Abstract
Wetlands are characterised by soils rich in organic matter that accumulate carbon, providing an important pathway for carbon dioxide sequestration. Nevertheless, not all the carbon fixed can be accumulated, and a proportion will decompose through microbial consumption and be partly released into the atmosphere. Rates of organic matter decomposition in tropical wetlands and the factors associated with this process are scarce. We conducted a 2-year field study in three Melaleuca wetlands in tropical and subtropical Australia using standardised tea litter substrates (green-labile and rooibos-recalcitrant) to measure organic matter decomposition and the microbial communities associated with this process. Decomposition rates were 4-fold higher in labile litter, which was low in carbon: nitrogen, compared to recalcitrant litter. The prokaryotic communities associated with the decomposing litter were unique at each site and different from the soil. They contained taxonomic groups adapted to anaerobic, high temperatures, acidic conditions and suggestive of slow anaerobic turnover. Microbial communities changed as decomposition progressed, with the latter characterised by taxa with cellulose-degrading functions. The decomposition of recalcitrant organic matter within Melaleuca soils was relatively slow, with half of the organic matter inputs remaining after two years, supporting long-term carbon sequestration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal wetlands are characterised by soils rich in organic carbon (C) and slow decomposition rates, favouring C sequestration. The rate at which wetlands store soil C is the net sum of litter accumulation and decomposition. Decomposition is variable among wetland types and varies with physicochemical characteristics (e.g. soil characteristics, salinity, nutrients), biological components (plant species, microbial community), environmental setting, disturbances and climate (Spivak et al., 2019). The highest decomposition rates are predicted in wetlands where the temperature and nutrients are high, salinity is low, and the soil has a microbial community capable of degrading compounds from the local litter (Rejmánková & Houdková, 2006). However, studies of decomposition rates in tropical wetlands, where many of these conditions are met, are scarce.
Tropical wetlands of Melaleuca trees, commonly known as “paper bark trees”, are widespread in Southeast Asia and Oceania. Only in Australia, Melaleuca forests cover an area of 6 million ha (Finlayson, 2005; Tran et al., 2015; ABARES, 2016). Melaleuca wetlands can also be found in the Caribbean, the United States and South America, where species such as M. quinquenervia have become invasive (Turner et al., 1998). In Australia, Melaleuca wetlands have been heavily lost to agriculture and urban development (Johnson et al., 1999). The deforestation of these ecosystems has led to the loss of the diversity and biogeochemical functions that support many ecosystem services they provide, including C storage and water quality improvement (Tran & Dargusch, 2016; Adame et al., 2019).
Melaleuca wetlands are highly productive (Finlayson et al., 1993) and have anoxic soils rich in C (Adame et al., 2020). Decomposition in Melaleuca wetlands varies with inundation frequency (Wallis & Raulings, 2011) and litter type (Rayamajhi et al., 2010). However, there is limited understanding of other factors that drive organic matter decomposition in these wetlands, compounded by a fundamental lack of knowledge on their microbial communities and functions.
Microbial communities of wetland soils are key for soil organic matter turnover and are variable among wetland types (Spivak et al., 2019). Microbial communities are also temporally variable, with community shifts during decomposition, reflecting changes in metabolic pathways, for instance, fermentation or sulphate reduction (Trevathan-Tackett et al., 2017). Communities also shift depending on the availability of organics, such as simple carbohydrates at the beginning of the decomposition versus lignocelluloses later in the decomposition process (Trevathan-Tackett et al., 2017). Changes in these microbial communities and metabolic pathways can be detected by changes in isotopic values (δ15N and δ13C of the litter), which change during decomposition (Kelleway et al., 2022).
Assessing biochemistry and microbiology responses is important to understand the causal mechanisms that support ecosystem services. For instance, the restoration of Melaleuca wetlands in Australia has gained momentum in the past five years, and obtaining carbon credits (ACCUs, Australian Carbon Credit Units) through restoring these wetlands is now possible (Lovelock et al., 2022). Restoration projects will benefit from understanding how the soil and microbial community change before and after restoration (Farrer et al., 2022).
In this study, we follow the decomposition process over two years in three Melaleuca wetlands in subtropical and tropical Queensland, northeast Australia. We used standardised substrate proxies for labile (green tea) and recalcitrant (rooibos or red tea) organic matter (Keuskamp et al., 2013). We measured changes in biomass, elemental C and nitrogen (N), isotope values (δ15N and δ13C) and the prokaryotic microbiome composition of the decomposing litter. We hypothesised that: (1) rates of organic matter decomposition will be faster in labile than recalcitrant litter, (2) changes in C, N, and isotopic values will reflect decomposition, with higher enrichment (increase in values) and an increase in C:N in substrates with faster decomposition (Fry, 2006), and (3) microbial communities associated with the tea litter will change as decomposition progresses, reflecting shifts in C and nutrient availability (Trevathan-Tackett et al., 2021). The goals were to fill in a knowledge gap on the biogeochemical cycling of Melaleuca wetlands (Adame et al., 2021), to understand soil C accumulation and losses (Wieder et al., 2013) and to provide foundational knowledge on the soil microbiota of these unique ecosystems.
