrophyte prevalence, flooding of lake margins, etc.) (Pla-Rabés & Catlan, 2018) and to some extent lake-level changes in response to hydroclimate, which use planktic/benthic ratios (Stone & Fritz, 2004; Wolin & Duthie, 1999). In most diatom-based reconstructions, it is assumed that the assemblages are a taphonomic mixture of different in-lake processes, local depositional processes, and communities associated with different habitats across the lake (Battarbee et al., 2002; Buchaca & Catlan, 2007). Pla-Rabés & Catlan (2018) addressed this assumption previously by characterizing diatoms from a variety of lake environments to refine potential ecological interpretations made from sediment records. In recent years, it has also been proposed that tychoplanktic assemblages (benthic or araphid diatoms suspended in the water column by mixing or turbulence) can be used to identify lake disturbances in response to landscape changes caused by human occupation (Velez et al., 2021). In this context, different diatom groups provide insights on a variety of ecological responses over the course of a lake’s history.
When assessing within-lake diatom deposition dynamics, there are several factors to be considered. Assemblages are composed of species with both benthic and planktic habitats, leading to an inherent spatio-temporal variability (Pla-Rabés et al., 2011). Additionally, benthic species may not be represented equally in the sediments, leading to an uneven preservation of the diatom assemblages that ultimately biases paleoenvironmental interpretations (Anderson & Battarbee, 1994). This limits palaeoecological analyses because more robust environmental reconstructions result from the most abundant species in the sediments (Pla-Rabés et al., 2011), which are commonly the planktic species. Yet, the importance of the benthic assemblage has been acknowledged, for example in studies that show ecological interpretations are more robust when the benthic diatoms are included in the reconstructions (Philibert & Prairie, 2002).
Diatom representation in the sediments can be linked to dispersion from the host habitat within the lake. It is generally understood that benthic and planktic diatoms have different dispersion patterns. Benthic diatoms are redistributed in the sediments as a function of turbulence and lake floor slope (Stone & Fritz, 2004), whereas planktic diatoms rain out into the sediments from the epilimnion, where they circulate by convective mixing. This depositional pattern creates a gradient from a dominance of benthic assemblages to a dominance of planktic assemblages as distance from the lake margin increases (Anderson, 1989). Additionally, Liu et al., (2013) found differences in the transport of benthic diatoms based on current intensity and how strongly attached the diatoms are to the substrate. In lakes, we interpret current intensity as exposure to wind and wave action, which effectively work in the same way as currents to transport attached diatoms. We infer that these differences play an important role in the diatom representation in the sediment record, which we hypothesize can be linked to substrate and attachment style.
In this paper, we aim to i) characterize the diatom deposition patterns in lake sediments ii) identify the driving mechanisms for diatom dispersion and deposition, and iii) analyze how diatom deposition patterns are recorded in sedimentary archives. We collected a series of sediment cores and surface sediment samples from Gull Lake, a small (0.32 km2) lake located at 2,332 m above sea level (m.a.s.l) with a maximum depth of ~ 19 m (Lyon et al., 2020) (Fig. 1A). The Gull Lake basin is characterized by a dominance in winter precipitation (~ 35 cm/year) originating from the Pacific Ocean, and drier summers (~ 1.5 cm/month) with occasional storms originating from the Gulf of California, where the prevailing wind direction is west-northwest (Lyon et al., 2020). The thermal mixing regime of Gull Lake has been defined as monomictic, with a mixing period after ice-off (Lyon et al., 2019). Both Gull and June Lakes are bordered by the June Lake Loop Road (CA-158 S) and highway 395, and fish restocking has occurred since the early nineteenth century (Lopera-Congote et al., 2024). Gull Lake (37°46′31.9"N 119°04′60.0"W) is located at the western edge of the Great Basin in the eastern Sierra Nevada of California, and the site is representative of glacial lakes in the region. The combination of its small size and heterogeneous surrounding benthic habitats (submerged macrophytes, wave-affected shorelines, flooded marsh, and fringing meadows) allows us to better constrain the variability in diatom communities and their transport and deposition into Gull Lake’s sediments. In a small lake, transport distances are short, and associations with the environment where the diatoms were originally produced can be more easily constrained. Additionally, the sediment record in this lake is dominated by planktic and tychoplanktic taxa (Lopera-Congote et al., 2024), which allows us to better characterize the differential preservation between these groups and the benthic taxa. Different microenvironments across the lake were sampled to identify variability among the assemblages recovered from sediments and the diatoms associated with microenvironments. We sought to use this information to identify patterns of diatom deposition in modern sediments to inform paleolimnological investigations in similar basin types.
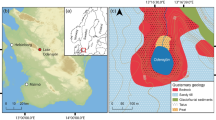
Map of study site and sample collection locations. (A) Location of study area in the Sierra Nevada, California. (B) Gull Lake and its outlet (Reversed Creek). Coring sites are denoted with circles (orange: GLCA-3A; red: GLCA-4A; green: GLCA-5A) and modern microenvironment samples are denoted by stars (blue: near-shore sediments; light yellow: meadow; dark yellow: marsh; green: submerged macrophytes). The tow net transect is denoted by a dashed line. (C) Bathymetric profile of Gull Lake (1-m intervals) showing sample collection location for each of the surface samples analyzed. 19 m depth and 9 m depth are shown in bold. Core collection sites are shown with hexagons that match their assigned colors
Methodology
Sample collection
Three short (30–50 cm) cores were collected from Gull Lake using a gravity corer. Cores GLCA-3A (47 cm; 37.77578, -119.08195), GLCA-4A (43 cm; 37.77649, -119.08107), and GLCA-5A (32 cm; 37.775607,—119.08507) were retrieved at water depths of 19.01 m, 19.53 m and 14 m, respectively (Fig. 1B). These cores capture the deepest part of the lake (depocenter) as well as a shallower area where the steep northern shore is dominated by submerged macrophytes, which were recovered in the grab samples taken from this section of the lake. For all the cores, the sediment–water interface was recovered and preserved using floral foam until ready for lab analysis. Cores were transported to the Continental Scientific Drilling Facility (CSD; University of Minnesota) where they were imaged, scanned, and subsampled at 0.5 cm intervals.
