Abstract
Techniques to control the phototactic behaviour of fish have expanded with progress in LED lights. However, the phototaxis direction of fish could be reversible at some light intensities, and thus it is necessary to evaluate the light-intensity levels that will induce repulsion or attraction behaviour to understand the transition. This study determined the light intensities of white LED light required to induce repulsion or attraction behaviour from a dark place, and the degree of dark-adapted visual sensitivity in juvenile of two salmon species. Oncorhynchus keta smolts showed negative phototaxis under intense light intensity, but positive phototaxis was not observed. The range of light intensities under which O. masou exhibited positive phototaxis changed with the life stage (from pre-smolts to smolts). Notably, the light intensities that elicited positive phototaxis were relatively low and narrow for pre-smolts, whereas smolts responded to a greater range of intensities. Positive phototaxis disappeared in O. masou pre-smolts under intense light intensity, but not in O. masou smolts under the most-intense light intensity tested here. Negative phototaxis was not observed in O. masou pre-smolts or smolts. The appropriate light intensity indicated here may be used to either guide juvenile O. masou from the dam reservoir to fishways or bypass channels, or to repel O. keta smolts from the water intakes of agricultural diversion weirs or hydropower dams.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Instream barriers to fish migration include dams and weirs constructed for irrigation, hydropower generation or disaster prevention. These structures hinder the longitudinal movement of fish and negatively impact aquatic biodiversity and ecosystem functioning (Dudgeon et al., 2006; Endou et al., 2006; Fukushima et al., 2007; Vorosmarty et al., 2010). Fishways that re-route a portion of the water around the obstacle, if properly designed, allow migratory fish to proceed upstream to spawn (Hayashida et al., 2015; Shimoda et al., 2019; Masumoto et al., 2022). However, when moving downstream, fish might fail to find the fishway because the size of the intake and the volume of water extracted by the intake facility are far larger than that of the fish-pass infrastructure. Consequently, a large proportion of fish might pass directly through the turbines of a hydropower dam, resulting in injury or death, and such cumulative loss is especially critical for migratory species like salmon fry (Coutant & Whitney, 2000).
Two important fisheries species in Japan are chum salmon Oncorhynchus keta (Walbaum 1792) and masu salmon Oncorhynchus masou (Brevoort 1856) (Hyodo et al., 1992; Katano et al., 2016), which perform downstream migration as juveniles, feed and grow at sea, and then swim up their natal river to spawn (anadromous and semelparous). Fishermen’s cooperatives and other public organisations actively participate in releasing hatchery-reared O. keta and O. masou juveniles into local rivers (Fisheries Agency, 2008; Hasegawa, 2019). O. keta undergo smoltification and migrate downstream to the ocean about 6 months after hatching, whereas O. masou remain in rivers longer, undergoing smoltification and downstream migration about 18 months after hatching (Hosoya, 2015). Available data indicate that many O. masou juveniles stray into the intakes of agricultural weirs and are lost during their downstream migration (Hyodo et al., 1992; Yoneyama et al., 1998). Likewise, O. keta juveniles probably enter diversion weirs (Seki, 2013) or the intakes of hydropower dams (Ministry of Land, Infrastructure, Transport and Tourism, 2022), though less information is available for this species.
Physical barriers have been used to direct fish movements (Gale et al., 2008; Mussen et al., 2015; Swanson et al., 2004; Walters et al., 2012), but they are largely limited to small-scale structures and are not practical around large-scale water intakes on rivers. Furthermore, physical barriers require substantial onsite engineering and frequent maintenance to unclog debris. Non-physical barriers are an alternative management approach for protecting valuable fish stocks or deterring biological invasions. In particular, non-physical barriers tested for their capacity to alter fish behaviour include electrical, visual, acoustic, chemical and hydrological deterrence, used in isolation or combinations (Jesus et al., 2021; Noatch & Suski, 2012; Wilkes et al., 2018). Frequencies of 150 and 300 Hz were effective in acoustically deterring O. keta juveniles (Matsuda, 2021). Practical techniques to guide O. keta and O. masou juveniles to secure fishways or bypasses in conjunction with this acoustic repelling technique are required.
One successful example used light to guide fish: juvenile Atlantic salmon Salmo salar (Linnaeus 1758) were attracted to mercury vapour lamps at the bypass near the intake of a dam reservoir (Tetard et al., 2019). Juvenile O. keta and O. masou exhibit positive phototaxis (Hoar et al., 1957; Funaki, 1996), and they move downstream mainly at night (Chapman et al., 2013; Hasegawa, 2019; Seki et al., 1981). Therefore, the use of LED floodlights in the field to attract and guide the fish from the dam reservoir to a fishway or bypass channel merits consideration. Conversely, O. masou showed repulsion behaviour towards light of a certain intensity (Hyodo et al., 1993); therefore, the use of LED floodlights in the field might repel the fish to prevent them from straying into the intakes of agricultural weirs or hydropower dams. Powerful LED floodlights are now available and are relatively inexpensive (see https://www.yanmar.com/media/news/2019/04/25065200/lb202_lb20_202003.pdf); they are also easy to install, have a long life and require little maintenance. In a controlled experiment, Hansen et al. (2019) found that chinook salmon Oncorhynchus tshawytscha (Walbaum 1792) fry were attracted to white LED lights at night.