Materials and methods
Study sites
We selected three sites in Queensland, Australia: Hull Heads National Park (HH), Insulator Creek (IC) and Toohey Forest (TF, Fig. 1). The site HH (− 18.0013°; 146.0465') has a humid tropical climate with a mean annual precipitation of 4,075 mm, and minimum and maximum mean temperatures of 19 and 28 °C (Tully Station, 1925–2021; and Cardwell Marine Parade, 1907–2021, Australian Bureau of Meteorology, ABM, 2021). The site IC (− 18.8957°; 146.2594') is drier, with mean annual precipitation of 2,145 mm and mean minimum and maximum temperatures of 19.0 and 29.3 °C, respectively (1968–2021, Ingham station, ABM, 2021). Finally, TF is in the subtropics (− 27.5513°; 153.0582') in a cooler and drier climate, with mean annual precipitation of 1,051 mm and 14.5 and 26.4 °C, for mean minimum and maximum annual temperatures, respectively (1929–2021, Archerfield Airport, ABM). HH and IC are located within the coastal zone; HH is permanently flooded except during very dry periods (e.g., the dry season of 2019); IC is flooded during the wet season (approximately December to April) and sporadically by high tides. TF is 16 km from the coast, within Mimosa Creek, a perennial freshwater creek. The forest in HH is dominated by trees of Melaleuca viridiflora mixed with rainforest species, while M. quinquenervia dominates IC and TH.
a Distribution of Melaleuca forests in Australia (wetlands and non-wetlands, ABARES, 2016); circles indicate the location of study sites in b tropical and c subtropical Queensland, Australia; d is Toohey forest and e Insulator Creek, and f is Hull Heads National Park; triangles are major cities within the study area
Litter decomposition
Rates of organic matter decomposition were measured using standardised tea litter bags from the Global TeaComposition H2O initiative (Trevathan-Tackett et al., 2021), a co-initiative to the TeaComposition program, with a modified method to the Tea Bag Index technique (Keuskamp et al., 2013; Djukic et al., 2018). The technique consists of burying tea bags as standardised and representative litter within wetlands and estimating exponential decay rates (k) from their biomass loss over time (Trevathan-Tackett et al., 2021). This technique can overcome natural plant litter variability among sites and provides information comparable to other ecosystems and studies using the same approach. However, this litter bag technique may provide rates different from those obtained from local litter and exclude large grazers from entering the teabag at the cost of retaining small particulate litter within the fine mesh. Thus, decomposition measured with this method may be underestimated if grazers are common (Bradford et al., 2002).
In January 2018, at the beginning of the wet season, 32 pre-weighed labile green and 32 recalcitrant rooibos teabags (hereafter “red tea”) were set at each site. The teabags were buried at 15 cm soil depth in two plots, three meters apart. Within each plot, 16 bags of green tea and 16 of red tea were buried (total n = 192 bags); half of the bags were pre-weighed. The tea bags were retrieved approximately at 3, 6, 12, and 24 months (TF at 97, 194 and 370 days; IC at 154, 281 and 505 days; HH at 154, 281, 505, and 672 days). Differences in sampling times among sites were due to limited accessibility during the summer when flooding is common and dangerous crocodiles (Crocodylus porosus) in IC and HH are present. At least three pre-weighed green and red bags were retrieved from each plot during each sampling. The bags were cleaned of soil and in-growth roots, oven-dried (60 °C), and weighed to estimate dry biomass loss. The remaining tea was analysed for elemental C, N, and δ13C and δ15N values (Elemental analyser coupled with a Mass Spectrometer, Griffith University) to estimate decomposition as indicated by the increase in C:N, δ13C and δ15N over time (Fry, 2006).
Additionally, three soil samples at each plot were taken with a graduated mini core at the beginning of the experiment. The samples of known volume were used to calculate bulk density. The soil was tested for organic C by adding HCl in five samples per site; none displayed bubbling, indicating minimal carbonate content in the soil. Consecutively, the soil was analysed for organic C, N, δ13C and δ15N in non-acidified samples, similar to the tea (above). From the changes in dry biomass, we calculated exponential decay rates (Wider & Lang, 1982):
where W0 is the initial mass, Wt is mass at time t (day), and k is the decay rate in proportion per day (day−1). Decay rates were calculated for each tea type, with all sites combined, since the variability in sampling among sites would confound inter-site decay rate comparisons.
