Abstract
The biological meaning of the parameters a and b of the empirical and in fisheries science and management omnipresent relationship Weight = a * Length b is still unclear; the depicting body size trajectory has been associated thus far with body form and with food/energy considerations. When I analyzed a 116 species large and 36 years long subset of the port biosampling program of the US Virgin Islands fishery, Eastern Caribbean, using regression prediction intervals and fish life traits, I found out that the power law model is not a perfect fit due to individual variation. Concurrent stunting and starvation are generated at marginal values of the a and b parameters; their frequency is independent of fish body form and habitat type; dependent on species, trophic level and life stage; and changes the fundamental fish body size growth model from (log transformed) linear to curvilinear for three demo species. Also, variation in the parameter b could not be explained by trophic level, body form or habitat type. An alternative explanation is proposed, where b is the “accelerator” and a is the “break”; it expresses the correspondence of fish condition to environmental condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Weight–Length functional relationship of fish body size is a well-established, empirical, an expected almost perfect fit power law function that describes population growth trajectory. In the equation, W = a * Lb, W is the Weight; L is the Length; a is the intercept coefficient; and b is the exponent coefficient of fish. Values of the a and b parameters may vary widely within and between species, yet remain within the ranges 0 < a < 1 and 2 < b < 4, respectively, for all fish species irrespective of body form / shape, trophic level, and asymptotic maximum size; their coupled values, however, are species-specific (Froese, 2006). Estimates of weight from length based on its linearized form, i.e., logW = loga + b * logL, are omnipresent in fisheries science and management: they are used in population dynamics, stock assessment, multi-stock and multi-regional comparisons, management measures, aquatic ecosystems management and modeling, fish condition, and relative weight estimates (King, 2007; Pauly et al., 2014) because body size is a fundamental life trait related to all processes that affect population size (fecundity, growth, mortality). Despite its fundamental importance in fish and fisheries biology, ecology, and management, the biological meaning of the parameters a and b is speculative; the latter has been associated thus far with fish body form (Froese, 2006) and with food / energy considerations (O’Brien, 2006).
The body form explanation views the biological parameters a and b as determinants of the way with which mass is allocated along the three dimensions of length, width, and depth so as a fish to fulfill its ontogenetically determined body form plan. As such, a value of b equal to three denotes isometric growth, while values of b lower or higher than three denote allometric growth (e.g., Froese et al., 2011). Isometric growth retains the body form / shape of an organism, while allometric growth enables change in the body form / shape through differential growth rate of body parts (tissues and organs) in relation to the growth rate of the whole body. Thus, parameter a is regarded as the coefficient of body shape, while parameter b is regarded as the coefficient balancing the dimensions of the equation. Indeed, it appears that the range of a body form factor metric (a3.0) decreases from eel-like through elongated and fusiform to short and deep body form; yet the trend is not significant (Froese, 2006: 250, Fig. 10; Czudaj et al., 2022) because there is a considerable overlap in values of the metric among different body forms so that fish species with distinctly different body forms may obtain exactly the same value of a (and b) parameter(s). Therefore, the a and b parameters only partly reflect body form / plan in fish. Indeed, significant ontogenetic changes in the body form of fish take place during only a very small fraction of the lifespan of a fish from birth as egg or larva to entry into juvenile stage (days) after which the same final body form / plan is maintained, while the Weight–Length relationship accounts for the entire life span of fish (years); consequently, adaptations in shape (plasticity) of the final body form / plan as a response to changes in fertility and / or hydro-dynamism and / or habitat use and / or even gender (e.g., Fruciano et al., 2012; Meyers & Belk, 2014; Bilici et al., 2016) are negligible compared to the orders of magnitude change in body size within the same fish species and body form/plan. In addition, the isometric growth of fish once the final body form has been acquired would require that b = 1 for a power law function between a body part and the whole body (Mansano et al., 2017), whereas the Weight–Length relationship in fish pivots around b = 3, which means that a onefold increase in weight requires a threefold increase in length; after all the Weight–Length relationship is not a relationship between two morphometric traits as is the typical morphometric relationship between the length of annual increments on fish sagittae / otoliths and the annual increments in their body length, which is used to age fish individuals; it is a functional (body size growth), empirical (we do not know exactly the underlying mechanism), phenological (correlative, goodness-of-fit evaluated) relationship between growth in biomass and growth in length that allows us to predict weight from length and vice versa. As yet, there are no other direct associations / correlations between the a and b parameters of the Weight–Length relationship and whole-organism morphometric indices to support or refute the morphometrics explanation. Even ecologically relevant, distinct patterns in the a and b parameters for different body forms, trophic habits and habitats could not be reliably established (Karachle & Stergiou, 2012), while a negative temporal trend in b for the European sardine Sardina pilchardus (Walbaum, 1792) from the same fished populations of the Aegean Sea has been detected (Voulgaridou & Stergiou, 2003).
On the other hand, the food / energy explanation views the biological parameters a and b as determinants of the rate with which body mass accumulates throughout a lifespan of indeterminate growth in fish. In that respect, the power law Weight–Length empirical functional relationship must be substantively related with the saturation type of growth functions in fish that is Weight at Age and Length at Age, because time, age and length are all unidirectional and highly correlated with one another. The amply demonstrated gender-, season-, place-, and year-associated variance in the a and b parameters of the Weight–Length relationship within species from empirical fisheries studies (e.g., Giacalone et al., 2010; Robinson et al., 2010; Al Nahdi et al., 2016) all culminate toward the food/energy alternative explanation; after all, field and laboratory experimental work has already established the causal relationship between food/energy and growth in fish with ensued practical applications in aquaculture (e.g., FAO, 2019). Nevertheless, fish body size growth throughout its lifespan is a surrogate of the internal and external environment that integrates processes from lower (bottom up) and higher (top down) levels of organization and thus, may be reflecting variation in parameters a and b of the Weight–Length relationship that goes beyond the food/energy explanation.
