Abstract
Freshwater green algae of the Haematococcus lacustris species complex are known for their ability to accumulate the secondary carotenoid astaxanthin, which has various industrial applications. Survival of H. lacustris in harsh environments is facilitated by the formation of desiccation-tolerant akinetes, which are thick-walled, resistant cells. In this study, we compared the desiccation tolerance of green and red akinetes and investigated the effect of different desiccation periods and extreme temperatures on their viability. We used the effective quantum yield of the photosystem II as an indicator of how akinetes respond to environmental stress. We also examined the ultrastructure of the akinetes using electron microscopy. Both green and red akinetes survived desiccation at all dehydration rates tested. The effective quantum yield of the green akinetes was generally higher than that of the red akinetes throughout the experiment. Moreover, desiccated red akinetes were able to survive different additional stresses, even exposure to extreme temperatures of − 80 °C and 55 °C. Red akinetes that had not been previously desiccated tolerated freezing better than high temperature. These findings contribute to our understanding of the desiccation tolerance of Haematococcus akinetes and have implications for the global distribution of this alga.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freshwater green algae of the Haematococcus lacustris (Girod-Chantrans) Rostafinski species complex (Allewaert et al., 2015) are among the most biotechnologically interesting microalgae due to their ability to accumulate large amounts of the secondary carotenoid astaxanthin. This red pigment is a natural source of coloration for shrimp and salmon cultures (Lorenz & Cysewski, 2000). In addition, astaxanthin is a very efficient antioxidant (Naguib, 2000) with broad pharmaceutical potential. The immunomodulatory (Okai & Higashi-Okai, 1996) and antitumor activity of astaxanthin (Jyonouchi et al., 2000) has been described, and it also prevents the development of Parkinson´s disease and other neurodegenerative disoders (Chan et al., 2009) and modulates age-related mitochondrial dysfunction (Park et al., 2013).
Until recently, Haematococcus lacustris was known as H. pluvialis, but both names were synonymized and H. lacustris was designated as the correct name of the type (Nakada & Ota, 2016). H. lacustris occurs in all biogeographic regions as a pioneer alga in shallow ephemeral pools (Genitsaris et al., 2016). These habitats are characterized by multiple abiotic stress conditions, primarily high radiation and periodic drought. Survival of H. lacustris in this harsh environment is facilitated by its complex life cycle consisting of four life stages: gametes, zoospores, green immotile round palmella stages, and akinetes. The thick-walled, resistant akinetes are the desiccation-tolerant stages. They allow H. lacustris to reach new habitats using aerial transport (Genitsaris et al., 2011) and on the legs and feathers of aquatic birds (Figuerola & Green, 2002).
Akinetes are formed in response to stressors such as high irradiance (Saha et al., 2013; Chekanov et al., 2014), temperature above 30 °C (Tjahjono et al., 1994), higher salt concentration in the medium (Sarada et al., 2002) and nutrient depletion (Boussiba et al., 1999; Saha et al., 2013). Cells undergo dramatic ultrastructural changes during akinete formation. Chloroplast volume is reduced, and partial degradation of thylakoids may occur (Wayama et al., 2013). Nevertheless, photosynthetic activity is maintained, and energy is likely used for lipid and astaxanthin synthesis (Gu et al., 2013). The young, green akinetes accumulate astaxanthin in lipid droplets that spread from the center to the periphery of the cell until the entire mature akinete appears red (Santos & Mesquita, 1984; Collins et al., 2011). In addition, a thick secondary cell wall forms, covered by a trilaminar sheath containing algaenans (Hagen et al., 2002). This layer probably contributes to the desiccation and UV light resistance of akinetes (Blokker, 2000).
Upon restoration of favorable conditions, akinetes increase their cell volume and undergo multiple divisions resulting in the release of 2–32 biflagellate daughter zoospores enclosed in a glycoprotein matrix (Hagen et al., 2002; Wayama et al., 2013). These zoospores then grow, multiply, and finally transform into immotile palmellas that can continue their cell division (Hazen, 1899; Elliott, 1934). Alternatively, instead of zoospores, akinetes can release up to 64 small biflagellate gametes (Triki et al., 1997) that fuse to form planozygotes with four flagella (Peebles, 1909; Pocock, 1960).
To simulate natural conditions and optimize the production of astaxanthin, different cultivation methods were applied to H. lacustris. Different cultivation media (Domínguez-Bocanegra et al., 2004), optimal irradiance, temperature, or pH (Sarada et al., 2002; Saha et al., 2013), and diverse types of photobioreactors and cultivation modes (Hata et al., 2001; Del Río, 2005; Park et al., 2014) have been extensively studied. However, there are still some gaps in our knowledge regarding the biology of H. lacustris. Despite a high number of biotechnologically oriented research, there is only a little knowledge about the ecology of the species and its survival strategies in natural conditions. One of the areas suitable for further investigation is the ability to survive unfavorable conditions in the desiccated state, which seems to be crucial for the worldwide distribution of this alga. Desiccation tolerance of H. lacustris has been studied recently. Roach et al. (2022a) conducted relatively short acclimation experiments (4–7 days) combining different desiccation rates, light regimes, and nitrogen availability and followed the synthesis of different stress markers (e.g., astaxanthin, α-tocopherol, glutathione). They concluded that exposure of liquid-cultivated cells in a growth phase to a humid atmosphere and light for several days is required for successful acclimation to desiccation. In another study, the authors investigated the longevity of dehydrated cells with respect to long-term storage and conservation of Haematococcus strains (Roach et al., 2022b).
The goal of the present study was to investigate the extent of desiccation tolerance of H. lacustris akinetes and the potential role of desiccation of akinetes as a strategic mechanism for survival at extreme temperatures. We asked the following questions: Is there a difference between the desiccation tolerance of the green and red akinetes? How does the length of the desiccation period affect the viability of the red akinetes and their temperature tolerance? Does the desiccation pretreatment affect the tolerance of the red akinetes to extreme temperatures? The results are discussed in the context of the global distribution of this alga.