Microbial community
Immediately after the tea bags (unweighted) were retrieved, one gram each of green and red tea and one gram of soil within each plot were transferred to 2 ml vials of Zymo DNA/RNA Shield. The samples were frozen until transportation to the laboratory and stored at − 80 °C until analyses. The DNA was extracted from each sample with the Zymo Mini Kit as per manufacturer instructions for soil (ZymoResearch, CA, USA). DNA amplification of the V4 16S rRNA gene region was performed before indexing and sequencing at the Deakin University Genomics Centre (Trevathan-Tackett et al., 2021). The amplicon sequences were analysed in the QIIME2 DADA2 pipeline (Callahan et al., 2016; Bolyen et al., 2018). The resulting amplicon sequence variants (ASVs) were normalised to 18,000 reads per sample and analysed for Beta diversity (weighted UniFrac). Sequences were classified using the Silva v132 database trained to the V4 hypervariable region at a threshold of 99% homology (Quast et al., 2012). Amplicon data are available at the European Nucleotide Archive under project accessions PRJEB50314 (tea litter) and PRJEB50315 (bulk soils).
Statistical analyses
Differences in Beta diversity were analysed with a three-way multivariate PERMANOVA, with site, substrate (green and red tea, and soil), and time, as fixed factors. The latter test was repeated with tea samples only to investigate changes in the microbial community as decomposition progressed within each site. Since the last sampling (2 years) at HH was about 6 months after the last sampling at IC and TF, we limited our statistical analysis interpretation to (a) the early sampling (3 to 5 months across sites) and (b) shifts through time within sites. SIMPER analysis was performed using the ASV table filtered at 0.001% and on significant results from the PERMANOVAs to identify the ASVs driving the differences. For the PERMANOVA analyses, a Monte Carlo tests (P(MC)) were used in cases where permutations were < 200 (PRIMER+ PERMANOVAv7; Anderson et al., 2008).
Results
Soil characteristics
The surface soil varied among the sites; IC had higher soil C and N (15.7 and 0.85%, respectively) compared to TH and HH (< 7 and < 0.3%). HH also differed in that it had higher C:N (33.8) and considerably lower soil δ13C and δ14N values (− 31.0 and − 0.05‰) compared to the other two sites (δ13C of − 27.2 to − 28.8‰, and δ14N of 2.33 and 1.69‰ for IC and TH, respectively, Table 1).
Litter decomposition: biomass, C and N losses
Despite differences in climate and location of the sampling sites, the trends of biomass, C and N were similar, with differences mostly explained by litter type (green or red) (Fig. 2). Biomass decay rates for the green tea litter were 4.3-fold higher (0.0073 ± 0.00061 day−1) than for red tea litter (0.0017 ± 0.0001 day−1). As a result of the higher decay rate, green tea samples had only 9.7 ± 0.12% of their biomass remaining at the end of the 2-year incubation, compared to 42.7 ± 1.4% biomass remaining of the red tea.
Proportion of a biomass, b carbon, and c nitrogen of green and red tea litter remaining after incubation in the soil of three Melaleuca wetlands (HH Hull Heads, IC Insulator Creek, TF Toohey Forest). Negative values in c indicate N gains. Biomass loss for the first 300 days has been previously published as site-level averages in Trevathan-Tackett et al. (2021)
The rates for C decay were 0.0015 ± 0.00009 day−1 for red tea and 0.0058 ± 0.0006 day−1 for green tea. The loss of C was similar among sites, ranging between 54.6 and 61.4% of the initial C for red tea and 77.6- to 90.9% for the green tea (Table S1; Fig. 2b). The changes in N were more variable, as N concentrations increased in the green and red tea in IC and TF from 3.8 ± 0.1% to 6.0 ± 0.1 and 6.0 ± 0.2% for the green and from 1.1 ± 1.1% to 1.5 ± 0.3 and 1.4 ± 0.1% for the red tea (Table S1).
The composition of organic matter also changed during the experiment. The C:N ratio of both teas decreased during decay; however, for the green tea, the decrease was limited to the first few months (Fig. 3). In comparison, the C:N of the red tea decreased throughout the incubation, especially in IC (Fig. 3a). For the stable isotopes, initial values were similar between the tea litter types for δ15N, while the δ13C of red tea was more enriched (higher) than green tea (Fig. 3b, c). As decomposition progressed, green tea litter became enriched (higher) for both δ13C and δ15N in TF and IC but not in HH, where δ13C remained constant (Fig. 3b, c). In contrast, the red tea litter became depleted (lower) in δ13C and δ15N within the first few months, with relatively little change after that, except for a further decrease at two years at HH (Fig. 3b, c).
