Abstract
The study focused on litter processing efficiency of the sesarmid crab, Parasesarma plicatum under experimental conditions and its possible role in carbon structuring in the mangrove habitats. The feeding experiment of crab with different stages of leaves of diverse mangrove species revealed that senescent brown leaves were preferred the most, due to their lesser tannin content. The leaf consumption differed significantly with leaf state as well as mangrove species and highest was for Avicennia officinalis brown leaves. The ingestion-egestion assay of P. plicatum revealed that it assimilated an average of 65.75 ± 10.30% of mangrove litter. The experimental study also revealed that rather than already reported C/N ratio, the inhibiting factors such as tannin and lignin like substances control the palatability of leaves by the crab species. The analysis of carbon and nitrogen variants in the experimental water indicated that handling of leaves by the crab helps in leaching of nutrients to the substratum. Therefore, the findings of litter processing efficiency of this sesarmid crab from the present study highlights its significance in structuring the carbon in natural mangrove habitat.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mangrove ecosystems are important blue carbon reservoirs, storing atmospheric carbon through their long-term carbon storage and sequestration efficiencies (Alongi, 2014) with 3–4 folds higher potential than tropical rain forests (Donato et al., 2011). Every year, the mangroves, salt marshes and seagrasses capture and store between 235 and 450 trillion tons of carbon (Nellemann et al., 2009). Mangrove plants have higher photosynthetic carbon fixation capacity than terrestrial forests (Christensen, 1978). This primary production of mangroves majorly as litterfall, is retained in the ecosystem through a series of biogeochemical processes (through herbivory especially by feeding mangrove litter as well as burrowing of leaves by mangrove fauna inhibits the early export of mangrove productivity as mangrove litter to adjacent water bodies) and associated physical factors such as topography, tidal inundation etc. (Kristensen, 2008). Few studies reported the importance of crabs in nutrient cycling and thereby reflected them as keystone species of mangrove ecosystem (Smith et al., 1991).
Brachyuran crabs, primarily fiddler crabs (family Ocypodidae) and leaf-eating sesarmid crabs (family Sesarmidae), dominating in number and biomass among the mangrove fauna, (Tan & Ng, 1994; Kristensen, 2008), are true ecosystem engineers (Kristensen, 2008), as they process, retain, macerate and ingest large amounts of litter thereby substantially reduces its export and shorten decomposition time, enhancing nutrient cycling in the mangrove habitat (Robertson, 1986). Burrowing activity and leaf consumption of mangrove crabs, removing 30–90% of the litterfall has been well documented (Slim et al., 1997; Lee, 1998; Schories et al., 2003; Lee et al., 2014), that helps in nutrient retention and recycling leading to long term burial of organic carbon in the sediment pool of the environment, which otherwise may be exported to adjacent water bodies. The interaction of mangrove crabs with mangrove species is actually bidirectional, that is it affects the structure and functioning of mangrove ecosystem as well as the population biology of crabs is also influenced by mangrove species (Lee, 1998; Lee & Kwok, 2002; Cannicci et al., 2008).
Although feeding experiments with mangrove crabs have been conducted in different regions around the world (Micheli, 1993; Kwok & Lee, 1995; Thongtham & Kristensen, 2005; Nordhaus & Wolff, 2007; Chen & Ye, 2008; Harada & Lee, 2016) there is still lack of feeding studies of crab, fed with all types of locally available mangrove species. The experimental crab selected for the present study was least studied in the past and those reported were mainly based on biological (growth related) and ecological aspects (Kwok & Lee, 1995; Shanij et al., 2016). In addition, there are few reports on feeding ecology which are based either on only one or two mangrove species as food source (Chen et al., 2016) or on other species under Parasesarma genus (Lee & Kwok, 2002). The literature also showed only very few reports on the crab activity on the litterfall processing (Shanij et al., 2016) from India. Therefore, there is a need for the understanding of dietary components and leaf preference of the selected experimental crab. Understanding the leaf preference of crab (species and state of leaf) especially its controlling factors, are important in the field to compare or correlate it with carbon dynamics and productivity of mangroves and its fate. A part of experimental design was adopted from Thongtham & Kristensen (2005), however, the present study focuses on the litter processing and carbon assimilation in mangrove ecosystems, selecting an abundant sesarmid crab population of the Kochi mangroves as well as using all mangrove species present in the field for the experiment. This will be helpful for checking the proportionality of crab population to the abundance of its preferred mangrove species. In addition, the feeding experiment checked the crab’s distribution in the field based on the nutritive value and inhibiting factors content like C/N ratio and tannin in different mangrove species. The results of this experimental study especially animal—plant interaction can be best utilised in the field for carbon sequestration enhancement studies in future. The mangrove management through restoration can be done while selecting the regeneration of those mangrove species which also favoured the crab density (through crab’s diet and other environmental conditions also should be checked), and therefore, their ecosystem service like soil carbon sequestration can be restored and thereby helping in ecological restoration as well as climate change mitigation. The litter processing by the crab and its ingestion as its diet will help to retain the organic matter within the ecosystem (Kristensen et al., 2008). The assimilation efficiency and egestion rate analysis of the selected abundant crab will help to understand their significant role in material flow in carbon-rich and nitrogen-limited mangrove habitats (Tongununui et al., 2021) and thereby help to understand carbon cycling phases especially soil carbon burial and organic carbon export. Thus, this study is very important in mangrove ecology and in carbon sequestration. The present study also made an attempt to compile the leaf feeding experimental data with already published field data on carb density, productivity and soil carbon burial and open up a novel future research to think about more on crab’s role in soil carbon sequestration which is usually underestimated so that it could be best utilised in climate change mitigation efforts.
Therefore, in the experiment our overarching aim was to understand the role of sesarmid crab in mangrove carbon cycling. A controlled feeding experiment was conducted to address the following questions: (1) How much of the crabs' diet is composed of mangrove leaves? (2) Which mangrove species are the preferred food source? (3) Which mangrove leaf state is preferred by the selected crab? (4) Is there any significant difference between the mangrove leaf ingestion according to species and leaf state? (5) What are the controlling factors for ingestion (6) What is the assimilation and egestion efficiency of this experimental crab for carbon and how much carbon is leaching to the substratum?
Materials and methods
Selection of the experimental crab
The selection of the experimental crab was based on field abundance and percentage of litter composition in its diet. The field collection and sampling of crabs were done from the mangrove habitats (Station 1, Aroor; Station 2, Malippuram-Vypin Island; Station3, Mangalavanam bird sanctuary) around the Cochin estuarine system (CES), South-West coast of India. The data on mangrove litterfall, crab density and soil carbon burial rate (sequestration) discussed with this experimental study were also based on the same area (Rani et al., 2016; Rani et al., 2021).