Records of 116 fish species from the port biosampling program of the US Virgin Islands coral reefs associated fishery in the Caribbean basin spanning 35 years of sampling provided the opportunity to delve into the biological meaning of the a and b parameters of the Weight–Length relationship in fish by analyzing its outlying data points. Focusing at the bounds of this empirical (non-causal but exact) functional relationship will help us elucidate the mechanism / underlying process, since the morphometric and food/energy interpretations appear insufficient, and, thus, increase our confidence in its use. The objectives of this study are to find out the effects of outlying yet valid individual fish on the L-W relationship and explain their patterns of occurrence. For this purpose, I tested for the significance of their frequency; their dependence on trophic level, life stage, habitat type, and fish body form; their temporal trends; and their affect on the growth model of fish. I also tested whether mean values of the b parameter vary among species when grouped by trophic level, habitat type, and body form.
Materials and methods
Database development
The original curated database of the port biosampling program of the US Virgin Islands fishery contained 386 850 records of: length measurements in mm; weight measurements in kg; weight type recorded as round whole, ungraded, or gutted-head on; length type recorded as fork length, standard length, or total length; year of sampling ranging from 1980 to 2015; Integrated Taxonomic Information System species’ code, common, and scientific name of unsexed vertebrate and invertebrates fishery resources. This is the longest available database on length and weight of fishery resources in the tropics that include target fish species with regional distribution (NOAA-NMFS, 2017; CFMC, 2017). I discerned and isolated records for 116 fish species of the same weight type, namely whole round, with at least seven individuals within a single year of sampling, and for at least one year of data (n = 276 171, nsp. ≥ 7, nyrs. ≥ 1, 1980–2015). Length types other than fork length were all converted into fork length using geographically relevant equations from FishBase (Froese & Pauly, 2014) (Online Resource Table S1). Weight measurements and length measurements were turned into measurements in g and in cm, respectively, for the development of Weight–Length regressions.
The database was expanded with variables on trophic level, habitat type, and body form of the 116 fish species derived from FishBase (Froese & Pauly, 2014) (Online Resource Table S2). Life stages delineated were: immature (L < Length at maturity); adult (Length at maturity < L < Length optimum for fishing + 10% of Length optimum); and megaspawner (L > Length optimum for fishing + 10% of Length optimum). To find out the Length at maturity and Length optimum for fishing for each species of interest, I first searched for geographically relevant, maximum length ever reported values for the species of interest, which were available in FishBase, other bibliographic sources, and in the current study (Online Resource Table S2). I then plugged these maximum length ever reported values into the Life History Tool in FishBase (Froese & Binohlan, 2000), which comprises robust empirical pairwise regressions of life traits derived from thousands of fish species that allow prediction of the life traits of the species of interest. Thus, I interpolated the values of length at maturity and length optimum for fishing for the species of interest from the life history empirical pairwise regressions available in the Life History Tool in FishBase.
Detection of outliers in the database
To identify outlying data points for each of the 116 fish species from the curated from human errors database, when natural variability in a and b parameters was the main interest, I had to devise a systematic and objective approach instead of subjectively deleting data points so as to attain a specific and acceptable or expected level of fitness of data on the linear model logW = loga + b * logL because the latter approach would subjectively define the range of natural variability. In addition, removing invalid outlying data points is a standard pre-requisite before constructing Weight–Length relationships in fisheries science. First, I identified outlying data points and then, I tested for their validity. To identify outlying data points, I developed 99% prediction intervals / lines above (upper boundary) and below (lower boundary) their logW = loga + b * logL linear function using their aggregate data, that is data from all years of sampling, with the ordinary least squares method for normally distributed data (Preston, 2000). Prediction intervals are estimates of (upper and lower boundary) intervals within which future observations are expected to lay with pre-defined confidence considering all available data points. Therefore, the 99% prediction intervals contain all expected future data points based on all available data points. I was gradually removing outlying data points until the 99% prediction intervals were stabilized, which was largely needed when there was a small sample size for a species, and the function’s line was parallel to the 99% prediction intervals; they are both prerequisites for correct prediction lines. Next, I tested whether the identified outlying data points above and below the 99% prediction intervals were valid data points. Thus, I first developed independent W = a * Lb relationships to derive paired a and b parameter values of the following groups of data: outlying above data, outlying below data, and non-outlying data. Then, I evaluated how well these derived paired loga and b values as well as referenced documented values from fishery independent surveys of the same species at the USVI and / or Puerto Rico, fit on the loga = c + d * b linear function for each species. Because the parameters a and b are species-specific, satisfactory fit of the outlying below and the outlying above data on their linear loga = c + d * b relationship, using both graphical inspection and the R2 coefficient of determination, means that the outlying individuals they correspond to are members of the same species or else valid data points (Froese, 2006: 249, Fig. 8). If so, then outlying data points are not incorrect data points due to species mis-identification, sampling, recording, and data processing errors (Kimmerer et al., 2005). Τhey lay, however, outside of the expected range of natural variability for a species, and this is indicative of pathology. Outlying data points above the 99% interval lines correspond to fish, which are too short for their weight (length impairment; stunting), while outlying data points below the 99% interval lines correspond to fish, which are too skinny for their length (weight impairment; starvation).