However, it remains to be determined how much light intensity is appropriate to illuminate the surface of the water to attract or repel juveniles of O. keta and O. masou. Too much light has been shown to be a repellent stimulus for salmon fry (Hoar et al., 1957; Nemeth & Anderson, 1992); therefore, it is important to determine the range of light intensity under which the target fish exhibit positive or negative phototaxis. Repulsion or attraction behaviour of fish in a dark place has been induced using various light intensities (including strobe lights) (Hoar et al., 1957; Hyodo et al., 1993; Nemeth & Anderson, 1992; Funaki, 1996; Mussen et al., 2014; Hansen et al., 2018, 2019; Ford et al., 2019; Tetard et al., 2019). Furthermore, the behaviour of fish in response to artificial lighting in a dark place has been shown to change with their developmental stage (Elvidge et al., 2019). To date, no studies have evaluated the light intensities that induce repulsion or attraction behaviour in a fish species in relation to the life stage. The primary objective of this study was to determine the light intensity required for inducing repulsion or attraction behaviour in a dark place (by behavioral approach) and the degree of dark-adapted visual sensitivity (by electrophysiological approach), and to understand any potential ontogenetic changes in behaviour in juveniles of O. keta and O. masou. I hypothesised that they will show positive phototaxis (Funaki, 1996; Hansen et al., 2019; Hoar et al., 1957; Tetard et al., 2019) towards moderate light intensities for them, but negative phototaxis (Hoar et al., 1957; Hyodo et al., 1993; Nemeth & Anderson, 1992) under a high light intensities for them.
Materials and methods
All experiments were conducted at the Fisheries Technology Institute, Nikko Field Station (FTINFS), National Research and Development Agency, Japan Fisheries Research and Education Agency (FRA). All experimental fish were kept in an environment with natural daylight, but with fluorescent lights (1.58–556 lx at the water surface) left on during the day for the keeper’s work, and under conditions of natural day length. The O. masou smolts (1 +) were reared outdoors from ~ 11 months after hatching. The O. keta were a wild strain from the Ishikari River in Hokkaido, and the O. masou were a hatchery strain originating from the Shiribetsu River in Hokkaido. O. keta eggs were obtained from the Fisheries Stock Assessment Center, Salmon Research Department of FRA, in autumn, and reared at ~ 10 °C. Oncorhynchus masou cultured at the FTINFS facility were used in this study. The fish used in experiments were O. keta smolts, O. masou pre-smolts (0 +) and O. masou smolts (1 +) (Table 1). All O. keta and O. masou smolts (1 +) used in this study had clearly begun smoltification. Because smoltification of O. keta begins 2 months after hatching (Kaeriyama, 1986), it would have been difficult to use small pre-smolts in the experiments; therefore, only smolts were used. Following the trials, fish were euthanised with an anaesthetic overdose of 0.5% phenoxyethanol, and the weight and standard length of each individual were measured. All animals used in this study were handled in accordance with the ethical guidelines established by the FRA.
Assessment of fish behaviour in response to white LED lights
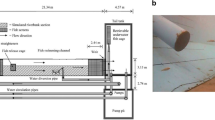
An indoor raceway tank (major axis 5 m, minor axis 1.6 m; tank depth 0.7 m, water depth 0.13 m) was used for the experiments (Fig. 1). Two submersible pumps were placed in the raceway tank to produce a constant flow and simulate rheophilic conditions. The raceway tank was surrounded with black plastic sheets to prevent incident light.
Four white LED devices (LRF-80/60W500G-USB; NS Lighting Co., Ltd, Osaka, Japan) were affixed to a transparent acrylic board (300 × 200 × 3 mm) fastened by a clip at one end of the major axis of the raceway tank, at a height of 0.7 m from the bottom of the tank (Fig. 1). The white LED was a combination of blue LED (peak of 446 nm) and yttrium aluminium garnet phosphor material, which is the most popular type of white LED. The intensity of the white LED devices was remotely controlled from outside the black plastic sheets using a USB LED controller (NSUBP4; NS Lighting Co., Ltd). Illumination was 0 lx (lx) at the base of the raceway tank without the white LED devices. The area in the range of < 1 m from the white LED devices was designated as the observation arena, and a camera (WAT-910HX/RC; Watec Co., Ltd, Japan) with infrared floodlights (S20D-IR, wavelength 850nm, Scene Electronics (HK) Co. Ltd, China) was set up above the raceway tank to record the position of fish in the arena throughout the experiments (Fig. 1). Spring-fed water from an upwelling at the FTINFS facility flowed into the raceway tank at a rate of ~ 0.6 l s−1; the water temperature during all experiments ranged from 9.5 to 10.4 °C. All trials were run between 07:00 a.m. and 10:30 a.m.
Ten fish were run in one trial, and a total of 10 trials were run in each test fish group, so 100 fish were used in each test fish group. On the day before the trial, 10 fish were released into the raceway tank by 11:00 a.m., to allow the fish to acclimatise. A few minutes after the fish were released into the raceway tank, the ceiling fluorescent lights (~ 200 lx at the water surface) were turned off and the fish were kept under darkness until 07:30 a.m. the next day. Therefore, the experiment started under conditions of an approximately 6-h longer dark environment than the normal rearing environment. It is possible that this may have affected the behaviour of the fish; however, since the fish were transferred from the rearing tank to the raceway tank the day before the experiment, it seemed that the longer acclimation period would be sufficient to allow the fish to settle in their new environment. Trials were run by increasing the light intensity of the white LED devices in six grades, in durations of 30 min at each grade, as follows: control: light intensity = dark → grade 1: 11.83 log photons cm–2 s–1 = 7.05 lx → grade 2: 12.48 log photons cm–2 s–1 = 31.7 lx → grade 3: 13.98 log photons cm–2 s–1 = 101 lx → grade 4: 14.59 log photons cm–2 s–1 = 418 lx → grade 5: 15.01 log photons cm–2 s–1 = 1 077 lx → grade 6: 15.60 log photons cm–2 s–1 = 4 217 lx.