Taxonomic identification of the crab samples was accomplished by morpho-taxonomic procedures (Chhapgar, 1957; Ng et al., 2008). In order to understand the most abundant crab in the field, crab density was estimated based on visual count method by mixed crab and burrow counting method by considering 25 m2 quadrat (5 quadrat in each site) for crab count and 1 m2 quadrat laid for burrow counting, respectively ,(Lee & Kwok, 2002). However, it may underestimate the crab density as we did not count the crabs present in the burrows. All crabs including adults and juveniles irrespective of sex, seen in sediment and on trees in the quadrat were counted during low tide and high tide periods in each season of a whole year. In order to check the highest mangrove litter consuming species, gut content analysis of the herbivorous mangrove crabs from the field (P. plicatum, Neosarmatium malabaricum, Pseudosesarma glabrum) and fiddler crab, Austruca annulipes (ten crabs from each species) was done according to Williams, 1981 and Ravichandran et al., 2006. Entire contents from the stomach and rectum were removed and stirred with distilled water at 1:2 volumes in a petri-dish. The contribution of each dietary item from the total diet is expressed in terms of the percentage of the different food categories under microscopic view. From this preliminary diet examination and abundance of the species in the field, Parasesarma plicatum was selected for the experiment.
Feeding ecology
Differential rates of leaf consumption experiment
This experiment was done to check the variation in ingestion rate of the experimental crab using different mangrove species leaves and in different leaf category (explained below), so that the results can be related with the litter processing in the field with the corresponding mangrove plant species. The experimental set up for this study was adopted from Thongtham & Kristensen (2005) with suitable modifications as described below. Carapace length, width, weight and sex of the selected crab species were adequately documented and care was taken to select crabs with approximately equal morphology representing both sex (carapace width of 1.5–1.7 cm, mean = 1.62 ± 0.13 cm, carapace length of 1.5–1.9 cm, mean = 1.73 ± 0.19 cm, mean weight of 2.66 ± 0.95 g). For one month, acclimatisation of the selected crab species was done prior to the experiment. Specific salinity (15ppt) suitable for the survival of the experimental crab (salinity was optimised according to the data from the field, laboratory observation and from the available literature) was maintained prior to the experiment. The salinity was measured using a Refractometer (Atago, Japan). The experimental set up consisted of aquarium of dimensions 16 × 16 × 10 cm, the aquaria was slightly tilted, elevating one side about 2 cm, to provide a dry refuge for the crabs. The aquarium was filled with sea water (UV filtered) with salinity as described before. Each aquarium was maintained with one crab. The experimental tanks were maintained in triplicates, having one crab in each leaf categories: fresh [green leaves freshly plucked from plants], yellow [yellow coloured matured leaves] and brown leaves [senescent brown coloured leaves] of 11 mangrove species [Acanthus ilicifolius (AC), Acrostichum aureum (ACR), Avicennia officinalis (AVO), A. marina (AVM), Rhizophora mucronata (RM), R. apiculata (RA), Bruguiera gymnorrhiza (BG), B. cylindrica (BC), B. sexangula (BS), Sonneratia caseolaris (SC), Excoecaria agallocha (EX)], collected from the study area. One control tank was also maintained without crab for each leaf category. Therefore, 4 experimental aquaria set up were maintained for each category of leaf for each mangrove species, summing to a total of 12 aquarium tank for each mangrove species. Since the study selected 11 mangrove species, a total of 132 experimental set up were retained for the study.
Before the experiment, the mangrove leaves were soaked for 24 h in 35 ppt saline sea waters for leaching of unpalatable substances like tannin (soaking is done to imitate the field condition where the crabs also preferred to eat leached leaves rather than fresh leaves from the plant. The leaching of leaves in the field was evident during tidal water fluctuation). The crabs were also starved for 24 h prior to the experiment. The selected leaves of each category with similar colour and morphology were divided into two halves along the midrib and labelled. One half was used for the feeding experiment, and the other half was used for determination of dry (D)/fresh (F) weight and (D/F) correlation factor. The experiment was started by feeding each crab with a pre-weighed, half portion of the mangrove leaves of the selected category. After 24 h, all uneaten leaf residue were collected, rinsed carefully with distilled water, dried and weighed. The ingestion rate was calculated as the difference between the estimated initial dry weight derived from the leached D/F ratios and the measured final dry weight of uneaten leaves.
Ingestion-egestion assay
Ingestion and egestion assay were performed based on the procedure of Thongtham & Kristensen (2005) to check the variation in carbon assimilation by the experimental crab using different mangrove species leaves. Based on the results of differential rate consumption of mangrove leaves by the experimental crab, green, yellow and brown leaves of mangrove species with maximum ingestion rate were chosen for the ingestion and egestion assay. All the leaves were pre-soaked at sea water salinity of 35 ppt for 96 h (the chemical analysis after 24 h showed negligible removal of tannin, therefore soaking period was extended) prior to experiment for removing leachates. Ingestion assay (six replicates with one control for each leaf type) for a period of 24 h was carried out as mentioned in the leaf choice experiment, and ingestion rate was calculated. All crabs from the ingestion experiment were kept in the experimental aquaria under the same conditions for another 24 h to defecate. Faeces left in the dry area of each aquarium was picked manually using forceps, while faeces in the water were collected by passing the water through a pre-combusted (520 °C) and pre-weighed GF/C filters. The dry weight of the collected faeces was measured by drying it in a hot air oven at 60 °C for 48 h. The weighed faecal matter was stored for later elemental carbon analysis. The egestion rate was calculated as the 24 h accumulated faecal material and expressed in dry weight, g C (gww)−1 day 1 (gww = the wet weight of the crab in g). Assimilation was calculated as the difference between ingestion and egestion rates and assimilation efficiency (%) was calculated by dividing assimilation with ingestion rate.
Chemical analysis
The dried leaves of each mangrove species in each leaf category, faecal material of crab from the various treatments and water sample from the tank were analysed for total carbon and nitrogen and its variants to check the leaching of nutrients to the substratum. The pre-soaked leaves were used for chemical analysis, as this will help to mimic the field condition where the fallen leaves are in halophytic condition (The fresh leaves were also soaked to check whether the fallen green leaves were chosen by the crab). The variants of carbon and nitrogen like Total carbon (TC), Total organic carbon (TOC), Particulate organic carbon (POC), Dissolved organic carbon (DOC), Total inorganic carbon (TIC), Dissolved inorganic carbon (DIC), Total Nitrogen (TN) and Dissolved Nitrogen (DN) were analysed in Analytik Jena 2100 S, TOC analyzer liquid module, HT 1300 solid module and CHN analyzer of the model Elementar Vario EL III, of STIC (Sophisticated Test & Instrumentation Centre), CUSAT and Kel plus KES 12 LR Digestion unit and Kjeldhal Nitrogen distillation unit (Kjeldahl method, AOAC, 2000). Tannin and lignin-like substances (TALLS) in leaf samples were estimated based on the Folin –Denis Method (APHA, 2005; Nair et al., 1989) for analysing the influence of inhibiting factors on feeding preference. The C/N ratio was also calculated from the measured TC and TN for understanding the food preference. The leaf in the control aquaria showed only negligible weight loss during the experiment and the concentration of leachable carbon and nitrogen fragments in the control tank was very negligible. However, calibration was done before calculation of concentration of each variable.
Statistical analysis
The variation in leaf choice of crabs among leaf state and mangrove species along with the variation in carbon and nitrogen leachates in the experimental water according to each mangrove species and category were statistically tested by two-way and one-way ANOVA and post hoc analysis was done using Tukey-HSD test using SPSS v.16.0. All the data were checked for normality and homogeneity of variance before ANOVA and the data that failed normality even after transformation was tested using Kruskal–Wallis (K-W) test. Spearman correlation analysis was also performed for checking the relation of ingestion of mangrove leaves with chemical composition of the leaves using the same statistical package.