Materials and methods
Algal cultures and desiccation treatments
The experimental strain Haematococcus lacustris (CCALA 357) was obtained from the Culture Collection of Autotrophic Organisms (Třeboň, Czech Republic). Cells were grown in 500 ml Erlenmeyer flasks in liquid BG11 medium (Kuhl & Lorenzen, 1964; Rippka & Herdman, 1992) at 22 °C under continuous illumination (~ 25 μmol m−2 s−1) and aeration. To induce the formation of akinetes, actively growing cultures were maintained for two months at 15 °C under continuous illumination without reinoculation or addition of fresh medium until they reached stationary phase. Red and green akinetes were obtained by applying different light intensities (95 and 2 μmol m−2 s−1, respectively). Representative images of both cultures used for the experiments can be found in Online Resource 1.
Four experimental setups were used, and the settings and conditions are summarized in the Table 1. The first experiment was conducted to compare the desiccation tolerance of green and red akinetes. A modified standardized setup developed for monitoring physiological performance during controlled dehydration and rehydration was used (Karsten et al., 2014; Pichrtová et al., 2014). Briefly, four samples of both akinete types (green and red) were filtered onto glass fiber filters (Whatman GF/C, particle retention 1.2 µm) using a manual vacuum pump. Diluted 10% BG11 medium (25 μl) was added to all samples to minimize salt stress. The filters were then placed on a grid in a polystyrene box sealed with a transparent lid prior to experimental drying. Different drying rates were achieved by desiccating the samples at different relative humidity levels (rh): slow drying rate at 86% rh over saturated KCl solution, medium drying rate at 43% rh over saturated K2CO3 solution (Greenspan, 1977), and fast drying rate at about 10% rh over partially dried silica gel (Silica Gel Orange, Carl Roth, Karlsruhe, Germany). After desiccation period of 24 h, the filters with dry biomass were placed in a 48-well cultivation plate (Greiner Bio-One) and rehydrated with 1 ml of fresh BG11 medium. The rehydrated samples were maintained at 22 °C with continuous illumination (~ 45 μmol m−2 s−1).
Experiments II and III were performed to investigate how desiccation pretreatment (30–50% rh) affects tolerance to low or high temperature. Only red akinetes were used in these experiments because they are more stable and easier to handle. Prior to the experiments, the old medium was replaced with 10% BG11 to minimize salt stress upon desiccation. For each experiment, 100 μl of this modified culture was placed in 96-well cultivation plate (Greiner Bio-One) in four replicates. Samples for experiments II and III were then exposed to desiccation under room conditions at approximately 30–50% rh at 25 °C and with continuous light (~ 30 μmol m−2 s−1) and the dry biomass on the bottom of the wells was kept desiccated for 1 to 12 weeks. Samples were then exposed to different temperatures for 1 to 3 h and then rehydrated with 300 μl of fresh BG11 medium and maintained at 22 °C and continuous light (~ 45 μmol m−2 s−1; Table 1). The experiment III additionally tested the effect of prolonged desiccation (8 and 12 weeks); Table 1). The refrigerator/freezer (Electrolux) was used for the − 1 °C, − 18 °C treatments, the vertical ultra-low temperature freezer (Kaltis International Co., Ltd.) for − 80 °C, and the drying oven (Memmert UNB 100) for 40 °C, 45 °C, 50 °C, 55 °C.
The experiment IV was also performed with red akinetes only. Samples on glass fiber filters were placed in the Petri dish and exposed to the temperatures of − 18 °C and 50 °C without the desiccation pretreatment.
Light and transmission electron microscopy
The physiological condition of the cultures was checked by Olympus CX22LED microscope before all experiments. After desiccation treatments and subsequent rehydration, the release of zoospores (evidence of viability of akinetes) was recorded by a non-invasive observation of the whole well content using Olympus SZ61 stereomicroscope. The exact number of released zoospores could not be quantified, and, therefore, we created a semi-quatitative scale describing their amount (Table 2).
Green and red akinetes collected from cultures prior to desiccation experiments were prepared for transmission electron microscopy using modification of the method described in Wayama et al. (2013). Briefly, samples were fixed in 2% glutaraldehyde at 4 °C for 8 h, rinsed three times with 0.05 M cacodylate buffer (pH 6.8), and post-fixed overnight in the same buffer containing 1% OsO4. Cells were then rinsed three times with 0.05 M cacodylate buffer (pH 7.8), once with 0.025 M cacodylate buffer (pH 7.8), and three times with distilled water. Samples were then dehydrated in increasing concentrations of ethanol (50% ethanol for several days and then 70%, 90%, 95% and 100% for 30 min each) and then incubated in ethanol-butanol solution (3:1), ethanol-butanol (1:1), ethanol-butanol (1:3) and finally 100% butanol (30 min each). The dehydrated samples were infiltrated with increasing concentrations of Spurr’s resin (Spurr, 1969) in butanol and finally with 100% Spurr’s resin (3 days in total). The ultrathin sections were cut on Ultracut E microtome with a diamond knife. The sections were counterstained with lead citrate and observed with an JEOL JEM-1011 transmission electron microscope. Images were captured with a Veleta CCD camera using Olympus Soft Imaging Solution GmbH acquisition software.
Measurement of the effective quantum yield
Effective quantum yield (ΦPSII) is a relative parameter used for monitoring of photochemical energy conversion effectivity in PSII in light-adapted state of cells (Roháček and Barták, 1999) and it is considered a good eco-physiological indicator of how plants respond to environmental stress (Rascher et al., 2000). It is computed as (FM′ − F)/FM′, where F is the steady state fluorescence state and FM′ is the maximum fluorescence in the light-adapted state measured after application of a saturation pulse. All measurements were performed non-invasively using an imaging modulated fluorimeter FluorCam (Photon System Instruments, Czech Republic). The box was continuously illuminated at 45 μmol m−2 s−1 between measurements. A saturating pulse of 600 ms was applied to the light-adapted samples to obtain the FM′ value.