The light intensity was measured at the bottom of the raceway tank in the area just below where the white LED devices were mounted. Light intensity was measured with a miniature fibre-optic spectrometer (USB4000; Ocean Optics, Florida, USA). This spectrometer was calibrated using a calibration light source (DH2000-CAL; Ocean Optics, Florida, USA). However, the weakest light intensity of grade 1 could not be measured by the USB4000 spectrometer; therefore, in that case, the light intensity of ‘lx’ was measured using a lux meter (LX-1108; Mother Tool Co., Ltd, Ueda, Japan), and the light intensity of ‘log photons cm–2 s–1’ was subsequently calculated by the relational expression (lx versus photons cm–2 s–1) with grade 2, since units of light can be mutually converted when the kind of light source is known (Thimijan & Heins, 1983). To maintain the 0.7-m distance between the bottom of the tank and the white LED devices, the position of the LED lights was adjusted at the time the measurement was taken with the USB4000 sensor.
The first 10 min at each light intensity was defined as an acclimation period, and the phototactic behaviour of the fish during the latter 20 min under each light intensity was analysed. The number of fish that were within the arena (i.e. within 1-m distance from the white LED devices) was counted at 30-s intervals by freezing the video images. The number of fish that responded phototactically was then summed across all 40 counts to obtain a total value for all light treatments.
Assessment of fish visual sensitivity
These experiments were conducted in a lightproof room, and the recording electrodes were covered by a case to exclude any noise during the electroretinogram (ERG). The methodology was based on the following papers (Frank, 2003; Horodysky et al., 2008, 2010; Matsuda & Wilder, 2013, 2014). Fish were anesthetised by submersion in a solution of 2-phenoxyethanol (0.5 ml l–1); they were then bound with a thin lead plate on the experimental table and perfused with freshwater (~ 10 °C) and 2-phenoxyethanol (0.25 ml l–1), with the room temperature maintained at 20 °C. The recording electrode (platinum wire, 0.3-mm diameter) was inserted into the cornea so that it rested on the retina. The top 3 mm of the recording electrode was bent manually into a U-shape to prevent damage to the retina. A reference electrode (platinum wire, 0.3-mm diameter) was placed under skin on the cranium, and the water bath was grounded with a silver wire (0.5-mm diameter). The recording electrode was positioned using a manual micromanipulator (MM-3; Narishige Group, Tokyo, Japan). Fish were left on the experimental table for 1 h to adapt to the dark conditions prior to testing. ERG responses to light stimuli were amplified with a bio-amplifier (AVB-11A; Nihon Kohden, Tokyo, Japan), displayed on an oscilloscope (VC-11; Nihon Kohden, Tokyo, Japan), digitised on an A/D PC Card (REX-5054U; Ratoc Systems, Tokyo, Japan), and finally stored on a computer. The two filter settings of the amplifier were low-cut (1.5 Hz) and high-cut (1 kHz).
Visual sensitivity was determined by exposing the dark-adapted eye to white-light flashes of varying irradiance for 100 ms, and then measuring the magnitude of the ERG in the 10 specimens tested. The peak b-wave to baseline magnitudes were measured for each stimulus response. White LED light (HLV2-22SW-3W; CCS Inc., Kyoto, Japan) was used as the lighting device. As mentioned, the white LED used was a combination of blue LED (peak of 442 nm) and yttrium aluminium garnet phosphor material, and was the same white LED used for the devices in the behavioural experiments. Flash duration was controlled using a Uniblitz electronic shutter (Vincent Associates, NY, USA). Light intensity was measured with the USB4000 miniature fibre-optic spectrometer.
To ensure that the eye remained in the same state of dark adaptation during the experiment, the response to a flash of white LED light with standard light intensity was tested periodically throughout the experiment. If the response to test flashes was variable for a given preparation, no further experimentation was conducted and the previous experimental data on that animal were not used in the analysis.
The data collected were normalised against the peak response (Vmax) for each trial, and the mean stimulus response data were plotted on a semi-logarithmic graph to generate V/log I curves. These curves were fitted using the Zettler modification of the Naka–Rushton equation, which describes the light-intensity response function of photoreceptors by using a least-squares fit (Naka & Rushton, 1966a, 1966b; Zettler, 1969), as follows: V/Vmax = Im/(Im + Km), where I = stimulus light intensity, V = response amplitude at light intensity I, Vmax = maximum response amplitude, m = slope of the linear part of the V/log I curve, and K = stimulus light intensity eliciting half the maximum response (half of the Vmax). The K value was used as an indicator of relative sensitivity. The dynamic range, defined as the log light-intensity range of 5% to 95% Vmax, was also calculated for each individual fish tested. The dynamic range is the range of light intensities over which the photoreceptor will operate, and it is directly related to the slope of the V/log I curve, with steeper slopes indicating smaller dynamic ranges (Frank, 2003). These procedures are commonly used to characterise the luminous sensitivity and operational range of the visual system (Frank, 2003; Horodysky et al., 2008, 2010; Matsuda & Wilder, 2013, 2014). All experiments were conducted between 07:00 a.m. and 05:00 p.m.
Statistical analysis
Counts of fish that were within the observation arena (within 1 m of the white LED devices: hereafter referred to as ‘close to’ the white LED devices) at each light intensity were converted to relative values (the number in trials at each light intensity/the number in the control), and the relative values among different light intensities were assessed using a Kruskal–Wallis test. Slope m, the K value, dynamic range, and 5% Vmax and 95% Vmax of the V/log I curves among the O. keta smolts, O. masou pre-smolts (0 +) and O. masou smolts (1 +) were assessed using a Kruskal–Wallis test. Multiple comparisons among significant values were assessed using a Steel–Dwass test. Results were considered significant at p < 0.05. All statistical tests were performed using BellCurve for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan).