Results
Crab density and gut content analysis
Seven species of crabs were identified from the study area, viz. Scylla serrata (Forskal, 1775), Scylla tranquebarica (Fabricius, 1798), Austruca annulipes (H. Milne Edwards, 1837) (Fiddler crab), Parasesarma plicatum (Latreille, 1803), Neosarmatium malabaricum (Henderson, 1893), Parasesarma bengalense (Davie, 2003) (previously Perisesarma bengalense, genus changed to Parasesarma) and including the newly discovered species Pseudosesarma glabrum Ng, Rani & Nandan, 2017 (Ng et al., 2017; since the holotype information of this new species was not published elsewhere it is given here for scientific and public information -holotype deposited in the type collections of the National Zoological Collections of Crustacea Division, Zoological Survey of India, Kolkata with registration no: C7956/2). St.1 was dominated by Parasesarma plicatum followed by Neosarmatium malabaricum. Parasesarma plicatum and fiddler crabs dominated in St.3 however, St.2 had less crab activity as the first three quadrats showed an absence of burrowing activities and did not observe any tree-dwelling or climbing crabs throughout the study period. Species of Scylla along with very few numbers of Parasesarma plicatum (noticed occasionally) were observed in this area. The crab density was high in St.1 with a total of 13.8 ind.m−2 (including juveniles and adults) followed by St.3 (11.8 ind m−2). The crab density was very low in St.2 with 0.35 ind.m−2 including adults and juveniles.
The results of gut content analysis of sesarmid crabs and fiddler crab present in the study area showed that Parasesarma plicatum was having the highest leaf litter amount in their gut (more than 75%) in the field and thereby indicates its role in nutrient cycling as it can turn over the energy into the ecosystem. The preliminary examination of gut content analysis results of different mangrove crabs from the field is shown in supplementary information, Fig. S1. The other materials found in the gut of Parasesarma plicatum included sand/silt/clay, ribbon worms, nematodes, fungal material, algae and other unidentified substances.
Differential rates of leaf consumption experiment
The feeding experiments with different mangrove species and with different state of leaves revealed that senescent partially degraded brown leaves were preferred by the crabs compared to green and yellow leaves (Fig. 1). All the eleven mangrove species showed the same trend for brown leaves. Ingestion rate of mangrove leaves varied significantly with mangrove species (N = 99, Kruskal–Wallis test, χ2(10) = 30.60, P = 0.001) and leaf state (N = 99, Kruskal- Wallis test, χ2(3) = 20.172, P < 0.001). A. officinalis was the most preferred mangrove leaf in brown leaf category with an ingestion rate of 0.271 ± 0.009 g crab−1 day−1 followed by Bruguiera cylindrica (0.249 ± 0.005 g crab−1 day−1), whereas fresh and brown leaves of A. ilicifolius and fresh leaves of A. aureum, B. gymnorrhiza, B. sexangula were the least preferred ones (not ingested by the crab).
Among green leaves, A. officinalis (0.18 ± 0.085 g crab−1 day−1) and A. marina (0.16 ± 0.047 g crab−1 day−1) were more preferred by the crabs compared to other species. The ingestion rate in other mangrove species under fresh green leaves are shown in Fig. 1 and described in supplementary Table 1. Among the yellow leaves, R. apiculata leaves (0.108 ± 0.028 g crab−1 day−1) were consumed highest, while in brown leaf category as stated before, A. officinalis was having highest ingestion.
Factors influencing leaf choice
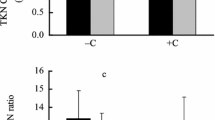
The total carbon in mangrove leaves used in the leaf choice experiment ranged from 205.8 ± 8.0 mg g−1 (B. cylindrica fresh leaves) to 414.4 ± 34.51 mg g−1 (S. caseolaris yellow leaves). There was no significant variation in carbon content with respect to species and leaf state. Total nitrogen in different mangrove leaves ranged from 3.15 ± 0.7 mg g−1 (R. mucronata brown) to 24.5 mg g−1 (A. ilicifolius green = 24.5 ± 2.25 mg g−1, E. agallocha green = 24.5 ± 1.6 mg g−1) (Fig. 2, Supplementary information, Table 2). There was no significant variation of nitrogen with mangrove species. However, it varied significantly with leaf state (F 2, 30 = 22.89, P < 0.001, N = 33). The C/N ratio of different mangrove leaves in different stages ranged from 11.04 (E. agallocha fresh green leaves) to 118.13 (R. mucronata brown). The C/N ratio did not vary significantly with mangrove species but marked significant variation with leaf state (F 2, 30 = 11.84, P < 0.001, N = 33). C/N ratio was high in brown senescent leaves compared to yellow and fresh green leaves (Fig. 3, Supplementary information, Table 3). In fresh green leaf category, the highest C/N ratio recorded was in R. apiculata (22.89 ± 4.1 mg g−1) and the least in A. ilicifolius (11.4 ± 1.7 mg g−1) leaves. In yellow leaf category, C/N ratio was maximum in B. gymnorrhiza (80.66 ± 6.5 mg g−1), and minimum in A. aureum (16.7 ± 3.6 mg g−1). C/N ratio was higher in R. mucronata but lower in A. aureum (15.55 ± 2.2 mg g−1) in brown leaf category.
The Tannin and Lignin like substances (TALLS) were higher in B. gymnorrhiza green leaves (61.2 ± 1.8 mg g−1) followed by B. gymnorrhiza yellow leaves (54.42 ± 1.6 mg g−1) and was lowest in A. marina brown leaves (2.17 ± 0.5 mg g−1). The tannin and lignin content significantly varied with the mangrove species (F 2, 20 = 2.672, P = 0.029, N = 33) and leaf state (F 2, 20 = 5.965, P = 0.009, N = 33). Tannin and lignin were high in fresh green leaves followed by yellow leaves and brown senescent leaves (Fig.4, supplementary information Table 4). In fresh green leaf category, the highest TALLS recorded was in B. gymnorrhiza and the least in A. officinalis (9.51 ± 3.2 mg g−1) leaves. In yellow leaf category, TALLS peaked in B. gymnorrhiza but was minimum in R. apiculata leaves (13.51 ± 1.2 mg g−1). Tannin and lignin were higher in E. agallocha (31.21 ± 1.1 mg g−1) but lower in A. marina in brown leaf category.
The correlation analysis of carbon, nitrogen, C/N ratio and tannin with ingestion rate indicated a significant negative correlation between tannin content and ingestion rate (rs = − 0.430, P = 0.015, N = 33), while carbon and nitrogen did not correlate significantly with the ingestion rate (Table 1). However, C/N ratio had a significant positive correlation (rs = 0.406, P = 0.019, n = 33) with ingestion rate.
