Abstract
We present a detailed study of spatial distribution of nutrient elements, phytoplankton and zooplankton in the Cheboksary Reservoir—a eutrophic artificial lake in Central Russia—during summer periods of 2017–2021 and review the relationships between these elements of the food chain to determine what shapes the trophic state of the lake. It is shown that vast differences in nutrient content of the reservoir’s tributaries cause its trophic state to change significantly in mouth areas of some larger inflowing rivers. However, in the largest part of the lake the relationship between nutrient content and chlorophyll becomes more subtle, whereas zooplankton community structure steps up as an important factor controlling the phytoplankton growth. It is clear that major efforts in nutrient loading reduction are needed to improve the ecological state of the reservoir, and nitrogen control may be more effective, than phosphorus control. However, described trophic interactions may reduce efficiency of nutrient control and require some biomanipulation measures to improve water quality of the reservoir.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
External nutrient loading as a factor controlling eutrophication in lakes and reservoirs is one of the most meticulously studied topics in modern limnology. In multiple experimental and monitoring studies, input of phosphorus and nitrogen have been shown to have direct impact on phytoplankton biomass (Elser et al., 1990; Ahoutou et al., 2022; Xie & Xie, 2002; Xu et al., 2010). These correlations have been used in environmental restoration projects on multiple natural lakes, ponds and reservoirs around the world: in most cases, decreasing TP and TN loads into a lake can noticeably mitigate manifestations of eutrophication, such as lowered water transparency, prolonged hypolimnetic hypoxia, high phytoplankton biomass and shifts towards cyanobacterial communities (Jeppesen et al., 2005; Zeng et al., 2016; Huang et al., 2019).
Control over nutrient input into rivers and lakes is now a key objective of water quality management around the world, with guidelines for nitrogen and phosphorus concentrations in surface waters being strictly enforced in most of the countries (Dodds & Welch, 2000; Carvalho et al., 2013; Poikane et al., 2019; Olokotum et al., 2020). However, the issue of excessive nutrient loading still exists even in countries with most rigorous wastewater management and water quality monitoring frameworks due to the poorly controllable nature of nutrient input from watersheds (Granlund et al., 2005; Carey et al., 2013), as a huge part of nitrogen and phosphorus comes into surface waters from non-point sources: fertilized agricultural lands, pastures, residential areas without centralized wastewater collection and treatment, and some other types of land use (Kronvang et al. 2005; Chambers et al., 2012). Moreover, thresholds of acceptable phosphorus and nitrogen inputs should be determined individually for each lake, and the effect of combined P and N limitation is still not entirely understood (Xu et al., 2015; Maberly et al., 2020; Poikane et al., 2022).
Nutrient loading in natural lakes overlaps and interacts with other in-lake processes and external impacts. Many physical factors, such as water temperature, flow velocities, mixing intensity and light availability may affect phytoplankton growth rates and spatial distribution and are defined by weather and hydrology of inflows, which can show high temporal variability but have little to none opportunities for management and control (Na & Park, 2006; Berger et al., 2010; Liu et al., 2016; Bouffard et al., 2018). In isolated bays of reservoir tributaries, intensity of water exchange, as well as wind mixing conditions, can differ significantly from the entire water body, causing accumulation of nutrients and increased sensitivity of bay areas to nutrient concentrations in inflow, while other parts of a reservoir can be much less affected by fluctuations in nutrient loading (Selge et al., 2016). After land cover alterations or during floods, phytoplankton communities in proximity to river mouths can become limited by light availability due to increased mineral turbidity or input of colored organic matter (Bergström & Karlsson, 2019; Gebrehiwot et al., 2020).
Interaction with higher levels of food chain also has a capacity to limit phytoplankton growth through zooplankton grazing and other interactions within the trophic cascade (Carpenter et al., 2001). Some species of zooplankton can even develop tolerance to toxic cyanobacteria (Gustafsson & Hansson, 2004; Ger et al., 2016). This provides biomanipulation opportunities for eutrophication control, such as reducing phytoplankton biomass by removing or introducing certain species of fish (Elser & Goldman, 1991; Xie & Liu, 2001; Kasprzak et al., 2007; Peretyatko et al., 2009), therefore decreasing predation pressure on grazing zooplankton and increasing phytoplankton removal by grazers, although this effect is also interconnected with external nutrient loading.
Accurate assessments of nutrient loading effect on productivity of freshwater ecosystems are further hindered by different responses to nutrient management by lakes with various initial trophic states. In oligotrophic lakes, various combinations of both bottom-up (related to resource limitation) and top-down (related to predatory control from higher levels of the food web) drivers of phytoplankton growth may be observed, whereas in eutrophic lakes sometimes no definitive effect of either factor can be clearly observed (McQueen et al., 1989; Rose et al., 2021). Historical development and trophic status of a lake also determine nutrient exchange between water column and bottom sediments, and release of phosphorus accumulated in sediments of eutrophic lakes can hinder de-eutrophication even as external nutrient loading is reduced (Lee et al., 2019).
In Russia, eutrophication is a widespread but not efficiently managed issue. There is evidence of overall deterioration of water quality linked to eutrophication occurring in all regions of Russia (Belykh et al., 2013; Ovaskainen et al., 2019; Namsaraev et al., 2020), but the most extreme situation, associated with harmful algal blooms and the most drastic changes in aquatic ecosystems is observed in densely populated regions of European Russia, including the Volga River Basin (Nikanorov & Khoruzhaya, 2014; Rumyantsev et al., 2018; Chernova et al., 2020). Several government programs have been introduced in attempts to improve the water quality of the Volga River reservoirs (Rozenberg et al., 2017), but federal water quality management mostly relies on standard control over pollutant discharges and concentrations (Venitsianov, 2019). At the same time, detailed ecological studies in this region are scarce, rarely go beyond chemical pollution, and only a handful of works are dedicated to more than one trophic level (Mineeva et al., 2008; Lazareva et al., 2014). We believe that a comprehensive study linking phytoplankton productivity of the Volga River reservoirs with physical and chemical factors and parameters of the zooplankton community would improve our understanding of environmental processes affecting its ecological state, reveal limitations of nutrient loading control measures in eutrophication management, and contribute to global understanding of in-lake processes in temperate lakes under high anthropogenic pressure.
Materials and methods
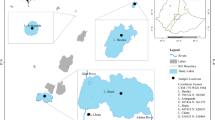
Study area
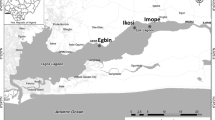
The Cheboksary Reservoir is a part of the Volga-Kama reservoir cascade, disconnected from the upstream Gorky Reservoir by an almost free-flowing part of the Volga River. It is traditionally considered that the transition from riverine to lacustrine conditions begins at the Volga’s confluence with the Oka River at Nizhniy Novgorod, and the typical lacustrine part of the reservoir extends from the mouth of the Sura River to the Cheboksary Dam in Novocheboksarsk (Fig. 1). For the transitional part, maximum depths of 10–15 m and width of 1–2 km are typical. During summer low flow of 2019, stream velocities of 0.4–0.5 m/s were observed in the reservoir downstream from the Oka River mouth, and by the confluence with Vetluga they decreased to 0.17–0.23 m/s (Kapustin et al., 2019). At the lacustrine part, depths can exceed 20 m and the maximum width reaches a little over 10 km.
Total surface area of the reservoir from Nizhniy Novgorod to Novocheboksarsk is 1213 km2, total watershed area—604,000 km2, mean annual flow at the dam is 3490 m3/s. The Oka River basin makes up about 40% of reservoir’s watershed area and total river inflow with watershed area of 244,000 km2 and mean annual discharge of 1255 m3/s. Rivers Sura and Vetluga each contribute to about 7% of river inflow, and other tributaries combined account for less than 3% of water inflow.