Microbial communities
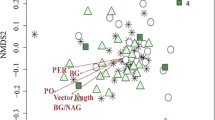
Beta diversity or community structure was primarily driven by site and substrate (red, green tea, and soil; 3-way interaction, Psuedo-F = 2.125, P (perm) = 0.002; Figs. 4 and S1; Table S2). Only at the early sampling were the tea-associated prokaryotic communities significantly different from the soil microbiome at TF and HH [TF P(MC) ≤ 0.008; IC P(MC) > 0.05; HH P(MC) ≤ 0.028; Fig. S1]. The tea-associated microbiomes were not significantly different from each other within each site and generally became more similar to the bulk soil microbiome as decomposition progressed (Fig. S1; Table S2).
Class-level changes in community composition across substrate type (green and rooibos/red tea, and soil, a–c) and time (early and late in the experiment; d–f) for Toohey Forest (a, d), Insulator Creek (b, e) and Hull Heads (c, f). Time points for Toohey Forests and Insulator Creek were three months (early) and 12 months (later), while for Hull Heads, the time points represent five months (early) and 22 months (later). Phylum is included in legend before class. Values are means
The differences among sites at the early sampling were attributed to TF being enriched in Bradyrhizobium sp. (ASV11), Candidatus udaeobacter, and Prosthecobacter (ASV95). In contrast, HH and IC were dominated by the Alpha- and Gammaproteobacteria, Myxococcota, Actinobacteriota and Planctomycetes taxonomic groups. There was a significant shift in the tea-associated microbiome through time within each site (main Site * Time interaction, Psuedo F = 2.3514, P(perm) = 0.005, Figs. 4 and 5; Table S2).
At TF, early communities were characterised by ASVs from the genus Bacteroidales, Citrifermentans (Geobacter), Burkholderiales and Rhizobiaceae. In contrast, later communities were dominated by Anaerolineae groups SBR1031 and A4b, Paenibacillus (Firmicutes) and a Bradyrhizobium ASVs [P(MC) = 0.031]. In IC, early communities were dominated by the Burkholderia–Caballeronia–Paraburkholderia group, Dyella sp., and Ktedonobacteraceae groups, and later communities by Paenibacillaceae (mostly Paenibacillus sp.), Myxococcus sp., Xanthobacteraceae, and Streptomyces (P(perm) = 0.004). Finally, at HH, the early and later communities had common orders, such as Acidobacteriales and Clostridiales, but different ASVs within these groups shifted over time [P(perm) = 0.003]. Early communities included Methanobacterium, Methanosarcina, the Burkholderia–Caballeronia–Paraburkholderia genus and Nevskia, while later communities comprised Rhodanobacteraceae, Rhizobiales, Syntrophobacter, Solirubrobacterales and WPS-2 ASVs.
Discussion
This long-term decomposition study provided insight into organic matter turnover in widespread tropical Melaleuca wetlands. The two standardised tea types roughly represent end-members for different types of litter (Trevathan-Tackett et al., 2021). The green tea litter, representing labile organic matter with low C:N (15), was mainly decomposed by the end of two years. Comparatively, red tea, representing recalcitrant material (C:N of 54) preserved half of its biomass at the end of the experiment. Thus, depending on the litter type, soil decomposition and storage pathways will differ vastly. For instance, the leaves and bark of Melaleuca trees are high in lignocellulose and C:N (> 50; Adame & Reef, 2020; Tjokorda et al. 2021). Thus, the red tea decay trends observed in this study indicate the likely fate of Melaleuca litter. Comparatively, the litter of grasses, typical of the Melaleuca forest understory (Fig. 1), or labile organic matter carried by floods, is likely to have a similar fate as green tea, with rapid decomposition, subsequent microbial colonisation and N immobilisation. These results are similar to those for mangrove forests, which found that litter composition, not climate, were the most important drivers of organic matter decomposition (Simpson et al., 2023).
The decay rates for red tea (mean k = 0.0017 day−1) were within the lower range of intertidal wetlands, such as mangroves, seagrass, and marshes, whose k values range between 0.001 and 0.005 d−1 during the first year of decomposition (Trevathan-Tackett et al., 2021). Slow decay rates in leaves of other Melaleuca species have previously been reported, with M. ericifolia, having a k = 0.001–0.002 (Wallis & Raulings, 2011) and M. argentea, M. leucadendra, with k = 0.005 and 0.007, respectively (Pettit et al., 2012). Biomass decay rates of red tea were similar to those of C decay, with a relatively rapid loss within the first months and a slow decline until the end of the two-year incubation. Similarly, in red tea, δ13C and C:N values changed within the first three months and stayed relatively constant throughout the rest of the experiment. Overall, this study supports the idea that Melaleuca litter and soils have conditions that support long-term soil C storage (Tran & Dargusch, 2016).