Ingestion-egestion assay
The ingestion rate (one way ANOVA, F3, 20 = 4.144, P = 0.019) and egestion rate (one way ANOVA, F3,20 = 4.382, P = 0.016, Table 5, supplementary file) of experimental crab varied significantly with the given mangrove species. The ingestion rate of the mangrove leaves by the experimental crab were highest for A. officinalis brown leaves (0.063 ± 0.02 g dw (gww)−1 day−1) followed by R. apiculata yellow (0.045 ± 0.03 g dw (gww)−1 day−1), A. officinalis green leaves (0.025 ± 0.01 g dw (gww)−1 day−1) and A. marina green leaves (0.046 ± 0.02 g dw (gww)−1 day−1). The egestion rate was also high for A. officinalis brown leaf category (0.021 ± 0.014 g dw (gww)−1 day−1) followed by A. marina green leaves (0.017 ± 0.015 g dw (gww)−1 day−1), R. apiculata yellow leaves (0.016 ± 0.005 g dw (gww)−1 day−1) and A. officinalis green leaves (0.0048 ± 0.003 g dw (gww)−1 day−1). The post hoc Tukey HSD analysis revealed that the A. marina green category and A. officinalis brown category contributed more to the variation (P = 0.011, P = 0.014, Table 6 and 7, supplementary file) in ingestion and egestion rate of crab for the mangrove leaves. The control aquaria showed only negligible weight loss during the ingestion assay. The carbon, nitrogen and C/N ratio of the mangrove leaves in the experiment is shown in Table 2. The carbon was higher in R. apiculata yellow leaf category (440.64 ± 20.5 mg g−1) but lower in A. officinalis green leaf category (368.6 ± 10.5 mg g−1), whereas the concentration of nitrogen was higher in A. officinalis green leaves (24.00 ± 2.2 mg g−1). The C/N ratio was highest (71.61 ± 1.7 mg g−1) for R. apiculata yellow leaves and lowest for A. officinalis green leaves (15.35 ± 1.4 mg g−1).
The tannin content of leaves used for ingestion–egestion assay revealed that 96 h pre-soaking of leaves removed a substantial quantity of tannin and lignin from the leaves thereby improved the ingestion rate. It was much reduced to 2.06 mg g−1 in A. officinalis brown leaves. The tannin content in A. officinalis green leaves was 9.54 mg g−1; R. apiculata yellow leaves was 13.51 mg g−1 while A. marina green leaves showed comparatively high tannin content (18.07 mg g−1) even after 96 h of pre-soaking. It was noticed that the additional soaking helped to reduce the tannin content in brown leaves only. The brown leaves of A. officinalis collected from the field without soaking also had low tannin content compared to the other leaf categories. Soaking them again in the laboratory helped to reduce tannin content and therefore in the field also more leached brown leaves may be preferred by the crab.
From the ingestion – egestion assay, the assimilation of mangrove leaves by the crab P. plicatum gave a high assimilation for A. officinalis brown leaves (40.89 ± 16.5 mg dw (gww)−1 day−1) and low assimilation for A. officinalis green leaves (20.36 ± 10.1 mg dw (gww)−1 day−1). The assimilation of P. plicatum for A. marina green leaves was 29.20 ± 16.50 mg dw (gww)−1 day−1, whereas that for R. apiculata yellow leaves was 28.27 ± 9.3 mg dw (gww)−1 day−1. However, the assimilation efficiency was higher for A. officinalis green leaves (80.4 ± 8.48%) compared to A. officinalis brown leaves (61.33 ± 20.05%). The assimilation efficiency of A. marina green leaves by P. plicatum was 64.63 ± 2 1.2% and for R. apiculata yellow leaves was 56.62 ± 15.2%.
Fate of carbon and nitrogen
Ingestion-egestion mechanism
The fate of carbon and nitrogen in terms of ingestion, egestion, assimilation and assimilation efficiency is shown in Table 3. The carbon returning to the environment through egestion was high for R. apiculata yellow leaves (6.15 ± 1.6 g C (gww)−1 day −1), which is 31.11% of the ingested carbon. This was followed by A. marina green leaves, as its egestion converted 24.29% of ingested carbon. The A. officinalis green leaf consumption resulted in the conversion of only 8.83% of carbon through egestion while A. officinalis brown leaf consumption removed 15.64% of ingested carbon through egestion. The carbon assimilation of P. plicatum for A. officinalis green leaves were lower (8.47 ± 1.7 g C (gww)−1 day−1) compared to other leaf categories; however, its assimilation efficiency was very high (91.21 ± 5.5%). A. officinalis brown leaves showed higher assimilation (22.93 ± 3.3 g C (gww)−1 day−1) with an efficiency of 84.37 ± 6.6% compared to other leaf categories. The carbon assimilation of P. plicatum for R. apiculata yellow leaves was 13.62 ± 2.7 g C (gww)−1 day−1 with carbon assimilation efficiency of 68.88 ± 6.3%. For A. marina green leaves, the carbon assimilation was 14.46 ± 3.3 g C (gww)−1 day−1 with carbon assimilation efficiency of 75.7 ± 6.8%.
The nitrogen was also cycled within the ecosystem by P. plicatum. It helps to bring nitrogen as one of the primary sources to mangrove ecosystem through its litter feeding activity. The highest removal of nitrogen (0.20 ± 0.06 g N(gww)−1 day −1, 71.43% of ingested leaf) through egestion mechanism of P. plicatum was exhibited when fed with R. apiculata yellow leaves (Table 3). The nitrogen assimilation efficiency was very high (95.30 ± 7.7%) for A. officinalis green leaves even though it was having lower assimilation (0.58 ± 0.33 g N (gww)−1 day −1).
Leaching of carbon and nitrogen
All carbon and nitrogen fragments (TOC, DOC, POC, TIC, DIC, TN, DN) leaching into the experimental aquaria during the feeding experiment showed significant variation with respect to mangrove species with the exception of dissolved nitrogen exhibiting no significant variation (Table 4). There was high leaching of particulate organic carbon than dissolved organic carbon (POC range = 0.3–59.88 mg L−1), and A. officinalis showed high DOC and POC compared to other species. The average leaching of carbon and nitrogen fragments from the experiments are shown in Fig. 5 and Fig. 6. The A. officinalis green leaf category showed an average leaching of TOC = 65.11 ± 3.2 mg L−1, DOC = 11.63 ± 1.44 mg L−1, POC = 53.48 ± 4.12 mg L−1, TIC = 1.45 ± 0.85 mg L−1, DIC = 1.36 ± 0.95 mg L−1, TN = 4.21 ± 3.08 mg L−1 and DN = 1.39 ± 1.27 mg L−1. A. officinalis brown leaves also showed leaching of carbon and nitrogen with: TOC = 19 ± 12.58 mg L−1, DOC = 5.12 ± 12.35 mg L−1, POC = 13.88 ± 11.73 mg L−1, TIC = 1.5 ± 0.9 mg L−1, DIC = 0.85 ± 0.6 mg L−1, TN = 3.1 ± 0.44 mg L−1, DN = 1.41 ± 0.48 mg L−1.
Discussion
Feeding ecology of P. plicatum
The stomach contents of P. plicatum in both the collection sites in the Cochin mangroves revealed that they are mainly detritivorous. This feeding choice from the field confirms the result of previous studies in related species of sesarmid crabs which are significant players in leaf degradation and nutrient regeneration in mangroves (Dahdouh-Guebas et al., 1999). He reported that sesarmid stomach contents comprised more than 85% of mangrove leaves and they removed 79–95% of mangrove leaf fall from the forest floor (Sheaves & Molony, 2000). The average percentage composition of mangrove litter in the stomach of P. plicatum in the present study was 79.25 ± 5.95%.