The data used in this article was obtained during annual field expeditions of the Russian Geographical Society on the Volga reservoir cascade in 2017–2021. Duration and scope of each expedition are listed in Table 1. The route started at the Oka River about 4 km above its mouth, and a unified sampling grid at the reservoir was maintained during each expedition (see Fig. 1), with some additional stations investigated in some years. Sampling on tributaries was carried out at upper parts of their mouth areas where little to no effect of mixing with reservoir water was observed.
Sampling and analytical methods
At each sampling station, water temperature, specific conductivity (SPC), pH and dissolved oxygen content were measured in situ with YSI ProODO, Pro30 and Pro10 sensors. The Secchi depth (SD) was determined by the same observer throughout the entire study. Water samples for chemical analyses were taken from 0.5 m depth and, in case of significant differences in physical parameters between surface and bottom, also from the bottom layer. About 0.5 L of each sample was filtered through a 0.45 μm membrane filter with magnesium carbonate for chlorophyll extraction. Another portion of water was filtered through a clean filter to separate dissolved forms of nutrient elements.
Samples for determination of phytoplankton quantity and species composition were taken from the surface layer and fixed by adding formaldehyde-iodine solution. Zooplankton samples were taken by dragging a 70 μm mesh net from bottom to the surface and fixed in 4% formaldehyde.
Concentrations of chlorophyll and nutrient elements were determined by photometric methods: chlorophyll-a concentration (Chl)—after acetone extraction, total and phosphate phosphorus (TP and DIP, respectively)—by the Murphy–Riley method, total nitrogen (TN)—by alkaline persulfate digestion. DIP and TN concentrations were measured beginning from 2018, TDN—from 2019.
Phytoplankton and zooplankton samples were examined in the Research Laboratory of Aquatic Ecosystems of the Lobachevsky Nizhny Novgorod State University’s Department of Ecology. Samples were analyzed under a Zeiss Stemi 2000C stereomicroscope (Carl Zeiss Microscopy, Germany), and an Olympus CX43 microscope (Olympus Corporation, Japan) was used for more detailed morphological analysis of species. Modern literary guides and keys were used in species identification (Hudec, 2010; Błędzki & Rybak, 2016; Rogers & Thorp, 2019; Korovchinsky et al., 2021).
Data processing methods
The trophic state index (TSI) was calculated from chlorophyll (Chl), total phosphorus (TP) and nitrogen (TN) concentrations, as well as (SD based on relations described in (Carlson, 1977):
In accordance with Carlson, waters with TSI < 40 were considered oligotrophic, 40–50—mesotrophic, 50–70—eutrophic, TSI > 70—hypertrophic.
A multivariate comparison of deviations between TSI(Chl), TSI(TP) and TSI(SD) (analysis of trophic state index deviation) was also made as suggested by Carlson (1992) to outline systematic patterns linked to phosphorus limitation and different genesis of suspended particles.
To assess spatial heterogeneity of chemical and biological parameters and outline various water masses and plankton communities within the reservoir, methods of cluster analysis were applied. Water masses were determined using centered and scaled vectors of SPC, TN, TP and Si concentrations with Euclidean distance as a distance metric. For zooplankton communities, the cosine of the angle between the species abundances vectors was used (Borcard et al., 2011). Average linkage hierarchical clustering was applied in both cases, and the optimal number of clusters was selected based on silhouette width analysis and Mantel cluster correlations. All calculations for cluster analysis were performed in R (packages “stats”, “cluster”, “vegan”).
Spearman rank correlation was used to determine statistical links between chlorophyll concentration and various chemical and physical parameters of water.
Results
Nutrient content and trophic state of reservoir’s tributaries
The upper part of the Cheboksary Reservoir from the Gorky Dam to the mouth of the Oka River (hereinafter referred to as the Volga) is characterized by relatively low concentrations of nutrient elements and chlorophyll compared to reservoir’s tributaries (Fig. 2). Its chemical profile is mostly shaped by biochemical processes in the Gorky Reservoir. At Nizhny Novgorod, concentrations of TP ranged between 0.053 and 0.077 mg/l, DIP—between 0.003 and 0.041 mg/l, TN—0.76 and 1.07 mg/l, Si—0.52 and 1.93 mg/l. TN/TP ratio in Volga water in 2019–2021 lied within the range of 10–15. Concentration of chlorophyll ranged between 5.72 and 18.4 μg/l, mostly classifying the Volga in mesotrophic category, and only exceeded 7 μg/l in 2021. Interannual variation in total nutrient content in the Volga was rather low, but DIP content ranged from negligible concentrations in 2018–2019 to about a half of TP in 2020–2021.
Small left-side tributaries mostly contained more nutrient elements than the Volga itself. The Kerzhenets River in 2017–2019 contained 0.044–0.161 mg/l of TP and 1.85–6.29 mg/l of Si. DIP concentration in 2018–2019 was 0.003–0.011 mg/l and TN concentration in 2019 was 0.585 mg/l. Concentration of chlorophyll ranged between 10.6 and 11.8 μg/l (mesotrophic). An even smaller Vatoma River was sampled in 2020 and contained even more nutrient elements: TP concentration reached 0.224 mg/l, DIP—0.052 mg/l, TN—1.56 mg/l, Si—10.1 mg/l. At the same time, chlorophyll concentration there was below 3 μg/l, ranging it in oligotrophic category.
The largest of left-side tributaries—the Vetluga River—had similar N and P concentrations as the Volga River: in 2019–2021, TP concentration was within 0.039–0.078 mg/l, DIP—0.003–0.021 mg/l, TN—0.48–0.89 mg/l. Concentration of Si was much higher than in the Volga—2.70–4.18 mg/l, and chlorophyll concentration had the highest range of all left-side tributaries—7.8–26.7 μg/l (meso-eutrophic). Like the Volga, the Vetluga River was characterized by DIP concentration close to detection limit in 2019, but in 2020–2021 its content was much higher (16–32% of TP). Interannual variation of total nitrogen and phosphorus content in all of the tributaries generally followed the same pattern, as far as our data allowed to see: higher nutrient content was observed in 2017 and 2020–2021, while in 2018–2019 it was noticeably lower.
The TN:TP ratio in Vetluga and Vatoma in 2020 was 7.0–8.7, but for Kerzhenets in 2019 and Vetluga in 2019 and 2021 that ratio was higher (11.6–13.3).
Right-side tributaries, including the Oka River, in general had even higher nutrient concentrations and chlorophyll content. In the Oka River, TP concentration was 0.115–0.217 mg/l, DIP—0.005–0.101 mg/l, TN—1.19–1.49 mg/l, Si—0.54–3.48 mg/l. TN:TP ratio was 10.3 in 2019 and 6.3–6.9 in 2020–2021, chlorophyll concentration varied between 37.4 and 65.1 μg/l (eutrophic-hypereutrophic). The Oka followed the same pattern of interannual variation of DIP as the Volga: in 2018–2019 its content was below 0.005–0.010 mg/l, whereas in 2020–2021 it accounted for 34–47% of TP.
In the Kudma River, TP content on 2017–2019 was within 0.087–0.174 mg/l, DIP—0.036–0.081 mg/l, Si—6.66–8.60 mg/l, TN concentration in 2019 was 2.0 mg/l. The Sundovik River (watershed area 1120 km2) was sampled only in 2019: TP concentration was 0.083 mg/l, DIP—0.036 mg/l, TN—0.59 mg/l, Si—6.58 mg/l. The Tsivil River was sampled in 2021: TP concentration was 0.130 mg/l, DIP—0.106 mg/l, TN—1.148 mg/l, Si—2.8 mg/l. Chlorophyll content in the Kudma River ranged within 9.0–26.6 μg/l (eutrophic), in the Sundovik its concentration was only 4.1 μg/l (mesotrophic), in the Tsivil—up to 50 μg/l (eutrophic). TN:TP ratio in the Sundovik and the Tsivil was within 4.9–7.1, in the Kudma in 2019—around 23.