I picked three species to demonstrate the pattern of outlying data points: Scarus taeniopterus (Lesson, 1829), Pomacanthus arcuatus (Linnaeus, 1758), and Epinephelus fulva (Linnaeus, 1758) (Online Resource Figs. S1, S2). These species were chosen for a number of reasons other than adequate data for robust linear regression estimation (Froese et al., 2011): they belong to different trophic levels; they contain data from different decades; they are coral-associated fish species; and none of them goes through diet change during their life cycle (Froese & Pauly, 2014). S. taeniopterus and E. fulva have a fusiform body form, while P. arcuatus has a short and deep body form.
Statistical analysis
To find out whether the outlying data points of the 116 fish species are a result of chance or not, I used χ2 tests of goodness of fit for each one of them. I also tested the dependence of outlying data points on trophic level, life stage, habitat type, and body form with χ2 tests of independence. To test for temporal trends in annual percentage of outlying individuals for the three species of the study and in the aggregate length and aggregate weight of the 116 species (n = 276 305), I used linear regressions; in the case of the annual percentage of outlying individuals for the three species of the study, I added a second explanatory variable to control for differences in sample size among years.
Fitting the linear function logW = loga + b * logL on the data available for each species entails a number of relevant considerations. First of all, because there has been repeated sampling of the same species at the same places during different times, data are subject to both spatial and temporal auto-correlations. Second, multiple comparisons in developing species’ logW = loga + b * logL regressions inflate Type I error. To account for the auto-correlations and multiple comparisons issues, I also employed a three level (hierarchical) model so that individuals (level 1) are nested within species by time groups (level 2), which, in turn, are nested within species (level 3). Such a model also accounts for the actual missing groups and unequal sample sizes in levels 2 and 3 (Rasbash et al., 2015a). Third, robust logW = loga + b * logL regressions require ranges of samples that correspond to at least a 25% of the asymptotic length of each species (Wang et al., 2013), which should also contain immature, mature, and megaspawner individuals (Froese, 2006); this requirement is met only by a subset of 46 species, and for only a subset of the years, they were sampled (n = 58 338). Fourth, the data are from truncated overfished populations, where megaspawners (1 369 of 276 171) and mature (13 215 of 276 171) fish are underrepresented, and thus, the distribution of weight cannot be properly assessed, though it is expected to increase with increase in weight under the power law model W = a * Lb because data variance is multiplicative (Kerkoff & Enquist, 2009). I assume that the lengths are measured without error compared to weights or, in the best case, measurements of length contain smaller errors compared to measurements of weight. All measurements are independent because they were obtained from different individual fish following a cross section, repeated quarterly, sampling design with haphazard sampling (Appeldoorn et al., 1992; CFMC, 2017). In addition, weight data are heteroscedastic and of unequal sample size across length. Fifth, the outlying but valid data points are expected to reduce the explanatory power of the linear model. For these reasons, I compared the results of fitting different models using different estimation methods, which require different assumptions about the data to be satisfied, based on the Akaike criterion, that evaluates both model parsimony and model fitness (Aho et al., 2014). Models fitted are a single level linear model with log-transformed data and with log-transformed binned data, a three level linear random intercept and random slope model, and a single level curvilinear model. Model estimation methods base on minimizing geometric distances of data points from the model (ordinary least squares, OLS), on maximizing probabilities that the data were generated by the model fitted (iterative generalized least squares (IGLS), on restricted iterative generalized least squares (RIGLS), and on simulation (Monte Carlo Markov Chain, MCMC). Not only data assumptions relax from OLS through maximum likelihood to simulation estimation methods but also the OLS method provides estimates of the regression parameters as fixed or species-specific, while the IGLS, RIGLS and MCMC methods provide estimates of the regression parameters as derivatives from a population of species, whose sampled portion constitutes the species of the subset analyzed (Newsom, 2017). The models were fit using the LibreOffice Calc and the MLWin 2.32 software (Rasbash et al., 2015b) on a subset of the 116 species that contained 46 species, which could provide robust estimates of the logW = loga + b * logL relationship for species by year groups; 34 of these 46 species, which could provide robust logW = loga + b * logL regressions, also contained significant outlying data points; three of these 34 species were S. taeniopterus, P. arcuatus, and E. fulva.
I also tested whether the b parameter of the logW-logL relationship of those 46 species for which I could develop robust relationships (RIGLS method) differs among species grouped into trophic level, habitat type and body form using one-way analyses of variance.
Results
A total of 276 171 records of the 116 fish species of the database contained 5 641 data points that lay outside the linear regression prediction intervals of each species’ linearized Weight–Length relationship. These outlying data points belong to 75 species (64.6%) and were found significant for 53 of them (46.6%) (Online Resource Table S3). Demonstrated upon S. taeniopterus, P. arcuatus, and E. fulva, notice that there are young / short overweight (stunting) and old/long underweight (starvation) fish above and below the prediction interval lines, respectively, of their temporally aggregated, linearized Length–Weight relationship (Online Resource Fig. S1 a–c). Such individuals are present during multiple years of sampling for the same species. They are individuals outside of the expected range; they do follow the almost perfect, species-specific relationship between the a and b parameters but are generated at marginal values of these parameters (Online Resource Fig. S2 a–c); note that normal growth is a bit faster for the herbivorous S. taeniopterus and slower for the omnivorous P. arcuatus and the carnivorous E. fulva compared to reported growth two or / and four decades ago, whereas impaired growth is consistently slower for all three species.