Differential rates of leaf consumption
Higher ingestion rates were observed for mangrove leaves in the category of brown leaves compared to green and yellow leaves. This higher ingestion rates established the strong tendency of crabs towards decomposed brown leaves. The difference in the diet intake by the experimental crab is analogous with many studies which reported high ingestion rate for partially decomposed brown leaves or senescent brown leaves of mangroves by sesarmid crabs (Thongtham & Kristensen, 2005; Nordhaus & Wolff, 2007; Chen & Ye, 2008). Kwok & Lee (1995) reported high growth and moulting rate of P. plicatum when fed with mangrove brown leaves. In the present study, the crab’s least preferred leaves were A. ilicifolius (fresh and brown), A. aureum (fresh), B. gymnorrhiza (fresh) and B. sexangula (fresh leaves) which indicates that there must be an important factor that restricted the herbivory of the crab for such leaf categories. P. plicatum showed low ingestion rate for the leaves of E. agallocha, while it was the most preferred leaf by another mangrove crab N. malabaricum (Shanij et al., 2016). In many studies, A. marina brown leaves were preferred by sesarmid crabs and herbivorous mangrove crabs (Werry & Lee, 2005; Ravichandran et al., 2006, 2007; Bui & Lee, 2014). However, these studies did not include A. officinalis leaves (except Shanij et al., 2016) in the experiment. Another study of leaf preference of P. plicatum with mature, senescent and decomposed leaves of Kandelia candel, B. gymnorrhiza and Aegiceras corniculatum showed maximum preference for K. candel leaves (Chen &Ye, 2008). Most of the studies mentioned above, used only 3–4 mangrove species or single mangrove species for feeding ecological experiment. However, the current study tested the differential rate consumption of 11 mangrove species, and therefore, showed a better comparative result. It showed that P. plicatum, showed high ingestion for A. officinalis leaves compared to A. marina. However, the preferences can be confirmed by mixing the diets in the experiment or experimental design as suggested by Olabarria et al., (2002) and Underwood et al. (2004). There is no reported study to compare the ingestion rate of P. plicatum for A. officinalis leaves. These crabs were abundant in habitats where A. officinalis mangrove species was abundant with high litterfall and less crab density was observed in areas where E. agallocha mangrove species are abundant (Rani et al., 2018, 2016).
Does C/N ratio determine leaf choice?
It was evident that certain factors greatly influenced the leaf preference of crab. Many studies revealed that the C/N ratio determined the palatability of mangrove leaves, however, the present study revealed that more than its nutritional value, crabs preferred leaves with less inhibiting factors like tannin (TALLS). It can be further justified by the ingestion- egestion assay, in which A. officinalis green leaves had low C/N ratio, but the most preferred leaf was A. officinalis brown leaves having comparatively higher C/N ratio with low tannin content. The current study results confirmed the observations of Feller (1995) and McKee & Feller (1995) which reported, inhibition of mangrove leaf grazing by the crabs due to high tannin content in the fresh green leaves compared to decomposed leaves. Another contrasting factor is that, in many studies (Ravichandran & Kannupandi, 2004; Ravichandran et al., 2006; Kathiresan & Ravi, 1990) reported, A. marina was having high ingestion rate due to low tannin content while the present study observed high tannin content (supplementary information, Table 4, low tannin content only in brown leaves) compared to other leaves even during the ingestion–egestion assay (96 h presoaking in 35 saline sea water reduced the tannin content to 18.07 ± 2.3 mg g−1).
The correlation between C/N ratio and ingestion rate revealed a negative correlation of food choice with C/N ratio, portraying a contrasting result while comparing it with majority of the feeding experimental studies revealed a negative correlation of food choice with C/N ratio (Feller, 1995; McKee & Feller, 1995; Nordhaus & Wolff, 2007; Chen & Ye, 2008). However, it was comparable with Erickson et al. (2004) which reported a positive correlation of grazing of a mangrove crab Aratus pisonii with C/N ratio and indicated that mangrove leaves are not a nitrogen source for the crab. Usually, marine invertebrates prefer food with a C/N ratio less than 17 (Russel-Hunter, 1970). However, C/N ratios in mangrove leaves reported by majority of studies far exceeded the Russel–Hunter ratio of 17. Leaves usually takes a very long duration to reach their lowest C/N values and even the most decayed leaves also had double the Russel–Hunter ratio for C/N (Skov & Hartnoll, 2002). Therefore, P. plicatum preferred other animal tissue and edaphic nitrogen as its nitrogen source. This coincides with field gut content results of the present study to that of Erickson et al. (2003), Nordhaus & Wolff (2007) which reported nematodes and other animal matters along with sediment in the stomach of herbivorous mangrove crabs. A work by Kristensen et al. (2010) on stable isotope studies for determining the source of food in the gut of mangrove crabs also suggested that many mangrove crabs fed animal tissues for meeting their nitrogen needs. The results of the present study and literature confirmed that there was a combination of multiple factors influencing the leaf preference by the mangrove crabs. The water content, crude fibre content, fatty acid content and nitrogen compound composition also contributed (Chen & Ye, 2008; Nordhaus et al., 2011) to mangrove leaf choice by the mangrove crabs in addition to tannin and C/N ratio.
Ingestion–egestion assay and fate of carbon and nitrogen
The crabs helped in the shredding of fresh or aged leaf litter and thereby making it small sized with an increased surface area to volume ratio. This fragmentation process enhanced microbial colonisation (which further enhanced decomposition) and leaching (Werry & Lee, 2005). Thus, these crabs act as an initial processor for converting low-quality mangrove leaf litter into biomass and eventually help in carbon storage in consumers. However, the ability of mangrove crab in nutrient cycling ultimately depends on crab’s ability to effectively digest and assimilate the low-quality mangrove leaf litter into its biomass.
The ingestion–egestion assay showed that A. officinalis brown leaves were most preferred by the mangrove crab, P. plicatum and egestion rate as well as assimilation was also high among the same species. However, assimilation efficiency was highest for A. officinalis green leaves followed by A. marina green leaves. The real physiological reason for this high assimilation for green mangrove leaves is unknown. However, a possible reason reported by Thongtham & Kristensen (2005) and Nordhaus & Wolff (2007) suggests that in laboratory condition, the crab may be trying to use its available food in the tank even though fresh leaves were not a preferred food in the field. The carb may be converting it into maximum biomass due to the absence of other preferred leaves or food items because of which the egestion rate became very low for this mangrove leaf category compared to other leaves resulting in higher assimilation for green leaf category.
The corresponding C and N assimilation efficiency was also high for Avicennia officinalis brown leaves and displayed evidence for the litter processing ability of mangrove crab for senescent leaves. R. apiculata yellow leaves had very low C and N assimilation efficiency compared to other leaves. This low assimilation may be owed to low digestion of Rhizophora spp. due to tough leaf morphology as reported in earlier study (Camilleri, 1989). This assimilated carbon is either respired as carbon dioxide or incorporated into crab biomass which eventually enters into sediment pool when the crab dies. The mangrove forest having species of high carbon assimilation efficiency helps in carbon storage majorly through crab biomass while the mangrove species having low assimilation efficiency (R. apiculata) brings the carbon to the ecosystem majorly through crab’s faeces and rest through crab biomass. In both instance, crab act as a helping agent for retaining the nutrients within the ecosystem and forms the keystone species.