Results
Fish behaviour in response to white LED lights
The total number of fish that were observed close to the white LED devices (i.e., within the arena) in each trial under each light intensity is presented by box-and-whisker plots for each group in Fig. 2a–c. Relative values (the number in trials at each light intensity/the number in the control) are presented by box-and-whisker plots for each group in Fig. 2d–f.
The total numbers of a O. keta smolts, b O. masou pre-smolts (0 +) and c O. masou smolts (1 +) that were observed close to the white LED devices (i.e. in the observation arena: see Fig. 1) during the control (dark) and under each light intensity for each trial (trials were run by increasing the light intensity of the white LED devices in six grades in durations of 30 min each). Relative values (the number in trials at each light intensity/the number in the control) are presented for d O. keta smolts, e O. masou pre-smolts (0 +) and f O. masou smolts (1 +). *,** Significantly different between groups at p < 0.05 and p < 0.01, respectively
There was a significant difference in the relative values of O. keta smolts close to the white LED devices among the different light intensities (Online Resource 1, combination 1). The relative values of O. keta smolts close to the white LED devices were lower at a light intensity of 15.01 log photons cm–2 s–1 (1 077 lx) or 15.60 log photons cm–2 s–1 (4 217 lx) when compared with the control (dark) (Fig. 2d; Online Resource 1, combination 6,7). Thus, the light intensities of 15.01 and 15.60 log photons cm–2 s–1 were defined as eliciting negative phototaxis because the relative values were significantly lower than that of the control. The relative values of O. keta smolts close to the white LED devices were lower with a light intensity of 15.60 log photons cm–2 s–1 (4 217 lx) compared with a light intensity of 11.83 log photons cm–2 s–1 (7.05 lx) (Fig. 2d; Online Resource 1, combination 12).
The relative values of O. masou pre-smolts (0 +) close to the white LED devices differed significantly among the trials at different light intensities (Online Resource 1, combination 23). The relative values of O. masou pre-smolts (0 +) close to the white LED devices were greater with a light intensity of 11.83 log photons cm–2 s–1 (7.05 lx) or 12.48 log photons cm–2 s–1 (31.7 lx) compared with the control (dark) (Fig. 2e; Online Resource 1, combination 24,25). The light intensities of 11.83 and 12.48 log photons cm–2 s–1 were defined as eliciting positive phototaxis because the relative values of the fish were significantly higher than that of the control. The relative values of O. masou pre-smolts (0 +) close to the white LED devices were lower with a light intensity of 14.59 log photons cm–2 s–1 (418 lx) than with a light intensity of 11.83 log photons cm–2 s–1 (7.05 lx) or 12.48 log photons cm–2 s–1 (31.7 lx) (Fig. 2e; Online Resource 1, combination 32,36). Under the light intensity of 14.59 log photons cm–2 s–1, at which the relative values were significantly lower than the light intensities at which the fish exhibited positive phototaxis, the fish’s positive phototaxis was considered to have disappeared.
The relative values of O. masou smolts (1 +) close to the white LED devices differed significantly among the different light intensities (Online Resource 1, combination 45). The relative values of O. masou smolts (1 +) close to the white LED devices were higher with light intensities of 12.48 log photons cm–2 s–1 (31.7 lx), 13.98 log photons cm–2 s–1 (101 lx), 14.59 log photons cm–2 s–1 (418 lx) and 15.01 log photons cm–2 s–1 (1 077 lx), compared with the control (dark) (Fig. 2f; Online Resource 1, combination 47–50). The light intensities of 12.48, 13.98, 14.59 and 15.01 log photons cm–2 s–1 were defined as eliciting positive phototaxis because the relative values of the fish were significantly higher than that of the control. There were no significant differences between the relative values of O. masou smolts (1 +) close to the white LED at the maximum light intensity of 15.60 log photons cm–2 s–1 (4 217 lx) and the relative values under the other light intensities tested (Fig. 2f; Online Resource 1, combination 51, 56, 60, 63, 65, 66).
Analysis of visual sensitivity
V/log I curves for dark-adapted specimens (Fig. 3) were modelled using the Naka–Rushton equation, and the practical values were fitted using the Zettler modification of the equation, which describes the light-intensity response function of photoreceptors. Parameters derived from the model-fit that quantified the visual sensitivity of O. keta smolts, O. masou pre-smolts (0 +) and O. masou smolts (1 +) were the slope m (0.31 rad, 0.26 rad and 0.23 rad, respectively) and the K value (14.2 log photons cm–2 s–1, 13.5 log photons cm–2 s–1 and 13.4 log photons cm–2 s–1, respectively). The dynamic ranges were 4.67 (equivalent to 12.03 to 16.70 log photons cm–2 s–1) in O. keta smolts, 5.36 (equivalent to 11.09–16.45 log photons cm–2 s–1) in O. masou pre-smolts and 6.11 (equivalent to 10.68–16.79 log photons cm–2 s–1) in O. masou smolts (Fig. 3). There were no significant differences in slope m (Kruskal–Wallis statistic = 2.0, df = 2, p = 0.367), K value (Kruskal–Wallis statistic = 5.4, df = 2, p = 0.067), dynamic range (Kruskal–Wallis statistic = 2.0, df = 2, p = 0.371), 5% Vmax (Kruskal–Wallis statistic = 3.5, df = 2, p = 0.175) and 95% Vmax (Kruskal–Wallis statistic = 3.1, df = 2, p = 0.213) among the O. keta smolts, O. masou pre-smolts and O. masou smolts. The ranges of light intensities under which the fish showed repulsion, attraction or other behaviour in response to white LED light (first experiment) are depicted in V/log I curves in Fig. 3.