The presence of these outlying individuals depends on trophic level; their incidence decreases with increase in trophic level so that 4 / 6 of the herbivores (13 out of 19 species), 3 / 6 of the omnivores (27 out of 60 species), and 2 / 6 of the carnivores (13 out of 37 species) show statistically significant presence of such outlying individuals (χ2 2,0.05 = 5.6290, P = 0.0599). The proportion of small size samples without a single impaired individual may show the reverse trend, namely 1.6 / 6 (5 out of 19 species) of the herbivores, 2.1 / 6 (21 out of 60 species) of the omnivores and 2.4 / 6 (15 out of 37 species) of the carnivores, yet impairment affects species within the same trophic level differently (both in nature and extent) regardless of sample size (Online Resource Table S3). For example, at the third trophic level, Haemulon plumierii (Lacepéde, 1801) and Balistes vetula (Linnaeus, 1758) have been sampled for comparable number of years, but H. plumierii has half the number of impaired individuals from double sample size compared to B. vetula. Also, Lutjanus analis (Cuvier, 1828) and Lutjanus synagris (Linnaeus, 1758) have comparable sample sizes during comparable number of years, yet L. analis has six times more impaired individuals compared to L. synagris. In addition, E. striatus has eight to nine times more impaired individuals for half number of years compared to Bodianus rufus (Linnaeus, 1758) although they have comparable sample sizes. Furthermore, among ten species with sample sizes in the range of 100–200 specimens for variable number of years providing specimens, there is Calamus calamus (Valenciennes, 1830) that presents significant number of impaired individuals during one-third of the number of years of the monitoring program. Similarly at the fourth trophic level, Pomadasys crocro (Cuvier, 1830) has more than double number of impaired individuals generated during half number of years compared to Coryphaena hippurus (Linnaeus, 1758) although they have comparable sample sizes. Also, Decapterus macarellus (Cuvier, 1833) has double number of impaired individuals from one year of sampling compared to Tylosurus crocodilus (Péron & Lesueur, 1821) although they have comparable sample sizes. In addition, Elagatis bipinnulata (Quoy & Gaimard, 1825) generated a significant number of impaired individuals compared to Thunnus albacores (Bonnaterre, 1788) for comparable number of years providing specimens and sample sizes. Indeed, the frequency of these outlying individuals is inversely related to trophic level so that carnivorous (average annual percentage ± SD = 4.95% ± 6.31) and omnivorous (average annual percentage ± SD = 2.74% ± 3.12) fish species have 3 and 1.5 times more outlying individuals compared to herbivorous (average annual percentage ± SD = 1.78% ± 1.69) fish species. Thus, target fish species with the highest mean annual percentage of outlying individuals during the 35 years of records are all carnivorous: Acanthocybium solandri (Cuvier, 1832) (31.4% of 477 data points, average annual percentage ± SD = 21% ± 19.4, 13 years data, trophic level 4.4); Lachnolaimus maximus (Walbaum, 1792) (13.3% of 279 data points, average annual percentage ± SD = 8.6% ± 16.4, 20 years data, trophic level 3.9); and L. analis (12.1% of 1 139 data points, average annual percentage ± SD = 7.2% ± 18.7, 29 years data, trophic level 3.9). Among the 53 species with significant presence of outlying individuals, immature individuals are susceptible to both length (stunting) and weight (starvation) impairment compared to mature and megaspawner individuals; yet, mature and megaspawner individuals are more susceptible to weight impairment rather than length impairment; both of these patterns occur irrespective of trophic level (χ2 2,0.05 = 430.7123, P = 2.9649 × 10–94). The presence of the outlying individuals depends neither on the body form (χ23,0.05 = 2.9210, P = 0.4040) nor on the habitat type (χ23,0.05 = 5.397, P = 0.1449) of fish species. There was found no temporal trend in mean annual percentage of outlying individuals for S. taeniopterus (a = 3.711(SE = 1.912), btime = − 0.114 (SE = 0.100), F = 0.6835, df = 26, P = 0.4159), P. arcuatus (a = 6.027 (SE = 4.078), btime = 0.091 (SE = 0.188), F = 0.1218, df = 25, P = 0.7300), and E. fulva (a = 4.362 (SE = 0.9945), btime = 0.065 (SE = 0.047), F = 2.0512, df = 28, P = 0.1632) after controlling for sample size differences in multiple linear regressions (Online Resource Table S4). Interestingly, neither the aggregate Length (a = 25.911 (SE = 0.029), b =−0.0178 (SE = 0.002), F = 5.9530, df = 276 302, P = 0.0001, R2 = 0.00020) nor the aggregate Weight (a = 499.621(SE = 2.909), b = – 3.398 (SE = 0.236), F = 206,3598, df = 276 302, P = 0.0001, R2 = 000075) of fish has changed during the sampling period in this stably truncated fishery (species’ mean percent of megaspawners ± SE = 8.3% ± 2.0; species’ mean annual percent of mature: 18.0% ± 2.0; n = 276 171) (Online Resource Table S3).
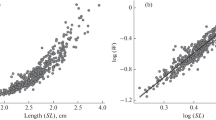
Growth impaired individuals grow more slowly under the power law model compared to normal individuals for all species (Online Resource Table S3). When combined, the second-order polynomial curvilinear model consistently outperforms all other models fitted on annual data available for S. taeniopterus, P. arcuatus, and E. fulva (Table 1; Fig. 1 a–c).