To aid in environmental interpretation and to characterize the modern environment, 41 lake floor “surface” samples were collected in a grid-like (~ 10 m between stations) pattern using a Peterson dredge (Table S1; Fig. 1C). Five tow net samples were collected in transects across the lake to characterize the modern living plankton community, and one of them was selected for analysis here (Fig. 1). Four modern microenvironments of the lake were sampled as well, to characterize the diatom assemblages associated with them. The meadow sample was taken by collecting a surface sediment sample from the flooded area. The marsh sample was taken by collecting surface sediments and organic debris (decaying plant matter) from the flooded area. The submerged macrophytes were collected using a Peterson dredge where these organisms were known to dominate the bottom of the lake. The shallow littoral samples were collected in three subsets. First emergent aquatic macrophytes were collected making sure the submerged part was collected in its entirety. Second, rocks exposed to wave action were scraped, collecting all the organic matter on the surface. Third, sand from the shoreline was collected, retrieving only the surface sediments. These samples were taken from the NW section of the east-facing shoreline (A) and SE-NW section of the east-facing shoreline (B) (Fig. 1B).
Age model
Bulk sediments from cores GLCA-3A and GLCA-4B were used for radioisotope dating. Visual correlation of stratigraphy and ages was used to determine the approximate age of the sediments in core GLCA-5A. 210Pb and 137Cs activities were measured on 0.5 g of sediments using alpha and gamma spectrometry according to Yeager et al. (2007) at the University of Kentucky (Supplementary information). The age models were generated using the R package Rplum (Blaauw et al., 2020). This Bayesian method assumes a constant rate of supply of unsupported 210Pb flux and assumes a constant amount of supported 210Pb.
Diatoms
Surface samples and core subsamples (0.5-cm intervals) were dried at 40 °C after which approximately 0.3 g were transferred to glass scintillation vials. Samples were processed with 30% H2O2 until the reaction stopped (Battarbee et al., 2002). A total of 236 core samples and 47 modern samples (surface samples and modern micro-environments) were analyzed.
An aliquot of each prepared sample was evaporated onto a coverslip and mounted with Naphrax for light microscopy analysis. Diatoms were identified using differential interference contrast (DIC) on a Leica DM2500 microscope at 1000 × magnification. Diatom identification was based on Lange-Bertalot & Krammer (1987), Krammer & Lange-Bertalot (1985), and Lange-Bertalot (2001). At least 400 diatom valves were counted per sample.
Scanning electron microscope (SEM) imaging was used to clarify the taxonomy of diatom species that could not be conclusively identified by light microscopy. A TESCAN Vega 3 SEM was used to perform these analyses at Indiana State University. Samples were evaporated onto aluminum stubs and coated with gold in a Denton Desk V at 50 amps for 75 s. For imaging, an accelerating voltage of 30 kV and a typical working distance between 5–10 mm were used.
Diatom ecological guilds
To refine ecological interpretations in the palaeoecological record, we propose analyzing the modern diatom distribution in the lake’s nearshore microenvironments and more broadly in the surface sediments with a focus on benthic diatoms. In this sense, diatoms can be categorized into ecological guild and life forms. Ecological guilds group diatoms based on attachment type and disturbance regime (Passy, 2007). Three benthic ecological guilds were defined: (1) low-profile diatoms include solitary adnate, prostrate and stalked diatoms that are adapted to low nutrient concentrations and high physical disturbance; (2) high-profile diatoms are large and tend to create filamentous or chain-forming colonies that thrive in nutrient-rich environments; and (3) motile diatoms are not anchored to a point on the substrate and are typically adapted to high disturbance environments where they are strong competitors for nutrients (Passy, 2007). Additionally, benthic diatoms can be classified according to their life form, which includes tube-forming, colonial, mobile, pedunculate, adnate, stalked, and pioneer species (characterized by their small size and fast colonization of substrates) (Berthon et al., 2011; Riato et al., 2017; Peszek et al., 2021). These ways of classifying the diatoms were chosen because they have been well established in the literature and allow for comparison with other studies. For the planktic assemblage, we have classified them into four guilds, according to their life form and attachment style: solitary, chain-forming, colonial and benthic attached to zooplankton (epizoic).
Statistical analysis
Diatom counts were converted to relative abundance prior to statistical analysis. To identify the zones of ecological change in the sediment cores, stratigraphically constrained cluster analysis (CONISS) was performed in R using the vegan (version 2.6–4) and rioja (version 1.0–6) packages, using the Bray–Curtis dissimilarity as the distance method (Oksanen et al., 2015; Juggins, 2020). Prior to all statistical analysis, diatom relative abundances were square root transformed to balance the dominant and less abundant species (Legendre & Gallagher, 2001). Taxa that had an abundance of less than 10% were plotted with exaggerated curves to clearly identify changes in abundances through time, and taxa with abundances lower than 3% in at least one sample were excluded from the analysis. The resulting dendrogram was plotted along with stratigraphic plots of diatom species relative abundance also using the rioja package. This was done to identify shifts in the diatom assemblages through time, as well as to identify the changes in dominance between benthic, tychoplanktic and planktic diatoms. A broken stick model was used to identify significant zones of ecological change inferred from the diatom composition.
To assess similarities between diatom assemblages found in the surface samples and lake microenvironments, a Bray–Curtis Dissimilarity Index was applied using the R package vegan (Oksanen et al., 2015). This analysis allows for identification of species that more commonly occur together. A multi-factor analysis (MFA) was performed using the R package FactoExtra (version 1.0.7) (Kassambara & Mundt, 2020) to aid in the identification of diatom assemblage associations among the lake microenvironments (sources) and the lake sediments. The categories included in the MFA were benthic, planktic and tychoplanktic diatom groups. Diatom distribution within the modern lake sediments was also assessed using their relative abundance (plotted as pie charts) and species richness on a map generated in Qgis 3.10.5. To identify distribution patterns of diatom assemblages, a principal components analysis (PCA) was performed on core sediment samples using the R package FactoExtra (Kassambara & Mundt, 2020).