The assimilation efficiency of the crab for the mangrove leaves had a significant role in retaining the carbon in mangrove ecosystem and thereby sequestering the carbon without releasing it to adjacent wetlands. However, this was questioned in some recent research works. Bui & Lee (2014) confirmed the role of grapsid crabs in assimilating low-quality mangrove litter into biomass, thereby playing a significant role in the food web and carbon cycling. With evidence from stable isotope analysis, Mazumder & Saintilan (2010) and Skov & Hartnoll (2002) questioned some of the misunderstandings of recent researches which claimed that mangrove litter is not the primary food of grapsid crabs. Later, Bui & Lee (2014) solved the anomaly in taking the stable isotope ratio in consumer level and confirmed that the primary diet of grapsid crab was mangrove litter even though it takes some other food items occasionally.
While comparing the ingestion, egestion and assimilation efficiency of Neoepisesarma versicolor and P. plicatum (Thongtham & Kristensen, 2005), P. plicatum was having high ingestion rate and low egestion rate with high assimilation efficiency. N. versicolor showed high assimilation efficiency for green leaves (68.7%) while very low for yellow leaves (25.9%) and brown leaves (6.5%). Bui & Lee, 2014 also reported low-assimilation efficiency for C and N in P. erythodactyla fed with A. marina (36% for C and 57% for N). However, the present assimilation efficiency of (82.44%) with consumption of A. marina leaves was comparable to Sesarma meinerti (Emmerson & Mc Gwynne, 1992; Ravichandran et al., 2006).
The analysis of TOC, DOC, POC, TIC, DIC, TN, DN in the experimental water including control tank (without crab) indicates that handling of leaves by the crab also helps in leaching of nutrients to the substratum. There was considerable amount of leaching of carbon and nitrogen to the water but significantly differed among mangrove species. However, it was comparable with Thongtham & Kristensen (2005). Among the mangrove species used in the experiment, A. officinalis species was having high leaching efficiency of carbon mainly in the form of DOC and POC compared to other species. More than these values, large amount of physical leaching of carbon from the mangrove leaves also occurs in the field from the uneaten crab processed litter fragments and also from the faeces.
Relation of mangrove crabs on mangrove primary production and fate
The experimental part showed high rate of leaf litter processing by the P. plicatum which was the abundant crab in the field (Kochi mangroves). While comparing the food in its natural habitat as litterfall production reported by Rani et al. (2016) it was found that the total litter production in St.1 (2413.36 ± 873.7 g DW m−2 y−1) was very high and almost two-fold compared to St.2 (1295.65 ± 401.1 g DWm−2 y−1) and St.3 (1263.28 ± 255.3 g DWm−2 y−1). Subsequently the primary productivity through litterfall was also high in St.1 (10.36 t C ha−1y−1) followed by St.2 (5.57 t C ha−1y−1) and St.3 (5.42 t C ha−1y−1). From this study, it could be seen that St.1 was having highest crab biodiversity with abundance of P. plicatum. The fate of this primary productivity through historic soil carbon burial (soil carbon sequestration upto a period of 80 years) was also reported by Rani et al. (2021) through radioisotope techniques, CRS model and carbon analysis and it averaged to a total carbon burial rate of 10.41 ± 2.50 t C ha−1 yr−1 at St.1. In St.3, it was 2.95 ± 0.79 t C ha−1 yr−1 and minimum burial rate was in St.2 (0.57 ± 0.24 t C ha−1 yr−1). The study also reported the major influencing factors like biomass, productivity, role of crabs as biotic factors and sediment grain size, sedimentation rate and topography as abiotic factors for such variation in carbon sequestration and highlighted that beyond abiotic factors, biotic factors are the primary controls for soil carbon burial/sequestration. The crabs played a major role in litter processing and trapping and found that even though two mangrove habitats were having similar productivity through litterfall, the carbon burial rate was negligible for mangrove habitat with very low crab density. The current experimental results of feeding ecology of P. plicatum and several other reported feeding experiments of crabs (Kwok & Lee, 1995; Lee & Kwok, 2002; Micheli, 1993; Thongthm & Kristensen, 2005) showed that, it can remove a large portion of mangrove litter and could significantly control organic matter availability or storage within the soils of the mangrove ecosystem. It was in 1991, Smith and his research team stated crab as ecosystem key stone species on the basis of their field study by removing crabs from the mangrove habitats (Smith et al., 1991). However, that study also did not check whether it reduced the carbon sequestration potential of that habitat. From the present study, the crab density could be related to the fate of primary productivity of mangrove habitats from the historical carbon burial rates of each habitat. Even though, the study area was small, it will be a revelation and more studies can be done in global level and regional level to check this phenomenon. Only limited studies compared feeding ecology of crabs with field data of soil carbon sequestration due to the lack of data. Andreetta et al. (2014) made an attempt to link the crab biomass from the field to soil organic carbon stock and found a good positive relation in the mangrove habitats of Gazi Bay, Kenya. Therefore, more field experimental studies on crab burrowing, community structure, related carbon stock and burial within each mangrove ecosystem around the globe will reveal exact role of crabs in carbon sequestration.
Conclusion
The study showed that P. plicatum is an efficient mangrove litter feeder (from field gut content and experimental study) and assimilate a large portion of mangrove primary production into its biomass thereby helping in the carbon and nitrogen cycling in the mangrove ecosystem. The handling and fragmentation of mangrove leaves by the crab also facilitates a large amount of carbon into the water. The crab’s faeces which are enriched with the nutrients (C, N), mixes with the substratum (water or sediment) thereby act as a major agent for sustaining the nutrients within the ecosystem helping in sediment carbon stock improvement and soil carbon sequestration. The finding of this study opens up new ventures in research to find out to what extent the crabs can control the carbon structuring with the help of other controlling factors. It also acts as a baseline information to find out: Whether the crabs or the detritus produced by the crabs is an important food source for other estuarine species—especially species that are of particular interest for conservation or commercial reasons (link between primary and secondary productivity)? Could the feeding and burrowing activities of the crabs help to encourage the establishment of mangroves in a deforested area or in a newly created wetland area? Could differential consumption of litter from different mangrove species have an influence on mangrove community structure in any way? Whether the mangrove community structure affects crab biodiversity depending upon the palatability of leaves? Another important research question to prove the observation of crab’s role in carbon structuring is that whether introducing herbivorous crabs into mangrove habitats increases the soil carbon stock and recent surface layer carbon burial so that we can strengthen the mangrove restoration strategies and plans and climate change mitigation policies to improve the natural carbon sink capacity of mangrove habitats. More investigations can be done in future to analyse the impacts of restoration efficiency of already degraded mangrove habitats by introducing the crabs into the environment.
Data availability
Enquiries about data availability should be directed to the authors.