Fit of a linear (continuous black line) and a third-order polynomial (intermittent black line) model on length and weight data for three species of the port biosampling database of the USVI fishery. Gray intermittent lines from left to right indicate species’ length at maturity, optimum length for fishing + 10%, and asymptotic length. a S. taeniopterus, n = 645, 1989, herbivore, b P. arcuatus, n = 183, 1985, omnivore, c E. fulva, n = 196, 1997, carnivore. Comparison of models in Table 1
Mean values of the b parameter in the L-W relationship of the 46 species for which robust relationships could be developed using hierarchical modeling with the RIGLS method did not differ among trophic levels (one-way ANOVA, F = 0.6293, d.f. = 2, P = 0.5379), habitat types (one-way ANOVA, F = 0.1228, d.f. = 3, P = 0.9461), and body form types (one-way ANOVA, F = 1.2727, d.f. = 2, P = 0.2906; eel-like group was not compared) (Online Resource Table S5).
Discussion
The species-, trophic level-, and life stage-dependent growth impairment of individual fish verified for 53 out of the 116 target species in the studied coral reef fishery based on the analysis of its 36 years long port biosampling database is a first documentation ever for any fishery, and thus, we cannot fully appreciate the biological / ecological significance of its magnitude for the studied fishery. As demonstrated on three selected species of the fishery, such impaired individuals are altering the fundamental growth trajectory in fish, are generated when the a and b parameters obtain marginal values, and point to a common cause for both types of impairment, namely stunting and starvation, albeit via different mechanisms. Length-impairment / stunting points to the nutritional value of fish diet whereas weight-impairment/starvation points to the calorific value of fish diet, thus reflecting both stoichiometry (materials) and energy (food) aspects of their environment. Young, overweight, stunted individuals may develop accelerated rates of grazing / predation in their effort to obtain essential but scarce nutrients. Older, underweight, starving individuals may not be able to obtain adequate amount of food.
Spatial and temporal differences in the a and b parameters among populations of the same species have been explicated with numerous factors that eventually affect energy availability, such as growth conditions (FAO, 2019), energy acquisition, such as inter- and intra-specific competition (Diana, 1987; Ylikarjula et al., 1999), energy assimilation, such as food quality and / or quantity (Duann, 1998), energy allocation, such as age and gender (Roff, 1983; Rana et al., 2013; Boukal et al., 2014), and energy distribution within the trophic web; yet, fishery ecosystems do conserve their aggregate size composition in highly networked food webs (Murawski & Idoine, 1992) as was also found in this study. Differences in the a and b parameters of species grouped by trophic level, body form, and habitat type could not be reliably established for populations in the N. Aegean Sea (Karachle & Stergiou, 2012) and the parameter b of populations of species of this study from the Eastern Caribbean did not differ among trophic levels, body form and habitat types. Because 0 < a < 1 and 2 < b < 4 regardless of fish species, length range, and body form and because parameter b is inversely and perfectly interdependent on its respective parameter a for any fish species that has been examined (Froese, 2006), the a and b parameters are the value expressions of the common body size growth trajectory regulation mechanism in all fish at the population level; parameter a is the value expression of a “brake” function, while parameter b is the value expression of an “accelerator” function of growth in all fish within limits; decreasing values of a reduce weight, while increasing values of b increase weight. Given the width of the estimated confidence intervals, the a and b parameters for a species are not “fixed” but “slide” along a species-specific exponential relationship since there can exist even marginal values of a and b parameters, which have been generating stunted and starved individual fish for half of the target fish in the studied fishery, which, in turn, indicate unfavorable conditions for fish body size growth. Finally, because stunting and starvation were found dependent on species, life stage, and trophic level, the parameters a and b must be reflecting adjustment of the population’s scope for growth of its body size; it is changes at lower (e.g., organism, cell; bottom up processes) and higher (e.g., community, ecosystem; top down processes) levels of organization that permeate into the population level thus increasing variance around an expected perfect fit of the power law model; when changes overwhelm the regulating feedbacks within and among levels of organization, then even the fundamental power law model changes incrementally or substantially as demonstrated in this study. Therefore, the demonstrated changing patterns of growth for S. taeniopterus, P. arcuatus, and E. fulva in this fishery indicate that this environment does not afford a consistent growth pattern for all species and / or all life stages through increase in individual variance toward growth impairment.
Such growth impaired individuals from a coastal tropical fishery prompt re-examination of how well the linear logW = loga + b * logL growth model may fit for a substantial number of the target species. Better fit of a curvilinear instead of a linear model weakens the expectation that the environment is adequately supportive of all life stages for half of the target species of this fishery system irrespective of their trophic level, bears important implications regarding accuracy of predictions of fisheries productivity, and motivates fisheries managers to elucidate whether affected species in this fishery participate in the same trophic chain/s and also consider the interaction of these chronically stably truncated and overfished populations (Dikou et al., 2018, 2021) with concurrent and aggravating ecosystemic shift from coral to algal reefs at local and regional scales (Bellwood et al., 2004; Rothenberger et al., 2008; Jackson et al., 2014; Lapointe et al., 2021). In this respect, it is important to confirm the relative importance of the ecological mechanisms of (i) food / mineral scarcity through replacement of edible by unpalatable phytoplankton and benthic algae as coral reefs shift toward algal reefs, (ii) aggravation of inter- and intra-species competition by fishing, and (iii) trophic stoichiometric elemental imbalance induced by catching biomass and exporting it for consumption on land and by alterations in terrestrial inputs, in the generation of fish growth impairment in this fishery by combining this dataset with datasets available from ecological monitoring programs of NOAA and other organizations. In other cases with lack of perfect fit of the Weight–Length power law model, there have been proposed model modification (Weight = a * Length2 * Height in Jones et al., 1999), estimation method change (least squares nonlinear instead of linear estimation method in Hayes et al., 1995) and piece-wise application on different size segments of the population (De Robertis & Williams, 2011) to reduce variance and improve the accuracy of predictions of Weight from Length with an efficient sample size but without consideration for plausible environmental change attesting to the perception of a and b parameters as expressions of a fixed rather than a flexible (adjusting) attribute.