The largest right-side tributary—the Sura River—had TP concentrations of 0.140–0.231 mg/l, DIP—0.032–0.144 mg/l, TN—0.73–1.27 mg/l, Si—7.9–11.1 mg/l. It had TN:TP ratio of 5.2–6.2 and chlorophyll content of 12.4–35.1 μg/l, which corresponds to a eutrophic state.
DIP concentrations in right-side tributaries (apart from the Oka) were rather high in 2018–2019 and did not drop to levels observed in left-side tributaries, but in 2020–2021 they were significantly higher. TN and TP concentrations were also lower in 2018–2019 and higher in 2017 and 2020–2021.
Spatial distribution of nutrients and chlorophyll in the reservoir
At the confluence of the Volga River with the Oka, due to their comparable flow rates and speeds, as well as differences in water density, lateral mixing is slow, allowing their water masses to not fully mix for dozens of kilometers (Table 2). At Nizhniy Novgorod, SPC in 2017–2021 was 2–3 times higher at the right bank than at the left bank, TP concentration was 1.8–3.7 times higher, DIP—1.3–11.6 times higher, TN—1.1–1.4 times higher, chlorophyll content—2.2–9.9 times higher. Ratio between Si concentration at right and left bank varied between years from 0.4 to 3.8.
At some 15 km below the Oka-Volga confluence, concentrations of nutrient elements in right and left parts of the reservoir barely changed compared to their content in both rivers upstream, showing little to no signs of mixing between their waters. However, chlorophyll content at left bank increased significantly to 4.7–18.8 μg/l and at right bank slightly decreased to 32.0–58.2 μg/l. Moving farther downstream, nutrient concentrations and chlorophyll content begin to even out between left and right banks. At km 35, TP concentration at right bank was only 1.5–2.2 times higher than at left bank, DIP—1.6–2.4 times higher, TN—1.3–1.8 times higher, Chl—1.8–3.2 times higher.
Differences in chlorophyll content between two banks of the reservoir become negligible at about 90 km downstream from the Oka (close to the Kerzhenets mouth). In 2018–2021, chlorophyll concentrations there were 9.1–28.1 μg/l. At the same time, nutrient concentrations at right bank remained noticeably higher: 1.1–1.4 times for TP, 1.1–1.3 times for TN, up to 1.6 times for DIP.
Sustainable differences between nutrient concentrations at right and left bank disappeared at 120 km mark. In the reservoir section from total Oka-Volga mixing to the mouth of the Sura River, TP concentrations were 0.072–0.158 mg/l, DIP—0.002–0.083 mg/l, TN—0.89–1.18 mg/l, Si—0.87–2.81 mg/l. Chlorophyll concentrations there ranged within 6.8–42.1 μg/l.
At the section from the Sura to Vetluga mouth, nutrient concentrations were higher: TP concentration was 0.088–0.243 mg/l, DIP—0.002–0.114 mg/l, TN—0.89–1.38 mg/l, Si—1.14–10.76 mg/l. In contrast, chlorophyll concentrations below the Sura dropped to 5.5–18.9 μg/l.
The last part of the reservoir (below the Vetluga mouth) was characterized by even higher mean nutrient concentrations and relatively high spatial variation of chemical composition of water, but no regular patterns were found. TP in this part ranged within 0.067–0.323 mg/l, DIP—0.013–0.121 mg/l, TN—0.84–2.99 mg/l, Si—1.26–3.06 mg/l. Range of chlorophyll concentration increased to 3.5–48.5 μg/l.
By hierarchical clustering, four parts of the reservoir were outlined in 2019–2021 based on SPC, TN, TP and Si concentrations (Figs. 3, 4). The first part covered a small right-side part of the reservoir below the mouth of the Oka River where its water did not mix with the Volga water. In 2019–2020 it reached 15 km below the Oka-Volga confluence, in 2021—dissipated almost immediately. Other three water masses were composed of gradually transformed Volga water. The limit between second and third parts in different years lied between 35 and 60 km downstream from the Oka mouth; the third water mass was characterized by generally higher salt and nutrient content, presumably indicating full Oka-Volga mixing. The limit between third and fourth masses shifted between ± 15 km from the Sura mouth, with largest differences observed in Si concentration and SPC. The three distinct sections of the Volga water were confidently outlined in all analyzed years, indicating important shifts between chemical profiles of different parts of the reservoir.
Trophic state and TN:TP ratio in the reservoir
The TSI value calculated by the cholorophyll content (TSI(Chl)) in the reservoir in 2017–2021 ranged from 42.9 to 72.2. The highest spatial contrast was observed at the confluence of Oka and Volga, where the left side was mesotrophic in all years (TSI 47.7–49.7), and the right side was eutrophic-hypereutrophic (TSI 56.9–72.2). At km 15 below the confluence, however, the left side of the reservoir was already characterized as eutrophic in all years except 2020, while right-side part remained strongly eutrophic and hypereutrophic. At 35–155 km, both sides almost always fell into the eutrophic category with few outliers (TSI 49.4–71.8).
A noticeable decline of TSI and its variability was observed below the Sura mouth, with TSI(Chl) dropping to 47.3–59.4. Below the Vetluga mouth, average TSI values remained about the same, but its range increased to 42.9–68.7.
The TN:TP ratio in the reservoir ranged from 4.9 to 14.6. Much like the trophic state, this ratio differed greatly between right and left bank just below the confluence of Oka and Volga (12.3–14.6 at left bank and 6.2–8.9 at right bank), but this difference was quickly evened out downstream. Starting at km 35 from the confluence and up to the dam, TN:TP values only differed slightly and typically lied within 5–10. Of all observations, 2019 stood out with TN:TP values reaching 9.0–13.9 throughout the reservoir.
The full analysis of trophic state deviations based on different trophic state indicators was performed for the entire reservoir on data of 2020–2021, and is presented in Table 3. As seen on Fig. 5, 94% of all observations were characterized by TSI(Chl) < TSI(TP), suggesting consistent phosphorus surplus in the reservoir ecosystem. In both years, this deviation tended to increase in downstream direction. The nitrogen TSI deviation tended to be the opposite: in majority of observations, TSI(Chl) was larger than TSI(TN). In 2020, TSI(Chl)–TSI(TN) difference in the section from the Oka to Sura mouth ranged within − 5.0 to 14.5 and in the section from the Sura to the dam—within − 13.1 to 0.2. In 2021, this difference ranged from − 6.1 to 19.8.
The 2 years differed greatly in SD TSI deviation. In 2020, over 60% of observations had TSI(Chl) < TSI(SD), with the TSI(Chl)–TSI(SD) difference spreading evenly between –10 and 12, indicating that on average, light attenuation was dominated by small algae. In 2021, this difference ranged from − 2.3 to 33.5, and over 87% of observations had TSI(Chl) > TSI(SD), suggesting domination of large algal particles in light attenuation.
Correlations between chlorophyll and nutrient concentrations
Statistical dependence between chlorophyll concentration and nutrient content, as well as water temperature, SPC and SD, varied greatly between different parts of the reservoir (Table 4). For the upper right-bank side (The Oka flow), only a slight positive correlation of chlorophyll with TP concentration was observed, while in the left-side (Volga) flow it correlated with TN, TP, DIP, TPP, and water temperature with high correlation coefficient values. In a large portion of the reservoir, including all of the transitional zone, no statistically significant correlations were detected with any of the studied factors, while in the lake section the situation was close to what was observed in the Volga water: relatively strong positive connection was found between Chl and water temperature, total and particulate nitrogen and phosphorus, and a negative correlation of Chl concentration with SD was found.
Spatial distribution of zooplankton in the reservoir
Based on hierarchical clusterization of 2020 and 2021 data, three distinctive clusters of zooplankton were delineated in the reservoir (Fig. 6). These clusters correspond to three plankton communities: the Oka, the Volga, and the limnic cenoses.