References
Alongi, D. M., 2014. Carbon Cycling and Storage in Mangrove Forests. Annual Review of Marine Science 6: 195–219. https://doi.org/10.1146/annurev-marine-010213-135020.
Andreetta, A., M. Fusi, I. Cameldi, F. Cimò, S. Carnicelli & S. Cannicci, 2014. Mangrove carbon sink. Do burrowing crabs contribute to sediment carbon storage? Evidence from a Kenyan mangrove system. Journal of Sea Research 85: 524–533. https://doi.org/10.1016/j.seares.2013.08.010.
AOAC, 2000. Official methods of analysis. Association of Official Analytical Chemists International. Maryland, USA
APHA, 2005. American Public Health Association. Standard methods for the examination of analysis of water and waste water, 21st edition.
Bui, T. H. H. B. & S. Y. Lee, 2014. Does ‘you are what you eat’ apply to mangrove Grapsid crabs? PLoS ONE 9: 1–12.
Camilleri, J. C., 1989. Leaf litter processing by invertebrates in a mangrove forest in Queensland. Marine Biology 114: 139–145.
Cannicci, S., D. W. Burrows, S. Fratini, T. J. Smith III., J. Offenberg & F. Dahdouh-Guebas, 2008. Faunal impact on vegetation structure and ecosystem function in mangrove forests: A review. Aquatic Botany 89: 186–200. https://doi.org/10.1016/j.aquabot.2008.01.009.
Chen, G. C. & Y. Ye, 2008. Leaf consumption by Sesarma plicata in a mangrove forest at Jiulongjiang Estuary, China. Marine Biology 154: 997–1007.
Chen, G.C., C. Lu, R. Li, B. Chen, Q. Hu & Y. Ye, 2016. Effects of foraging leaf litter of Aegiceras corniculatum (Ericales, Myrsinaceae) by Parasesarma plicatum (Brachyura, Sesarmidae) crabs on properties of mangrove sediment: a laboratory experiment. Hydrobiologia 763: 125–133. https://doi.org/10.1007/s10750-015-2367-1
Chhapgar, B. F., 1957. Marine crabs of Bombay state. Contribution No. 1 of the Taraporevala, Marine Biological Station. 1–129.
Christensen, B., 1978. Biomass and primary production of Rhizophora apiculata in a mangrove forest in southern Thailand. Aquatic Botany 4: 43–52.
Nellemann, C., E. Corcoran & C. M. Duarte, et al. 2009. Blue carbon. A rapid response assessment. GRID-Arendal: United Nations Environment Programme. ISBN: 978–82–7701–060–1.
Dahdouh-Guebas, F., Giuggioli, A. Olouch, M. Vannini & S. Cannicci, 1999. Feeding habits of non-ocypodid crabs from two-mangrove forest in Kenya. Bulletin of 52 Islam et al. /The Agriculturists 6: 43–53 (2008) Marine Science 64: 291–297.
Donato, D.C., J. B. Kauffman, S., Kurnianto, M. Stidham & D. Murdiyarso, 2011. Mangroves among the most carbon-rich forests in the tropics. Nature Geoscience 4: 293–297. https://doi.org/10.1038/NGEO1123.
Emmerson, W. D. & L. E. Mc Gwynne, 1992. Feeding and assimilation of mangrove leaves by the crab Sesarma meinarti de Man in relation to leaf litter production in Magzana, a warm -temperature in southern African mangrove swamp. Journal of Experimental Marine Biology and Ecology 157: 41–53.
Erickson, A. A., M. Saltis, S. S. Bell & C. J. Dawes, 2003. Herbivory feeding preferences as measured by leaf damage and stomatal ingestion: a mangrove crab example. Journal of Experimental Marine Biology and Ecology 289: 123–138.
Erickson, A. A., S. S. Bell & C. J. Dawes, 2004. Does mangrove leaf chemistry help explain crab herbivory patterns? Biotropica 36: 333–343.
Feller, I. C., 1995. Effects of nutrient enrichment on growth and herbivory of dwarf red mangrove (Rhizophora mangle). Ecological Monograph 65: 477–505.
Harada, Y. & S. Y. Lee, 2016. Foraging behavior of the mangrove sesarmid crab Neosarmatium trispinosum enhances food intake and nutrient retention in a low-quality food environment. Estuarine Coastal Shelf Science 174: 41–48. https://doi.org/10.1016/j.ecss.2016.03.017.
Kathiresan, K. & A. V. Ravi, 1990. Seasonal Changes in Tannin Content of Mangrove leaves. Indian Forester 116(5): 390–392.
Kristensen, E., S. Bouillon, T. Dittmar & C. Marchand, 2008. Organic carbon dynamics in mangrove ecosystems: A review. Aquatic Botany 89: 201–219.
Kristensen, D. K., E. Kristensen & P. Mangion, 2010. Food partitioning of leaf-eating mangrove crabs (Sesarminae): Experimental and stable isotope (13C and 15N) evidence. Estuarine Coastal and Shelf Science 87: 583–590.
Kwok, W. P. W. & S. Y. Lee, 1995. The growth performances of two mangrove crabs, Chiromanthes bidens and Parasesarma plicata under different leaf litter diets. Hydrobiologia 295: 141–148. https://doi.org/10.1007/BF00029121.
Lee, S. Y., 1998. Ecological role of grapsid crabs in mangrove ecosystems: a review. Marine Freshwater Research 49: 335. https://doi.org/10.1071/MF97179.
Lee, S. Y. & W. P. Kwok, 2002. The importance of mangrove species association to the population biology of the sesarmid crabs Parasesarma affinis and Perisesarma bidens. Wetlands Ecology and Management 10: 215–226. https://doi.org/10.1023/A:1020175729972.
Lee, S. Y., J. H. Primavera, F. Dahdouh-Guebas, K. L. McKee, J. O. Bosire, S. Cannicci, K. Diele, F. Fromard, N. Koedam, C. Marchand, I. A. Mendelssohn, N. Mukherjee & S. Record, 2014. Ecological role and services of tropical mangrove ecosystems: a reassessment. Global Ecology and Biogeography 23: 726–743. https://doi.org/10.1111/geb.12155.
Mazumder, D. & N. Saintilan, 2010. Mangrove Leaves are Not an Important Source of Dietary Carbon and Nitrogen for Crabs in Temperate Australian Mangroves. Wetlands 30: 375–380.
McKee, K. L. & I. C. Feller, 1995. Interactions among nutrients, chemical and structural defense, and herbivory in mangroves at Rookery Bay, Florida. Final Report. Office of Ocean and Coastal Resource Management, National Oceanic and Atmospheric Administration, Silver Spring, Maryland.
Micheli, F., 1993. Feeding ecology of mangrove crabs in North Eastern Australia: mangrove litter consumption by Sesarma messa and Sesarma smithii. Journal of Experimental Marine Biology and Ecology 171: 165–186. https://doi.org/10.1016/0022-0981(93)90002-6.
Nair, S. M., A. N. Balchand & P. N. K. Nambisan, 1989. On the determination and distribution of hydroxylated aromatic compounds in estuarine waters. Toxicology and Environmental Chemistry 23: 203–213.
Ng, P. K., D. Guinot, & P. J. Davie, 2008. Systema Brachyurorum: Part I. An annotated checklist of extant brachyuran crabs of the world. The Raffles Bulletin of Zoology 17 (1): 1–286.