This is the first communication demonstrating fish starvation and stunting at an altered tropical coral reef environment in the Caribbean though reporting from other localities in the world is available (MacKenzie, 2019). Analysis of these outlying individual fish weakens the morphometrics explanation of the a and b parameters of the Weight–Length power law relationship. It confirms their wide adjusting flexibility within a regulating (not allometric) function of fish body size growth trajectory. It shows that they constitute an “accelerator”—“break” type of paired adjustors of growth at the population level to changes in their environment that goes beyond the food/energy explanation. It supports their explanation as integrative of top down and bottom up processes from multiple levels of organization.
Data availability
All data analyzed are available by the South East Fisheries Science Center, National Oceanic and Atmospheric Administration upon request (joshua.bennett@noaa.gov) or at https://www.fisheries.noaa.gov/inport/q?keywords=USVI.
References
Aho, K., D. Derryberry & T. Peterson, 2014. Model selection for ecologists: the worldviews of AIC and BIC. Ecology 95: 631–636.
Al Nahdi, A., C. Garcia de Leaniz & A. J. King, 2016. Spatio-temporal variation in length-weight relationships and condition of the ribbonfish Trichiurus lepturus (Linnaeus, 1758): implications for fisheries management. PLoS ONE 11: e0161989. https://doi.org/10.1371/journal.pone.0161989.
Appeldoorn, R., J. Beets, J. Bohnsack, S. Bolden, D. Matos, S. Meyers, A. Rosario, Y. Sadovy & W. Tobias, 1992. Shallow water reef fish stock assessment for the US Caribbean, NOAA Fisheries. Silver Spring, MD:
Bellwood, D. R., T. P. Hughes, C. Folke & M. Nyström, 2004. Confronting the coral reef crisis. Nature 429: 827–833. https://doi.org/10.1038/nature02691.
Bilici, S., A. Kaya & M. Y. Dörtbudak, 2016. A survey on Luciobarbus mystaceus (Pallas, 1814) by geometric morphometric methods depend on gender, age and season variations. Survey in Fisheries Sciences 3: 40–49. https://doi.org/10.18331/SFS2017.3.2.5.
Bohnsack, J.A. & D.E. Harper, 1988. Length-weight relationships of selected marine reef fishes from the southeastern United States and the Caribbean. NOAA, NMFS: Technical Memoir, NMFS-SEFC-215. https://repository.library.noaa.gov/view/noaa/3027/noaa_3027_DS1.pdf
Boukal, D. S., U. Dieckmann, K. Enberg, M. Heino & C. Jørgensen, 2014. Life-history implications of the allometric scaling of growth. Journal of Theoretical Biology 359: 199–207. https://doi.org/10.1016/j.jtbi.2014.05.022.
Burton, M. L. & J. C. Potts, 2015. Age, growth and natural mortality of coney (Cephalopholis fulva) from the southeastern United States. PeerJ 3: e825. https://doi.org/10.7717/peerj.825.
Choat, J. H., D. R. Robertson, J. L. Ackerman & J. M. Posada, 2003. An age-based demographic analysis of the Caribbean stoplight parrotfish Sparisoma viride. Marine Ecology Progress Series 246: 265–277.
Caribbean Fishery Management Council, 2017. US Virgin Islands Commercial Fishery Data Collection Programs. https://www.caribbeanfmc.com/After_the_Meeting_Documents/157th_After_the_Meet_Docs/8_CFMC_presentation_USVI_data_collection.pdf
De Robertis, A. & K. Williams, 2011. Weight-Length relationships in fisheries studies: the standard allometric model should be applied with caution. Transanctions of the American Fisheries Society 137: 707–719. https://doi.org/10.1577/T07-124.1.
Diana, J. S., 1987. Simulation of mechanisms causing stunting in northern pike populations. Transanctions of the American Fisheries Society 116: 612–617. https://doi.org/10.1577/1548-8659(1987)116%3c612:SOMCSI%3e2.0.CO;2.
Dikou, D., T. Corneille, K. Carmody, 2018. Life-traits based coral reef fisheries management – More support from the US Virgin Islands fishery. Poster presented at the 4th Guam coral reef symposium, March 27, 2018, Guam Bureau of Statistics and Plans. www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwi1pJ-m-671AhXRSfEDHTy_DR8QjBB6BAgJEAE&url=https%3A%2F%2Fbsp.guam.gov%2Freports%2F&usg=AOvVaw02f9XlWKVYhmnSibbcn1dD
Dikou, D., T. Corneille & K. Carmody, 2021. Sustainability of a tropical, multispecies, multigear, coral-reef-associated fishery system is efficiently inferred with the direct use of long-term port biosampling length records and life-history traits, US Virgin Islands. Aquatic Conservation: Marine and Freshwater Ecosystems 31: 567–589. https://doi.org/10.1002/aqc.3047.