Results
Age model
The GLCA-3A age-depth model reveals that 210Pb decreases exponentially with depth, reaching supported levels at 26 cm, after which the dates are modeled, resulting in higher uncertainty in the ages for this section. The base of the core dates to ~ 1730s CE (38 cm), with a sedimentation rate of 0.22 cm/yr (Figure S1). The GLCA-4A age-depth model reveals that 210Pb decreases exponentially with depth, reaching supported levels at 32 cm. The base of the core dates to ~ 1750s CE (39 cm), with a sedimentation rate of 0.22 cm/yr (Figure S2).
Sediment core diatom assemblages
GLCA-3A
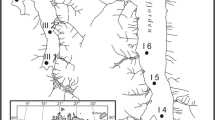
A total of 92 samples were analyzed for core GLCA-3A, with 82 diatom taxa identified. Of the total diatom assemblage, 18 diatom species were included in the stratigraphic analysis. The CONISS analysis (Fig. 2A) revealed three zones of ecological change. Zone 1 (ca. 1736 (30 cm)–1880 (26.5 cm) CE) is defined by the dominance of Stephanodiscus coruscus Stone and the presence of Stephanodiscus hantzschii Cleve & Grunow; in lower abundance, Lindavia ocellata (Pant.) Nakov, Guillory, Julius, Ther & Alverson is restricted to this zone. This zone is also defined by low abundances of Stephanodiscus minutulus (Kütz.) Round and Fragilaria crotonensis Kitton. Zone 2 (ca. 1886 (26 cm) –1943 (19.5 cm) CE) is characterized by the increase in abundance of S. minutulus and F. crotonensis. It is also defined by a decrease in the abundance of S. coruscus, leading to the disappearance of this species by the end of this zone. Less abundant taxa such as Stauroneis spp. Ehrenb. and Synedra cyclopum Brutschy are restricted to this zone. Pseudostaurosira parasitica (W.Sm.) E.Morales and Pseudostaurosira brevistriata (Grunow) D.M.Williams & Round appear for the first time during this zone in low abundances. Zone 3 (1945 (19 cm)–2021 (0 cm) CE) is characterized by Tabellaria flocculosa (Roth) Kütz, which is restricted to this zone, in high abundance. In lower abundance, this zone is defined by the presence of P. parasitica, P. robusta and P. brevistriata, as well as planktic species Lindavia intermedia (Manguin ex Kociolek and Reviers) Nakov et al. ex Daniels et al.
CONISS diatom stratigraphic analysis from three cores retrieved from Gull Lake. GLCA-3A has 3 zones of ecological change (Zone 1: 1736 (30 cm)–1880 (26.5 cm) CE; Zone 2: 1886 (26 cm) –1943 (19.5 cm) CE; Zone 3: 1945 (19 cm)–2021 (0 cm) CE). GLCA-4A has three zones of ecological change (Zone 1: 1756 (39.5 cm)–1908 (25.5 cm) CE; Zone 2: 1913 (25 cm)–1961 (17.5 cm) CE; Zone 3: 1964 (17 cm)–2021 (0 cm) CE). GLCA-5A has 2 zones of ecological change (Zone 1: 1813–1900 CE; Zone 2: 1913 (25 cm)–2021 (0 cm) CE). Red denotes planktonic diatoms, blue denotes tychoplanktic, and green denotes benthic (attached) diatoms
GLCA-4A
A total of 80 samples were analyzed for core GLCA-4A, with 114 diatom taxa identified. Of the total diatom assemblage, 18 diatom species were included in the stratigraphic analysis. The CONISS (Fig. 2B) analysis revealed three zones of ecological change. Zone 1 (ca. 1756 (39.5 cm)–1908 (25.5 cm) CE) is defined by the dominance of S. coruscus; L. ocellata is restricted to this zone, in low abundance, with a peak in abundance at the bottom of the core. Asterionella formosa Hassall also appears in its highest abundance in this zone. This zone is also characterized by lower relative abundances of S. minutulus and F. crotonensis. Zone 2 (1913 (25 cm)–1961 (17.5 cm) CE) is characterized by the increase in abundance of S. minutulus and F. crotonensis. It is also defined by a decrease in the abundance of S. coruscus, leading to the disappearance of this species by the end of this zone as well as the decrease in abundance of A. formosa. Peaks of Aulacoseira ambigua (Grunow) Simonsen and Aulacoseira granulata var. angustissima (O.Müll.) Simonsen are characteristic of this zone, as well as the appearance of P. brevistriata and Staurosirella martyi (Hérib.) E.Morales and Manoylov for the first time in the record. Zone 3 (1964 (17 cm)–2021 (0 cm) CE) is characterized by high abundances of T. flocculosa, which is restricted to this zone. P. parasitica and P. robusta are likewise restricted to this zone and are present in lower abundances, as is the planktic species L. intermedia.
GLCA-5A
A total of 63 samples were analyzed for core GLCA-4A, with 87 diatom taxa being identified. Of the total diatom assemblage, 16 diatom species were included in the stratigraphic analysis. The CONISS (Fig. 2C) analysis revealed two zones of ecological change. Zone 1 (1813 (31 cm)–1900 (26 cm) CE) is defined by the dominance of S. coruscus and L. ocellata; S. hantzschii is present through this zone in low abundance. A peak in Stauroneis spp. defines the end of this zone. Zone 2 (1913 (25 cm)–2021 (0 cm) CE) is characterized by the increase in abundance of S. minutulus, A. formosa and F. crotonensis. Nitzschia spp. and Stauroneis spp. peak in abundance at the beginning of the zone. Zone 2 is defined by the appearance of T. flocculosa, P. parasitica, P. brevistriata, P. robusta and S. pinnata. Lindavia intermedia is present in this zone but is restricted to the top of the core.