Average dark-adapted light intensity/response functions in a O. keta smolts, b O. masou pre-smolts (0 +) and c O. masou smolts (1 +). Circles represent mean values of V/Vmax for 10 specimens. Lines represent fits to the Naka–Rushton equation. Vertical bars indicate standard error. Shaded boxes indicate the dynamic range (5% to 95% Vmax), with the numbers designating its breadth (in log units). Dashed vertical lines and adjacent number indicate the K point (illumination at 50% Vmax); m = slope of curve (rad). The red line with red arrow in panel a shows the lower bound of light intensity under which O. keta smolts showed negative phototaxis (result of fish behaviour in response to white LED lights). The green line with green arrow in panel b shows the lower bound of light intensity under which positive phototaxis disappeared in O. masou pre-smolts (0 +) (result of fish behaviour in response to white LED lights). The blue boxes with blue stippling in panels b and c show the range of light intensities under which each specimen showed positive phototaxis (result of fish behaviour in response to white LED lights)
Discussion
When the dark-adapted eyes of fish experience light, the eyes will change to a light-adapted state depending on the exposure time, and their visual sensitivity will also change (Vetter et al., 2019). Therefore, the eyes of each fish used in my test of their behaviour under the white LED devices would change from a dark-adapted to light-adapted state with proximity to the white LED lights. The V/log I curves obtained in my analysis of visual sensitivity show dark-adapted visual sensitivity—a state approximating their visual sensitivity at night. There were no statistical differences among V/log I curves (in slope m, K value and dynamic range) for the O. keta smolts, O. masou pre-smolts (0 +) and O. masou smolts (1 +). Therefore, it seems unlikely that the rearing environment of the test fish had a significant effect on their visual sensitivity.
This work is the first to uncover a relation between light intensity (to induce repulsion or attraction behaviour) and dark-adapted visual sensitivity in juvenile salmon. The most notable discovery was that the range of light intensities of white LED under which the fish exhibited positive phototaxis changed with the developmental stage of O. masou (from 0 + pre-smolts to 1 + smolts), and the light intensities that elicited positive phototaxis were relatively low and narrow for pre-smolts, whereas smolts responded to a greater range of intensities. The light-intensity range under which O. masou pre-smolts (0 +) showed positive phototaxis was < 10-times the K value, which is within a relatively narrow range of light intensity (11.83–12.48 log photons cm–2 s–1 [7.05–31.7 lx]), denoting a rather dark environment for them, judging from the V/log I curve (Fig. 3b). However, at > 14.59 log photons cm–2 s–1 (418 lx) (> 10-times the K value; Fig. 3b), the O. masou pre-smolts (0 +) lost positive phototaxis and could not be attracted. In contrast, O. masou smolts (1 +) showed positive phototaxis under a wide range of light intensities (12.48–15.01 log photons cm–2 s–1 [31.7–1 077 lx]) including the K value, indicating that O. masou smolts exhibit a keen visual sense in this range, judging from the V/log I curve (Fig. 3c). At the maximum light intensity tested in the experiment (15.60 log photons cm–2 s–1 [4 217 lx]), the smolts did not lose positive phototaxis nor show negative phototaxis. Habitat cover is important for juvenile salmon in rivers, as it provides shelter from predators and protection from changes in streamflow (Inoue & Nakano, 1998; Shirvell, 1990). Furthermore, juvenile coho salmon Oncorhynchus kisutch (Walbaum, 1792) and rainbow trout Oncorhynchus mykiss (Walbaum, 1792) preferred locations in a river with reduced light intensities (Shirvell, 1990). This may explain why river-dwelling O. masou pre-smolts (0 +) may show positive phototaxis to rather dark light intensities judging from their visual sensitivity, whereas O. masou smolts (1 +), which migrate downstream to the open ocean (where cover is scarce), may prefer bright light intensities under which they can see the most and better evade predators. The present results coincide with assorted results from previous behaviour experiments that examined the effect of light on the movements of O. masou. Funaki (1996) reported that O. masou pre-smolts and smolts both showed positive phototaxis, but the author did not specify the light-intensity level. Hyodo et al. (1993) reported that O. masou (0 +) moved to a darker area in the experimental tank when the light intensity near the water surface exceeded 500 lx, which is in agreement with the present results. The cause of mixed results for O. masou might be attributable to different growth stages between studies (O. masou 0 + or 1 +) as well as to different light intensities used in the experiments. A light intensity of 12.48 log photons cm–2 s–1 (31.7 lx) near the water surface seems the best option for attracting both pre-smolts and smolts of O. masou at night. In addition, it may be possible to use different light intensities to selectively induce the phototactic attraction of O. masou pre-smolts or smolts.