On the contrary, green tea was rapidly lost, probably due to the fast leaching of labile C, especially in sites with frequent flooding (Wallis & Raulings, 2011). HH is usually flooded, and green tea at this site was lost the fastest within the first months of incubation. Our results are consistent with other long-term tea litter studies showing that after the first few months, the green tea is exhausted of most of its organic resources, and what remains is likely inaccessible to further breakdown (Duddigan et al., 2020; Trevathan-Tackett et al., 2021). As green tea biomass was rapidly lost, the elemental N content of litter increased, and the δ15N increased after the first year of incubation. The changes in N are the net balance between ammonification (breakdown of organic N to ammonium) and immobilisation (transformation of inorganic N into organic through microbial consumption; Reddy & DeLaune, 2008). Our results suggest that N was immobilised in green tea due to microbial colonisation, results previously observed in other coastal wetlands (Benner et al., 1991; Macko et al., 1994).
Most prokaryotic groups associated with the tea litter and soil were characteristic of freshwater ecosystems or terrestrial forests, with significant differences among sites. While we only characterised the community profile, we could use the assigned taxonomy to infer putative functions. Many of the abundant taxa were adapted to anaerobic and acidic conditions, such as those in these wetlands where organic matter is abundant, and soils are waterlogged (Finlayson, 2005). The conditions at TF were different due to its location upstream with no tidal influence and in a subtropical climate. In contrast, HH and IC are on the tropical coast and experiencing sporadic tidal influece. Within the TF site, two groups were dominant, the freshwater decomposer Prosthecobacter (Staley, 1981) and Candidatus udaeobacter, a common bacterium in acidic soils (Willms et al., 2021). The genera Bradyrhizobium, a common root symbiont known to survive harsh soil environments, was also abundant (Vaninsberghe et al., 2015).
Comparatively, HH was the site most regularly flooded and had the highest soil C:N, which is a key driver of microbial community composition (Peralta et al., 2013). Thus, this site had a unique microbial community dominated by two groups within the Actinobacteriota phylum: Acidothermus sp., tolerant to acidic conditions, and Thermoleophilia ASVs, tolerant to very high temperatures (> 60 °C; Kristjansson & Alfredsson, 1981), suggesting soil communities enriched in taxa tolerant to hot and low redox and pH conditions. Finally, IC was dominated by alpha- and gammaproteobacterial groups, typical soil families. These include Burkholderiaceae, associated with roots in forest soils (Zwetsloot et al., 2020) and Rhizobiaceae, important for denitrification and N fixation (Wang et al., 2012).
Temporal changes in microbial composition varied among sites. Early in the study, fermenters Citrifermentans, Burkholderiaceae, and Rhizobiaceae were common in TF. While later, methane oxidisers and cellulolytic fermenters (Anaerolineae groups SBR1031; Xia et al., 2016), Bradyrhizobium, and Paenibacillus (Firmicultes), a potential cellulose degrader in tropical freshwater swamps (Kanchanadumkerng et al., 2017) dominated. Similarly, in HH, Methanobacterium, Methanosarcina, prokaryotes involved in the methane cycle, the Burkholderia–Caballeronia–Paraburkholderia genus (Burkholderiaceae family), and Nevskia, common in stagnant freshwater (Cypionka et al., 1981), were common. Later in the study, dominant groups included Rhodanobacteraceae, Rhizobiales, Syntrophobacter, an anaerobic group with fermentative metabolism, and Solirubrobacterales, which includes bacteria that break down leaves in tropical forests (Donald et al., 2020). Finally, in IC, early communities were found of Dyella sp, which can degrade aromatic compounds (Kong et al., 2013), and the aerobic Ktedonobacteraceae. While later in the incubation, Paenibacillus (Firmicutes), a potential cellulose degrader in tropical soils, Myxococcus sp. ASVs and Streptomyces ASV, essential for cellulose and chitin breakdown (Chater, 2016), were common. While these microbial communities differed in the taxa associated with the tea litter within each site, their putative abilities to break down recalcitrant organic matter (cellulose) and use methane and fermentation pathways suggest a degree of functional redundancy.