Modern diatom distribution
The diatom assemblage characterization of the modern lake floor sediment samples revealed considerable spatial variability in species distributions (Fig. 3A). In general, the samples near the northern and eastern shores of Gull Lake are dominated by P. brevistriata and P. parasitica, whereas samples along the southern shore show greater heterogeneity. Samples from the center of the lake are dominated by S. minutulus and T. flocculosa. The data revealed that sediment samples near the shores of Gull Lake have a higher diatom species richness (~ 37 species), whereas samples near the center of the lake are the least diverse (~ 12 species) (Fig. 3B).
(A) Diatom assemblage characterization for the modern sediment samples using the relative abundance of each species on each of the samples. (B) Species richness from the diatom assemblages characterized from the modern sediments. Bubble size and shade denote species richness binned values (12–17, 18–23, 24–19, 32–37)
The species identified in the surface sediments were classified into their corresponding ecological guilds (Table 1) and life form (Table 2), according to Passy, (2007) & Berthon et al., (2011), Riato et al., (2017) & Peszek et al., (2021), respectively.
The species identified in the surface sediments were classified into their corresponding ecological guilds (Table 1) and life form (Table 2), according to Passy, (2007) & Berthon et al., (2011), Riato et al., (2017) & Peszek et al., (2021), respectively.
PCA performed on the core sediment samples shows that Dimension 1 explains 28.2% of the variability in the data, while Dimension 2 explains 15.5% (Fig. 4A). Variability associated with Dimension 1 can be interpreted as changes in the dominance between planktic and benthic diatom assemblages. Here, the positive values represent the planktic/free floating assemblage, while negative values represent the benthic and tychoplanktic assemblages. There is a clear exception in the diatom clusters, where S. hantzschii, a planktic diatom, clusters with the benthic assemblage. This can be explained through the ecological preference of S. hantzschii, as it has been associated with near-shore environments exposed to wave action (Reavie & Kireta, 2015). Dimension 2 can be associated with diatoms and their sub-environments. Positive values represent assemblages associated with exposed (sub-aerial) and more unstable environments, while negative values represent assemblages that are associated with more stable environments, such as submerged macrophytes. The spatial variance of PC1 scores across the lake floor sediment samples of Gull Lake shows that lower values (reflecting the benthic assemblage) are distributed among the samples near the periphery of Gull Lake, while higher values (planktic assemblage) are associated with sediment samples found farther from the shores and near the center of the lake (Fig. 4B).
(A) PCA biplot of the diatom assemblages characterized from modern sediments. Three clusters were identified based on the diatom ecological preferences: cluster one is characterized by benthic and attached diatoms associated with submerged environments (macrophytes and algae), cluster two is characterized by a benthic and tychoplanktic assemblage associated with exposed habitats, and cluster three is characterized by the planktic assemblage. (B) Spatial representation of the PC1 scores where higher values (light blue) are associated with the planktic assemblage and lower values (dark blue) are associated with the benthic and tychoplanktic assemblage
The Bray–Curtiss Dissimilarity Index revealed two distinct clusters: one composed of the lake floor sediment sample assemblages (samples 1–38) and the other one composed of the microenvironment assemblages (marsh, meadow, submerged macrophytes, sediment, tow transect and algae) (Fig. 5A). The first cluster is characterized by T. flocculosa, P. brevistriata, P. parasitica, A. formosa, F. crotonensis and S. minutulus. Analysis of the second cluster allowed for the identification of distinct diatom species associated with each microenvironment. Aquatic macrophytes are characterized by Epithemia turgida (Ehrenb.) Kütz, sediments from location A are characterized by the abundance of Nitzschia soratensis Morales and Vis, epilithic algae (location A) is associated with Epithemia sorex Kütz. Additionally, sediments from location B are characterized by Karayevia clevei (Grunow) Bukht, while epilithic algae from the same location is characterized by Fragilaria vaucheriae (Kütz.) Petersen, and submerged macrophytes are dominated by Sellaphora atomoides (Grunow) Wetzel & Van de Vijve. The marsh is associated with Tabellaria flocculosa, and the meadow by Nanofrustulum cataractarum (Hust.) C.E.Wetzel, E.Morales & Ector. Finally, the plankton net sample is characterized by Synedra cyclopum. Despite the fact S. cyclopum is a benthic high-profile diatom, we consider it as plankton because this diatom lives attached to planktic copepods (Bahls, 2012). We assume that the dispersion of this diatom throughout the lake follows the same pattern as true planktic taxa.
(A) Bray–Curtiss Dissimilarity Index performed on the modern sediment and microenvironment assemblages. The cluster dendrogram shows the similarity among samples, and it distinguishes between the assemblages from the sediments and the microenvironments. The heatmap allows for the identification of the most abundant (red) and least abundant (yellow) species on each sample. (B) Multi-factor analysis (MFA) performed on modern sediments and microenvironment assemblages. Benthic diatoms are represented in green, planktic diatoms are represented in red and tychoplanktic diatom are represented in blue
The MFA (Fig. 5B) allowed for the identification of three clusters, one dominated by the planktic assemblage (red), clustered on the negative side of Dimension 1. Dimension 2 describes the variability in the planktic and tychoplanktic assemblages, where the true planktic/free floating diatoms are associated with positive values along this axis, while the tychoplanktic assemblage is associated with the negative values.