Duann, C., 1998. Nutritional and developmental regulation of insulin-like growth factors in fish. The Journal of Nutrition 128(Suppl. 2): 306S-314S. https://doi.org/10.1093/jn/128.2.306S.
Food and Agriculture Organization of the United Nations, 2019. Milk-fish growth. Growth: species profiles. www.fao.org/fishery/affris/species-profiles/milkfish/growth/en/
Froese, R., 2006. Cube law, condition factor and weight–length relationships: history, meta-analysis and recommendations. Journal of Applied Ichthyology 22: 241–253. https://doi.org/10.1111/j.1439-0426.2006.00805.x.
Froese, R. & C. Binohlan, 2000. Empirical equations to estimate various life history characters of fishes (Linf, Lmat, Lopt, tmax). FishBase. www.fishbase.org/Download/
Froese, R. & D. Pauly, 2014. FishBase. www.fishbase.org
Froese, R., A. C. Tsikliras & K. I. Stergiou, 2011. Editorial note on weight-length relations of fishes. Acta Ichthyologica et Piscatoria 41: 261–263.
Fruciano, C., C. Tigano & V. Ferrito, 2012. Body shape variation and colour change during growth in a protogynous fish. Environmental Biology of Fish 94(4): 615–622. https://doi.org/10.1007/s10641-011-9968-y.
Giacalone, V. M., G. Danna, F. Badalamenti & C. Pipitone, 2010. Weight-length relationships and condition factor trends for thirty-eight fish species in trawled and untrawled areas off the coast of northern Sicily (central Mediterranean Sea). Journal of IED Ichthyology. https://doi.org/10.1111/j.1439-0426.2010.01491.x.
Gobert, B., A. Guillou, P. Murray, P. Berthou, M. D. Oqueli Turcios, E. Lopez, P. Lorance, J. Huet, N. Diaz & P. Gervain, 2003. Biology of queen snapper (Etelis oculatus: Lutjanidae) in the Caribbean. Fisheries Bulletin 103: 417–425.
Guabiroba, H. C. & J.-C., Joyeux, 2018. Length-weight relationships for reef fishes in a southwestern Atlantic tropical oceanic island. Pan-American Journal of Aquatic Sciences 13(1): 84–87.
Hayes, D. B., J. K. T. Brodziak & J. B. O’Gorman, 1995. Efficiency and bias of estimators and sampling designs for determining length-weight relationships of fish. Canadian Journal of Fisheries and Aquatic Sciences 52: 84–92. https://doi.org/10.1139/f95-008.
Jackson, J., M. Donovan, K. Cramer & V. Lam, 2014. Status and trends of Caribbean coral reefs: 1970–2012, Global Coral Reef Monitoring Network, Washington DC, USA:
Jones, R. E., R. J. Petrell & D. Pauly, 1999. Using modified length–weight relationships to assess the condition of fish. Aquaculture Engineering 20: 261–276. https://doi.org/10.1016/S0144-8609(99)00020-5.
Kadison, E., E. K. D’Alessandro, G. O. Davis & P. B. Hood, 2010. Age, growth, and reproductive patterns of the great barracuda, Sphyraena barracuda, from the Florida Keys. Bulletin of Marine Science 86: 773–784. https://doi.org/10.5343/bms.2009.1070.
Karachle, P. K. & K. I. Stergiou, 2012. Morphometrics and allometry in fishes. In Wahl, C. (ed), Morphometrics InTech: 65–86.
Kerkoff, A. J. & B. J. Enquist, 2009. Multiplicative by nature: why logarithmic transformation is necessary in allometry. Journal of Theoretical Biology 257: 519–521. https://doi.org/10.1016/j.jtbi.2008.12.026.
Kimmerer, W., S. R. Avent & S. M. Bollens, 2005. Variability in length-weight relationships used to estimate biomass of estuarine fish from survey data. Transanctions of the American Fisheries Society 134: 481–495. https://doi.org/10.1577/T04-042.1.
King, M., 2007. Fisheries biology assessment and management, Wiley-Blackwell, Oxford:
Lapointe, B. E., R. A. Brewton, L. W. Herren, M. Wang, C. Hu, D. J. McGillicuddy Jr., S. Lindell, F. J. Hernandez & P. L. Morton, 2021. Nutrient content and stoichiometry of pelagic Sargassum reflects increasing nitrogen availability in the Atlantic Basin. Nature Communications 12: 3060. https://doi.org/10.1038/s41467-021-23135-7.
MacKenzie, D. 2019. The starving ocean. www.fisherycrisis.com/index.html
Mansano, C. F. M., B. I. Macente, K. Khan, T. MTd. Nascimento, EPd. Silva, N. Sakomura & J. B. K. Fernandes, 2017. Morphometric growth characteristics and body composition of fish and amphibians. In Pares-Casanova, P. M. (ed), New insights into morphometry studies. IntechOpen, London.
Marks, K. W. & K. D. Clomp, 2003. Appendix II fish biomass conversion equations. In Lang, J. C. (ed), Atlantic and gulf rapid reef assessment status of coral reefs in the western Atlantic: results of initial surveys, Atlantic and gulf rapid reef assessment (AGRRA) Program National Museum of Natural History, Washington DC: 625–630.