The first cluster was formed by samples that were collected in the mouth area of the Oka River and the right bank of the reservoir below it. In 2020, the zone affected by the Oka water extended for 35 km below the Oka and Volga confluence, in 2021—only for several kilometers. Apparently, this is due to differences in hydrological conditions of those years: in 2020 during the study period the water level in the lower reaches of the Oka River was on average 19.0 ± 0.7% higher than in the study period in 2021. The complex of dominant zooplankton species in the Oka stream for many years remains unchanged: rotifers of the genus Brachionus Pallas, 1766 and, in particular, such species as Brachionus calyciflorus Pallas, 1766 and Brachionus angularis Gosse, 1851. In 2020, the share of these two species in the total zooplankton population was 68.2%, and in 2021 it was 52.1%. In some years, such as in 2021, the dominant species include immature forms of copepods, especially nauplii (14.4% of the total zooplankton abundance). Species pool of zooplankton in the Oka stream was represented by 46 species in 2020, and by 45 species in 2021. The ratio of species of rotifers and crustaceans (Cladocera, Copepoda) can vary greatly: in 2020, the share of rotifers in the total species richness was 52.3%, and in 2021—60.0%. Quantitative development of zooplankton also varied greatly in different years: in 2020, the average number of zooplankton in the Oka stream was 16.5 ± 6.5 ind./l, while in 2021 it was more than 5 times higher and was 96.6 ± 18.7 ind./l.
The second cluster was formed by samples collected in the left bank and right bank of the reservoir up to 155 km below the confluence of the Oka River. The species structure of this zooplankton community is quite variable and often depends on the length/intensity of the Oka flow. In 2020, the complex of dominant species was formed largely by the large limnic filter-feeding crustacean Daphnia galeata G.O. Sars, 1864, whose proportion in the total number of zooplankton was 41.6%. The dominant species also included rotifers Euchlanis dilatata Ehrenberg, 1832 (25.8%) and Keratella quadrata (Müller, 1786) (20.8%). In 2021, the complex of dominant species underwent substantial changes including rotifers B. calyciflorus (21.9%), Conochilus unicornis Rousselet, 1892 (21.5%) and E. dilatata (14.5%), and nauplial stages of crustaceans (13.2%). Species richness of zooplankton in the study period of 2020 counted 35 species (the lowest of all zooplankton communities in both years of observations). However, in 2021, the maximum species richness was recorded in this zooplankton community with 79 species. The ratio of rotifer to crustacean species numbers in both years was similar: the proportion of rotifers in the total species richness was 54.3% in 2020 and 54.4% in 2021. The difference between the quantitative development of zooplankton in different years in this community was even greater: the average zooplankton abundance in 2020 was 4.4 ± 0.1 ind./l and in 2021 it was more than 20 times higher at 105.8 ± 9.1 ind./l.
The third cluster was formed by the samples collected from the left bank and right bank of the reservoir in the area downstream of the Sura River, Vetluga River and directly in the lacustrine part of the reservoir. The complex of dominant zooplankton species in the limnic cenosis remains unchanged for many years: species of the genus Daphnia Müller, 1785, as well as nauplial and copepodite copepod stages. However, different species of Daphnia may occupy key positions in this community in different years. In particular, in 2020, the crustacean D. galeata played the leading role and its proportion in the total zooplankton abundance was 25.6%, and in 2021, this position was occupied by a smaller species of this genus, Daphnia cucullata G.O. Sars, 1862 (31.9%). Apparently, this was due to the food preferences of these two species and changes in their food supply. In addition, dominant species in 2020 included nauplial (23.3%) and copepodite (19.4%) stages of copepods as well as D. cucullata (12.6%). In 2021, the role of nauplial and copepodite stages decreased significantly (11.9% and 15.7%, respectively), and the proportion of D. galeata in the total zooplankton population was 13.2%. A total of 43 zooplankton species were identified in 2020 and 56 species—in 2021. The proportion of rotifers to total species richness in the limnic zooplankton community was the lowest—41.9% in 2020 and 51.8% in 2021. It should be noted that the quantitative development of zooplankton in the limnic cenosis in 2020 (18.39 ± 1.12 ind./l) was 3.5 times lower than in 2021 (65.39 ± 6.53 ind./l).
Dominant algal and cyanobacterial species of selected reservoir areas
In the Oka cenosis in 2020 diatoms, green algae, and cyanobacteria (Cyclotella meneghiniana Kütz., 1844 (7–11%), Dactylosphaerium jurisii Hindák, 1977 (11–25%), Aphanocapsa sp.) dominated in terms of phytoplankton abundance. However, only the diatom algae (C. meneghiniana (58–70%) and Stephanodiscus hantzschii Grunow, 1880 (11–13%)) dominated in terms of biomass. In 2021, there were significant changes in the complex of dominant species with prevailing abundance of cyanobacteria [Chroococcus minutus (Kütz.) Nägeli, 1849 (10%), Aphanocapsa sp. (53%), Microcystis aeruginosa (Kütz.) Kütz., 1846 (11%)]. Only Aulacoseira granulata (Ehrenb.) Simonsen, 1979 (20–36%) was among the dominant diatom algae. However, the complex of dominant species in terms of biomass did not change, as C. meneghiniana (13–20%) and A. granulata (67–74%) still dominated.
In the Volga cenosis, diatoms A. granulata, Aphanocapsa sp. and C. meneghiniana were dominant in both number and biomass. Their proportion in the total abundance and biomass ranged from 14 to 81%.
In the lake cenosis in 2020, cyanobacteria exclusively dominated in abundance (Aphanocapsa sp. (12–64%), M. aeruginosa (11%), Aphanizomenon flos-aquae Ralfs ex Bornet & Flahault, 1888 (15–21%), Dolichospermum flos-aquae (Bréb. ex Bornet & Flahault) Wacklin, L.Hoffm. & Komárek, 2009 (11%)). In terms of biomass, only D. flos-aquae (17%) was the dominant cyanobacteria, but diatoms (A. granulata (17–40%), S. hantzschii (10–11%)) were still dominant. In 2021, the dominance of cyanobacteria increased in plankton abundance (M. aeruginosa (33–61%), Microcystis wesenbergii (Komárek) Komárek in N.V.Kondrat., 1968 (21%), A. flos-aquae (13–20%), D. flos-aquae (17–34%)). Diatom algae disappeared from the biomass-dominant species complex and were replaced by cryptophyte algae (Cryptomonas sp. (23–27%) and Komma caudata (Geitler) Hill, 1991 (10%)). The role of cyanobacteria also increased in total biomass (M. aeruginosa (13–43%), M. wesenbergii (18%), A. flos-aquae (13–23%), D. flos-aquae (23–28%)).
Discussion
Response of the reservoir’s environmental state to spatial heterogeneity of external nutrient loading
The peculiarity of the Cheboksary Reservoir’s ecological zoning is rooted in high diversity of its tributaries. As the Oka and Volga merge, their almost equal water discharge but vastly different water chemistry create a rather extensive transition zone, separating the Cheboksary reservoir from other parts of the cascade upstream. This vast area of incomplete lateral mixing is well visible on satellite images during the spring flood due to increased water turbidity (Ermakov et al., 2019), and is observable in other seasons via differences in chemical composition of water between left and right banks (Kozhara, 1997; Nemirovskaya, 2012). Several studies, including this one, find that the Volga River reaches its highest biological productivity (as measured by chlorophyll content) specifically at the confluence with the Oka, which causes the Cheboksary Reservoir to stand out from the overall trend of chlorophyll decrease from upper to lower reservoirs of the cascade (Mineeva, 2022).