Discussion
The sedimentary diatom assemblages in cores GLCA-3A, GLCA-4A and GLCA-5A show that the transition from Zone 1 to Zone 2 is defined by the shift in abundance from S. coruscus to S. minutulus, and both species are categorized as solitary plankton (Table 1). Lopera-Congote and collaborators (2024) have suggested that this species shift is related to thermal stratification and disrupted nutrient cycling. In the transition from Zone 2 to Zone 3, the dominance of F. crotonensis, A. formosa and T. flocculosa are typically associated with increasing spring temperatures and increased allochthonous nutrient input into the lake (Saros et al., 2003, 2005; Hallstan et al., 2013). Additionally, this zone is characterized by an increase in tychoplanktic diatoms such as P. brevistriata, P. robusta and P. parasitica. This assemblage is characterized by a dominance in colonial planktic and tychoplanktic species (Table 1). Such assemblages have been related to ecosystem disturbance, in particular landscape changes related to human occupation, deforestation and changes in land use (Velez et al., 2021). The diatom assemblage reconstruction of three sediment cores from the same lake allowed us to take a first step in characterizing the inherent variability associated with recovered diatom assemblages. The most notable difference identified in the diatom assemblage reconstruction was that in core GLCA-5A only two zones of ecological change were identified, according to the broken stick model. Although similar patterns are observed in this record, like the increase in abundance of A. granulata and Stauroneis spp., the magnitude of change in this record is muted. We hypothesize that this might be because this core is located closest to the northern shore, where macrophytes cover most of the lake’s bottom. This might affect diatom deposition rates in the sediments, as aquatic macrophytes can act as sediment traps (Schulz et al., 2003). Alternatively, this difference could be attributed to the uneven distribution of the submerged macrophytes across the lake, resulting in a greater representation of the attached epiphytic diatoms that are associated with them (Fig. 2). Given that attached benthic diatoms are deposited by gravity (Stone & Fritz, 2004), they would not travel a great distance, resulting in the observed heterogeneity. When analyzing the diatom assemblage shift record, this might mean that the assemblages recovered were not a representation of the living diatoms at the time, but rather an assemblage resulting from the reworking of particles trapped by macrophyte mats (Horpila et al., 2005). Additionally, the three paleoenvironmental reconstructions show different timings for initiation of a new diatom ecological zone. As such, the identification of whole-lake ecological response may be biased by diatom assemblages recovered from different parts of the lake. For example, the transition from Zone 1 to Zone 2 is identified around 1880 for core GLCA-3A, 1908 for core GLCA-4A and 1900 for core GLCA-5A. The transition to Zone 3 happens around 1943 for core GLCA-3A and 1961 for core GLCA-4A, but it cannot be identified in core GLCA-5A. To further constrain the age differences between the transition zones in the cores, we accounted for the age uncertainties associated with the age models at those intervals, while also having into account the difference in age for the transition zones in each core (Table 3). Because the age difference we calculated is greater than the age uncertainties, we are able to rule out age uncertainties as the main factor driving the observed differences.
We speculate that the inherent variability in diatom deposition across Gull Lake’s sediments is the factor driving the differences observed. First, the characterization of the modern sediments (Fig. 3A) shows that diatom representation is variable across the lake. Additionally, the species richness assessment indicates that there are differences in the number of species represented in surface samples (Fig. 3B). Here, we identified a depositional pattern where sediments closer to the shore/benthic areas have a higher diversity than those farther away/closer to the center of the lake. These results support the pattern identified by Heggen et al. (2012), in which assemblages recovered from near-shore sediments are expected to be more variable and more diverse, while the assemblages from the deepest part of the lake are expected to present compositional stability but represent less taxa.
In this case, we identified the ecotone between planktic dominance and benthic/tychoplanktic assemblages. Our data show that according to the diatom assemblage distribution, the boundary between a benthic/tychoplanktic dominance and a planktic dominance occurs at ~ 9 m water depth. It is important to note that the maximum depth of Gull Lake is ~ 19 m. The identified threshold in the depth-diatom diversity relationship becomes a very important factor when considering core locations, if the benthic assemblage is the focus of the research. This approach would have the caveat mentioned above, that the samples will be more diverse and have a higher representation of the benthic taxa, but variability among samples might increase, causing discrepancies in the paleoenvironmental interpretation (Heggen et al., 2012). For a more thorough understanding of lake dynamics ranging across different ecological drivers, we suggest that analyzing several cores within the lake will yield the best results (Davis & Brubaker, 1973). This approach is also useful when the ecotone in diatom deposition can be used to reconstruct specific aspects of lake ecology such as lake-level response to drought (Laird et al., 2011). In this sense, a sediment core taken from the benthic-dominated zone would be most useful in reconstructing changes in lake’s near-shore habitats. A core from the planktic-dominated area would be most appropriate for reconstructing nutrient cycling and availability as well as changes in the lake’s physical and chemical conditions (i.e., increased thermal stratification and disruption of the nutrient cycling within the lake). Finally, a core taken for the identified ecotone would be a sensitive record that would better integrate the benthic and planktic assemblages, allowing for a more comprehensive environmental reconstruction.
Several factors can explain diatom frustule distribution in the subfossil assemblage. Firstly, distance to the shore is interpreted to be the primary control. It has been proposed previously that assemblages recovered from the sediment record have a high degree of connection to the original source, in this case the lake’s microenvironments, and the deposition site (Zhao et al., 2006). Here the slope of the lake bottom does not seem to alter this assumption, because benthic assemblages are found primarily near the lake borders where the slope is the highest. Further, these results support a gravity-driven sediment focusing model, as proposed by Stone & Fritz, (2004), where the plankton settles from the water column vertically, and the benthic/tychoplanktic assemblages settle near their habitats where they are living, with limited resuspension and transport. Additionally, our data are in accordance with the model proposed by Passy, (2001) (Table 1), where diatom transport is mainly mediated by currents, and diatom frustules travel smaller distances when currents are weak or absent.