Meyers, P. J. & M. C. Belk, 2014. Shape variation in a benthic stream fish across flow regimes. Hydrobiologia 738: 147–154. https://doi.org/10.1007/s10750-014-1926-1.
Molina-Ureña, H. 2009. Towards an ecosystem approach for non-target reef fishes: habitat uses and population dynamics of South Florida parrotfishes (Perciformes: Scaridae). PhD Thesis, University of Miami.
Munro, J. L., 1983. Caribbean coral reef fishery resources, ICLARM-Manila, The Philippines:
Murawski, S. A. & J. S. Idoine, 1992. Multispecies size composition: a conservative property of exploited fishery systems? Journal of Northwest Atlantic Fishery Science 14: 79–85.
Newsom, T.J., 2017. Sample size issues and power. Psy 510/610 multilevel regression. web.pdx.edu/~newsomj/mlrclass/ho_sample%20size.pdf
NOAA-NMFS, 2017. www.fisheries.noaa.gov/inport/q?keywords=USVI
O’Brien, L., 2006. Trends in observed mean weight and length of several groundfish stocks. Northeast fisheries science center, woods hole, garm maturity and weight working group. archive.nefmc.org/press/council_discussion_docs/Mean%20Weight%20and%20Length%20Review%20OBRIEN_nov06.pdf
Opitz, S., 1996. Trophic interactions in Caribbean coral reefs, ICLARM-Makati City, The Philippines:
Pauly, D., Froese, R., Palomares, M.L., Stergiou, K. & C. Apostolidis, 2014. Fish online a guide to learning and teaching Ichthyology using the FishBase information system. Version 3. www.fishbase.in/FishOnLine/English/index.htm
Preston, S., 2000. Teaching prediction intervals. Journal of Statistics Education. https://doi.org/10.1080/10691898.2000.12131297.
Rana, R., Staron, M., Berger, C., Hansson, J., Nilsson, M. & F. Törner, 2013. Comparing between maximum likelihood estimator and non-linear regression estimation procedures for NHPP software reliability growth modeling. In: The Institute of Electrical and Electronics Engineers (ed.), Joint conference of the 23rd international workshop on software measurement and the 8th international conference on software process and product measurement. Ankara, Turkey, pp. 213–218.
Rasbash, J., Charlton, C., Browne, W.J., Healy, M. & B. Cameron, 2015a. MLwiN Version 2.32. Center for Multilevel Modeling, University of Bristol. www.bristol.ac.uk/cmm/software/mlwin/download/
Rasbash, J., Charlton, C., Browne, W. J., Healy, M., Cameron, B., 2015b. MLwiN version 2.32, University of Bristol, UK, Centre for Multilevel Modelling, Bristol.
Robinson, L. A., S. P. R. Greenstreet, H. Reiss, R. Callaway, J. Craeymeersch, I. de Boois, S. Degraer, S. Ehrich, H. M. Fraser, A. Goffin, I. Kröncke, L. Lindal Jorgenson, M. R. Robertson & J. Lancaster, 2010. Length–weight relationships of 216 North Sea benthic invertebrates and fish. Journal of the Marine Biological Association UK 90: 95–104. https://doi.org/10.1017/S0025315409991408.
Roff, D., 1983. An allocation model of growth and reproduction in fish. Canadian Journal of Fisheries and Aquatic Sciences 40: 1395–1404. https://doi.org/10.1139/f83-161.
Rothenberger, P., Blondeau, J., Cox, C., Curtis, S., Fisher, W.S., Garrison, V., Hillis-Starr, Z., Jeffrey, C., Kadison, E., Lundgren, I., Miller, W.J., Muller, E., Nemeth, R., Paterson, S., Rogers, C., Smith, T., Spitzack, A., Taylor, M., Toller, W. & Waddell, J., 2008. The state of coral reef ecosystems of the U.S. Virgin Islands. In: NOAA (ed.), The state of coral reef ecosystems of the United States and Pacific freely associated states: 2008. NOAA, Silver Spring, NOAA Technical Memorandum NOS NCCOS 73, pp 29–73. hppt://repository.library.noaa.gov/view/noaa/17794
Smith, M., D. Warmolts, D. Thoney & R. Hueter, 2004. The elasmobranch husbandry manual: captive care of sharks rays and their relatives, Ohio Biological Survey:
Voulgaridou, P. & K. I. Stergiou, 2003. Trends in various biological parameters of the European sardine, Sardina pilchardus (Walbaum, 1972), in the Eastern Meditteranean Sea. Scientia Marina 67: 269–280. https://doi.org/10.3989/scimar.2003.67s1269.
Wang, X., Y. Xue & Y. Ren, 2013. Length–weight relationships of 43 fish species from Haizhou Bay, central Yellow Sea. Journal of Applied Ichthyology 29: 1183–1187. https://doi.org/10.1111/jai.12200.
Ylikarjula, J., M. Heino & U. Dieckmann, 1999. Ecology and adaptation of stunted growth in fish. Evolution and Ecology 13: 433–453. https://doi.org/10.1023/A:1006755702230.
Acknowledgements
I thank the South East Fisheries Science Center, National Oceanic and Atmospheric Administration (joshua.bennett@noaa.gov) for the 1980–2015 data extracted from the USVI port biosampling monitoring program.
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has no relevant financial or non-financial interests to disclose.
Additional information
Handling editor: Grazia Pennino
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dikou, A. Weight–length relationship in fish populations reflects environmental regulation on growth. Hydrobiologia 850, 335–346 (2023). https://doi.org/10.1007/s10750-022-05072-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-05072-8