Positive phototaxis by O. keta smolts was not observed in this study; however, Hoar et al. (1957) reported that O. keta fry show positive phototaxis before smoltification. Therefore, positive phototaxis of O. keta likely weakens with the developmental stage, and consequently, it might be difficult to use artificial lighting at night to attract smolts in the field. This study demonstrated that O. keta smolts show negative phototaxis when the light intensity near the water surface exceeds 15.01 log photons cm–2 s–1 (1 077 lx) (about 10-times the K value; Fig. 3a); this result suggests that white LED devices targeted at O. keta smolts could be used for a repellent effect during their migration downstream. When salmon exhibit positive phototaxis and are free-swimming, positive phototaxis seems to persist in a constant light environment over a long period of time (Tabor et al., 2017). Therefore, it was assumed that O. keta smolts exhibited negative phototaxis due to changes in light intensity. The light intensity applied to near the water surface needs to be practically established by actually introducing white LED floodlights in the field—to either guide juvenile O. masou from the dam reservoir to fishways or bypass channels, or to repel O. keta smolts from the water intakes of agricultural diversion weirs or hydropower dams; eventually, this knowledge could become standard. For O. keta smolts, a combination of white LED devices at the water surface (> 15.01 log photons cm–2 s–1 [1 077 lx]) and sound (150–300 Hz tone) (Matsuda, 2021) is expected to have a stronger repellent effect. Confirmation of the repellent effect of sound on juvenile O. masou is still needed.
Hansen et al. (2019) tested LED light devices with various colours at night to repel salmon and found that the repulsion effect differed according to the colour. Maximum spectral sensitivity in the rods of Pacific salmon smolts is in the range of 504–531 nm, with less sensitivity at longer wavelengths (Novales-Flamarique, 2005). Therefore, the reason that the repulsion effect would differ by colour may be not only because of different wavelengths, but also because of differences in visual sensitivity to various wavelengths among salmon species. Future work should concentrate on manipulating various light intensities of various wavelengths and then evaluate the fish behaviour at the given developmental stage, as well as their visual sensitivity to the various wavelengths.
Bright lights induced a “torpor-like” state or a sensory distraction that led to a reduced escape response in salmon (Novales-Flamarique et al., 2006). In this study, the light-intensity level was gradually increased in six grades from darkness. Therefore, I cannot exclude the possibility that the previous light-intensity grade affected the behaviour of the fish when it was changed to the next light-intensity grade. However, because the fish swam freely in the raceway tank, the light-intensity level received by their eyes would vary greatly depending on the distance from the LED devices, regardless of the light-intensity grade. Therefore, during the experiment, the light-intensity level taken in by the fish’s eyes was not necessarily decided only by the light-intensity grade. It is possible that the fish’s eyes became accustomed to the strong light-intensity level as they approached the white LED devices, even if the white LED devices were set to a weak grade. Conversely, even when the white LED devices were set to the maximum grade, the light intensity received by their eyes would have become weaker as the fish moved away from the LED devices. Therefore, in this study, the fish behaviour in response to the light source was in the context of their free behaviour under a certain light-intensity level.
Conclusions
To date, no study has evaluated the light intensities that induce repulsion or attraction behaviour in a fish species in relation to the life stage. In this study, it was revealed that the range of light intensities of white LED under which the fish exhibited positive phototaxis changed with the developmental stage of O. masou (from 0 + pre-smolts to 1 + smolts), and the light intensities that elicited positive phototaxis were relatively low and narrow for pre-smolts, whereas smolts responded to a greater range of intensities. Positive phototaxis disappeared in O. masou pre-smolts under intense light intensity, but not in O. masou smolts under the most-intense light intensity tested here. Negative phototaxis was not observed in O. masou pre-smolts or smolts. O. keta smolts showed negative phototaxis under intense light intensity, but positive phototaxis was not observed. The light intensity applied to near the water surface needs to be practically established by actually introducing white LED floodlights in the field—to either guide juvenile O. masou from the dam reservoir to fishways or bypass channels, or to repel O. keta smolts from the water intakes of agricultural diversion weirs or hydropower dams; eventually, this knowledge could become standard.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Chapman, E. D., A. R. Hearn, C. J. Michel, A. J. Ammann, S. T. Lindley, M. J. Thomas, P. T. Sandstrom, G. P. Singer, M. L. Peterson, R. B. MacFarlane & A. P. Klimley, 2013. Diel movements of out-migrating Chinook salmon (Oncorhynchus tshawytscha) and steelhead trout (Oncorhynchus mykiss) smolts in the Sacramento/San Joaquin watershed. Environmental Biology of Fishes 96: 273–286.
Coutant, C. C. & R. R. Whitney, 2000. Fish behavior in relation to passage through hydropower turbines: a review. Transactions of the American Fisheries Society 129: 351–380.
Dudgeon, D., A. H. Arthington, M. O. Gessner, Z. I. Kawabata, D. J. Knowler, C. Leveque, R. J. Naiman, A. H. Prieur-Richard, D. Soto, M. L. J. Stiassny & C. A. Sullivan, 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews 81: 163–182.
Elvidge, C. K., C. H. Reid, M. I. Ford, M. Sills, P. H. Patrick, D. Gibson, S. Backhouse & S. J. Cooke, 2019. Ontogeny of light avoidance in juvenile lake sturgeon. Journal of Applied Ichthyology 35: 202–209.
Endou, S., J. Tsuboi & T. Iwata, 2006. Effects of damming on the persistence of white-spotted charr and red-spotted masu salmon populations. Japanese Journal of Conservation Ecology 11: 4–12.
Fisheries Agency, 2008. Release manual of the freshwater fish [online]. http://www.jfa.maff.go.jp/j/enoki/naisuimeninfo.html [last accessed 4 August 2022]. [in Japanese]
Ford, M. I., C. K. Elvidge, P. H. Patrick, M. Sills & S. J. Cooke, 2019. Coloured LED light as a potential behavioural guidance tool for age 0 and 2 year walleye Sander vitreus. Journal of Fish Biology 95: 1249–1256.
Frank, T. M., 2003. Effects of light adaptation on the temporal resolution of deep-sea crustaceans. Integrative and Comparative Biology 43: 559–570.