Conclusions
The fate or organic matter in Melaleuca wetlands will significantly differ depending on the type of litter that enters the soil. Local Melaleuca litter (leaves, barks and roots), which have high lignocellulose content and high C:N, will follow a similar trend as red tea, with low decomposition rates, with half of the C remaining in the soil after two years of incubation. Comparatively, grass, shrubs and labile organic matter transported during floods may follow a similar pattern as green tea. The biomass and C of litter low in C:N will be consumed through colonising microbial communities, immobilising N. The microbial community was characterised by groups of taxa common to freshwater ecosystems or terrestrial forests, with many groups adapted to anaerobic, high-temperature and acidic conditions. There was an evident change in these communities as decomposition progressed, with communities at later experiment stages characterised by cellulose degraders. The microbiome data also suggested metabolic pathways, such as the slow anaerobic turnover of organic C. Overall this study has shown that Melaleuca wetlands have conditions that support microbial degradation of organic matter along with lon-term sequestration of recalcitrant carbon.
Data availability
Amplicon data are available at the European Nucleotide Archive under project accessions PRJEB50314 (tea litter) and PRJEB50315 (bulk soils). Soil physical and chemical properties and organic matter decomposition data will be available open access at Griffith Data Repository (https://research-repository.griffith.edu.au) upon acceptance of the manuscript.
References
ABARES, 2016. Australian Forest Profiles: Melaleuca. Australian Bureau of Agricultural and Resource Economics and Sciences, Department of Agriculture and Water Resources, Australian Government [available on internet at: http://www.agriculture.gov.au/abares/research-topics/forests]
Adame, M. F. & R. Reef, 2020. Potential pollution sources from agricultural activities on tropical forested floodplain wetlands revealed by soil eDNA. Forests 11: 1–12. https://doi.org/10.3390/F11080892.
Adame, M. F., H. Franklin, N. Waltham, et al., 2019. Nitrogen removal by tropical forested wetlands through denitrification. Marine and Freshwater Research 70: 1513–1521.
Adame, M. F., R. Reef, V. N. L. Wong, et al., 2020. Carbon and nitrogen sequestration of Melaleuca floodplain wetlands in tropical Australia. Ecosystems 23: 454–466. https://doi.org/10.1007/s10021-019-00414-5.
Adame, M. F., M. P. Vilas, H. Franklin, et al., 2021. A conceptual model of nitrogen dynamics for the Great Barrier Reef catchments. Marine Pollution Bulletin 173: 112909. https://doi.org/10.1016/j.marpolbul.2021.112909.
Anderson, M. J., R. N. Gorley & K. R. Clarke, 2008. PERMANOV +for PRIMER: Guide to Software and Statistical Methods, PRIMER-ET Ltd, Devon:
Benner, R., M. L. Fogel & E. K. Sprague, 1991. Diagenesis of belowground biomass of Spartina alterniflora in salt-marsh sediments. Limnology and Oceanography 36: 1358–1374.
Bolyen, E., J. R. Rideout, M. R. Dillon, et al., 2018. Reproducible, interactive, scalable, and extensible microbiome data science using QIIME2. Nature Biotechnology 37: 852–857.
Bradford, M., G. Tordoff, T. Eggers, et al., 2002. Microbiota, fauna, and mesh size interactions in litter decomposition. Oikos 99: 317–323.
Callahan, B. J., P. J. Mcmurdie, M. J. Rosen, et al., 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nature Methods 13: 581–587. https://doi.org/10.1038/nmeth.3869.
Chater, K. F., 2016. Recent advances in understanding Streptomyces. F1000Research 5: 2795. https://doi.org/10.12688/f1000research.9534.1.
Cypionka, H., H.-D. Babenzien, F. Glockner & R. Amann, 1981. The Genus Nevskia. In Starr, M. P., H. Stolp, H. G. Trüper & A. S. H. G. Balows (eds), The Prokaryotes. Springer, Berlin.
Donald, J., S. Manzi, M. Roy, et al., 2020. A test of community assembly rules using foliar endophytes from a tropical forest canopy. Journal of Ecology 108: 1605–1616. https://doi.org/10.1111/1365-2745.13344.
Duddigan, S., L. J. Shaw, P. D. Alexander & C. D. Collins, 2020. Chemical underpinning of the Tea Bag Index: an examination of the decomposition of tea leaves. Applied and Environmental Soil Science 2020: 6085180.
Farrer, E. C., S. A. Van Bael, K. Clay & M. K. Smith, 2022. Plant–microbial symbioses in coastal systems: their ecological importance and role in coastal restoration. Estuaries and Coasts 45: 1805–1822. https://doi.org/10.1007/s12237-022-01052-2.