Measurements in Experiment I were made through the transparent polyester lid of the desiccation chambers. The probe was placed at a constant distance of 11 cm from the samples. The first measurement was taken immediately after placing the filters with biomass in the chambers. Subsequent measurements were performed every 30 min for samples desiccated at 86% rh, every 20 min for samples desiccated at 43% rh, and every 10 min for samples desiccated rapidly at 10% rh. The intervals were selected based on the results of pilot experiments. The measurement was stopped when the value of ΦPSII dropped under 0.1. The effective quantum yield was also measured immediately after addition of fresh BG11 to the samples and then 1, 6, 12, 24, 48, and 72 h after rehydration.
Measurements after desiccation in Experiments II, III, and IV followed a similar pattern as described above. The probe was placed at a constant distance of 16 cm from the samples. The difference in distance between the samples and the probe was caused by the different diameters of the cultivation plate compared to the desiccation chamber.
Results
Ultrastructural examination of green akinetes revealed chloroplasts with starch grains localized to the periphery of the cell. The electron-dense lipid droplets containing astaxanthin were present only in the central part of the cell around the nucleus (Fig. 1a). In contrast the chloroplast of astaxanthin-rich red akinetes was considerably reduced and had a reticular appearance. Lipid droplets containing astaxanthin filled almost the entire cell (Fig. 1b). Both types of akinetes were surrounded by a thick (4–4.5 µm) fibrillar cell wall.
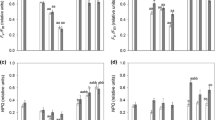
Green and red akinetes were desiccated at three different drying rates (86%, 43% and 10% rh) for 24 h (Experiment I). The effective quantum yield (ΦPSII) of green akinetes was generally higher than that of red akinetes throughout the experiment (Fig. 2). However, the temporal changes in the measured ΦPSII values were nearly identical for both cell types at all three desiccation rates. After transfer to the desiccation chamber, the values of ΦPSII increased steadily from 0.1–0.2 to 0.21–0.28 and dropped abruptly to 0.03–0.05 once the cells were completely dried. However, the values of FM′ and F decreased steadily throughout the desiccation process (data not shown). Lower relative humidity in the desiccation chamber resulted in faster desiccation. Samples were desiccated after 7 h at 86% rh (Fig. 2a), after 160 min at 43% rh (Fig. 2c), and after 90 min at 10% rh (Fig. 2e). A gradual increase in ΦPSII values after rehydration was observed in all samples (Fig. 2b, d, f). The ΦPSII values measured at 48 h after rehydration in some treatments were even higher than in the cultures at the beginning of the experiment. The first released zoospores (Table 2) were observed in all samples 24 h after rehydration, which confirmed at least partial viability of Haematococcus lacustris akinetes even after the strongest desiccation stress tested.
Changes in the effective quantum yield values during desiccation and subsequent rehydration using three different dehydration rates (Experiment I). A, B: samples desiccated at 86% rh over saturated KCl solution; C, D: samples desiccated at 43% rh over saturated K2CO3 solution; E, F: samples desiccated at 10% rh over partly dried silica gel. Circles and dotted lines represent green akinetes, triangles and dashed lines represent red akinetes. Means ± SD of four independent replicates
The Experiment II was performed to investigate the extent to which desiccation pretreatment at 30–50% rh for a period 1, 2, and 4 weeks affects the tolerance of red akinetes to different temperature exposures (− 1 °C, 25 °C and 40 °C for one hour). Red akinetes survived all combinations of the two factors tested: desiccation time vs. one-hour exposure to different temperatures; no mutual effect of the two factors was observed. The recovery of ΦPSII followed a similar pattern for all samples; only the samples maintained in the desiccated state for the longest time (4 weeks) showed slower recovery (Fig. 3). The individual treatments did not show any differences in the amount of released zoospores (Table 2).
Since the results of Experiment II showed almost no effect of moderate temperature stress on the recovery of the desiccated samples, the next series of experiments (Experiment III) was conducted to test the effects of extreme temperatures or very long desiccation times (see the summary of applied protocols in Table 1). Similarly, all samples survived, and the measured increase in ΦPSII values after rehydration showed an almost identical trend for all samples compared to the control sample (Fig. 4). The only observed difference was the apparently lower number of zoospores released in the samples exposed to 55 °C (Table 2). In addition, recovery of ΦPSII in samples kept in the desiccated state for 8 and 12 weeks demonstrated the ability of Haematococcus lacustris akinetes to survive very long periods of desiccation (Fig. 4c). However, the results show that the longer desiccation, the slower the increase in the levels of ΦPSII values. Moreover, the number of zoospores released was lower in samples desiccated for 12 weeks than after 8 weeks (Table 2).
Effective quantum yield values during recovery after various extreme conditions (Experiment III). A: the effect of high temperature; B: low temperature; C: prolonged period of desiccation, all treatments at 25 °C. Temperature stress was applied on the dessicated material for 1 h. Means ± SD of four independent replicates
Finally, the non-dessicated red akinetes removed from the liquid medium were exposed to temperatures of − 18 °C and 50 °C for one hour (Experiment IV). Short-term exposure to freezing at − 18 °C showed no signs of severe impact on Haematococcus lacustris akinetes (Fig. 5). The ΦPSII values measured after replacing the old medium with fresh BG-11 were even higher than the values measured for the samples desiccated and rehydrated at 25 °C. The observed effect of short-term exposure of non-desiccated samples to higher temperature (50 °C) was completely different. The values of ΦPSII increased only slightly, and no released zoospores were observed even 72 h after addition of the fresh medium (Table 2).