Fukushima, M., S. Kameyama, M. Kaneko, K. Nakao & E. A. Steel, 2007. Modelling the effects of dams on freshwater fish distributions in Hokkaido, Japan. Freshwater Biology 52: 1511–1524.
Funaki, J., 1996. Research on the diversion of migrating-down cherry salmon in a dam reservoir. Monthly Report, Civil Engineering Research Institute 522: 103–107.
Gale, S. B., A. V. Zale & C. G. Clancy, 2008. Effectiveness of fish screens to prevent entrainment of westslope cutthroat trout into irrigation canals. North American Journal of Fisheries Management 28: 1541–1553.
Hansen, M. J., D. E. Cocherell, S. J. Cooke, P. H. Patrick, M. Sills & N. A. Fangue, 2018. Behavioural guidance of Chinook salmon smolts: the variable effects of LED spectral wavelength and strobing frequency. Conservation Physiology 6: 1–12.
Hansen, M. J., A. E. Steel, D. E. Cocherell, P. H. Patrick, M. Sills, S. J. Cooke, K. J. Carr, M. L. Kavvas & N. A. Fangue, 2019. Experimental evaluation of the effect of a light-emitting diode device on Chinook salmon smolt entrainment in a simulated river. Hydrobiologia 841: 191–203.
Hasegawa, K., 2019. The time downstream migration behavior of juvenile chum salmon Oncorhynchus keta becomes active. Salmon Information 13: 14–19. http://salmon.fra.affrc.go.jp/kankobutu/srr/srr013_p14-19.pdf [last accessed 4 August 2022]. [in Japanese]
Hayashida, K., K. Watanabe, H. Mizuno, M. Hayashi, T. Miyazaki, H. Mogi, H. Nii & H. Mayama, 2015. Evaluation of the long-term impact of dam and fishway construction on fish inhabitation. Advances in River Engineering 21: 43–48.
Hoar, W. S., M. H. A. Keenleyside & R. G. Goodall, 1957. Reactions of juvenile Pacific salmon to light. Journal of the Fisheries Research Board of Canada 14: 815–830.
Horodysky, A. Z., R. W. Brill, E. J. Warrant, J. A. Musick & R. J. Latour, 2008. Comparative visual function in five sciaenid fishes inhabiting Chesapeake Bay. Journal of Experimental Biology 211: 3601–3612.
Horodysky, A. Z., R. W. Brill, E. J. Warrant, J. A. Musick & R. J. Latour, 2010. Comparative visual function in four piscivorous fishes inhabiting Chesapeake Bay. Journal of Experimental Biology 213: 1751–1761.
Hosoya, K., 2015. Freshwater fishes of Japan, Yama-kei Publishers Co., Ltd, Tokyo:, 232–274.
Hyodo, N., Y. Seki, K. Tsukamoto, M. Oya & H. Okubo, 1992. Entrainment of masu salmon through agricultural diversion in the Kaji River. Report of Niigata Prefectural Inland Water Fisheries Experimental Station 18: 21–30.
Hyodo, N., M. Hoshino & H. Okubo, 1993. Negative phototaxis used to discourage juvenile masu salmon, Oncorhynchus masou, from being entrained by water diversions of agriculture. Report of Niigata Prefectural Inland Water Fisheries Experimental Station 19: 1–7.
Inoue, M. & S. Nakano, 1998. Effects of woody debris on the habitat of juvenile masu salmon (Oncorhynchus masou) in northern Japanese streams. Freshwater Biology 40: 1–16.
Jesus, J., R. Cortes & A. Teixeira, 2021. Acoustic and light selective behavioral guidance systems for freshwater Fish. Water 13: 745.
Kaeriyama, M., 1986. Ecological study on early life of the chum salmon, Oncorhynchus keta (Walbaum). Scientific Reports of the Hokkaido Salmon Hatchery 40: 31–92.
Katano, O., H. Hakoyama & S. S. Matsuzaki, 2016. Japanese inland fisheries and aquaculture: Status and trends. In Craig, J. F. (ed), Freshwater fisheries ecology Wiley-Blackwell, Chichester: 231–240.
Masumoto, T., M. Nakai, T. Asaeda & M. Rahman, 2022. Effectiveness of new rock-ramp fishway at Miyanaka intake dam compared with existing large and small stair-type fishways. Water 14: 1991.
Matsuda, K., 2021. A comparison of avoidance to acoustic stimuli in fish with different auditory capabilities: juvenile chum salmon (Oncorhynchus keta) and common carp (Cyprinus carpio). Journal of Fish Biology 98: 1459–1464.
Matsuda, K. & M. N. Wilder, 2013. Differences in sensitivity and temporal resolution between juveniles and sub-adults of the whiteleg shrimp Litopenaeus vannamei. Fisheries Science 79: 55–57.
Matsuda, K. & M. N. Wilder, 2014. Eye structure and function in the giant freshwater prawn Macrobrachium rosenbergii. Fisheries Science 80: 531–541.
Ministry of Land, Infrastructure, Transport and Tourism (2022) The 33rd conference. https://www.hrr.mlit.go.jp/shinano/shinanogawa_info/mizukan/giji_r03.html [last accessed 12 January 2022]. [in Japanese]
Mussen, T. D., O. Patton, D. Cocherell, A. Ercan, H. Bandeh, M. L. Kavvas, J. J. Cech & N. A. Fangue, 2014. Can behavioral fish-guidance devices protect juvenile Chinook salmon (Oncorhynchus tshawytscha) from entrainment into unscreened water-diversion pipes? Canadian Journal of Fisheries and Aquatic Sciences 71: 1209–1219.