Not only the Oka itself, but all smaller right-side tributaries as well act as major sources of nutrient inflow into the reservoir, due to their elevated average nutrient concentrations and their large variation. These differences are caused by vastly different land-use structure of the two sides of the watershed: the left side remains mostly covered by forests, while the right side is dominated by cultivated fields and animal farms and has more urbanized areas. Based on fertilizer input data and other landscape-based estimations, Yasinskii et al. (2020) calculated that the right side receives over 10 times more inorganic nitrogen and phosphorus than the left side (total direct anthropogenic load on the watershed almost 38,000 tons N/year and almost 9700 tons P/year on the right bank vs 3000 tons N/year and 780 tons P/year on the left side). At the same time, authors of that study suggested that due to nutrient retention on the watershed, over two thirds of phosphorus and one third of nitrogen do not reach the reservoir, and due to unequal water runoff total nutrient loading of the two sides on the reservoir should become almost equal. However, our study suggests that right-side tributaries do play a much larger role in the reservoir’s nutrient budget. In some years, when DIP concentration in most parts of the reservoir and most of the tributaries, including the Oka, falls below detection limit, small right side tributaries, such as the rivers Kudma, Sundovik and Sura, may play a crucial role in replenishing the supply of biologically available phosphorus in their mouth areas. Right-side tributaries usually rate worse on most chemical and biological water quality indexes than left tributaries, and some of them, especially the Kudma and Sundovik, have phytoplankton community structures typical for eutrophic waters with chronic nutrient pollution (Kulizin et al., 2020; Vodeneyeva et al., 2020).
TN and TP content in the reservoir increases several times after the Oka confluence and keeps slightly rising further downstream due to external loading from other rivers, but respective changes in chlorophyll content are often non-linear, diverging from positive relation to nutrient content. This may imply that the ecosystem cannot assimilate all of the nutrients and achieve its maximum productivity because of some other limiting factor other than nutrient elements (Chorus & Spijkerman, 2021). Mineeva et al. (2008) hypothesized that due to extreme anthropogenic nutrient loading, the relationship between productivity of the reservoir and nutrient concentrations becomes too complicated, with P and N not acting as strictly limiting elements and phytoplankton growth depending more on water temperature and color and flow velocities defined by the dam’s regulation regime.
In the entire reservoir, TN:TP ratio generally stays within limits of 5 to 10, rarely reaching 10–15, which indicates the shift from phosphorus limitation to nitrogen or co-limitation, which can be typical for agricultural and urban temperate lakes (Graham et al., 2004; Elser et al., 2007). In these conditions, control over nitrogen input can be more crucial for environmental protection and restoration efforts than control over phosphorus (Bogard et al., 2020).
By several accounts, the reservoir is shown to be split into parts with evidently different mechanisms controlling phytoplankton growth. In the upper part mostly unaffected by the Oka inflow, as well as in the lower widest part of the reservoir, chlorophyll content is correlated with many nutrient parameters, which is typical for lakes (Quinlan et al., 2021), and water temperature. However, these two sections are characterized by widely different plankton composition, and are separated by an extensive area where no such correlations are observed. TSI deviation analysis suggests that phytoplankton composition itself may affect its productivity in some years, as large-sized cyanobacteria can alter light attenuation, shifting the balance in physical factors. Since phytoplankton species composition depends on many chemical, hydrological and meteorological factors, any environmental changes may affect the entirety of phytoplankton interaction with other components of the ecosystem.
Top-down control of phytoplankton productivity
Presence of distinctive zooplankton communities in middle and lower parts of the reservoir suggests their possible interference with nutrient elements as a factor of phytoplankton productivity control. Whether or not cyanobacteria are edible for zooplankton and especially for species of the genus Daphnia Müller, 1785 is a matter of much discussion (Burns, 1987; Haney, 1987; Lampert, 1987; Gliwicz, 1990; Gliwicz & Lampert, 1990). However, cyanobacteria are still considered to be an important food item for daphnids (Repka, 1998). It is known that D. galeata feeds on large diatom algae, possessing three large teeth in its mandible to crush skeletons of diatom algae. Among filter-feeding daphnids, D. galeata can filter cyanobacteria to a lesser extent, but D. cucullata can show preference in cyanobacteria as a food object, which can explain why massive development of D. cucullata is often observed in eutrophic water bodies with intensive cyanobacterial blooms, and D. galeata gravitate towards more mesotrophic conditions and develops en masse when diatom and green algae are prevalent (Gulati, 1990; Lammens et al., 1992; Monakov, 2003).
Moving away from the Oka River inflow into the Cheboksary reservoir, zooplankton communities are change and their species structure is being restructured. Predominantly diatom complexes of upper parts of the reservoir are replaced by cryptophyte, green algae, and finally cyanobacterial ones downstream. Accordingly, the proportion of rotifers decreases and the proportion of large limnic crustaceans increases. The trophic relationships between algae and rotifers downstream are balanced. However, the key component of the ecosystem, cyanobacteria, may be limited by the massive development of some limnic filter-feeders. In 2020 and 2021 we observed not only different intensity of "blooming", but also a change of dominant species in zooplanktocenosis of the lacustrine section of the reservoir. In 2020, the key role was played by the crustacean D. galeata and development of cyanobacteria was not catastrophic. In 2021, we observed mass development of cyanobacteria, and the key role among zooplankton was represented by the crustacean D. cucullata. Mass development of the latter may be just a response to the increased nutrient load on the reservoir waters and, as a consequence, increased cyanobacterial blooms. In experiments it has been shown that larger Daphnia species experience greater interfering effects of cyanobacteria on the food collection and ingestion processes (Gulati et al., 2001). Thus, the smaller D. cucullata has a competitive advantage over the larger D. galeata. In addition, it seems that weather conditions also played a significant role. Water temperature in 2021 was higher than in 2020 and in the most part of the water area was within 23–25 °C. It is known that maximum filtration rate in daphnids is observed at water temperature 23–24 °C. When algal biomass exceeds 8 g/m3, crustaceans are unable to reduce filtration rate to an acceptable level, turning to overconsumption of algae with decreased food use efficiency (Monakov, 2003), which can subsequently lead to top-down control of phytoplankton. For zooplankton communities the functioning of top-down control can be easily explained with the size-efficiency hypothesis (Brooks & Dodson, 1965; Low et al., 2010). It is important to keep in mind the protective morphological (size) and chemical (toxins) mechanisms against filter-feeders (Kirk & Gilbert, 1992). In addition, it is important to consider the size structure of the cyanobacterial communities. Gulati et al. (2001) found that long filaments and large cells of cyanobacteria are prone to clumping and, therefore, can be rejected by Daphnia during food collection and absorption.
Conclusion
Due to the extremely high nitrogen and phosphorus content in the eutrophic Cheboksary Reservoir, multi-year fluctuations of its productivity are not sufficiently explained by changes in nutrient inflow. Other complicating factors include short residence time, low depths and disparity in phytoplankton community composition in different parts of the reservoir. For a significant part of its area, nutrient content seemingly plays no significant part in variation of phytoplankton productivity. It is still clear that major efforts in nutrient loading reduction are needed to improve the ecological state of the reservoir, and nitrogen control may be more effective, than phosphorus control. At the same time, most urban areas in the Volga basin are still facing the problem of obsolete domestic wastewater treatment facilities, which do not include a treatment unit for nutrients. As a result, up to 90% of Russian cities still discharge wastewater with a very high nitrogen level. The results of our study show that reduction of nitrogen content in wastewater is a key step to improve the ecological condition of both the Cheboksary reservoir and other reservoirs in the Volga basin.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Change history
25 December 2023
The footnote was missing three of the guest editors’ names
References
Ahoutou, M. K., E. K. Yao, R. Y. Djeha, M. Kone, K. Tambosco, C. Duval, S. Hamlaoui, C. Bernard, M. Bouvy, B. Marie, B. Montuelle, M. Troussellier, F. K. Konan, J. K. Coulibaly, M. Dosso, J.-F. Humbert & C. Quiblier, 2022. Impacts of nutrient loading and fish grazing on the phytoplankton community and cyanotoxin production in a shallow tropical lake: results from mesocosm experiments. MicrobiologyOpen 11: e1278. https://doi.org/10.1002/mbo3.1278.