Discussion
Desiccation tolerance of green and red akinetes
Both green and astaxanthin-rich red akinetes recovered well from desiccation stress at all desiccation rates tested (at different relative humidity conditions). Desiccation tolerance is usually defined as the ability to survive desiccation up to 10% of the previous water content in the cells, which corresponds to the condition at 50% relative air humidity (Alpert, 2006). We did not measure the water content of the cells, but both red and green specialized cells survived desiccation even at 10% relative air humidity for 24 h. The desiccation tolerance of H. lacustris is probably primarily due to the thick cell wall containing algaenan (Montsant et al., 2001), which is present in both akinete types (green and red). Algaenan belongs to a group of aliphatic, nonhydrolyzable hydrocarbons found in the cell walls of various microalgae. It may be present in the cell walls of vegetative cells, e.g., in some members of the class Chlorophyceae (Blokker et al., 1998) or, more commonly, in certain stages of the life cycle that ensure survival under stressful conditions, e.g., in the zygospores of Mougeotia (Permann et al., 2021) or Chlamydomonas (Blokker et al., 1999).
Recently, Roach et al. (2022a) investigated desiccation tolerance of a non-motile Haematococcus strain during the exponential growth phase in liquid medium supplied with excessive nitrogen. Thus, green cells investigated in their study can be described as actively proliferating palmella stages, not akinetes. It was shown that such cells are not constitutively desiccation tolerant, but desiccation tolerance was acquired later by slow desiccation combined with nitrogen limitation (Roach et al., 2022a). On the other hand, green akinetes used in our study showed similar desiccation tolerance to red akinetes. In contrast to palmella stages, akinetes have a fully developed thick cell wall composed of algaenans. We obtained them from a two-month-old culture in the stationary growth phase, where we can assume nutrient limitation. Thus, our results suggest that formation of desiccation-tolerant green akinetes was induced in nitrogen limited medium. Similar results were observed in Zygnema, where nitrogen starvation induced the formation of desiccation-tolerant pre-akinetes, even in liquid cultures without acclimation by slow desiccation (Pichrtová et al., 2014).
The main difference between green and red cells is the lipid composition and astaxanthin content (Roach et al., 2022a). Astaxanthin accumulates in response to nitrogen deficiency in combination with high irradiation (Boussiba, 2000; Gwak et al., 2014; Roach et al., 2022a). Therefore, astaxanthin is also mainly involved in protection against high irradiation and coupled oxidative stress rather than desiccation tolerance (Hagen et al., 1993; Wang et al., 2003). A very similar life strategy is known from snow algae of the order Chlamydomonadales. Their life cycle also includes green motile stages and red spores with thick cell walls and a large accumulation of secondary carotenoids (Hoham & Remias, 2020). Red snow algal cysts were remarkably resistant to osmotic stress and formed aggregates with increased absorbance after drying (Holzinger et al., 2016).
Green akinetes exhibited visibly better preserved chloroplasts, resulting in slightly higher ΦPSII values than red akinetes, in all treatments. On the other hand, the ultrastructure of red akinetes showed considerable reduction of chloroplasts. Similarly, nitrogen-depleted pre-akinetes of Zygnema had clearly reduced chloroplast lobes and lower photophysiological performance than young vegetative cells (Herburger et al., 2015; Pichrtová et al., 2016). Interestingly, the addition of fresh medium led very quickly to the transition into the vegetative stage, the rapid release of zoospores, and increase of ΦPSII.
The effect of various combined stress conditions
The resistance of desiccated Haematococcus lacustris akinetes to different stress factors is very high. Desiccated akinetes were able to survive all treatments, even exposure to extreme temperatures of − 80 °C and 55 °C. Recovery of photosynthetic activity after rehydration was comparable at all temperatures studied, which may indicate that the stress levels we selected are still far above the actual limits of akinetes survival. The increase of the effective quantum yield also reflects the physiological activity of freshly released zoospores. Desiccation treatment may serve as a factor that increases resistance to extreme temperatures, as was previously shown for cells of Botryococcus braunii Kützing (Demura et al., 2014). Roach et al. (2022b) showed that Haematococcus akinetes desiccated at 50% rh maintained viability longer than those desiccated at higher humidity levels (80 and 92.5% rh). However, an oxygen-rich atmosphere, which promotes the production of reactive oxygen species, and temperature also influenced longevity.
The lower number of zoospores released from akinetes exposed to 55 °C (Experiment III) suggests that high temperature affects cell viability more severely than freezing. This was also confirmed by the results of the Experiment IV, where even the non-desiccated akinetes were more tolerant to freezing than to heat stress. Desiccation has similar effects on cells as freezing, since both lead to water loss and osmotic stress (Smirnoff, 1993). Acclimation strategies (e. g. specific cell wall composition) against one of these stresses might also help to tolerate the other one (Permann et al., 2022). On the other hand, the main stress effect of high temperatures is protein misfolding, which can be counteracted by specific molecular chaperones, also known as heat shock proteins (Kotak et al., 2007). For diatoms, exposure to heat also appeared to be more stressful than exposure to freezing temperatures (Souffreau et al., 2010).
Haematococcus akinetes also showed the ability to survive up to three months in the desiccated state at ambient temperature of 25 °C. However, such a long period proved to be very stressful and lethal for a large proportion of the cells. Therefore, recovery after rehydration and release of zoospores were also slower than in the other treatments. Drought is an important stress factor affecting the survival of various green algae (Gray et al., 2007; Lüttge & Büdel, 2010; Lewis & Trainor, 2012). However, Roach et al. (2022b) calculated that subzero storage of dried Haematococcus akinetes equilibrated to 50% rh should ensure their viability for centuries. The maintenance of cell viability in the dried state may be due to the accumulation of low molecular weight antioxidants (Roach et al., 2022a). Nevertheless, even a very small number of surviving cells can ensure the continuation of the algal population for the next vegetation period (Pichrtová et al., 2016).