Mussen, T. D., D. E. Cocherell, O. Patton, D. Jauregui, A. Ercan, H. Bandeh, D. Meier, S. Thomas, M. L. Kavvas, J. J. Cech & N. A. Fangue, 2015. Modified water diversion structures can behaviourally deter juvenile chinook salmon from entrainment. Transactions of the American Fisheries Society 144: 1070–1080.
Naka, K. I. & W. A. H. Rushton, 1966a. S-potentials from colour units in the retina of fish (Cyprinidae). Journal of Physiology 185: 536–555.
Naka, K. I. & W. A. H. Rushton, 1966b. S-potentials from luminosity units in the retina of fish (Cyprinidae). Journal of Physiology 185: 587–599.
Nemeth, R. S. & J. J. Anderson, 1992. Response of juvenile coho and chinook salmon to strobe and mercury vapor lights. North American Journal of Fisheries Management 12: 684–692.
Noatch, M. R. & C. D. Suski, 2012. Non-physical barriers to deter fish movements. Environmental Reviews 20: 71–82.
Novales-Flamarique, I., 2005. Temporal shifts in visual pigment absorbance in the retina of Pacific salmon. Journal of Comparative Physiology A 191: 37–49.
Novales-Flamarique, I., S. Hiebert & J. Sechrist, 2006. Visual performance and ocular system structure of Kokanee and Sockeye salmon following strobe light exposure. North American Journal of Fisheries Management 26: 453–459.
Seki, J., 2013. Development of hatchery techniques for releasing juvenile chum salmon in Japan. Journal of Fisheries Technology 6: 69–82.
Seki, Y., F. Kaneko & Y. Tominaga, 1981. The migration of artificially incubated smolt transformed masu salmon in Uono-gawa. Report of Niigata Prefectural Inland Water Fisheries Experimental Station 9: 10–15.
Shimoda, K., H. Urabe & H. Kawamula, 2019. Recovery process of masu salmon stock after the construction of fish ways. Nippon Suisan Gakkai Shi 85: 305–313.
Shirvell, C. S., 1990. Role of instream rootwads as juvenile coho salmon (Oncorhynchus kisutch) and steelhead trout (O. mykiss) cover habitat under varying streamflows. Canadian Journal of Fisheries and Aquatic Sciences 47: 852–861.
Swanson, C., P. S. Young & J. J. Cech, 2004. Swimming in two-vector flows: performance and behavior of juvenile Chinook salmon near a simulated screened water diversion. Transactions of the American Fisheries Society 133: 265–278.
Tabor, R. A., A. T. C. Bell, D. W. Lantz, C. N. Gregersen, H. B. Berge & D. K. Hawkins, 2017. Phototaxic behavior of subyearling Salmonids in the Nearshore area of two urban lakes in western Washington State. Transactions of the American Fisheries Society 146: 753–761.
Tetard, S., A. Maire, M. Lemaire, E. De Oliveira, P. Martin & D. Courret, 2019. Behaviour of Atlantic salmon smolts approaching a bypass under light and dark conditions: importance of fish development. Ecological Engineering 131: 39–52.
Thimijan, R. W. & R. D. Heins, 1983. Photometric, radiometric, and quantum light units of measure: a review of procedures for interconversion. Horticultural Science 18: 818–822.
Vetter, B. J., L. S. Rogers & A. F. Mensinger, 2019. The effect of light stimuli on dark-adapted visual sensitivity in invasive silver carp Hypophthalmichthys molitrix and bighead carp H. nobilis. Journal of Fish Biology 95: 256–262.
Vorosmarty, C. J., P. B. McIntyre, M. O. Gessner, D. Dudgeon, A. Prusevich, P. Green, S. Glidden, S. E. Bunn, C. A. Sullivan, C. R. Liermann & P. M. Davies, 2010. Global threats to human water security and river biodiversity. Nature 467: 555.
Walters, A. W., D. M. Holzer, J. R. Faulkner, C. D. Warren, P. D. Murphy & M. M. McClure, 2012. Quantifying cumulative entrainment effects for chinook salmon in a heavily irrigated watershed. Transactions of the American Fisheries Society 141: 1180–1190.
Wilkes, M. A., M. Mckenzie & J. A. Webb, 2018. Fish passage design for sustainable hydropower in the temperate Southern Hemisphere: an evidence review. Reviews in Fish Biology and Fisheries 28: 117–135.
Yoneyama, Y., K. Tsukamoto & S. Kitada, 1998. Rate of entrainment of the juvenile masu salmon Oncorhynchus masou through the intake of agricultural diversion weirs during downstream migration in the Kaji River. Nippon Suisan Gakkai Shi 64: 398–405.
Zettler, F., 1969. Course of output–input ratio in relation to frequency and state of adaptation measured at a single photo-receptor in compound eye of Calliphora erythrocephala. Zeitschrift Für Vergleichende Physiologie 64: 432–449.
Acknowledgements
The author thanks K. Mutou, H. Nakamura and H. Morino of FTINFS for providing technical support with fish rearing and the experimental facilities. The author is grateful to the laboratory staff of Fisheries Stock Assessment Center, Salmon Research Department for sharing their technical skills. The author thanks Cynthia Kulongowski from Edanz (https://jp.edanz.com/ac) for editing the language of a draft of this manuscript.
Funding
This work was supported by the National Research and Development Agency, Japan Fisheries Research and Education Agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has not disclosed any competing interests.
Additional information
Handling editor: Fernando M. Pelicice
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Matsuda, K. How much light intensity to induce repulsion or attraction behaviour in juvenile salmon?. Hydrobiologia 851, 2237–2248 (2024). https://doi.org/10.1007/s10750-023-05452-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05452-8