Belykh, O. I., A. S. Gladkikh, E. G. Sorokovikova, I. V. Tikhonova, S. A. Potapov & G. A. Fedorova, 2013. Microcystin-producing cyanobacteria in water reservoirs of Russia, Belarus and Ukraine. Chemistry for Sustainable Development 21: 347–361.
Berger, S. A., S. Diehl, H. Stibor, G. Trommer & M. Ruhenstroth, 2010. Water temperature and stratification depth independently shift cardinal events during plankton spring succession. Global Change Biology 16: 1954–1965. https://doi.org/10.1111/j.1365-2486.2009.02134.x.
Bergström, A. K. & J. Karlsson, 2019. Light and nutrient control phytoplankton biomass responses to global change in northern lakes. Global Change Biology 25: 2021–2029. https://doi.org/10.1111/gcb.14623.
Błędzki, L. & J. I. Rybak, 2016. Freshwater Crustacean Zooplankton of Europe: Cladocera & Copepoda (Calanoida, Cyclopoida) Key to Species Identification, with Notes on Ecology, Distribution, Methods and Introduction to Data Analysis. Springer, Germany: 918 pp. https://doi.org/10.1007/978-3-319-29871-9.
Borcard, D., Gillet, F. & P. Legendre, 2011. Numerical Ecology with R. Springer, New York: 306 pp.
Bogard, M. J., R. J. Vogt, N. M. Hayes & P. R. Leavitt, 2020. Unabated nitrogen pollution favors growth of toxic cyanobacteria over chlorophytes in most hypereutrophic lakes. Environmental Science & Technology 54: 3219–3227. https://doi.org/10.1021/acs.est.9b06299.
Bouffard, D., I. Kiefer, A. Wüest, S. Wunderle & D. Odermatt, 2018. Are surface temperature and chlorophyll in a large deep lake related? An analysis based on satellite observations in synergy with hydrodynamic modelling and in-situ data. Remote Sensing of Environment 209: 510–523. https://doi.org/10.1016/j.rse.2018.02.056.
Brooks, J. L. & S. I. Dodson, 1965. Predation, body size, and composition of plankton. The effect of a marine planktivore on lake plankton illustrates theory of size, competition, and predation. Science 150: 28–35. https://doi.org/10.1126/science.150.3692.28.
Burns, C. W., 1987. Insights into zooplankton–cyanobacteria interactions derived from enclosures studies. New Zealand Journal of Marine and Freshwater Research 21: 477–482. https://doi.org/10.1080/00288330.1987.9516243.
Carey, R. O., G. J. Hochmuth, C. J. Martinez, T. H. Boyer, M. D. Dukes, G. S. Toor & J. L. Cisar, 2013. Evaluating nutrient impacts in urban watersheds: challenges and research opportunities. Environmental Pollution 173: 138–149. https://doi.org/10.1016/j.envpol.2012.10.004.
Carlson, R. E., 1977. A trophic state index for lakes. Limnology and Oceanography 22: 361–369. https://doi.org/10.4319/lo.1977.22.2.0361.
Carlson, R. E., 1992. Expanding the trophic state concept to identify non-nutrient limited lakes and reservoirs. In Proceedings of a National Conference on Enhancing the States’ Lake Management Programs: 59–71.
Carpenter, S. R., J. J. Cole, J. R. Hodgson, J. F. Kitchell, M. L. Pace, D. Bade, K. L. Cottingham, T. E. Essington, J. N. Houser & D. S. Schindler, 2001. Trophic cascades, nutrients, and lake productivity: whole-lake experiments. Ecological Monographs 71: 163–186. https://doi.org/10.1890/0012-9615(2001)071[0163:TCNALP]2.0.CO;2.
Carvalho, L., C. McDonald, C. de Hoyos, U. Mischke, G. Phillips, G. Borics, S. Poikane, B. Skjelbred, A. L. Solheim, J. Van Wichelen & A. C. Cardoso, 2013. Sustaining recreational quality of European lakes: minimizing the health risks from algal blooms through phosphorus control. Journal of Applied Ecology 50: 315–323. https://doi.org/10.1111/1365-2664.12059.
Chambers, P. A., D. J. McGoldrick, R. B. Brua, C. Vis, J. M. Culp & G. A. Benoy, 2012. Development of environmental thresholds for nitrogen and phosphorus in streams. Journal of Environmental Quality 41: 7–20. https://doi.org/10.2134/jeq2010.0273.
Chernova, E., S. Sidelev, I. Russkikh, L. Korneva, V. Solovyova, N. Mineeva, I. Stepanova & Z. Zhakovskaya, 2020. Spatial distribution of cyanotoxins and ratios of microcystin to biomass indicators in the reservoirs of the Volga, Kama and Don Rivers, the European part of Russia. Limnologica 84: 125819–21. https://doi.org/10.1016/j.limno.2020.125819.
Chorus, I. & E. Spijkerman, 2021. What Colin Reynolds could tell us about nutrient limitation, N:P ratios and eutrophication control. Hydrobiologia 848: 95–111. https://doi.org/10.1007/s10750-020-04377-w.
Dodds, W. K. K. & E. B. Welch, 2000. Establishing nutrient criteria in streams. Journal of the North American Benthological Society 19: 186–196. https://doi.org/10.2307/1468291.
Elser, J. J. & C. R. Goldman, 1991. Zooplankton effects on phytoplankton in lakes of contrasting trophic status. Limnology and Oceanography 36: 64–90. https://doi.org/10.4319/lo.1991.36.1.0064.
Elser, J. J., E. R. Marzolf & C. R. Goldman, 1990. Phosphorus and nitrogen limitation of phytoplankton growth in the freshwaters of North America: a review and critique of experimental enrichments. Canadian Journal of Fisheries and Aquatic Sciences 47: 1468–1477. https://doi.org/10.1139/f90-165.
Elser, J. J., M. E. S. Bracken, E. E. Cleland, D. S. Gruner, W. S. Harpole, H. Hillebrand, J. T. Ngai, E. W. Seabloom, J. B. Shurin & J. E. Smith, 2007. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecology Letters 10: 1135–1142. https://doi.org/10.1111/j.1461-0248.2007.01113.x.
Ermakov, S. A., A. A. Molkov, I. A. Kapustin, T. N. Lazareva, O. A. Danilicheva, O. V. Shomina, M. V. Smirnova & O. Y. Lavrova, 2019. Satellite and in-situ observations of a river confluence zone. Remote Sensing of the Ocean, Sea Ice, Coastal Waters, and Large Water Regions 2019(11150): 384–390. https://doi.org/10.1117/12.2533470.
Gebrehiwot, M., D. Kifle & L. Triest, 2020. Partitioning the influence of hydrodynamics-induced physical variables and nutrients on phytoplankton assemblages in a shallow tropical reservoir (Koka, Ethiopia). Limnology 21: 269–274. https://doi.org/10.1007/s10201-020-00611-5.
Ger, K. A., P. Urrutia-Cordero, P. C. Frost, L.-A. Hansson, O. Sarnelle, A. E. Wilson & M. Lürling, 2016. The interaction between cyanobacteria and zooplankton in a more eutrophic world. Harmful Algae 54: 128–144. https://doi.org/10.1016/j.hal.2015.12.005.
Gliwicz, Z. M., 1990. Why do cladocerans fail to control algal blooms? Hydrobiologia 200: 83–97. https://doi.org/10.1007/BF02530331.
Gliwicz, Z. M. & W. Lampert, 1990. Food thresholds in Daphnia species in the absence and presence of blue-green filaments. Ecology 71: 691–702. https://doi.org/10.2307/1940323.
Graham, J. L., J. R. Jones, S. B. Jones, J. A. Downing & T. E. Clevenger, 2004. Environmental factors influencing microcystin distribution and concentration in the Midwestern United States. Water Research 38: 4395–4404. https://doi.org/10.1016/j.watres.2004.08.004.
Granlund, K., A. Räike, P. Ekholm, K. Rankinen & S. Rekolainen, 2005. Assessment of water protection targets for agricultural nutrient loading in Finland. Journal of Hydrology 304: 251–260. https://doi.org/10.1016/j.jhydrol.2004.07.033.