The role of desiccation tolerance in Haematococcus ecology
Very high desiccation tolerance observed in Haematococcus lacustris akinetes is likely a key factor enabling a global distribution of this organism in different types of habitats, including flooded shallow depressions in rocks, so-called lithotelms (Hazen, 1899; Proctor, 1957; Pocock, 1960; Chekanov et al., 2014), cemeteries (Hazen, 1899; Proctor, 1957), birdbaths (Proctor, 1957; Pocock, 1960), or gutters (Pocock, 1960). All of these sites represent relatively isolated environments with island-like character. Haematococcus therefore requires well-developed dispersal mechanisms, such as desiccation resistance of akinetes, to successfully colonize. One of the means of dispersal is transport on the legs or feathers of birds, possibly also in their digestive tracts (Figuerola & Green, 2002; Cellamare et al., 2010). Transport by wind is probably very common; akinetes have been repeatedly found in samples taken from the air (Genitsaris et al., 2014).
Therefore, the main factor limiting the distribution of H. lacustris is probably its low competition abilities. This alga is observed only in exceptional cases in the phytoplankton of larger waterbodies (Proctor, 1957; Genitsaris et al., 2016). Desiccation tolerance thus allows the species to survive in environments that are limiting for many other algae. The desiccated akinetes of this alga survive the effects of both low and high extreme temperatures, and they survive in the desiccated state for a long time. Haematococcus lacustris can thus survive in both cold and warm, relatively dry areas, as long as there is at least occasional rainwater.
When pools of H. lacustris dry out, a large portion of the akinetes accumulates as a dense red biofilm on the pool walls (a phenomenon observed under both natural and laboratory conditions; Hazen, 1899; Peebles, 1909; Droop, 1956; Wan et al., 2014). One might think that it would be more benefitial for the akinetes to sink to the bottom of the tank and thus remain in the aquatic environment as long as possible. However, on hot, sunny days the water in the shallow pools may warm to temperatures too high for non-desiccated akinetes. Therefore, the formation of a dense biofilm of akinetes formed from zoospores that have previously accumulated on the surface due to their phototaxis could be critical for survival under these conditions. In addition, the formation of a biofilm could be beneficial due to slower desiccation rate and reduced exposure of cells in the inner layers of the biofilm to high radiation.
Akinetes of H. lacustris, with their ability to survive unfavorable conditions, assume the role of resistant life stage, which in other algae is usually represented by sexually produced spores. The comparatively uncomplicated asexual origin of akinetes is a major advantage for H. lacustris because conditions in ephemeral pools can change very rapidly and unpredictably (Pocock, 1960).
Conclusion
In this study, we have shown that Haematococcus lacustris survives desiccation at the stage of akinetes. Both green and red akinetes were found to be desiccation tolerant at all dehydration rates tested. Moreover, desiccated akinetes can cope very well with temperature extremes, and a certain proportion of cells even survive very long periods of desiccation. These features, together with probable low competition ability, predestine Haematococcus as a typical inhabitant of small ephemeral pools and basins.
The high desiccation resistance of H. lacustris akinetes could also prove useful in the laboratory. For example, if the culture is contaminated with less resistant algal species, rapid drying of a portion of the culture at low relative humidity over silica gel could potentially lead to elimination of the contamination. Another advantage is the possibility of cultivation on a moistened membrane with lower water consumption compared to other cultivation methods. Furthermore, mild desiccation stress combined with higher irradiation resulted in faster accumulation of astaxanthin in the cells (Wan et al., 2014; Zhang et al., 2014).
References
Allewaert, C. C., P. Vanormelingen, T. Pröschold, P. I. Gómez, M. A. González, G. Bilcke, S. D’Hondt & W. Vyverman, 2015. Species diversity in European Haematococcus pluvialis (Chlorophyceae, Volvocales). Phycologia 54(6): 583–598. https://doi.org/10.2216/15-55.1.
Alpert, P., 2006. Constraints of tolerance: why are desiccation-tolerant organisms so small or rare? Journal of Experimental Biology 209(9): 1575–1584. https://doi.org/10.1242/jeb.02179.
Blokker, P., 2000. Structural analysis of resistant polymers in extant algae and ancient sediments, Vol. 193. Utrecht University, Utrecht.
Blokker, P., S. Schouten, H. van den Ende, J. W. de Leeuw, P. G. Hatcher & J. S. S. Damsté, 1998. Chemical structure of algaenans from the fresh water algae Tetraedron minimum, Scenedesmus communis and Pediastrum boryanum. Organic Geochemistry 29(5–7): 1453–1468. https://doi.org/10.1016/s0146-6380(98)00111-9.
Blokker, P., S. Schouten, J. W. de Leeuw, J. S. S. Damsté & H. van den Ende, 1999. Molecular structure of the resistant biopolymer in zygospore cell walls of Chlamydomonas monoica. Planta 207: 539–543. https://doi.org/10.1007/s004250050515.
Boussiba, S., 2000. Carotenogenesis in the green alga Haematococcus pluvialis: cellular physiology and stress response. Physiologia Plantarum 108(2): 111–117. https://doi.org/10.1034/j.1399-3054.2000.108002111.x.
Boussiba, S., W. Bing, J. P. Yuan, A. Zarka & F. Chen, 1999. Changes in pigments profile in the green alga Haematococcus pluvialis exposed to environmental stresses. Biotechnology Letters 21(7): 601–604. https://doi.org/10.1023/A:1005507514694.
Cellamare, M., M. Leitão, M. Coste, A. Dutartre & J. Haury, 2010. Tropical phytoplankton taxa in Aquitaine lakes (France). Hydrobiologia 639(1): 129–145. https://doi.org/10.1007/s10750-009-0029-x.
Chan, K. C., M. C. Mong & M. C. Yin, 2009. Antioxidative and anti-inflammatory neuroprotective effects of astaxanthin and canthaxanthin in nerve growth factor differentiated PC12 cells. Journal of Food Science 74(7): 225–231. https://doi.org/10.1111/j.1750-3841.2009.01274.x.