Ng, P. K. L., Varghese Rani & S. Bijoy Nandan, 2017. A new species of Pseudosesarma Serène and Soh, 1970 (Crustacea: Brachyura: Sesarmidae) from Cochin in southwestern India. Zootaxa 4311(2): 263–270.
Nordhaus, I. & M. Wolff, 2007. Feeding ecology of the mangrove crab Ucides cordatus (Ocy-podidae): food choice, food quality and assimilation efficiency. Marine Biology 151: 1665–1681.
Nordhaus, I., T. Salewski & T. C. Jennerjahn, 2011. Food preferences of mangrove crabs related to leaf nitrogen compounds in the Segara Anakan Lagoon, Java, Indonesia. Journal of Sea Research 65: 414–426.
Olabarria, C., A. Underwood & M. Chapman, 2002. Appropriate experimental design to evaluate preferences for microhabitat: an example of preferences by species of microgastropods. Oecologia 132(2): 159–166.
Pati, S. K., V. Rani, P. S. Sujila & S. Bijoy Nandan, 2019. First confirmed record of the sesarmid crab, Parasesarma bengalense (Davie, 2003) (Decapoda: Brachyura) in Indian waters. Nauplius 27: e-2019012. https://doi.org/10.1590/2358-2936e2019012.
Rani, V., S. Sreelekshmi, C. M. Preethy & S. Bijoy Nandan, 2016. Phenology and litterfall dynamics structuring Ecosystem productivity in a tropical mangrove stand on South West coast of India. Regional Studies in Marine Science 8: 400–407. https://doi.org/10.1016/j.rsma.2016.02.008
Rani, V., S. Sreelekshmi, C.V. Asha & S. Bijoy Nandan, 2018. Forest Structure and Community Composition of Cochin Mangroves, South-West Coast of India. Proceedings of National academy of Science, Section B: Biological Science. India 88(1): 111–119. https://doi.org/10.1007/s40011-016-0738-7.
Rani, V., S. Bijoy Nandan & P.T. Schwing, 2021. Carbon source characterisation and historical carbon burial in three mangrove ecosystems on the South West coast of India. Catena 197: 104980. https://doi.org/10.1016/j.catena.2020.104980
Ravichandran, S. & T. Kannupandi, 2004. Biochemical changes in decomposing leaves and crabs of Pichavaram mangroves. Biochemical and Cellular Archives 4(2): 79–86.
Ravichandran, S., T. Kannupandi, & K. Kathiresan, 2006. Mangrove leaf litter processing by sesarmid crabs, Cry. Journal of Science (Bio.Sci) 35(2): 107–114.
Ravichandran, S., A. Anthonisamy, T. Kannupandi & T. Balasubramanian, 2007. Leaf choice of Herbivorous mangrove crabs. Research Journal of Environmental Science 1: 26–30.
Robertson, A. I., 1986. Leaf-burying crabs: their influence on energy flow and export from mixed mangrove forests (Rhizophora spp.) in northeastern Australia. Journal of Experimental Marine Biology and Ecology 102: 237–248.
Russell-Hunter, W. D., 1970. Aquatic Productivity: An Introduction to Some Basic Aspects of Biological Oceanography and Limnology, MacMillan, New York:, 306.
Schories, D., A. Barletta-Bergan, M. Barletta, U. Krumme, U. Mehlig & V. Rademaker, 2003. The keystone role of leaf-removing crabs in mangrove forests of North Brazil. Wetland Ecology and Management 11: 243–255.
Shanij, K., V. P. Praveen, S. Suresh, M. Mathew & Oommen & T. S. Nayar, 2016. Leaf litter translocation and consumption in mangrove ecosystems: the key role played by the sesarmid crab Neosarmatium malabaricum. Current Science 110(10): 1969–1976.
Sheaves, M. & B. Molony, 2000. Short-circuit in the mangrove food chain. Marine Ecology Progress Series 199: 97–109.
Skov, M. W. & R. G. Hartnoll, 2002. Paradoxical selective feeding on a low-nutrient diet: why do mangrove crabs eat leaves? Oecologia 131: 1–7.
Slim, F. J., M. A. Hemminga, C. Ochieng, N.T. Cocheret. Jannick & de la Morinie`re & E.G. van der Velde, 1997. Leaf litter removal by the snail Terebralia palustris (Linneaus) and sesarmid crabs in an East African mangrove forest (Gazi Bay, Kenia). Journal of Experimental Marine Biology and Ecology 215: 35–48.
Smith, T. J., K. G. Boto, S. D. Frusher & R. L. Giddins, 1991. Keystone species and mangrove forest dynamics: the influence of burrowing by crabs on soil nutrient status and forest productivity. Estuarine Coastal and Shelf Science 33: 419–432.
Tan, C. G. S. & P. K. L. Ng, 1994. An Annoted checklist of mangrove brachyuran crabs from Malaysia and Singapore. Hydrobiologica 285: 75–84.
Thongtham, N. & E. Kristensen, 2005. Carbon and nitrogen balance of leaf-eating sesarmid crabs (Neoepisesarma versicolor) offered different food sources. Estuarine Coastal and Shelf Science 65: 213–222.
Tongununui,P, Y. Kuriya, M. Murata, H. Sawada, M. Araki, M. Nomura, K. Morioka, T. Ichie, K. Ikejima & K. Adachi, 2021. Mangrove crab intestine and habitat sediment microbiomes cooperatively work on carbon and nitrogen cycling. PLoS One 16(12): e0261654. https://doi.org/10.1371/journal.pone.0261654.
Underwood, A. J., M. G. Chapman & T. P. Crowe, 2004. Identifying and understanding ecological preferences for habitat or prey. Journal of Experimental Marine Biology and Ecology 300(1–2): 161–187.
Werry, J. & S. Y. Lee, 2005. Grapsid crabs mediate link between mangrove litter production and estuarine planktonic food chains. Marine Ecology Progress Series 293: 165–176.
Williams, W. T., J. S. Bunt & N. C. Duke, 1981. Mangrove litter fall in NE Australia II. Periodicity. Australian Journal of Botany 29: 555–563.
Acknowledgements
The first author is thankful to DST-INSPIRE, Govt. of India with grant number (IF110502) which financially supported the present research work. The authors are thankful to Dr. Ng. K. Peter, National University of Singapore for his support in crab identification.
Funding
The Research was carried out with the funding from DST-INSPIRE, Govt. of India with grant number (IF110502).
Author information
Authors and Affiliations
Contributions
VR: contributed to the conception and design of the work, acquisition, analysis, interpretation of data and manuscript drafting. BN: contributed in conception and edited the manuscript. SC: helped in experimental part and field collection of crabs and leaves. KSS: helped in molecular analysis of crabs and also assisted in experimental analysis. CMPp: helped in editing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Additional information
Handling editor: Emily M. Dangremond
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rani, V., Sreelakshmi, C., Nandan, S.B. et al. Feeding ecology of Parasesarma plicatum and its relation to carbon structuring in mangrove ecosystem. Hydrobiologia 850, 911–927 (2023). https://doi.org/10.1007/s10750-022-05133-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-05133-y