Finlayson, C. M., 2005. Plant ecology of Australia’s tropical floodplain wetlands: a review. Annals of Botany 96: 541–555. https://doi.org/10.1093/aob/mci209.
Finlayson, C. M., I. D. Cowie & B. J. Bailey, 1993. Biomass and litter dynamics in a Melaleuca forest on a seasonally inundated floodplain in tropical, northern Australia. Wetlands Ecology and Management 2: 177–188. https://doi.org/10.1007/BF00188152.
Fry, 2006. Stable Isotope Ecology, Springer, New York:
Johnson, A. K. L., S. P. Ebert & A. E. Murray, 1999. Distribution of coastal freshwater wetlands and riparian forests in the Herbert River catchment and implications for management of catchments adjacent the Great Barrier Reef Marine Park. Environmental Conservation 26: 229–235.
Kanchanadumkerng, P., M. Sakka, K. Sakka & C. Wiwat, 2017. Characterisation of endoglucanase from Paenibacillus sp. M33, a novel isolate from a freshwater swamp forest. Journal of Basic Microbiology 57: 121–131. https://doi.org/10.1002/jobm.201600225.
Kelleway, J. J., S. M. Trevathan-Tackett, J. Baldock & L. P. Critchley, 2022. Plant litter composition and stable isotope signatures vary during decomposition in blue carbon ecosystems. Biogeochemistry 158: 147–165. https://doi.org/10.1007/s10533-022-00890-3.
Keuskamp, J. A., B. J. J. Dingemans, T. Lehtinen, et al., 2013. Tea Bag Index: a novel approach to collect uniform decomposition data across ecosystems. Methods in Ecology and Evolution 4: 1070–1075. https://doi.org/10.1111/2041-210X.12097.
Kong, C., L. Wang, P. Li, et al., 2013. Genome sequence of Dyella ginsengisoli Strain LA-4, an efficient degrader of aromatic compounds. Genome Announcements 6: e00961-e1013. https://doi.org/10.1007/s00253-013-4814-0.7.
Kristjansson, J., & G. Alfredsson, 1981. The heterotrophic, Thermophilic Genera Thermomicrobium, Rhodothermus, Saccharococcus, Acidothermus, and Scotothermus. In: Thermophilic Bacteria. CRC Press, Boca Raton.
Lovelock, C. E., M. F. Adame, J. Bradley, S. Dittmann, V. Hagger, S. M. Hickey, L. B. Hutley, A. Jones, J. J. Kelleway, P. S. Lavery, P. I. Macreadie, D. T. Maher, S. McGinley, A. McGlashan, S. Perry, L. Mosley, K. Rogers & J. Z. Sippo, 2022. An Australian blue carbon method to estimate climate change mitigation benefits of coastal wetland restoration. Restoration Ecology 31: e13739.
Macko, S. A., et al., 1994. Early diagenesis and organic matter preservation – a molecular stable carbon-isotope perspective. Chemical Geology 114: 365–379.
Peralta, R. M., C. Ahn & P. M. Gillevet, 2013. Characterisation of soil bacterial community structure and physicochemical properties in created and natural wetlands. Science of the Total Environment 443: 725–732. https://doi.org/10.1016/j.scitotenv.2012.11.052.
Pettit, N. E., T. Davies, J. B. Fellman, et al., 2012. Leaf litter chemistry, decomposition and assimilation by macroinvertebrates in two tropical streams. Hydrobiologia 680: 63–77. https://doi.org/10.1007/s10750-011-0903-1.
Rayamajhi, M. B., P. D. Pratt, T. D. Center & T. K. Van, 2010. Exotic tree leaf litter accumulation and mass loss dynamics compared with two sympatric native species in south Florida, USA. European Journal of Forest Research 129: 1155–1168. https://doi.org/10.1007/s10342-010-0404-1.
Reddy, K. R. & R. D. DeLaune, 2008. Biogeochemistry of Wetlands, CRC Press, Boca Raton:
Spivak, A. C., et al., 2019. Global-change controls on soil-carbon accumulation and loss in coastal vegetated ecosystems. Nature Geoscience 12: 685–692.
Staley, J., 1981. The Genus Prosthecobacter, The Prokaryotes Springer, Berlin: 477–479.
Tjokorda, I. I., B. Khairul Hadi, R. Manurung & A. Widiana, 2021. Enhancement of essential oil yield from Melaleuca leucadendra L. leaves by lignocellulose degradation pre-treatment using filamentous fungi. Journal of Bioresources and Bioproducts 6: 379–386. https://doi.org/10.1016/j.jobab.2021.02.010.