Chekanov, K., E. Lobakova, I. Selyakh, L. Semenova, R. Sidorov & A. Solovchenko, 2014. Accumulation of astaxanthin by a new Haematococcus pluvialis strain BM1 from the White Sea coastal rocks (Russia). Marine Drugs 12(8): 4504–4520. https://doi.org/10.3390/md12084504.
Collins, A. M., H. D. T. Jones, D. Han, Q. Hu, T. E. Beechem & J. A. Timlin, 2011. Carotenoid distribution in living cells of Haematococcus pluvialis (Chlorophyceae). PLoS ONE 6(9): e24302. https://doi.org/10.1371/journal.pone.0024302.
Del Río, E., F. G. Acién, M. C. García-Malea, J. Rivas, E. Molina-Grima & M. G. Guerrero, 2005. Efficient one-step production of astaxanthin by the microalga Haematococcus pluvialis in continuous culture. Biotechnology and Bioengineering 91(7): 808–815. https://doi.org/10.1002/bit.20547.
Demura, M., M. Ioki, M. Kawachi, N. Nakajima & M. M. Watanabe, 2014. Desiccation tolerance of Botryococcus braunii (Trebouxiophyceae, Chlorophyta) and extreme temperature tolerance of dehydrated cells. Journal of Applied Phycology 26(1): 49–53. https://doi.org/10.1007/s10811-013-0059-7.
Domínguez-Bocanegra, A. R., I. G. Legarreta, F. M. Jeronimo & A. Campocosio, 2004. Influence of environmental and nutritional factors in the production of astaxanthin from Haematococcus pluvialis. Bioresource Technology 92(2): 209–214. https://doi.org/10.1016/j.biortech.2003.04.001.
Droop, M. R., 1956. Haematococcus pluvialis and its allies. I. the Sphaerellaceae. Revue Algologique 2: 53–71.
Elliott, A. M., 1934. Morphology and life history of Haematococcus pluvialis. Archiv Für Protistenkunde 82: 250–272.
Figuerola, J. & A. J. Green, 2002. Dispersal of aquatic organisms by waterbirds: a review of past research and priorities for future studies. Freshwater Biology 47: 483–494. https://doi.org/10.1046/j.1365-2427.2002.00829.x.
Genitsaris, S., M. Moustaka-Gouni & K. A. Kormas, 2011. Airborne microeukaryote colonists in experimental water containers: diversity, succession, life histories and established food webs. Aquatic Microbial Ecology 62(2): 139–152. https://doi.org/10.3354/ame01463.
Genitsaris, S., K. A. Kormas, U. Christaki, S. Monchy & M. Moustaka-Gouni, 2014. Molecular diversity reveals previously undetected air-dispersed protist colonists in a Mediterranean area. Science of the Total Environment 478: 70–79. https://doi.org/10.1016/j.scitotenv.2014.01.071.
Genitsaris, S., N. Stefanidou, M. Katsiapi, E. Vardaka, K. A. Kormas, U. Sommer & M. Moustaka-Gouni, 2016. Haematococcus: a successful air-dispersed colonist in ephemeral waters is rarely found in phytoplankton communities. Turkish Journal of Botany 40(4): 427–438. https://doi.org/10.3906/bot-1509-8.
Gray, D. W., L. A. Lewis & Z. G. Cardon, 2007. Photosynthetic recovery following desiccation of desert green algae (Chlorophyta) and their aquatic relatives. Plant, Cell & Environment 30(10): 1240–1255. https://doi.org/10.1111/j.1365-3040.2007.01704.x.
Greenspan, L., 1977. Humidity fixed points of binary saturated aqueous solutions. Journal of Research of the National Bureau of Standards. Section A Physics and Chemistry 81(1): 89–96. https://doi.org/10.6028/jres.081a.011.
Gu, W., X. Xie, S. Gao, W. Zhou, G. Pan & G. Wang, 2013. Comparison of different cells of Haematococcus pluvialis reveals an extensive acclimation mechanism during its aging process: from a perspective of photosynthesis. PLoS ONE 8(6): e67028. https://doi.org/10.1371/journal.pone.0067028.
Gwak, Y., Y. S. Hwang, B. Wang, M. Kim, J. Jeong, C. G. Lee, Q. Hu, D. Han & E. Jin, 2014. Comparative analyses of lipidomes and transcriptomes reveal a concerted action of multiple defensive systems against photooxidative stress in Haematococcus pluvialis. Journal of Experimental Botany 65(15): 4317–4334. https://doi.org/10.1093/jxb/eru206.
Hagen, C., W. Braune & F. Greulich, 1993. Functional aspects of secondary carotenoids in Haematococcus lacustris [Girod] Rostafinski (Volvocales) IV. Protection from photodynamic damage. Journal of Photochemistry and Photobiology B: Biology 20(2–3): 153–160. https://doi.org/10.1016/1011-1344(93)80145-y.
Hagen, C., S. Siegmund & W. Braune, 2002. Ultrastructural and chemical changes in the cell wall of Haematococcus pluvialis (Volvocales, Chlorophyta) during aplanospore formation. European Journal of Phycology 37(2): 217–226. https://doi.org/10.1017/s0967026202003669.
Hata, N., J. C. Ogbonna, Y. Hasegawa, H. Taroda & H. Tanaka, 2001. Production of astaxanthin by Haematococcus pluvialis in a sequential heterotrophic-photoautotrophic culture. Journal of Applied Phycology 13(5): 395–402. https://doi.org/10.1023/A:1011921329568.
Hazen, T. E., 1899. The life history of Sphaerella lacustris. Memoirs of the Torrey Botanical Club 6: 211–244. https://doi.org/10.5962/bhl.title.97555.