Gulati, R. D., 1990. Zooplankton structure in the Loosdrecht lakes in relation to trophic status and recent restoration measures. Hydrobiologia 191: 173–188. https://doi.org/10.1007/978-94-009-0467-5_20.
Gulati, R. D., M. Bronkhorst & E. Van Donk, 2001. Feeding in Daphnia galeata on Oscillatoria limnetica and on detritus derived from it. Journal of Plankton Research 23: 705–718. https://doi.org/10.1093/plankt/23.7.705.
Gustafsson, S. & L. A. Hansson, 2004. Development of tolerance against toxic cyanobacteria in Daphnia. Aquatic Ecology 38: 37–44. https://doi.org/10.1023/B:AECO.0000020985.47348.5e.
Haney, J. F., 1987. Field studies on zooplankton-cyanobacteria interactions. New Zealand Journal of Marine and Freshwater Research 21: 467–475. https://doi.org/10.1080/00288330.1987.9516242.
Huang, J., Y. Zhang, G. B. Arhonditsis, J. Gao, Q. Chen, N. Wu, F. Dong & W. Shi, 2019. How successful are the restoration efforts of China’s lakes and reservoirs? Environment International 123: 96–103. https://doi.org/10.1016/j.envint.2018.11.048.
Hudec, I., 2010. Anomopoda, Ctenopoda, Haplopoda, Onychopoda (Crustacea: Branchiopoda). Fauna Slovenska III. VEDA, Bratislava, Slovakia: 496 pp.
Jeppesen, E., M. Soendergaard, J. P. Jensen, K. E. Havens, O. Anneville, L. Carvalho, M. F. Coveney, R. Deneke, M. T. Dokulil & B. Foy, 2005. Lake responses to reduced nutrient loading – an analysis of contemporary long-term data from 35 case studies. Freshwater Biology 50: 1747–1771. https://doi.org/10.1111/j.1365-2427.2005.01415.x.
Kapustin, I. A., A. A. Molkov, S. A. Ermakov & M. V. Smirnova, 2019. General characteristic and features of the current structure in the Cheboksary Reservoir from Nizhny Novgorod to Kozmodemyansk. In Proceedings of the 4th All-Russian Scientific Conference “Problems of the ecology of the Volga basin” (“Volga 2019”) (in Russian)
Kasprzak, P., J. Benndorf, T. Gonsiorczyk, R. Koschel, L. Krienitz, T. Mehner, S. Hülsmann, H. Schultz & A. Wagner, 2007. Reduction of nutrient loading and biomanipulation as tools in water quality management: Long-term observations on Bautzen Reservoir and Feldberger Haussee (Germany). Lake and Reservoir Management 23: 410–427. https://doi.org/10.1080/07438140709354027.
Kirk, K. L. & J. J. Gilbert, 1992. Variation in herbivore response to chemical defences – zooplankton foraging on toxic cyanobacteria. Ecology 73: 2208–2217. https://doi.org/10.2307/1941468.
Korovchinsky, N. M., A. A. Kotov, A. Yu. Sinev, A. N. Neretina & P. G. Garibyan, 2021. Branchy-Billed Crustaceans (Crustacea: Cladocera) of Northern Eurasia. Vol. II. Association of Scientific Editions KMK, Moscow: 544 pp. (in Russian)
Kozhara, V. L., 1997. Cartographic classification using large sets of variables. Mapping Sciences and Remote Sensing 34: 247–263. https://doi.org/10.1080/07493878.1997.10642066.
Kronvang, B., M. Bechmann, H. Lundekvam, H. Behrendt, G. H. Rubaek, O. F. Schoumans, N. Syversen, H. E. Andersen & C. C. Hoffmann, 2005. Phosphorus losses from agricultural areas in river basins: effects and uncertainties of targeted mitigation measures. Journal of Environmental Quality 34: 2129–2144. https://doi.org/10.2134/jeq2004.0439.
Kulizin, P., E. Vodeneeva & A. Okhapkin, 2020. The experience of using the functional classification of phytoplankton to assess the water quality of some left-bank tributaries of the Volga River (basin of the Cheboksary Reservoir). Principy ekologii 9: 3−15. https://doi.org/10.15393/j1.art.2020.9582 (in Russian)
Lammens, E. H. R. R., N. Boesewinkel-De Bruyn, H. Hoogveld & E. Van Donk, 1992. P-Load, phytoplankton, zooplankton and fish stock in Loosdrecht Lake and Tjeukemeer – confounding effects of predation and food availability. Hydrobiologia 233: 87–94. https://doi.org/10.1007/BF00016098.
Lampert, W., 1987. Laboratory studies on zooplankton–cyanobacteria Interactions. New Zealand Journal of Marine and Freshwater Research 21: 483–490. https://doi.org/10.1080/00288330.1987.9516244.
Lazareva, V. I., N. M. Mineeva & S. M. Zhdanova, 2014. Spatial distribution of plankton from the Upper and Middle Volga reservoirs in years with different thermal conditions. Biology Bulletin 41: 869–878. https://doi.org/10.1134/S1062359014100070.
Lee, H. W., Y. S. Lee, J. Kim, K. J. Lim & J. H. Choi, 2019. Contribution of internal nutrients loading on the water quality of a reservoir. Water 11: 1409. https://doi.org/10.3390/w11071409.
Liu, X., B. G. Liu, Y. W. Chen, & J. F. Gao, 2016. Responses of nutrients and chlorophyll a to water level fluctuations in Poyang Lake. Huan Jing ke Xue = Huanjing Kexue 37: 2141–2148. https://doi.org/10.13227/j.hjkx.2016.06.017.
Low, E. W., E. Clew, P. A. Todd, Y. C. Tai & P. K. L. Ng, 2010. Top-down control of phytoplankton by zooplankton in tropical reservoirs in Singapore? The Raffles Bulletin of Zoology 52: 311–322. https://doi.org/10.5281/zenodo.5342886.
Maberly, S. C., J. A. Pitt, P. S. Davies & L. Carvalho, 2020. Nitrogen and phosphorus limitation and the management of small productive lakes. Inland Waters 10: 159–172. https://doi.org/10.1080/20442041.2020.1714384.
McQueen, D. J., M. R. Johannes, J. R. Post, T. J. Stewart & D. R. Lean, 1989. Bottom-up and top-down impacts on freshwater pelagic community structure. Ecological Monographs 59: 289–309. https://doi.org/10.2307/1942603.
Mineeva, N., 2022. Chlorophyll and its role in freshwater ecosystem on the example of the Volga River reservoirs. In: Chlorophylls. IntechOpen, London. https://doi.org/10.5772/intechopen.105424.
Mineeva, N. M., A. S. Litvinov, I. E. Stepanova & M. Y. Kochetkova, 2008. Chlorophyll content and factors affecting its spatial distribution in the Middle Volga reservoirs. Inland Water Biology 1: 64–72. https://doi.org/10.1007/s12212-008-1010-5.
Monakov A.V., 2003. Feeding of Freshwater Zooplankton Invertebrates. Kenobi Productions, Ghent, Belgium: 373 pp.
Na, E. H. & S. S. Park, 2006. A hydrodynamic and water quality modeling study of spatial and temporal patterns of phytoplankton growth in a stratified lake with buoyant incoming flow. Ecological Modelling 199: 298–314. https://doi.org/10.1016/j.ecolmodel.2006.05.008.
Namsaraev, Z., A. Melnikova, A. Komova, V. Ivanov, A. Rudenko & E. Ivanov, 2020. Algal bloom occurrence and effects in Russia. Water 12: 285. https://doi.org/10.3390/w12010285.
Nemirovskaya, I. A., 2012. Variations in different compounds in Volga water, suspension, and bottom sediments in the summer of 2009. Water Resources 39: 533–545. https://doi.org/10.1134/S0097807812030062.