Tran, D. B. & P. Dargusch, 2016. Melaleuca forests in Australia have globally significant carbon stocks. Forest Ecology and Management 375: 230–237. https://doi.org/10.1016/j.foreco.2016.05.028.
Tran, D. B., T. V. Hoang & P. Dargusch, 2015. An assessment of the carbon stocks and sodicity tolerance of disturbed Melaleuca forests in Southern Vietnam. Carbon Balance and Management 10: 15. https://doi.org/10.1186/s13021-015-0025-6.
Trevathan-Tackett, S. M., J. R. Seymour, D. A. Nielsen, P. I. Macreadie, T. C. Jeffries, J. Sanderman, et al., 2017. Sediment anoxia limits microbial-driven seagrass carbon remineralisation under warming conditions. FEMS Microbiology Ecology 93: fix033.
Trevathan-Tackett, S. M., S. Kepfer-Rojas, A. H. Engelen, et al., 2021. Ecosystem type drives tea litter decomposition and associated prokaryotic microbiome communities in freshwater and coastal wetlands at a continental scale. Science of the Total Environment 78: 146819. https://doi.org/10.1016/j.scitotenv.2021.146819.
Turner, C. E., T. D. Center, D. W. Burrows & G. R. Buckingham, 1998. Ecology and management of Melaleuca quinquenervia, an invader of wetlands in Florida, U.S.A. Wetlands Ecology and Management 5: 165–178.
Vaninsberghe, D., K. R. Maas, E. Cardenas, et al., 2015. Non-symbiotic Bradyrhizobium ecotypes dominate North American forest soils. ISME Journal 9: 2435–2441. https://doi.org/10.1038/ismej.2015.54.
Wallis, E. & E. Raulings, 2011. Relationship between water regime and hummock-building by Melaleuca ericifolia and Phragmites australis in a brackish wetland. Aquatic Botany 95: 182–188. https://doi.org/10.1016/j.aquabot.2011.05.006.
Wang, C., G. Zhu, Y. Wang, et al., 2012. Nitrous oxide reductase gene (nosZ) and N2O reduction along the littoral gradient of a eutrophic freshwater lake. Journal of Environmental Science 25: 44–52. https://doi.org/10.1016/S1001-0742(12)60005-9.
Wider, R. K. & G. E. Lang, 1982. A critique of the analytical methods used in examining decomposition data obtained from litter bags. Ecology 63: 1636–1642.
Wieder, W. R., G. B. Bonan & S. D. Allison, 2013. Global soil carbon projections are improved by modelling microbial processes. Nature Climate Change 3: 909–912. https://doi.org/10.1038/nclimate1951.
Willms, I. M., S. H. Bolz, J. Yuan, et al., 2021. The ubiquitous soil Verrucomicrobial clade ‘Candidatus udaeobacter’ shows preferences for acidic pH. Environmental Microbiology Reports 13: 878–883. https://doi.org/10.1111/1758-2229.13006.
Xia, Y., Y. Wang, Y. Wang, et al., 2016. Cellular adhesiveness and cellulolytic capacity in Anaerolineae revealed by omics-based genome interpretation. Biotechnology and Biofuels 9: 1–13. https://doi.org/10.1186/s13068-016-0524-z.
Zwetsloot, M., J. Munoz Ucros, K. Wickings, et al., 2020. Prevalent root-derived phenolics drive shifts in microbial community composition and prime decomposition in forest soil. Soil Biology and Biochemistry 145: 107797. https://doi.org/10.1016/j.soilbio.2020.107797.
Acknowledgements
We thank the Traditional Owners of the land in which this research took place, especially the Nywaigi and Gulnay people. We also thank Sam and Santo Lamari for allowing us to work in their property and sharing with us their knowledge on wetlands. We are grateful to the Wetlands Team at the Department for Environment and Science for guidance, and to Bahar Farahani and Emad Kavehei for field assistance. Thanks to Deakin University's Genomics Centre, particularly Dr Stella Loke.
Funding
STT was supported by the ARC DECRA DE210101029, and MFA was supported by an Advance Queensland Industry Research Fellowship.
Author information
Authors and Affiliations
Contributions
MFA, SMTT and PM contributed to the conception and design of the study; MFA and NI conducted fieldwork and laboratory work, MFA, NI and STT analysed the data, MFA wrote the first draft and all co-authors contributed to the writing of the final draft of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Handling editor: Stefano Amalfitano.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adame, M.F., Iram, N., Macreadie, P. et al. Organic matter decomposition and associated microbial communities in wetlands: insights from tropical and subtropical Melaleuca forests in Australia. Hydrobiologia 851, 1577–1588 (2024). https://doi.org/10.1007/s10750-023-05407-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05407-z