Herburger, K., L. A. Lewis & A. Holzinger, 2015. Photosynthetic efficiency, desiccation tolerance and ultrastructure in two phylogenetically distinct strains of alpine Zygnema sp. (Zygnematophyceae, Streptophyta): role of pre-akinete formation. Protoplasma 252(2): 571–589. https://doi.org/10.1007/s00709-014-0703-3.
Hoham, R. W. & D. Remias, 2020. Snow and glacial algae: a review. Journal of Phycology 56(2): 264–282. https://doi.org/10.1111/jpy.12952.
Holzinger, A., M. C. Allen & D. D. Deheyn, 2016. Hyperspectral imaging of snow algae and green algae from aeroterrestrial habitats. Journal of Photochemistry and Photobiology B: Biology 162: 412–420. https://doi.org/10.1016/j.jphotobiol.2016.07.001.
Jyonouchi, H., S. Sun, K. Iijima & M. D. Gross, 2000. Antitumor activity of astaxanthin and its mode of action. Nutrition and Cancer 36(1): 59–65. https://doi.org/10.1207/s15327914nc3601_9.
Karsten, U., K. Herburger & A. Holzinger, 2014. Dehydration, temperature, and light tolerance in members of the aeroterrestrial green algal genus Interfilum (Streptophyta) from biogeographically different temperate soils. Journal of Phycology 50(5): 804–816. https://doi.org/10.1111/jpy.12210.
Kotak, S., J. Larkindale, U. Lee, P. von Koskull-Döring, E. Vierling & K. D. Scharf, 2007. Complexity of the heat stress response in plants. Current Opinion in Plant Biology 10(3): 310–316. https://doi.org/10.1016/j.pbi.2007.04.011.
Kuhl, A. & H. Lorenzen, 1964. Handling and culturing of Chlorella. In Methods in Cell Biology, Vol. 1. Academic Press, Cambridge, 159–187.
Lewis, L. A. & F. R. Trainor, 2012. Survival of Protosiphon botryoides (Chlorophyceae, Chlorophyta) from a Connecticut soil dried for 43 years. Phycologia 51(6): 662–665. https://doi.org/10.2216/11-108.1.
Lorenz, R. T. & G. R. Cysewski, 2000. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends in Biotechnology 18(4): 160–167. https://doi.org/10.1016/s0167-7799(00)01433-5.
Lüttge, U. & B. Büdel, 2010. Resurrection kinetics of photosynthesis in desiccation-tolerant terrestrial green algae (Chlorophyta) on tree bark. Plant Biology 12(3): 437–444. https://doi.org/10.1111/j.1438-8677.2009.00249.x.
Montsant, A., A. Zarka & S. Boussiba, 2001. Presence of a nonhydrolyzable biopolymer in the cell wall of vegetative cells and astaxanthin-rich cysts of Haematococcus pluvialis (Chlorophyceae). Marine Biotechnology 3: 515–521. https://doi.org/10.1007/s1012601-0051-0.
Naguib, Y. M. A., 2000. Antioxidant activities of astaxanthin and related carotenoids. Journal of Agricultural and Food Chemistry 48(4): 1150–1154. https://doi.org/10.1021/jf991106k.
Nakada, T. & S. Ota, 2016. What is the correct name for the type of Haematococcus Flot (Volvocales, Chlorophyceae)? Taxon 65(2): 343–348.
Okai, Y. & K. Higashi-Okai, 1996. Possible immunomodulating activities of carotenoids in in vitro cell culture experiments. International Journal of Immunopharmacology 18(12): 753–758. https://doi.org/10.1016/s0192-0561(97)85558-0.
Park, J. S., B. D. Mathison, M. G. Hayek, J. Zhang, G. A. Reinhart & B. P. Chew, 2013. Astaxanthin modulates age-associated mitochondrial dysfunction in healthy dogs. Journal of Animal Science 91(1): 268–275. https://doi.org/10.2527/jas.2012-5341.
Park, J. C., S. P. Choi, M. E. Hong & S. J. Sim, 2014. Enhanced astaxanthin production from microalga, Haematococcus pluvialis by two-stage perfusion culture with stepwise light irradiation. Bioprocess and Biosystems Engineering 37: 2039–2047. https://doi.org/10.1007/s00449-014-1180-y.
Peebles, F., 1909. The life history of Sphaerella lacustris with special reference to the nature and behavior of the zoospores. Centralblatt Für Bacteriologie, Parasitenkunde Und Infektionskrankheiten 2(24): 511–521.
Permann, C., K. Herburger, M. Niedermeier, M. Felhofer, N. Gierlinger & A. Holzinger, 2021. Cell wall characteristics during sexual reproduction of Mougeotia sp. (Zygnematophyceae) revealed by electron microscopy, glycan microarrays and RAMAN spectroscopy. Protoplasma 258(6): 1261–1275. https://doi.org/10.1007/s00709-021-01659-5.
Permann, C., B. Becker & A. Holzinger, 2022. Temperature- and light stress adaptations in Zygnematophyceae: the challenges of a semi-terrestrial lifestyle. Frontiers in Plant Science 13: 945394. https://doi.org/10.3389/fpls.2022.945394.
Pichrtová, M., J. Kulichová & A. Holzinger, 2014. Nitrogen limitation and slow drying induce desiccation tolerance in conjugating green algae (Zygnematophyceae, Streptophyta) from polar habitats. PLoS ONE 9(11): e113137. https://doi.org/10.1371/journal.pone.0113137.
Pichrtová, M., E. Arc, W. Stöggl, I. Kranner, T. Hájek, H. Hackl & A. Holzinger, 2016. Formation of lipid bodies and changes in fatty acid composition upon pre-akinete formation in Arctic and Antarctic Zygnema (Zygnematophyceae, Streptophyta) strains. FEMS Microbiology Ecology 92: fiw096. https://doi.org/10.1093/femsec/fiw096.