In this study, we were able to characterize the diatom assemblages as local signals, meaning that long-distance transport by external forces (i.e., wind or wave action) can be ignored as a plausible mechanism. For instance, our data suggest that benthic diatoms are more dominant where there is more benthic substrate available (Fig. 4). Additionally, we also see a higher diatom species richness in these settings (Fig. 3B). The higher species richness and benthic representation on the eastern shoreline could be explained by wind transport in a W-E axis, but this would not explain why this pattern is also present in the NW shoreline of the lake, where the meadow and marsh benthic environments are present. Additionally, we expect that if wind was the primary transport mechanism, the diatom assemblages throughout the lake sediments would be homogenous, yet we see spatial variation and changes in the diatom composition (Fig. 3A) that do not follow a homogenization pattern,
Here, we infer that the diatom distribution in the sediments is a function of attachment style and availability of benthic substrates (Table 1; Table 2). Diatoms representative of each microenvironment are underrepresented in the assemblages recovered from the surface sediments (Fig. 5). This could be an effect arising from the nature of the samples, where the sediment samples are time-averaged, and the living samples represent a snapshot in time. Yet, the observed patterns in diatom deposition suggest that attachment style is an important factor to be taken into account. We attribute this to the fact that the diatoms representative of each microenvironment are classified as benthic and attached to a substrate (i.e., low-profile or stalked solitary diatoms) (Table 1). On the other hand, the cores taken from the deeper parts of the lake are dominated by planktic and tychoplanktic diatoms that are characterized as mainly colonial, free floating and high-profile (epizoic). Diatom assemblages recovered from the surface sediments record a gradient in dominance between diatom life forms, with planktic diatoms dominating toward the center of the lake and benthic/tychoplanktic assemblages being more abundant near the lake edges.
Differences in diatom deposition across the lake’s sediments is further supported by the PCA, where variation along PC1 allowed us to characterize the benthic/planktic depositional dynamics within the lake (Fig. 4), where benthic assemblages are more prominent within the 9 m depth threshold identified. The benthic assemblage is generally more diverse, a pattern that could be explained by the diversity that benthic areas harbor in relation to the different niches available (Moos et al., 2005). In this sense, heterogeneous environments near shore, where light penetration, wave action, nutrient availability, and substrate availability allow for the benthic (mainly attached) diatoms to thrive (Kingsbury et al., 2012; Tan et al., 2014). In contrast, the planktic environment is characterized by low light availability, so the diatoms in these areas are limited to those that can efficiently occupy the area of the water column with sufficient light penetration (Kingsbury et al., 2012). In this case, Gull Lake has a Secchi depth of 6 m, with a total depth of 19 m, meaning that a third of the water column has sufficient light penetration. These variations of the diatom assemblages across the lake explain the differences found in the cores we analyzed. These results support the idea that coring location can be an important factor to consider, having into account the target diatom assemblage and the lake processes being reconstructed (Davidson et al., 2005).
Further, our results suggest that the benthic assemblage found in the sediments is dissimilar to the diatom community sampled from modern lake environments (Fig. 5A). Additionally, the MFA allowed us to understand these patterns. Our results suggest that the distribution of the benthic assemblages is related to distance to the source, where transport is limited by energy in the system and attachment style. The clustering pattern in Fig. 5B supports this hypothesis, as diatoms with similar life form cluster together, meaning that life form determines the diatom representation in the sediments. In the case of the lake microenvironments, the diatoms found are representatives of the low-profile diatom guild, benthic and strongly attached to the substrate, meaning that they are adapted to limited resources and high disturbance stress (Passy, 2007). Because of their design, colonial tychoplankton can become fragmented and continue living as plankton, where they may be subjected to convective mixing in the epilimnion, similar to plankton, allowing them to be distributed more widely. In this regard, it is important to note that the characteristic species found in plankton net samples, S. cyclopum, is not well represented in either the modern sediment samples or the cores. This could be explained by the association with copepods (epizoic), and their highly seasonal nature. The copepod peak in abundance happens between May and June (Perhar et al., 2012), during the period when these samples were collected. Additionally, plankton nets concentrate samples across the whole transect, which means that a high biomass is not required for a high concentration of diatoms (McNabb, 1960). Our results build on previous research that has highlighted the variability and complexity of diatom deposition and the interpretation of sediment records. In general, our results suggest that substrate type, exposure to wind and waves, and association to lake environments are the main factors that determine the diatom distribution through the lake. Mainly, attachment type will determine how easily the diatoms get detached from their substrate, with wind and waves acting as abrasive agents that contribute to short distance transport and deposition. Likewise, the ability to relate diatom assemblages to a source habitat has been proven to aid in environmental change interpretations, where the benthic diatom assemblage is the focus (Reavie & Smol, 1997). Additionally, sediment resuspension, and in-lake processes that control this variable, have a significant effect on the interpretations made from the sediment core samples (Yang et al., 2009).
Conclusions
The results of this study have implications for paleoenvironmental reconstructions and interpretations. Mainly, even for a small glacial lake, there is a high spatial heterogeneity when it comes to diatom deposition and preservation. Our results suggest that variations in the diatom assemblages collected from the cores were controlled by two main processes: distance to the source and the diatom attachment style. These two factors combined determine the transport of the benthic assemblages from their source environments and into the lake’s sediments. This distribution pattern explains the offset observed between the sediment cores analyzed, making it clear that core site selection could greatly affect the paleolimnological interpretation because of a diatom distribution bias. It is important to highlight that we found considerable variability in the distribution of diatoms across Gull Lake’s sediments and microenvironments despite the small size of the basin. We expect that in larger lakes, this variability will be enhanced due to the presence of more microhabitats and increased spatial complexity induced by slope gradients, substrate texture, patterns of light penetration, and relative wave energy. In particular, we highlight the importance heterogeneous diatom assemblages have when trying to reconstruct various environmental variables. This is because different diatom assemblages are sensitive to specific lake processes (i.e., benthic substrate availability, water clarity, pH, nutrient status), and there are instances where a more planktic (i.e., nutrient availability/cycling) or a more benthic (i.e., lake level changes) focus might be needed. Additionally, we highlight that the modern benthic diatom communities are underrepresented in the paleo records as an effect of dispersal mechanisms.
Data Availability
Information about an individual Neotoma Dataset including the dataset ID is accessible in the URL: https://data.neotomadb.org/} GLCA-3A- 57858, GLCA-4A—57859, GLCA-5A—57860, Aquatic Macrophytes—57968, Sed A – 57969, Algae A – 57970, Sed B – 57971, Algae B – 57972.