Nikanorov, A. M. & T. A. Khoruzhaya, 2014. Intra-waterbody processes in large reservoirs of southern Russia (pollution, eutrophication, toxification). Geography and Natural Resources 35: 135–142. https://doi.org/10.1134/S1875372814020048.
Olokotum, M., Mitroi, V., Troussellier, M., Semyalo, R., Bernard, C., Montuelle, B., Okello, W., Quiblier, C., & J.-F. Humbert, 2020. A review of the socioecological causes and consequences of cyanobacterial blooms in Lake Victoria. Harmful Algae 96: 101829. https://doi.org/10.1016/j.hal.2020.101829.
Ovaskainen, O., B. Weigel, O. Potyutko & Y. Buyvolov, 2019. Long-term shifts in water quality show scale-dependent bioindicator responses across Russia-Insights from 40 year-long bioindicator monitoring program. Ecological Indicators 98: 476–482. https://doi.org/10.1016/j.ecolind.2018.11.027.
Peretyatko, A., S. Teissier, S. De Backer & L. Triest, 2009. Restoration potential of biomanipulation for eutrophic peri-urban ponds: the role of zooplankton size and submerged macrophyte cover. Hydrobiologia 634: 125–135. https://doi.org/10.1007/s10750-009-9888-4.
Poikane, S., M. G. Kelly, F. S. Herrero, J. A. Pitt, H. P. Jarvie, U. Claussen, W. Leujak, A. L. Solheim, H. Teixeira & G. Phillips, 2019. Nutrient criteria for surface waters under the European Water Framework Directive: current state-of-the-art, challenges and future outlook. Science of the Total Environment 695: 133888. https://doi.org/10.1016/j.scitotenv.2019.133888.
Poikane, S., M. G. Kelly, G. Várbíró, G. Borics, T. Erős, S. Hellsten, A. Kolada, B. A. Lukács, A. Lyche Solheim, J. P. López, N. J. Willby, G. Wolfram & G. Phillips, 2022. Estimating nutrient thresholds for eutrophication management: novel insights from understudied lake types. Science of the Total Environment 827: 154242. https://doi.org/10.1016/j.scitotenv.2022.154242.
Quinlan, R., A. Filazzola, O. Mahdiyan, A. Shuvo, K. Blagrave, C. Ewins, L. Moslenko, D. K. Gray, C. M. O’Reilly & S. Sharma, 2021. Relationships of total phosphorus and chlorophyll in lakes worldwide. Limnology and Oceanography 66: 392–404. https://doi.org/10.1002/lno.11611.
Repka, S., 1998. Effects of food type on the life history of Daphnia clones from lakes differing in trophic state. II. Daphnia cucullata feeding on mixed diets. Freshwater Biology 38: 685–695. https://doi.org/10.1046/j.1365-2427.1997.00243.x.
Rogers, D. C. & J. H. Thorp (eds.), 2019. Keys to Palaearctic Fauna. Thorp and Covich’s Freshwater. Invertebrates, Vol. IV: 4th edition. Academic Press, Oxford: 920 pp.
Rose, V., G. Rollwagen-Bollens, S. M. Bollens & J. Zimmerman, 2021. Effects of grazing and nutrients on phytoplankton blooms and microplankton assemblage structure in four temperate lakes spanning a eutrophication gradient. Water 13: 1085. https://doi.org/10.3390/w13081085.
Rozenberg, G. S., A. V. Vasilyev, A. G. Zibarev, G. E. Kudinova, V. I. Popchenko, A. G. Rozenberg, M. V. Rubanova, S. V. Saksonov & G. R. Hasaev, 2017. From “Revival of the Volga” to the “Improvement of the Volga”: comparing the target Federal Programs Passports. In Proceedings of the Sixth International Environmental Congress (Eighth International Scientific-Technical Conference) “Ecology and Life Protection of Industrial-Transport Complexes” ELPIT 2017: 248–259.
Rumyantsev, V. A., A. V. Izmailova, V. G. Drabkova & S. A. Kondrat’ev, 2018. The current status and problems of the lake fund of European Russia. Herald of the Russian Academy of Sciences 88: 230–240. https://doi.org/10.1134/S1019331618030140.
Selge, F., E. Matta, R. Hinkelmann & G. Gunkel, 2016. Nutrient load concept-reservoir vs. bay impacts: a case study from a semi-arid watershed. Water Science & Technology 74: 1671–1679. https://doi.org/10.2166/wst.2016.342.
Venitsianov, E. V., 2019. Modern problems of water protection in Russia. IOP Conference Series: Earth and Environmental Science 321: 012033. https://doi.org/10.1088/1755-1315/321/1/012033.
Vodeneyeva, Y. L., K. Y. Kolomina, Y. M. Sharagina, P. V. Kulizin & A. G. Okhapkin, 2020. Water quality assessment of some right-bank tributaries of the Volga River (the Cheboksary Reservoir catchment) using phytoplankton functional classification. Hydrobiological Journal 56: 50–62. https://doi.org/10.1615/HydrobJ.v56.i2.50.
Xie, P. & J. Liu, 2001. Practical success of biomanipulation using filter-feeding fish to control cyanobacteria blooms: a synthesis of decades of research and application in a subtropical hypereutrophic lake. The Scientific World 1: 337–356. https://doi.org/10.1100/tsw.2001.67.
Xie, L. & P. Xie, 2002. Long-term (1956–1999) dynamics of phosphorus in a shallow, subtropical Chinese lake with the possible effects of cyanobacterial blooms. Water Research 36: 343–349. https://doi.org/10.1016/S0043-1354(01)00198-1.
Xu, H., H. W. Paerl, B. Qin, G. Zhu & G. Gao, 2010. Nitrogen and phosphorus inputs control phytoplankton growth in eutrophic Lake Taihu, China. Limnology and Oceanography 55: 420–432. https://doi.org/10.4319/lo.2010.55.1.0420.
Xu, H., H. W. Paerl, B. Qin, G. Zhu, N. S. Hall & Y. Wu, 2015. Determining critical nutrient thresholds needed to control harmful cyanobacterial blooms in eutrophic Lake Taihu, China. Environmental Science & Technology 49: 1051–1059. https://doi.org/10.1021/es503744q.
Yasinskii, S. V., E. A. Kashutina, M. V. Sidorova & A. N. Narykov, 2020. Anthropogenic load and the effect of drainage area on the diffuse runoff of nutrients into a large water body: case study of the Cheboksary reservoir. Water Resources 47: 810–827. https://doi.org/10.1134/S009780782005022X.
Zeng, Q., L. Qin, L. Bao, Y. Li & X. Li, 2016. Critical nutrient thresholds needed to control eutrophication and synergistic interactions between phosphorus and different nitrogen sources. Environmental Science and Pollution Research 23: 21008–21019. https://doi.org/10.1007/s11356-016-7321-x.
Funding
Most of the fieldwork for this study was supported by the Russian Geographical Society («The Floating University of the Volga Basin», project no. 02/2019-P, 07/2020-p, 17/2021-P). Research of plankton communities of the reservoir and its tributaries was supported by the federal academic leadership program «Priority 2030» of the Ministry of Science and Higher Education of the Russian Federation (subject-matter H-477-99_2021-2023). Studies of nutrient limitation were performed as a part of the State Assignment no. 121051400038-1 of the LMSU Department of Hydrology.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MT, OE, VZ, EV, DS and DG. GS conceptualized and supervised the study and provided funding acquisition. The first draft of the manuscript was written by MT and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Helmut Habersack, Marcel Liedermann, Martin Schletterer, Markus Eder & Michael Tritthart / Biodiversity and Bioindication in Large Rivers
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tereshina, M., Erina, O., Zhikharev, V. et al. Nutrients and zooplankton as factors controlling phytoplankton growth in the Cheboksary Reservoir. Hydrobiologia (2023). https://doi.org/10.1007/s10750-023-05367-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10750-023-05367-4