Pocock, M. A., 1960. Haematococcus in Southern Africa. Transactions of the Royal Society of South Africa 36: 5–55. https://doi.org/10.1080/00359196009519031.
Proctor, V. W., 1957. Some controlling factors in the distribution of Haematococcus pluvialis. Ecology 38(3): 457–462. https://doi.org/10.2307/1929890.
Rascher, U., M. Liebig & U. Lüttge, 2000. Evaluation of instant lightresponse curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. Plant, Cell & Environment 23: 1397–1405. https://doi.org/10.1046/j.1365-3040.2000.00650.x.
Rippka, R. & M. Herdman, 1992. Pasteur culture collection of cyanobacterial strains in axenic culture. Catalogue and taxonomic handbook, catalogue of strains 1992/1993, 1: 1–103.
Roach, T., N. Böck, N. Rittmeier, E. Arc, I. Kranner & A. Holzinger, 2022a. Acquisition of desiccation tolerance in Haematococcus pluvialis requires photosynthesis and coincides with lipid and astaxanthin accumulation. Algal Research 64: 102699. https://doi.org/10.1016/j.algal.2022.102699.
Roach, T., A. Fambri & D. Ballesteros, 2022b. Humidity and light modulate oxygen-induced viability loss in dehydrated Haematococcus lacustris cells. Oxygen 2(4): 503–517. https://doi.org/10.3390/oxygen2040033.
Roháček, K. & M. Barták, 1999. Technique of the modulated chlorophyll fluorescence: basic concepts, useful parameters, and some applications. Photosynthetica 37(3): 339–363. https://doi.org/10.1023/a:1007172424619.
Saha, S. K., E. McHugh, J. Hayes, S. Moane, D. Walsh & P. Murray, 2013. Effect of various stress-regulatory factors on biomass and lipid production in microalga Haematococcus pluvialis. Bioresource Technology 128: 118–124. https://doi.org/10.1016/j.biortech.2012.10.049.
Santos, M. F. & J. F. Mesquita, 1984. Ultrastructural study of Haematococcus lacustris (Girod.) Rostafinski (Volvocales) I. Some aspects of carotenogenesis. Cytologia 49: 215–228. https://doi.org/10.1508/cytologia.49.215.
Sarada, R., U. Tripathi & G. Ravishankar, 2002. Influence of stress on astaxanthin production in Haematococcus pluvialis grown under different culture conditions. Process Biochemistry 37: 623–627. https://doi.org/10.1016/s0032-9592(01)00246-1.
Smirnoff, N., 1993. Tansley Review No. 52 the role of active oxygen in the response of plants to water deficit and desiccation. New Phytologist 125: 27–58. https://doi.org/10.1111/j.1469-8137.1993.tb03863.x.
Souffreau, C., P. Vanormelingen, E. Verleyen, K. Sabbe & W. Vyverman, 2010. Tolerance of benthic diatoms from temperate aquatic and terrestrial habitats to experimental desiccation and temperature stress. Phycologia 49(4): 309–324. https://doi.org/10.2216/09-30.1.
Spurr, A. S., 1969. A low viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructure Research 26: 31–43. https://doi.org/10.1016/s0022-5320(69)90033-1.
Tjahjono, A. E., Y. Hayama, T. Kakizono, Y. Terada, N. Nishio & S. Nagai, 1994. Hyper-accumulation of astaxanthin in a green alga Haematococcus pluvialis at elevated temperatures. Biotechnology Letters 16(2): 133–138. https://doi.org/10.1007/bf01021659.
Triki, A., P. Maillard & C. Gudin, 1997. Gametogenesis in Haematococcus pluvialis Flotow (Volvocales, Chlorophyta). Phycologia 36(3): 190–194. https://doi.org/10.2216/i0031-8884-36-3-190.1.
Wan, M., D. Hou, Y. Li, J. Fan, J. Huang, S. Liang, W. Wang, R. Pan, J. Wang & S. Li, 2014. The effective photoinduction of Haematococcus pluvialis for accumulating astaxanthin with attached cultivation. Bioresource Technology 163: 26–32. https://doi.org/10.1016/j.biortech.2014.04.017.
Wang, B., A. Zarka, A. Trebst & S. Boussiba, 2003. Astaxanthin accumulation in Haematococcus pluvialis (Chlorophyceae) as an active photoprotective process under high irradiance. Journal of Phycology 39(6): 1116–1124. https://doi.org/10.1111/j.0022-3646.2003.03-043.x.
Wayama, M., S. Ota, H. Matsuura, N. Nango, A. Hirata & S. Kawano, 2013. Three-dimensional ultrastructural study of oil and astaxanthin accumulation during encystment in the green alga Haematococcus pluvialis. PLoS ONE 8(1): e53618. https://doi.org/10.1371/journal.pone.0053618.
Zhang, W., J. Wang, J. Wang & T. Liu, 2014. Attached cultivation of Haematococcus pluvialis for astaxanthin production. Bioresource Technology 158: 329–335. https://doi.org/10.1016/j.biortech.2014.02.044.
Acknowledgements
This work was supported by the institutional grant of the Charles University, Prague "Cooperatio Biology", by Charles University Research Centre Program No. 204069 and by the Czech Science Foundation Project No. 22-20989S.
Funding
Funding was provided by Grantová Agentura České Republiky (22-20989S), Charles University Research Centre (204069), and Univerzita Karlova v Praze (Cooperatio).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Handling editor: Judit Padisak
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Online resource 1
Representative microscopic images of the green and red akinetes used in this study (JPG 1501 kb)
Supplementary file1 (JPG 1501 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vávrová, K., Nemcova, Y. & Pichrtová, M. Desiccation and temperature tolerance of green and red Haematococcus lacustris (Chlamydomonadales, Chlorophyta) akinetes. Hydrobiologia 851, 1169–1181 (2024). https://doi.org/10.1007/s10750-023-05381-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05381-6