References
Anderson, N. J., 1989. A whole-basin diatom accumulation rate for a small eutrophic lake in Northern Ireland and its palaeoecological implications. J Ecol 77: 926–946.
Anderson, N. J. & R. W. Battarbee, 1994. Aquatic community persistence and variability: a palaeolimnological perspective. In Giller, P. S., A. G. Hildrew & D. G. Raffaelli (eds), Aquatic ecology scale, patterns and process Blackwell Scientific Publications, Oxford: 233–259.
Battarbee, R. W., V. J. Jones, R. J. Flower, N. G. Cameron, H. Bennion, L. Carvalho & S. Juggins, 2002. Diatoms. In Smol, J. P., H. John, B. Birks, W. M. Last, R. S. Bradley & K. Alverson (eds), Tracking environmental change using lake sediments Kluwer Academic Publishers, Dordrecht: 155–202. https://doi.org/10.1007/0-306-47668-1_8.
Berthon, V., A. Bouchez & F. Rimet, 2011. Using diatom life-forms and ecological guilds to assess organic pollution and trophic level in rivers: a case study of rivers in south-eastern France. Hydrobiologia 673(1): 259–271.
Blaauw, M., Christen, J.A. & M.A Aquino-Lopez, 2020. rplum: Bayesian age-depth modelling of ‘210Pb’-dated cores. R package version 0.1. 4.
Buchaca, T. & J. Catalan, 2007. Factors influencing the variability of pigments in the surface sediments of mountain lakes. Freshw Biol 52: 1365–1379.
Davidson, T. A., C. D. Sayer, H. Bennion, C. David, N. Rose & M. P. Wade, 2005. A 250 year comparison of historical, macrofossil and pollen records of aquatic plants in a shallow lake. Freshw Biol 50: 1671–1686.
Davis, M. B. & L. B. Brubaker, 1973. Differential sedimentation of pollen grains in lakes Limnology. Oceanography 18: 635–646.
DeNicola, D. M., 2000. A review of diatoms found in highly acidic environments. Hydrobiologia 433: 111–122.
Hallstan, S., C. Trigal, K. S. Johansson & R. K. Johnson, 2013. The impact of climate on the geographical distribution of phytoplankton species in boreal lakes. Oecologia 173: 1625–1638.
Heggen, M. P., H. H. Birks, O. Heiri, J. A. Grytnes & H. J. B. Birks, 2012. Are fossil assemblages in a single sediment core from a small lake representative of total deposition of mite, chironomid, and plant macrofossil remains? J Paleolimnol 48: 669–691.
Horppila, J. & L. Nurminen, 2005. Effects of different macrophyte growth forms on sediment and P resuspension in a shallow lake. Hydrobiologia 545: 167–175.
Juggins, S., 2020. rioja: Analysis of Quaternary Science Data. R package version 0.9–26, https://cran.r-project.org/package=rioja
Kassambara, A. & Mundt, F., 2020. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. https://CRAN.R-project.org/package=factoextra
Kingsbury, M. V., K. R. Laird & B. F. Cumming, 2012. Consistent patterns in diatom assemblages and diversity measures across water-depth gradients from eight Boreal lakes from north-western Ontario (Canada). Freshw Biol 57: 1151–1165.
Krammer, K. & H. Lange-Bertalot, 1985. Naviculaceae: Neue und wenig bekannte taxa Neue Kombinationen und synonyme Sowie Bemerkungen zu einigen gattungen, J. Cramer:
Laird, K. R., M. V. Kingsbury, C. M. Lewis & B. F. Cumming, 2011. Diatom-inferred depth models in 8 Canadian boreal lakes: inferred changes in the benthic: planktonic depth boundary and implications for assessment of past droughts. Quat Sci Rev 30: 1201–1217.
Lange-Bertalot, H., 2001. Navicula sensu stricto, 10 genera separated from Navicula sensu lato, Frustulia. Diatoms Eur: Diatoms Eur Inl Waters Comp Habitat 2: 526.
Lange-Bertalot, H., and K. Krammer, 1987. 'Bacillariaceae', 'Epithemiaceae', 'Surirellaceae': Neue und wenig bekannte taxa, Neue Kombinationen und synonyme Sowie Bemerkungen und ergänzungen zu den 'Naviculaceae'. J. Cramer
Legendre, P. & E. D. Gallagher, 2001. Ecologically meaningful transformations for ordination of species data. Oecologia 129: 271–280.
Liu, J., J. Soininen, B. P. Han & S. A. Declerck, 2013. Effects of connectivity, dispersal directionality and functional traits on the metacommunity structure of river benthic diatoms. J Biogeogr 40: 2238–2248.
Lopera-Congote, L., M. M. McGlue, K. S. Westover, K. Yeager, L. Streib & J. R. Stone, 2024. Detecting climate-driven ecological changes in high-altitude lakes in the Sierra Nevada California. Holocene 34: 870.
Lyon, E. C., M. M. McGlue, E. W. Woolery, S. L. Kim, J. R. Stone & S. R. Zimmerman, 2019. Sublacustrine geomorphology and modern sedimentation in a glacial scour basin, June Lake, eastern Sierra Nevada, USA. J Sediment Res 89(10): 919–934.
Lyon, E. C., M. M. McGlue, A. M. Erhardt, S. L. Kim, J. R. Stone & S. R. Zimmerman, 2020. Late Holocene hydroclimate changes in the eastern Sierra Nevada revealed by a 4600-year paleoproduction record from June Lake. CA. Quat Sci Rev 242: 106432.
McNabb, C. D., 1960. Enumeration of freshwater phytoplankton concentrated on the membrane filter. Limnology and Oceanography 5: 57–61.
Moos, T.M., Laird, K.R. & Cumming, B.F., 2005. Diatom assemblages and water depth in Lake 239 (Experimental Lakes Area, Ontario): implications for paleoclimatic studies. Journal of Paleolimnology, 34: 217-227.
Oksanen, J., F. G. Blanchet, R. Kindt, P. Legendre, P. R. Minchin, R. B. O’hara, G. Simpson, P. Solymos, M. Stevens & H. J. W. Wagner, 2015. Vegan: community ecology package. R Packag Veg, Version 2: 2–1.
Passy, S. I., 2001. Spatial paradigms of lotic diatom distribution: a landscape ecology perspective. J Phycol 37: 370–378.
Passy, S. I., 2007. Diatom ecological guilds display distinct and predictable behavior along nutrient and disturbance gradients in running waters. Aquat Bot 86: 171–178.
Perhar, G., G. B. Arhonditsis & M. T. Brett, 2012. Modelling the role of highly unsaturated fatty acids in planktonic food web processes: a mechanistic approach. Environ Rev 20: 155–172.
Peszek, Ł, A. Zgrundo, T. Noga, N. Kochman-Kędziora, A. Poradowska, M. Rybak, C. Puchalski & J. Lee, 2021. The influence of drought on diatom assemblages in a temperate climate zone: A case study from the Carpathian Mountains. Poland Ecol Indic 125: 107579.
Philibert, A. & Y. T. Prairie, 2002. Is the introduction of benthic species necessary for open-water chemical reconstruction in diatom-based transfer functions? Canadian J Fisheries Aquat Sci 59: 938–951.
Pla-Rabes, S., R. J. Flower, E. M. Shilland & A. M. Kreiser, 2011. Assessing microbial diversity using recent lake sediments and estimations of spatio-temporal diversity. J Biogeogr 38: 2033–2040.
Pla-Rabés, S. & J. Catalan, 2018. Diatom species variation between lake habitats: implications for interpretation of paleolimnological records. J f Paleolimnol 60: 169–187.
Reavie, E. D. & A. R. Kireta, 2015. Centric Araphid and Eunotioid Diatoms of the Coastal Laurentian Great Lakes (Bibliotheca Diatomologica), Vol. 41. J. Cramer:
Riato, L., Della Bella, V., Leira, M., Taylor, J.C. & P. J. Oberholster, 2017. A diatom functional-based approach to assess changing environmental conditions in temporary depressional wetlands. Ecological indicators, 78: 205-213.
Rühland, K. M., A. M. Paterson & J. P. Smol, 2015. Lake diatom responses to warming reviewing the evidence. J Paleolimnol 54: 1–35.
Saros, J. E., S. J. Interlandi, A. P. Wolfe & D. R. Engstrom, 2003. Recent changes in the diatom community structure of lakes in the Beartooth Mountain Range, USA. Arct Antarct Alp Res 35: 18–23.
Saros, J. E., T. J. Michel, S. J. Interlandi & A. P. Wolfe, 2005. Resource requirements of Asterionella formosa and Fragilaria crotonensis in oligotrophic alpine lakes: implications for recent phytoplankton community reorganizations. Can J Fish Aquat Sci 62: 1681–1689.
Schulz, M., H. P. Kozerski, T. Pluntke & K. Rinke, 2003. The influence of macrophytes on sedimentation and nutrient retention in the lower River Spree (Germany). Water Res 37: 569–578.
Smol, J. P. & E. F. Stoermer, 2010. The diatoms: applications for the environmental and earth sciences, Cambridge University Press:
Stone, J. R. & S. C. Fritz, 2004. Three-dimensional modeling of lacustrine diatom habitat areas: Improving paleolimnological interpretation of planktic: benthic ratios. Limnol Oceanogr 49: 1540–1548.
Tan, X., P. Ma, X. Xia & Q. Zhang, 2014. Spatial pattern of benthic diatoms and water quality assessment using diatom indices in a subtropical river, China. Clean–soil Air, Water 42: 20–28.
Winder, M., J. E. Reuter & S. G. Schladow, 2009. Lake warming favours small-sized planktonic diatom species. Proc Royal Soc B 276: 427–435.
Wolin, J. A. & H. C. Duthie, 1999. Diatoms as indicators of water-level change. In Smol, J. & E. Stoermer (eds), The Diatoms. Cambridge University Press Cambridge, Cambridge.
Velez, M.I., Salgado, J., Brenner, M., Hooghiemstra, H., Escobar, J., Boom, A., Bird, B., Curtis, J.H., Temoltzin-Loranca, Y., Patino, L.F. & C. Gonzalez-Arango, 2021. Novel responses of diatoms in neotropical mountain lakes to indigenous and post-European occupation. Anthropocene, 34, p.100294.
Yang, H., Flower, R.J. & R. E. Battarbee, 2009. Influence of environmental and spatial variables on the distribution of surface sediment diatoms in an upland loch, Scotland. Acta Botanica Croatica, 68: 367–380.
Zhao, Y., C. D. Sayer, H. H. Birks, M. Hughes & S. M. Peglar, 2006. Spatial representation of aquatic vegetation by macrofossils and pollen in a small and shallow lake. J Paleolimnol 35: 335–435.
Acknowledgements
This work was partially funded by the National Science Foundation, grant # 1829093. We would like to thank Bailee Hodelka for supporting the field work and Adam Benfield, Susan Zimmerman and Sarah Ivory for the aid in core and sample collection during the field season (2021), and the Continental Scientific Drilling (CSD) Facility, University of Minnesota, for facilitating the initial core description and subsampling.
Funding
This study was funded by NSF, # 1829093, Michael M McGlue.
Author information
Authors and Affiliations
Contributions
LLC analyzed the diatom samples and wrote the manuscript, LLC, JRS and MMM designed the study, LLC and MMM collected the samples in the field, and KY provided the age model. All authors edited and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We declare no conflict of interests.
Additional information
Handling editor: Jasmine Saros
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lopera Congote, L., McGlue, M.M., Yeager, K.M. et al. Diatom spatial variations in Gull Lake (California) sediments: implications for improving paleolimnological interpretations in small glacial lakes. Hydrobiologia (2024). https://doi.org/10.1007/s10750-024-05670-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10750-024-05670-8